Abstract
Introduction
Cancer is the leading cause of death worldwide. Current cancer treatments in the clinic mainly include chemotherapy, radiotherapy and surgery, with chemotherapy being the most common.
Areas covered
Cancer treatments based on the single ‘magic-bullet’ concept are often associated with limited therapeutic efficacy, unwanted adverse effects, and drug resistance. The combination of multiple drugs is a promising strategy for effective cancer treatment due to the synergistic or additive effects. Small interfering RNA (siRNA) has the ability to knock down the expression of carcinogenic genes or drug efflux transporter genes, paving the way for cancer treatment. Treatment with both a chemotherapeutic agent and siRNA based on nanoparticle (NP)-mediated co-delivery is a promising approach for combination cancer therapy.
Expert opinion
The combination of chemotherapeutic agents and siRNA for cancer treatment offers the potential to enhance therapeutic efficacy, decrease side effects, and overcome drug resistance. Co-delivery of chemical drug and siRNA in the same NP would be much more effective in cancer therapy than application of chemical agent or siRNA alone. With the development of material science, NPs have come to be the most widely used platform for co-delivery of chemotherapeutic drugs and siRNAs.
Keywords: co-delivery, chemical drug, siRNA, nanoparticle, combination therapy, cancer
1. Introduction
Cancer is a common malignant disease that is a serious threat to human health and life. A recent investigation predicted a surge in new cancer patients worldwide, from 12.7 million in 2008 to 22.2 million by 2030, posing an enormous challenge in medical care [1]. Current strategies for cancer therapy mainly include radiotherapy, surgery and chemotherapy. Generally, surgery and radiotherapy are used to treat local and non-metastatic cancers in which the tumor tissue is removed but the peripheral part cannot be cleared completely [2–4]. Seriously, surgery- and radiotherapy-induced acceleration of tumor and metastatic growth have been noticed, probably due to inflammatory response during wound healing [5]. On the other hand, chemotherapy is essential for killing cancer cells that have metastasized to distant organs in the whole body [6,7]. Thus, chemotherapy has become the most common strategy in the clinic.
Chemotherapy involves the application of chemotherapeutic drugs to inhibit or control the growth of cancer cells [8–10]. Tumors are thought to arise and develop as a result of the combined effects of many factors. Thus, cancer treatment strategies based on a single ‘magic bullet’ are inevitably suboptimal [11,12]. In contrast, multiple drugs applied in a combination therapy regimen could function synergistically to achieve better therapeutic efficacy compared with single chemical drug-based chemotherapy [13]. RNA interference (RNAi) is a powerful technology for post-transcriptional downregulation of genes that, when specifically applied to the expression of carcinogenic gene expression, is an efficient cancer therapy strategy. Accordingly, the combination of chemotherapy and RNAi technology holds great promise as a new strategy for cancer treatment. However, the chemotherapeutic agents and siRNAs present many limitations that hinder their efficacy. In the context of chemotherapeutic agents, they are generally hydrophobic in aqueous solutions, non-specificity, toxic to healthy tissue and limited cancer cell-killing capacity [14, 15]. As to siRNAs, they are easily cleared by the renal system, lack of selectivity to the targeting tissue, and poor cell uptake [16]. Nanoparticles (NPs) are expected to achieve efficient drug encapsulation, easy drug administration, enhanced drug accumulation in tumor tissues, maximized therapeutic efficacy and minimized adverse effect [17, 18]. Thus, they have been used for the co-delivery of chemotherapeutic agent and siRNA in the recent years.
2. Chemotherapy
Chemotherapy based on a single chemical drug was introduced to treat cancer in the 1940s, and has become a mainstay in the oncology field despite accompanying serious side effects. The common anticancer drugs have already been reviewed previously [19]. With advances in genomics and proteomics, it has become clear that cancer is the result of a combination of interconnected disease pathways, and exhibits characteristics of heterogeneity and complexity [20]. Therefore, inhibition of a specific target pathway by monotherapy often leads to low therapeutic efficacy, serious adverse effects and the emergence of drug resistance, largely because of the activation of compensatory pathways in cancer cells [21]. Combination chemotherapy, first proposed by Free et al. in the 1960s, has recently become a standard regimen for treating cancer in the clinic [22]. Since combination therapeutics impact multiple targets or the same target through different pathways simultaneously, it can more effectively exert maximal anticancer effects with acceptable side effect, while reducing the likelihood of adaptive drug resistance, reflecting the fact that tumor cells/tissues are less able to compensate for the simultaneous action of multiple drugs [23,24].
In one such combination regimen, Kolishetti et al. [25] conjugated a platinum (IV) [Pt(IV)] prodrug to a hydroxyl group-appended polylactide (PLA) to obtain the polymer, PLA-Pt(IV). This polymer was further used to fabricate nanoparticles (NPs) containing the poly(lactic-co-glycolic acid)-block-poly(ethylene glycol) copolymer (PLGA-PEG) and the anticancer agent docetaxel (Dtxl) using microfluidic channels. In vitro experiments showed that Pt(IV)/Dtxl-loaded NPs exhibited superior efficacy compared with single-drug NP analogues, suggesting the potential of combination chemotherapy. In addition, Xiao et al. recently [26] fabricated a series of camptothecin (CPT)/curcumin (CUR)-loaded polymeric NPs with various weight ratios of CPT to CUR. The resultant cationic, spherical CPT/CUR-NPs had a desirable particle size (193–224 nm) and exhibited a simultaneous, sustained release profile for both drugs throughout the study period. Importantly, the combined delivery of CPT and CUR in a single NP synergistically enhanced the effects of the individual drugs. Among the five cationic CPT/CUR-NPs tested, NPs with a CPT/CUR weight ratio of 4:1 showed the highest anticancer activity, resulting in a combination index of approximately 0.46.
