Abstract
The recently demonstrated radiation-induction of chronic lymphocytic leukemia (CLL) raises the question as to whether the amount of radiation exposure influences any of the clinical characteristics of the disease. We evaluated the relationship between bone marrow radiation doses and clinical characteristics and survival of 79 CLL cases diagnosed during 1986–2006 in a cohort of 110,645 male workers who participated in the cleanup work of the Chornobyl nuclear accident in Ukraine in 1986. All diagnoses were confirmed by an independent International Hematology Panel. Patients were followed up to the date of death or end of follow-up on October 31, 2010. The median age at diagnosis was 57 years. Median bone marrow dose was 22.6 milligray (mGy) and was not associated with time between exposure and clinical diagnosis of CLL (latent period), age, peripheral blood lymphocyte count or clinical stage of disease in univariate and multivariate analyses. A significant increase in the risk of death with increasing radiation dose was observed (P=0.03, hazard ratio=2.38, 95% confidence interval: 1.11,5.08 comparing those with doses ≥22 mGy to doses <22 mGy). After adjustment for radiation dose, survival of CLL cases was significantly shorter among those with younger age at first exposure, higher peripheral blood lymphocyte count, more advanced clinical stage of disease and older age at diagnosis (all P<0.05). This is the first study to examine association between bone marrow radiation doses from the Chornobyl accident and clinical manifestations of the CLL in Chornobyl cleanup workers. The current study provides new evidence on the association of radiation dose and younger age at first radiation exposure at Chornobyl with shorter survival after diagnosis. Future studies are necessary with more cases in order to improve the statistical power of these analyses and to determine their significance.
Keywords: Chronic lymphocytic leukemia, Chornobyl, Chernobyl, radiation, cleanup worker
Introduction
Our recent case-control study covering 20 years of follow-up (1986–2006) of the cohort of 110,645 Chornobyl cleanup workers from Ukraine reported, for the first time in a large population, statistically significant evidence that chronic lymphocytic leukemia (CLL) may be induced from exposure to ionizing radiation [1]. A study of Chornobyl cleanup workers from Belarus, Russia and the Baltic countries, which used a study design similar to ours and an identical radiation dose estimation method, demonstrated a similar, though not statistically significant, radiation risk for CLL [2]. In contrast, the recent study of Russian cleanup workers based on the official reported doses and Chernobyl Registry-based CLL diagnoses had negative findings [3].
It has been known, since the early 1950’s from the Hiroshima and Nagasaki atomic bomb (A-bomb) survivor studies, that radiation exposure may induce most types of leukemia, but it generally has been accepted that radiation does not induce CLL [4]. However, the most recent follow-up of this population showed a significant linear dose-response for incident CLL [5]. Additional supportive evidence of a dose-related association between exposure to ionizing radiation and increased risk of incident CLL comes from some [6–8] but not all studies of occupationally exposed workers [9,10].
The emergence of CLL as a radiation-induced disease has raised questions as to whether these cases demonstrate any unusual clinical characteristics that might differ from idiopathic CLL. No unusual effects of ionizing radiation exposure on the clinical manifestations of leukemia have been observed in the A-bomb survivors [11]. That study, however, did not include any cases of CLL. We, therefore, thought important to determine if the radiation from the Chornobyl accident had influenced any of the measurable clinical manifestations and survival of the CLL in the Chornobyl radiation-exposed cleanup workers.
Methods
Study population and case ascertainment
Study methods have been previously described [1,12,13]. Briefly, a cohort of 110,645 Ukrainian men who were 20–60 years of age during various cleanup activities in the 30-kilometer zone around the 1986 nuclear reactor accident site at Chornobyl in Ukraine was formed from the list of workers registered in the State Chornobyl Registry. Cleanup workers resided in one of five oblasts (an oblast is an administrative area similar in size to a state or province), or in the city of Kyiv. Leukemia cases diagnosed in the cohort between the time of the reactor accident in 1986 and the year 2000, were identified by an intensive search of all regional hospitals, outpatient clinics, regional tumor clinics and other health agencies in the target areas [12]. Leukemia cases occurring between the years 2001 and 2006, were identified by linkage of the cohort file with the Ukrainian National Cancer Registry [1,14]. The medical records were available for 100% of the cases and bone marrow aspiration smears, bone marrow biopsies and/or a peripheral blood smears for approximately 70 % of the cases. The diagnosis of CLL was based on the criteria established by the U.S. National Cancer Institute (NCI) Working Group, within the constraints of lymphocyte phenotype information from various outside laboratories which was available for 46% of the cases [15]. All cases were reviewed by study hematologists (I.D., T.L., and V.B.), and later confirmed by an independent International Hematology Panel (see Acknowledgements). The current analysis is based on 79 of the 89 cases confirmed by the Panel for whom it was possible to reconstruct bone marrow radiation doses. One of the 79 cases included in the study was classified as small lymphocytic lymphoma (SLL).
Study protocol was approved by the institutional review boards of the NCI (Bethesda, MD, USA), the University of California, San Francisco (San Francisco, CA, USA) and the National Research Center for Radiation Medicine (Kyiv, Ukraine). All participants gave written informed consent.
Clinical characteristics
The following clinical characteristics were considered for a possible radiation effect: First: the latent period (interval of time in years between the date of first exposure and the date of diagnosis of CLL). The date of diagnosis for each individual is the first date recorded when the absolute number of lymphocytes in the peripheral blood exceeded 5,000/microliter. For the SLL case, the date of the histological diagnosis was used for the date of diagnosis. Second: age at the time of diagnosis. Third: stage of the disease, at the time of diagnosis of CLL, as measured by the Rai criteria [16]. Fourth: absolute number of peripheral blood lymphocytes at time of diagnosis, as calculated from the percent of lymphocytes in the differential blood count and the total leukocyte count. Fifth: survival, as measured in years following the date of diagnosis to the date of death or termination of the follow-up.
All cleanup workers registered in the State Chornobyl Registry were eligible for an annual health examination, which included a differential blood count and consultation with a physician. These examinations usually were conducted at a regional health facility.
Estimation of bone marrow radiation dose
Individual bone marrow doses were estimated from questionnaires and extensive environmental measurements taken immediately after the Chornobyl accident by means of the validated RADRUE method (Realistic Analytical Dose Reconstruction with Uncertainty Estimation) [17] This method of dose estimation is dependent on such information as dates of work within the 30-kilometer zone around Chornobyl, type of jobs performed, transportation routes, and availability of official work history records. At no time, was dose information released to any clinician or case reviewer involved in the study.
Statistical methods
Regressions of estimated doses with clinical variables were performed using un-lagged doses. Analyses also were performed with 2-, 5-, 10-, and 15 year lags and gave similar results (not shown).
Bone marrow radiation dose latent periods were not normally distributed. Therefore, all further univariate tests of these variables were done using a Wilcoxon-Mann-Whitney test. Mean age at first exposure had a normal distribution and all further univariate tests were done using one-way analysis of variance (ANOVA). Additional multivariate analyses of latent period were conducted using analysis of covariance (ANCOVA) and included categorical as well as continuous predictors.
Survival analyses were conducted using the Cox proportional hazards models [18]. The partial likelihood method was used to estimate the hazard of death from CLL and the 95% confidence interval around the estimate. For each case, we calculated the time interval from the diagnosis of CLL until death, the date last known to be alive, or the termination of follow-up date on October 31, 2010, whichever occurred first.
All P values presented were two-sided. The best fitting models were chosen by using the likelihood ratio test and Akaike Information Criterion [19]. Independent predictors were retained in the model if they changed hazard ratio by more than 20% (survival analysis) or if they significantly improved the fit of the model at P=0.10 (survival and ANCOVA analyses). All multivariate analyses excluded those with missing vital status (1), chemotherapy (2) and demographic characteristics (3), such as urban/rural status, alcohol consumption and smoking, reducing the final set for analysis to 73 CLL cases. All analyses were conducted using the SAS statistical software package [20].