Although some combination chemotherapy strategies are effective, they are still far from perfect. Differences in pharmacokinetics, biodistribution and chemical-physical properties among various chemical drugs make optimization of dosing and scheduling extremely difficult [27]. These challenges have driven researchers to develop alternative, more suitable strategies.
3. RNAi technology
RNAi has become an attractive technology to silence the gene expression in most eukaryotic cells, in which small RNAs exert their function in a complementarity-dependent manner [28]. There are three main types of small RNA: siRNA, microRNAs and PIWI interacting RNAs (piRNAs) [29]. However, since most of the RNAi-related researches are conducted with siRNA rather than miRNA and piRNA [30], the focus of this review is mainly on siRNA-based therapy. By activating RNAi, siRNA can silence the expression of virtually any gene with high efficiency and specificity [31]. Advancements in RNAi technology have provided highly specific therapeutic options for silencing target genes related to cancer treatment, thus it has come to be utilized as a new potential therapeutic strategy [32, 33]. An important application of RNAi-based medicine is targeting proteins that are related to certain diseases but cannot be targeted using conventional molecules because of their lack of enzymatic function or inaccessibility.
Xu et al. [34] synthesized a block copolymer of PEG with PLGA (PEG-Dlinkm-PLGA) linked via a tumor pH-labile bridge, and used it for delivery of siRNA targeting polo-like kinase1 (Plk1), a mitotic kinase essential for cell proliferation. The resulting dPEGNPPLGA/siPlk1 containing PEG-Dlinkm-PLGA achieved an efficiently prolonged circulation time, preferential accumulation in tumor sites, and detachment of the PEG surface layer in the acidic tumor environment. In vivo results demonstrated that dPEGNPPLGA/siPlk1 markedly inhibited the growth of MDA-MB-231 tumors after intravenous injection. Furthermore, dPEGNPPLGA/siPlk1 inhibited tumor growth at a much lower dose of siPlk1 compared with NPPLA/siPlk1 and NPPLGA/siPlk1.
4. Combination therapy based on chemotherapeutic agents and siRNAs
Cancer occurrence is often accompanied by mutations of numerous core proteins involved in tumor growth, metastasis, and survival. Thus, in combination with chemical drugs, siRNAs can play a secondary role in sensitizing cancer cells or inhibiting multidrug-resistance genes [35, 36].
White and coworkers [37] recently combined a high-throughput, cell-based, “one-well/one-gene” screening platform with a genome-wide synthetic library of siRNA to systematically interrogate the molecular underpinnings of NCI-H1155 cancer cell sensitivities. They found that downregulation of several genes sensitized these lung cancer cells to a paclitaxel concentration 1,000-fold lower than that otherwise required for a significant response. Following incubation for an additional 24–48 h, some treatment groups responded to paclitaxel concentrations that were even 10,000-fold lower than otherwise required. Moreover, these authors also demonstrated that the observed decrease in cell number was attributable to cell death rather than to a transient delay in proliferation. This finding implies that the combination of chemotherapy and RNAi technology could be a promising strategy for substantially improving the therapeutic efficacy of chemotherapy through synergistic effects.
Importantly, numerous recent reports indicate that the combination of chemical drug and siRNA offers superior anticancer effects compared with chemical drugs or siRNA alone [38–41]. Downregulation of the expression of B-cell lymphoma 2 (Bcl-2), an important anti-apoptotic protein, has been shown to inhibit tumor growth. Zheng et al. [42] found that the combination of Dtxl and Bcl-2 siRNA clearly downregulated Bcl-2 and enhanced antitumor activity, resulting in significant inhibition of tumor growth in an MCF-7 breast cancer cell murine xenograft model compared with individual Dtxl or siRNA treatment.
5. NPs for co-delivery of a chemotherapeutic agent and siRNA
To exert optimal synergistic effects, chemical drugs and siRNAs need to be temporally localized in the same tumor cell. NPs are capable of simultaneously encapsulating both chemic drug and siRNA through physical or chemical interaction and co-delivering to the same cells, and are thus emerging as a promising delivery platform in combination therapy [43,44]. Numerous additional advantages of NPs have also been recognized, including improvement of the solubility of hydrophobic drugs, prolongation of circulation time, delivery of drug to tumors to minimize systemic side effects through passive or active targeting, and controlled drug release [25,45,46]. The following section reviews the commonly used NPs for the co-delivery of chemical drugs and siRNAs (Table 1). Furthermore, the advantages and disadvantages of these NPs have been summarized in Table 2.
Table 1.