Results
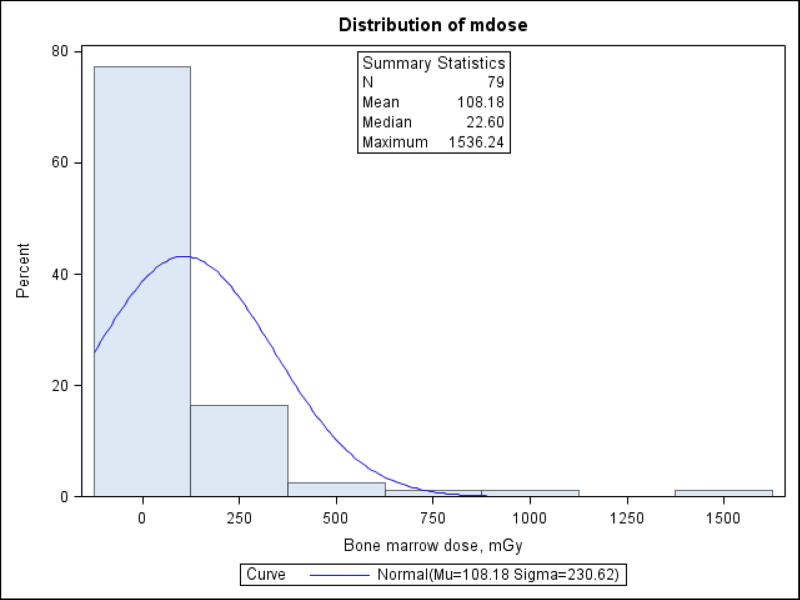
The estimated bone marrow radiation doses for the 79 CLL cases ranged from 0 to 1,536.2 mGy with a median of 22.6 mGy (Table 1). The distribution was heavily skewed toward lower doses (Figure 1), with 56 (70.9%) of CLL cases having doses <100 mGy. Median age at first exposure was 45 years and median age at diagnosis was 57 years. The majority of the cases (68.4%) were diagnosed more than 10 years after exposure in the Chornobyl zone (Table 1). Cases were predominately urban residents and reported relatively heavy smoking and alcohol consumption.
Table 1.
Descriptive characteristics of CLL cases.
| Characteristics | All CLL cases (1986–2006) N=79 (%) |
Reduced set of CLL casesa N=65 (%) |
P- Valued |
|---|---|---|---|
| Bone marrow dose, median, mGy (range) | 22.6 (0–1,536.2) | 23.5 (0–1,536.2) | 0.51 |
| Age at first exposure, years, median (range) | 45 (22–63) | 46 (29–63) | 0.50 |
| 22–34 | 11 (13.9) | 8 (12.3) | |
| 35–39 | 15 (19.0) | 12 (18.5) | |
| 40–44 | 14 (17.7) | 9 (13.9) | |
| 45–49 | 17 (21.5) | 16 (24.6) | |
| 50–54 | 11 (13.9) | 10 (15.4) | |
| 55–63 | 11 (13.9) | 10 (15.4) | |
| Age at diagnosis, years, median (range) | 57 (42–78) | 57 (43–76) | 0.94 |
| 42–44 | 6 (7.6) | 5 (7.7) | |
| 45–54 | 25 (31.7) | 20 (30.8) | |
| 55–64 | 35 (44.3) | 28 (43.1) | |
| 65–74 | 9 (11.4) | 9 (13.9) | |
| 75–78 | 4 (5.1) | 3 (4.6) | |
| Latent period, years, median (range) | 14 (1–20) | 12 (1–20) | 0.29 |
| 0–4 | 7 (8.9) | 7 (10.8) | |
| 5–9 | 18 (22.8) | 17 (26.2) | |
| 10–14 | 22 (27.9) | 20 (30.8) | |
| 15–20 | 32 (40.5) | 21 (32.3) | |
| Urban / rural statusb | 0.72 | ||
| Urban | 62 (81.6) | 52 (83.9) | |
| Rural | 11 (14.5) | 8 (12.9) | |
| Mixed | 3 (4.0) | 2 (3.2) | |
| Smokingc | 0.80 | ||
| Never/ former | 36 (48.0) | 28 (45.9) | |
| 10–20 cigarettes per day | 24 (32.0) | 20 (32.8) | |
| More than 20 cigarettes per day | 15 (20.0) | 13 (21.3) | |
| Alcohol consumptionb | 0.99 | ||
| Never | 20 (26.3) | 16 (25.8) | |
| 1–3 times per month | 41 (54.0) | 34 (54.8) | |
| Once per week – every day | 15 (19.7) | 12 (19.4) |
Abbreviations: mGy, milligray.