The application of NPs in co-delivery of chemical drug and siRNA in cancer therapy.
| System | Drug | siRNA | Encapsulation strategy | Particle size (nm) |
Targeting | Targeting moiety | Cell line | Dose (mg/kg) | Injection procedure | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Drug | siRNA | Drug | siRNA | |||||||||
| TLPD (cationic liposome) | ADR | RRM2 | Physical encapsulation | Physical encapsulation | 150–200 | Activea | EGFR antibody | HCC cells | 0.5 | 1.2 | Single injection via tail vein | [50] |
| G(4)-PAMAM-PEG-DOPE (dendrimers) | DOX | GFP | Physical encapsulation | Electrostatic interaction | 20±5.2 | Passiveb | - | C166 cells | - | - | - | [58] |
| PEG-PLL-PLLeu (micelles) | Docetaxel | BCL-2 | Physical encapsulation | Electrostatic interaction | 121.3 ± 1.9 | Passive | - | MCF-7 human breast cancer | 0.5 | 0.2 | Every 3 days within 24 days via intravenous (i.v.) injection | [53] |
| HSOP (Redox-sensitive micelles) | PTX | AURKA | Physical encapsulation | Electrostatic interaction | 135–220 | Active | HA | MDA-MB-231 cell | 5 | 1 | Every 3 days for 5 times via i.v. injection | [39] |
| PEG-b-Pasp(DET)-b-PCL (Trilayer micelles) | Rapamycin | Y-box binding protein-1 | Physical encapsulation | Electrostatic interaction | 108 | Passive | - | PC3 prostate cancer cells | 30 | 2 | Retro-orbital injections on days 1, 3 and 5 | [52] |
| PLGA-PEI-Biotin (Cationic NPs) | PTX | P-gp | Physical encapsulation | Electrostatic interaction | 200–250 | Active | Biotin | JC breast adenocarinoma | 20 | 20 µg/animal | Single injection via tail vein | [66] |
| PAMA-Silica (Inorganic carrier) | DOX | BCL-2 | Physical encapsulation | Electrostatic interaction | - | Passive | - | A2870/AD ovarian carcinoma | - | - | - | [41] |
| MSNP-PEI-PEG (Inorganic carrier) | DOX | P-gp | Physical encapsulation | Electrostatic interaction | 71 | Passive | - | MDR breast cancer cell | 4 | 1.2 | Six times via i.v. injection | [71] |
“-” The content which are marked with this symbol are not mentioned in the article.
“a” Surface functionalization of nanotherapuetics with targeting ligands such as hyaluronic (HA), folic acid (FA) can facilitate their cellular uptake into target cells via receptor-mediated endocytosis.
“b” Nanotherapeutics tend to accumulate in tumor tissues via the enhanced permeability and retention effect (EPR).
Table 2.
Comparison of the advantages and disadvantages of different NPs for co-delivering chemical drug/siRNA in cancer therapy.
| NPs | Advantages | Disadvantages |
|---|---|---|
| Liposome | Readily self-assembled in one step Versatility in fluidity, size, zeta-potential High entrapment efficiency Narrow size distribution Drug controlled release | Lack of storage stability Toxicity of some liposomal contents High cost of liposome production |
| Micelle | Multifunctional design Excellent blood stability Passive targeting to tumor Drug controlled release | Lack of storage stability Concerns over nanotoxicity Chemical reaction required |
| PAMAM | Homogenous structure Easy surface modification for active targeting, drug attachment or Pegylation Host–guest entrapment property | Chemical reaction with many steps required Concerns over nanotoxicity |
| Polyester NPs | Well-established techniques for fabrication Good reproducibility FDA-approved carrier materials Easy to be scale-up | High-energy input Surfactant needed Lack of controlled release |
| Chitosan-based NPs | Biocompatibility and low toxicity Biodegradable Easy chemical modification Excellent cellular uptake profile | High-energy input Lack of storage stability |
| Mesoporous silica NPs | Low toxicity Biodegradable Easy surface functionalization High pore volume Uniform and tunable pore size | Chemical reaction with many steps required Residual organic solvent Potential hemolysis |
5.1 Liposomes
Liposomes have attracted huge interest after Doxil® was approved by FDA to deliver doxorubicin for cancer treatment [47]. They are artificially fabricated drug carriers in which the inner aqueous core is covered by outer lipid bilayers. Thus, they can be used to simultaneously encapsulate both hydrophilic and hydrophobic drugs, and protect the loaded drugs against degradation [48,49]. Their surface can be further modified to provide a longer circulatory lifetime and site-specific delivery to tumor tissues. The size, charge, and other parameters of liposomes can be altered according to the drug and the desired site of action [45]. Liposomes provide a great opportunity to co-deliver therapeutic agents and siRNAs for cancer therapy, and have been widely used for this purpose (Table 1).
Recently, Gao et al. [50] developed an anti-EGFR (epidermal growth factor receptor) Fab’-functionalized liposome-polycation-DNA (LPD) complex. This LPD complex was used for targeted co-delivering of Adriamycin (ADR) and siRNA targeting ribonucleotide reductase M2 (RRM2) to achieve combined therapeutic effects in human hepatocellular carcinoma (HCC). They found that the resulting ADR-RRM2-TLPD complex specifically and efficiently co-delivered Dox and RRM2 siRNA to EGFR-overexpressing HCC cells both in vitro and in vivo, resulting in enhanced therapeutic effects (cytotoxicity, apoptosis and senescence-inducing activity) compared with single-drug–loaded or non-targeted controls, including ADR-NC-TLPD (targeted LPD co-delivering ADR and negative control siRNA), RRM2-TLPD (targeted LPD delivering RRM2 siRNA), and ADR-RRM2-NTLPD (non-targeted LPD co-delivering ADR and RRM2 siRNA).
5.2 Micelles
Polymeric micelles—nanoscopic core/shell structures formed by amphiphilic block copolymers—have received growing attention as chemical drug/siRNA co-carriers for tumor therapy [51]. Polymeric micelles not only possess good capacities for solubilizing hydrophobic drugs and providing sustained release of siRNA, they also exhibit other attractive properties, such as a distinctive core/shell structure, passive tumor localization through the enhanced permeability and retention (EPR) effect, and increased protection of encapsulated drugs from degradation and metabolism [52–54].
Yin et al. [36] synthesized the hyaluronic acid-based, amphiphilic conjugate, HA-ss-(OA-g-bPEI) (HSOP), and then fabricated a redox-responsive micelle for tumor-targeted co-delivery of paclitaxel (PTX) and aurora kinase A (AURKA)-specific siRNA (si-AURKA). They found that HSOP micelles had excellent PTX and siAURKA loading efficiencies with adjustable dosing ratios, as well as desirable redox sensitivity. Moreover, these micelles could co-deliver PTX and siRNA into breast cancer cells via HA receptor-mediated endocytosis. Importantly, further in vitro and in vivo experiments demonstrated that HSOP micelles possessed improved anticancer efficacy compared with redox-sensitive single drug controls and non-sensitive co-delivery controls.
5.3 Poly (amido amine) (PAMAM)
Dendrimers are monodispersed, highly branched, three-dimensional synthetic polymeric macromolecules synthesized by controlled polymerization reactions that allow a high level of control over their architecture [55,56]. By regulating the chemical synthesis process, it is possible to adjust their biocompatibility and pharmacokinetics. The surface of dendrimers can be further functionalized to modify their toxicity and allow the simultaneous conjugation of multiple molecules, such as chemotherapeutic agents, targeting moieties and PEG, to increase water solubility and prolong the lifetime of the drugs in the bloodstream [57]. PAMAM dendrimers, a promising NP, can be used to co-deliver a chemotherapeutic agent and siRNA [58]. Low-molecular weight hydrophobic drugs mainly interact with the PAMAM dendrimer, while negatively charged nucleic acids interact through electrostatic force with cavities in PAMAM dendrimers provided by the large number of surface primary amine groups [45]. Therefore, PAMAM dendrimers are readily applicable as drug-delivery carriers for the co-delivery of hydrophobic drugs and siRNAs (Table 1).
Biswas et al. [59] synthesized the triblock copolymer, PAMAM (generation 4)-PEG- 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (G(4)-D-PEG-DOPE), and then complexed it with siRNA. Subsequently, they evaluated its siRNA-delivery profile and further engineered a mixed micellar system consisting of G(4)-D-PEG-DOPE and PEG-DOPE (1:1) for co-delivery of a chemical drug and siRNA. The combination of dendrimer and polymeric micelles in a single NP resulted in a truly multifunctional nanomedicine that could potentially address the challenges of co-delivery of chemical drugs and siRNAs for therapeutic purposes.
5.4 Polyester NPs
Polymeric NPs have been used as delivery systems for individual chemotherapeutic agent or nucleotide [60]. They also have been used for the co-delivery of chemotherapeutic agents and siRNAs owing to their biocompatibility, stability, narrow size distribution, controllable drug-release profile, and highly efficient cellular uptake [27]. In addition, polymeric NPs have the ability to stabilize drugs in the systemic circulation and provide sustained release of drugs at the site of action, while minimizing side effects [61]. Polyester and FDA-approved biodegradable copolymers such as PLGA and PLA can efficiently encapsulate hydrophobic and hydrophilic drugs to form NPs, and thus have been widely used for drug delivery [62,63]. The drug release from polyester NPs is based on the combination of degradation and erosion of polymers, and the detailed mechanisms have been extensively reviewed elsewhere [64, 65].
Patil et al. [66] fabricated biotin-functionalized NPs loaded with PTX and siRNA targeting MDR1 (multidrug resistance gene 1), also known as P-glycoprotein 1 (P-gp), and further investigated their antitumor efficacy. In vitro results indicated that these dual-agent–loaded NPs were significantly more cytotoxic than PTX-loaded NPs, suggesting that silencing the expression of MDR1 increased the accumulation of PTX in drug-resistant tumor cells. Further in vivo experiments demonstrated that biotin-functionalized PTX/P-gp siRNA-loaded NPs inhibited tumor growth to a significantly greater extent than biotin-functionalized NPs loaded with the same amount of PTX only.
Polyester has also been fabricated into polymersomes for co-delivery of a chemical drug and siRNA. Kim et al. [67] encapsulated doxorubicin (DOX) and Bcl-xL siRNA into PEG-b-PLA-based polymersomes. The in vitro results indicated that polymersomes co-loaded with DOX/Bcl-xL siRNA exerted much stronger anticancer efficacy than those loaded with only drug or siRNA, indicating that polymersomes are efficient NPs for combined cancer therapy.
5.5 Chitosan-based NPs
Chitosan, a linear, cationic copolymer of glucosamine and N-acetyl-gluocosamine, is a deacetylated derivative of chitin—the second-most abundant natural polysaccharide. Chitosan has to be widely investigated as a drug carrier owing to its biocompatibility, biodegradability and gene-binding ability, as well as its ease of chemical modification [68, 69]. Co-delivery of chemotherapeutic drugs and siRNA using chitosan derivatives has recently been evaluated.
Wei et al. [70] prepared uniform-sized N-([2-hydroxy-3-trimethylammonium] propyl) chitosan chloride (HTCC) NPs using a Shirasu porous glass membrane emulsification technique for oral delivery of PTX and telomerase reverse transcriptase siRNA (siTERT) to tumors. HTCC not only protected drugs from degradation in the gastrointestinal (GI) tract but also improved drug permeability into the circulatory system from the GI tract. Further in vitro and in vivo experiments indicated that the resulting NPs could simultaneously deliver PTX and siTERT to cancer cells and increase local drug concentrations. Moreover, they were much more effective in suppressing tumor growth than conventional cocktail therapy, suggesting that HTCC NPs are powerful carriers for co-delivery of chemical drugs and siRNAs.