Based on 65 cases with <2 years from start of chemotherapy to interview.
Among 76 subjects with known information on alcohol consumption and urban/rural status.
Among 75 subjects with known information on on smoking.
P-value for heterogeneity from the Wilcoxon Rank Sums test or one-way analysis of variance (ANOVA) for continuous variables and Kruskal Wallis tests for multi-level variables.
Figure 1.
Distribution of bone marrow doses among CLL cases (n=79).
Kolmogorov-Smirnov Goodness-of-Fit test for normal distribution p<0.010.
Previous analyses of this study population suggested possibly unreliable information for 14 of the 79 cases because interviews were conducted at the time when they were receiving or recovering from chemotherapy [1]. There were no appreciable differences in the distribution of disease characteristics in the two samples (all P-values>0.2, Table 1) and all further analyses were conducted for all 79 cases.
In univariate analyses of 79 CLL cases (Table 2), median bone marrow radiation dose was not associated with Rai stage at diagnosis (P=0.25) and peripheral blood lymphocyte count at the time of diagnosis (P=0.53), although there was a monotonic trend to lower lymphocyte counts at higher doses. Latent period (P=0.82), and the age at diagnosis (P=0.41) also were not associated with median bone marrow radiation dose. However, latent period was associated with the mean age at first exposure, with those older at exposure being diagnosed sooner (P=0.01). The administration of chemotherapy was not significantly related to either the median bone marrow radiation dose (P=0.39) or the mean age at first exposure (P=0.27). Radiation dose was strongly associated with the average frequency of visits to the doctor prior to CLL diagnosis (P<0.01); the higher the dose the more frequent the visits. Those with more than one visit every two years, had an almost three-fold higher median bone marrow dose, compared to those with no doctor visits, prior to diagnosis (median bone marrow doses of 56 and 18 mGy, respectively).
Table 2.
Bone marrow dose and age at first exposure in the 30-km Chornobyl zone by categories of various characteristics.
| Characteristics | N cases=79 |
Median bone marrow dose, mGy |
DOF |
P- valuea |
Mean age at first exposure, years |
P- Valueb |
|---|---|---|---|---|---|---|
| Rai stage | 4 | 0.25 | 0.15 | |||
| 0 | 4 | 12.8 | 40 | |||
| 1 | 25 | 43.7 | 44 | |||
| 2 | 33 | 15.8 | 46 | |||
| 3 | 10 | 3.9 | 41 | |||
| 4 | 7 | 22.6 | 51 | |||
| Absolute lymphocyte count at diagnosis, per mm3, c | 3 | 0.53 | 0.42 | |||
| <10,000 | 18 | 49.9 | 44 | |||
| 10,000–19,000 | 17 | 36.7 | 42 | |||
| 20,000–39,000 | 21 | 19.8 | 46 | |||
| >=40,000 | 21 | 4.1 | 46 | |||
| Latent period, years | 3 | 0.82 | 0.01 | |||
| 0–4 | 7 | 43 | 51 | |||
| 5–9 | 18 | 22.7 | 47 | |||
| 10–14 | 22 | 14.1 | 46 | |||
| 15–20 | 32 | 37.4 | 41 | |||
| Age at diagnosis, years | 4 | 0.41 | <0.001 | |||
| 42–44 | 6 | 14.2 | 33 | |||
| 45–54 | 25 | 38.2 | 39 | |||
| 55–64 | 35 | 22.7 | 46 | |||
| 65–74 | 9 | 21.9 | 56 | |||
| 75–78 | 4 | 3.1 | 59 | |||
| Chemotherapyd | 1 | 0.39 | 0.27 | |||
| Yes | 66 | 20.0 | 44 | |||
| No | 11 | 33.6 | 47 | |||
| Average frequency of visits to the doctor prior to diagnosis | 2 | <0.01 | 0.05 | |||
| 0 | 22 | 18.0 | 49 | |||
| Once per two years | 29 | 4.2 | 44 | |||
| More than once per two years | 28 | 56.0 | 43 | |||
Abbreviations: DOF, degrees of freedom; ANOVA, analysis of variance.
P-value for heterogeneity from the Wilcoxon Rank Sums test for chemotherapy and Kruskal Wallis tests for multi-level variables.