5.6 Mesoporous silica NPs
Mesoporous silica NPs (MSNPs) have been used for co-delivery of chemotherapeutic agents and siRNAs because of their enormous specific surface area, large pore volume, regular pore channels, adjustable pore size, easy modifiability of interior and exterior surfaces to encapsulate higher amounts of drugs, and improved stability associated with their inorganic oxide framework.
Meng et al. [71] fabricated MSNPs co-loaded with DOX and P-gp siRNA as a strategy for overcoming DOX resistance in a multidrug-resistant human breast cancer xenograft. The resulting NPs were further functionalized with a polyethyleneimine-PEG (PEI-PEG) copolymer, providing protected delivery of stably bound DOX and P-gp siRNA to tumor sites. In vivo studies showed that this co-delivery system achieved significantly improved inhibition of tumor growth compared with free DOX or NPs loaded with either DOX or siRNA alone.
6. Conclusion
In practice, chemotherapy has become a standard regimen for treating cancer patients. However, it currently represents a bottleneck in the path toward improved cancer therapies. RNAi has the ability to influence cellular pathways by silencing or inhibiting certain proteins, thereby sensitizing cancer cells or enhancing the accumulation of anticancer drugs at the tumor site. The co-delivery of chemotherapeutic agent and siRNA using NPs is significantly superior in cancer therapy compared with NPs loaded with either drug or siRNA alone. Various NPs have been developed to co-deliver drug and siRNA, including liposomes, dendrimers, micelles, and inorganic NPs. The ideal NP system should share some unique beneficial features, including: (1) good biocompatibility for the carrier materials; (2) easy scale-up with good quality control; (3) excellent drug loading and encapsulation efficiency; (4) high therapeutic efficacy; (5) reduced unwanted side effect.
7. Expert opinion
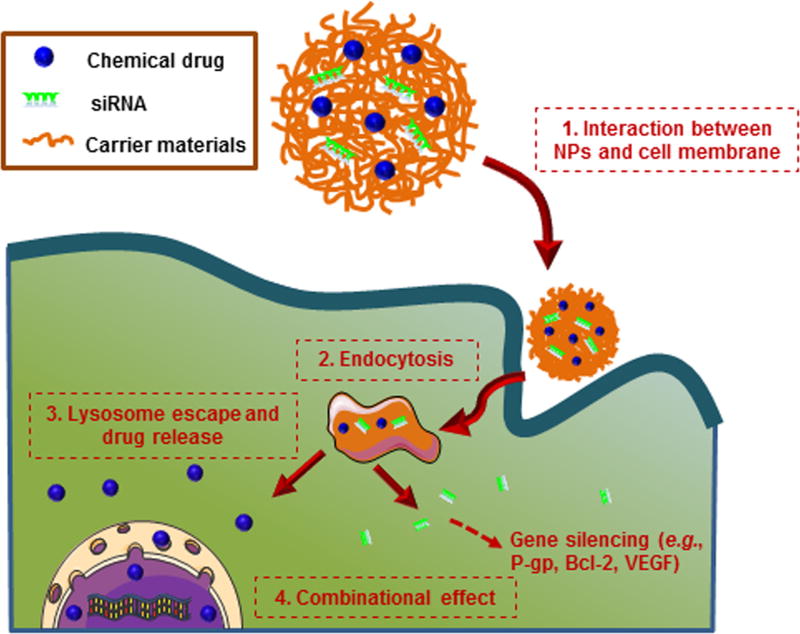
Combination therapies based on chemotherapy and RNAi technology greatly improve the therapeutic efficacy of cancer therapy. The combined application of multiple drugs (e.g., chemotherapeutic drug, siRNA) targeting different cellular signal pathways can raise the genetic barriers for cancer cell mutations [72–74]. Thus, this strategy can delay the cancer adaptation process, and increase the therapeutic efficacy [75]. One of the prerequisites for the combination of chemotherapy and RNAi technology is to select a proper gene target. Generally, the candidate genes should have the following features: (1) be important for the drug resistance of tumor (e.g., gene encoding P-gp) [76]; (2) be critical in the tumor survival pathways (e.g., gene encoding Bcl-2) [77]; (3) be preferentially expressed in the tumor tissues (e.g., gene encoding vascular endothelial growth factor (VEGF)) [35]. There is also a concern for siRNA as a drug. siRNA, a type of exogenous oligonucleotide, can trigger the innate immune system, and it is not as safe as originally expected. Fortunately, the chemical modifications or optimized sequence design of siRNA can reduce the immunogenicity [78]. To achieve synergistic effects of such combination, chemical drugs and siRNAs need to be delivered to the same tumor cells (Figure 1) [16]. NP-based nanotherapeutics have emerged as one of the most promising strategies for drug delivery in recent years. Chemical drugs are generally encapsulated into NPs through the “like dissolves like” principle, covalent binding, or physical restriction, whereas siRNA are often loaded into NPs based on the interaction between positive-charged polymer and negative-charged siRNA, or physical restriction. Various types of NPs have dual-capacity to encapsulate both chemical drug and siRNA, thus they have been used for the co-delivery of anticancer drug and siRNA so as to synergistically enhance their individual anticancer effects.