P-value for heterogeneity from the one-way ANOVA.
Among 77 subjects with known information about lymphocyte count.
Among 77 subjects with known information about chemotherapy.
Multivariate ANCOVA analyses of latent period (Table 3) indicated that it was strongly associated with age at first exposure, smoking frequency and average frequency of visits to the doctor prior to diagnosis (all P<0.05). As in the univariate analyses, latent period showed little association with bone marrow dose (P=0.84). Overall, these four variables explained a third of the variation in time since exposure (R2=0.33, not shown).
Table 3.
Analysis of factors associated with latent period.a
| Source | DOF | Type I SSb | Mean Square | F Value | P-valuec |
|
| |||||
| Age at first exposure, years | 1 | 231.46 | 231.46 | 13.52 | <0.01 |
| Smoking | 2 | 172.08 | 86.04 | 5.02 | <0.01 |
| Average frequency of visits to the doctor, per 2 years | 1 | 160.10 | 160.10 | 9.35 | <0.01 |
| Bone marrow dose, mGy | 1 | 0.69 | 0.69 | 0.04 | 0.84 |
| Source | DOF | Type III SSd | Mean Square | F Value | P-valuee |
| Age at first exposure, years | 1 | 135.86 | 135.86 | 7.93 | <0.01 |
| Smoking | 2 | 126.85 | 63.43 | 3.70 | 0.03 |
| Average frequency of visits to the doctor, per 2 years | 1 | 158.40 | 158.40 | 9.25 | <0.01 |
| Bone marrow dose, mGy | 1 | 0.69 | 0.69 | 0.04 | 0.84 |
Abbreviations: DOF, degrees of freedom; SS, sum of squares.
For 74 CLL cases with available information on smoking.
Indicates the reduction in the sequential error sum of squares with addition of each variable.
P-value from the analysis of covariance adjusted for all variables above the test variable.
Indicates error sum of squares computed by comparing the full model to the model without the test variable.
P-value from the analysis of covariance adjusted for all other factors in the Table.
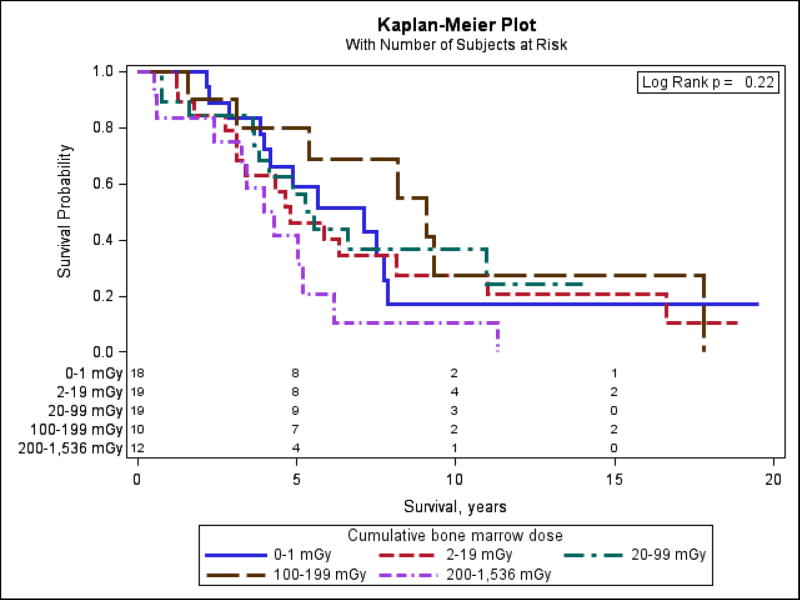
Median survival of 78 patients with complete follow-up information was 4.8 years (range 0.5–19.5). Figure 2 demonstrates Kaplan-Meier survival curves for 5 dose categories and number of subjects at risk. Those with the highest cumulative bone marrow doses (200–1,536 mGy) had the shortest survival, although the differences in survival between various dose categories was not statistically significant (Log Rank P=0.22). Table 4 presents the results of Cox regression analyses and shows that survival of CLL cases was significantly related to bone marrow radiation dose (P=0.03) with a hazard ratio (HR) of 2.38 comparing survival of those with doses above the median dose of 22 mGy to those with doses below. When the dose variable was split into 5 categories with approximately even number of cases in each, HRs for survival monotonically increased with increasing bone marrow radiation dose, with HRs ranging from 1.79 for those with doses 2–19 mGy to 4.21 for those with doses 200–1,536 mGy, compared to those with doses below 2 mGy (not shown). However, the test for linear trend was not statistically significant (P=0.19, not shown). Use of the Akaike Information Criterion suggests that the dichotomous model is optimal among the three dose-response models considered (linear, dichotomous, 5-level categorical). Other clinical characteristics associated with significantly poorer survival included later age at time of diagnosis, higher absolute lymphocyte count at time of diagnosis, Rai stage at time of diagnosis, and the administration of chemotherapy (all P<0.05, Table 4). Overall, 86% of the CLL patients had received chemotherapy by the end of follow-up on October 31, 2010. Survival was significantly shorter among those who were younger at first exposure (P=0.02). Although not statistically significant, we observed that cleanup workers who were heavy smokers (P=0.06) or alcohol drinkers (P=0.14) had relatively poorer survival.