Figure 1.
Schematic illustration of co-delivery process of chemical drug and siRNA by NPs to cancer cell.
Ideal NPs for co-delivery of drugs should have the advantages of high capacity to load multiple drugs, long stability in the circulation, and targeted drug delivery without an initial burst release. In order to achieve the maximal effect of the combination of chemotherapeutic drug and siRNA, the ideal carrier will also be multifunctional, possessing the ability to release its contents in a controlled ratio and at an accurate dose. Therefore, further studies should pay more attention to the interaction of chemical drugs and siRNA, as well as the interaction between therapeutic agents and carriers. Another remaining challenge for combination cancer therapy is minimizing systemic side effects. Several strategies can be applied, such as optimization of size and surface physicochemical property, as well as careful selection of the surface composition materials [18]. Surface functionalization of NPs with the targeting ligands (e.g., antibodies, peptides or small molecules) can promote drug accumulation in the tumor cells via receptor-mediated endocytosis and decrease the systemic distribution [79], which offers a promising approach to reduce the adverse effect and enhance the treatment efficacy. Additionally, the stimuli-responsive DDS may represent another promising alternative for tumor-targeted and further organelle-targeted drug delivery. This DDS may be sensitive to specific endogenous stimuli, such as a lowered interstitial pH, a higher glutathione concentration or an increased concentration of certain enzymes in the tumor tissue (e.g., matrix metalloproteinase) [80].
The application of RNAi to chemotherapy will require evaluation of therapeutic efficacy and safety from the lab to the clinic through preclinical trials. In coming years, more and more RNAi-based combination therapeutics are expected to enter into clinical trials, with some products successfully transitioning to commercial markets. Since the field of nanomedicine is relatively young, the long-term health effects of NPs are largely unknown. Combination therapies based on chemotherapy and RNAi are still evolving, and novel co-delivery approaches that bring combination therapy to the clinic will ultimately be found.
Article highlights box.
The combination of multiple ‘magic-bullets’ is a promising approach for cancer therapy.
Combination therapy based on chemotherapy and RNAi technology can modulate different cellular pathways in tumor cells, thus maximizing the therapeutic efficacy and minimizing the unwanted adverse effect.
Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA is more effective in treating cancer than the application of drugs or siRNA alone.
Acknowledgments
Funding
This work was supported by grants from the Department of Veterans Affairs (BX002526), the National Institutes of Health of Diabetes and Digestive and Kidney by the grant (RO1-DK-071594), the National Natural Science Foundation of China (51503172 and 81571807), the Fundamental Research Funds for the Central Universities (SWU114086 and XDJK2015C067) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars (State Education Ministry).
Footnotes
Declaration of Interest
D Merlin is a recipient of a Career Scientist Award from the Department of Veterans Affairs. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. The lancet oncology. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 2.Chabner BA, Roberts TG., Jr Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5(1):65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 3.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(3):573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 5.Coffey JC, Wang JH, Smith MJ, et al. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4(12):760–8. doi: 10.1016/s1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Zhao G, Liu J, et al. Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J Control Release. 2009;140(3):277–83. doi: 10.1016/j.jconrel.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Tekade RK, Dutta T, Tyagi A, et al. Surface-engineered dendrimers for dual drug delivery: a receptor up-regulation and enhanced cancer targeting strategy. J Drug Target. 2008;16(10):758–72. doi: 10.1080/10611860802473154. [DOI] [PubMed] [Google Scholar]
- 8.Malmstrom P-U, Rintala E, Wahlqvist R, et al. Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial 1. J urology. 1996;155(6):1903–1906. [PubMed] [Google Scholar]
- 9.Steinberg JL, Yeo W, Zhong S, et al. Hepatitis B virus reactivation in patients undergoing cytotoxic chemotherapy for solid tumours: precore/core mutations may play an important role. J medical virology. 2000;60(3):249–255. [PubMed] [Google Scholar]
- 10.Chabner BA, Roberts TG. Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5(1):65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 11.Hu CM, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83(8):1104–11. doi: 10.1016/j.bcp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Song S, Wientjes MG, Walsh C, et al. Nontoxic doses of suramin enhance activity of paclitaxel against lung metastases. Cancer Res. 2001;61(16):6145–6150. [PubMed] [Google Scholar]
- 13.Lehár J, Krueger AS, Avery W, et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat biotechnol. 2009;27(7):659–666. doi: 10.1038/nbt.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mu L, Feng SS. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J Control Release. 2003;86(1):33–48. doi: 10.1016/s0168-3659(02)00320-6. [DOI] [PubMed] [Google Scholar]
- 15.Primeau AJ, Rendon A, Hedley D, et al. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res. 2005;11(24):8782–8. doi: 10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- 16.Creixell M, Peppas NA. Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance. Nano Today. 2012;7(4):367–379. doi: 10.1016/j.nantod.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 18.Hu Q, Sun W, Wang C, et al. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv Drug Deliv Rev. 2016;98:19–34. doi: 10.1016/j.addr.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nussbaumer S, Bonnabry P, Veuthey JL, et al. Analysis of anticancer drugs: a review. Talanta. 2011;85(5):2265–89. doi: 10.1016/j.talanta.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 20.Woodcock J, Griffin JP, Behrman RE. Development of novel combination therapies. New England Journal of Medicine. 2011;364(11):985–987. doi: 10.1056/NEJMp1101548. [DOI] [PubMed] [Google Scholar]
- 21.Jia J, Zhu F, Ma X, et al. Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug discovery. 2009;8(2):111–128. doi: 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- 22.Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Molecular interventions. 2007;7(4):216. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann GR, Lehar J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug discovery today. 2007;12(1):34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30(7):679–692. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 25•.Kolishetti N, Dhar S, Valencia PM, et al. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc Natl Acad of Sci. 2010;107(42):17939–17944. doi: 10.1073/pnas.1011368107. This original research demonstrates the superiority of dual-drug combination NPs over NPs with single drug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao B, Si X, Han MK, et al. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J. Mater. Chem. B. 2015;3(39):7724–7733. doi: 10.1039/c5tb01245g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C-MJ, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Therapeutic delivery. 2010;1(2):323–334. doi: 10.4155/tde.10.13. [DOI] [PubMed] [Google Scholar]
- 28.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–33. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457(7228):405–12. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- 30.Daka A, Peer D. RNAi-based nanomedicines for targeted personalized therapy. Adv Drug Deliv Rev. 2012;64(13):1508–21. doi: 10.1016/j.addr.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Xuan B, Qian Z, Tan C, et al. esiRNAs purified with chromatography suppress homologous gene expression with high efficiency and specificity. Mol Biotechnol. 2005;31(3):203–9. doi: 10.1385/MB:31:3:203. [DOI] [PubMed] [Google Scholar]
- 32.de Fougerolles A, Vornlocher H-P, Maraganore J, et al. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug discovery. 2007;6(6):443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8(3):173–84. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 34.Xu C-F, Zhang H-B, Sun C-Y, et al. Tumor acidity-sensitive linkage-bridged block copolymer for therapeutic siRNA delivery. J Biomaterials. 2016;88:48–59. doi: 10.1016/j.biomaterials.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 35.Lee SY, Yang CY, Peng CL, et al. A theranostic micelleplex co-delivering SN-38 and VEGF siRNA for colorectal cancer therapy. Biomaterials. 2016;86:92–105. doi: 10.1016/j.biomaterials.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 36.Yin T, Wang L, Yin L, et al. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials. 2015;61:10–25. doi: 10.1016/j.biomaterials.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 37••.Whitehurst AW, Bodemann BO, Cardenas J, et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature. 2007;446(7137):815–819. doi: 10.1038/nature05697. A important report focuses on the combination of RNAi and chemotherapy. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z-Z, Li J-Q, Wang Z-Z, et al. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35(19):5226–5239. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Yin T, Wang P, Li J, et al. Tumor-penetrating codelivery of siRNA and paclitaxel with ultrasound-responsive nanobubbles hetero-assembled from polymeric micelles and liposomes. Biomaterials. 2014;35(22):5932–5943. doi: 10.1016/j.biomaterials.2014.03.072. [DOI] [PubMed] [Google Scholar]
- 40.Beh CW, Seow WY, Wang Y, et al. Efficient delivery of Bcl-2-targeted siRNA using cationic polymer nanoparticles: downregulating mRNA expression level and sensitizing cancer cells to anticancer drug. Biomacromolecules. 2009;10(1):41–8. doi: 10.1021/bm801109g. [DOI] [PubMed] [Google Scholar]
- 41.Chen AM, Zhang M, Wei D, et al. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5(23):2673–7. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng C, Zheng M, Gong P, et al. Polypeptide cationic micelles mediated co-delivery of docetaxel and siRNA for synergistic tumor therapy. Biomaterials. 2013;34(13):3431–3438. doi: 10.1016/j.biomaterials.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 43.Sun T-M, Du J-Z, Yao Y-D, et al. Simultaneous delivery of siRNA and paclitaxel via a"two-in-one” micelleplex promotes synergistic tumor suppression. ACS nano. 2011;5(2):1483–1494. doi: 10.1021/nn103349h. [DOI] [PubMed] [Google Scholar]
- 44.Xiong X-B, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS nano. 2011;5(6):5202–5213. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Gu F, Chan J, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharm Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 46.Xiao B, Han MK, Viennois E, et al. Hyaluronic acid-functionalized polymeric nanoparticles for colon cancer-targeted combination chemotherapy. Nanoscale. 2015;7(42):17745–17755. doi: 10.1039/c5nr04831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barenholz Y. Doxil(R)--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 49.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug discovery. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 50••.Gao J, Chen H, Yu Y, et al. Inhibition of hepatocellular carcinoma growth using immunoliposomes for co-delivery of adriamycin and ribonucleotide reductase M2 siRNA. Biomaterials. 2013;34(38):10084–10098. doi: 10.1016/j.biomaterials.2013.08.088. A key paper demonstrates that chemical agent/siRNA-co-loaded NPs are much more effective in cancer therapy than application of NPs loaded with chemical agent or siRNA alone. [DOI] [PubMed] [Google Scholar]
- 51.Itaka K, Kataoka K. Progress and prospects of polyplex nanomicelles for plasmid DNA delivery. Curr gene Ther. 2011;11(6):457–465. doi: 10.2174/156652311798192879. [DOI] [PubMed] [Google Scholar]
- 52.Zeng S, Xiong MP. Trilayer micelles for combination delivery of rapamycin and siRNA targeting Y-box binding protein-1 (siYB-1) Biomaterials. 2013;34(28):6882–92. doi: 10.1016/j.biomaterials.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng C, Zheng M, Gong P, et al. Polypeptide cationic micelles mediated co-delivery of docetaxel and siRNA for synergistic tumor therapy. Biomaterials. 2013;34(13):3431–8. doi: 10.1016/j.biomaterials.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 54.Matsumura Y. Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv Drug Deliv Rev. 2011;63(3):184–92. doi: 10.1016/j.addr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Crooks RM, Zhao M, Sun L, et al. Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc Chem Res. 2001;34(3):181–190. doi: 10.1021/ar000110a. [DOI] [PubMed] [Google Scholar]
- 56.Dwivedi P, Kumar Tekade R, Kumar Jain N. Nanoparticulate carrier mediated intranasal delivery of insulin for the restoration of memory signaling in Alzheimer's disease. Current Nanoscience. 2013;9(1):46–55. [Google Scholar]
- 57.Cheng Y, Zhao L, Li Y, et al. Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem Soc Rev. 2011;40(5):2673–703. doi: 10.1039/c0cs00097c. [DOI] [PubMed] [Google Scholar]
- 58.Biswas S, Deshpande PP, Navarro G, et al. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials. 2013;34(4):1289–301. doi: 10.1016/j.biomaterials.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biswas S, Deshpande PP, Navarro G, et al. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials. 2013;34(4):1289–1301. doi: 10.1016/j.biomaterials.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C-Z, Fu Y-C, Jian S-C, et al. Synthesis and characterization of cationic polymeric nanoparticles as simvastatin carriers for enhancing the osteogenesis of bone marrow mesenchymal stem cells. Journal of colloid and interface science. 2014;432:190–199. doi: 10.1016/j.jcis.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 61.Xiao B, Zhang M, Viennois E, et al. Inhibition of MDR1 gene expression and enhancing cellular uptake for effective colon cancer treatment using dual-surface-functionalized nanoparticles. Biomaterials. 2015;48:147–60. doi: 10.1016/j.biomaterials.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panyam J, Sahoo SK, Prabha S, et al. Fluorescence and electron microscopy probes for cellular and tissue uptake of poly(d,l-lactide-co-glycolide) nanoparticles. International Journal of Pharmaceutics. 2003;262(1):1–11. doi: 10.1016/s0378-5173(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 63.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2012;64:61–71. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 64.Kamaly N, Yameen B, Wu J, et al. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ford Versypt AN, Pack DW, Braatz RD. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres--a review. J Control Release. 2013;165(1):29–37. doi: 10.1016/j.jconrel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patil YB, Swaminathan SK, Sadhukha T, et al. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31(2):358–65. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim HO, Kim E, An Y, et al. A biodegradable polymersome containing Bcl-xL siRNA and doxorubicin as a dual delivery vehicle for a synergistic anticancer effect. Macromolecular bioscience. 2013;13(6):745–754. doi: 10.1002/mabi.201200448. [DOI] [PubMed] [Google Scholar]
- 68.Xiao B, Wan Y, Wang X, et al. Synthesis and characterization of N-(2-hydroxy)propyl-3-trimethyl ammonium chitosan chloride for potential application in gene delivery. Colloids Surf B Biointerfaces. 2012;91:168–74. doi: 10.1016/j.colsurfb.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 69.Xiao B, Wang X, Qiu Z, et al. A dual-functionally modified chitosan derivative for efficient liver-targeted gene delivery. J Biomed Mater Res A. 2013;101(7):1888–97. doi: 10.1002/jbm.a.34493. [DOI] [PubMed] [Google Scholar]
- 70.Wei W, Lv P-P, Chen X-M, et al. Codelivery of mTERT siRNA and paclitaxel by chitosan-based nanoparticles promoted synergistic tumor suppression. Biomaterials. 2013;34(15):3912–3923. doi: 10.1016/j.biomaterials.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 71.Meng H, Mai WX, Zhang H, et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS nano. 2013;7(2):994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li G, Song S, Zhang T, et al. pH-sensitive polyelectrolyte complex micelles assembled from CS-g-PNIPAM and ALG-g-P(NIPAM-co-NVP) for drug delivery. Int J Biol Macromol. 2013;62:203–10. doi: 10.1016/j.ijbiomac.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 73.Lehar J, Krueger AS, Avery W, et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol. 2009;27(7):659–66. doi: 10.1038/nbt.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao B, Si X, Han MK, et al. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J Mater Chem B Mater Biol Med. 2015;3(39):7724–7733. doi: 10.1039/c5tb01245g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao B, Han MK, Viennois E, et al. Hyaluronic acid-functionalized polymeric nanoparticles for colon cancer-targeted combination chemotherapy. Nanoscale. 2015;7(42):17745–55. doi: 10.1039/c5nr04831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang CG, Zhu WJ, Liu Y, et al. Novel polymer micelle mediated co-delivery of doxorubicin and P-glycoprotein siRNA for reversal of multidrug resistance and synergistic tumor therapy. Sci Rep. 2016;6:23859. doi: 10.1038/srep23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee E, Oh C, Kim IS, et al. Co-delivery of chemosensitizing siRNA and an anticancer agent via multiple monocomplexation-induced hydrophobic association. J Control Release. 2015;210:105–14. doi: 10.1016/j.jconrel.2015.05.262. [DOI] [PubMed] [Google Scholar]
- 78.Judge A, MacLachlan I. Overcoming the innate immune response to small interfering RNA. Hum Gene Ther. 2008;19(2):111–24. doi: 10.1089/hum.2007.179. [DOI] [PubMed] [Google Scholar]
- 79.Shi J, Votruba AR, Farokhzad OC, et al. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10(9):3223–30. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]