Figure 2.
Kaplan-Meier plot for categories of bone marrow dose among CLL cases with survival information until 10/31/2010 (n=78).
Table 4.
| Variable | Value | N cases = 73 |
Hazard ratio (95% CI)c |
DOF | P- valued |
|---|---|---|---|---|---|
| Bone marrow dose, mGye | 0–21 | 36 | 1 | 1 | 0.03 |
| 22–1,536 | 37 | 2.38 (1.11, 5.08) | |||
| Age at diagnosis, years | per 10 years increase | 2.50 (1.09, 5.73) | 1 | 0.03 | |
| Age at first exposure, years | per 10 years increase | 0.41 (0.19, 0.88) | 1 | 0.02 | |
| Absolute lymphocyte count at diagnosis, per mm3 | 1,533–10,000 | 17 | 1 | 3 | <0.01 |
| 10,000–19,000 | 17 | 0.83 (0.27, 2.56) | |||
| 20,000–39,000 | 19 | 1.79 (0.62, 5.18) | |||
| 40,000–1,089,000 | 20 | 5.02 (1.69, 14.9) | |||
| Rai stage | Stage 0–2 | 57 | 1 | 1 | <0.01 |
| Stage 3–4 | 16 | 2.98 (1.32, 6.75) | |||
| Chemotherapy | No | 9 | 1 | 1 | 0.01 |
| Yes | 64 | 13.5 (1.63, 112) | |||
| Smoking | Never/ former | 34 | 1 | 2 | 0.06 |
| 10–20 cigarettes per day | 24 | 0.83 (0.39, 1.78) | |||
| More than 20 cigarettes per day | 15 | 2.46 (1.01, 5.98) | |||
| Alcohol consumption | Never | 19 | 1 | 2 | 0.14 |
| 1–3 times per month | 39 | 1.16 (0.52, 2.62) | |||
| Once per week – every day | 15 | 2.79 (0.88, 8.85) | |||
| Urban/rural status | Urban | 59 | 1 | 2 | 0.20 |
| Rural | 11 | 0.43 (0.17, 1.12) | |||
| Mixed | 3 | 1.25 (0.19, 8.38) |
Abbreviations: CI, confidence interval; DOF, degrees of freedom, mGy, milligray.
For 73 CLL cases with known vital status and available information on chemotherapy, smoking and alcohol consumption and urban/rural status.
Survival is defined as time from diagnosis to death or end of follow-up on 10/31/2010.
Maximum likelihood confidence limits.
P-value from the likelihood ratio test comparing full model with all variables to the nested model without the variable of interest.
Unlagged bone marrow doses; similar risks were estimated for doses lagged by 2, 5, 10, and 15 years.
Discussion
This is the first study to examine association between bone marrow radiation doses from the Chornobyl accident and clinical manifestations of the CLL which had developed in the cleanup workers. Our previous study based in a cohort of 110,645 male cleanup workers of the Chornobyl accident in Ukraine in 1986 showed a significant dose-related increase in the risk of CLL in analyses comparing CLL cases and controls and estimated that about 20% of CLL cases could be attributed to radiation exposure from cleanup work [1]. In the current study, we analyzed 79 cases of CLL which have been confirmed by the International Hematology Panel. We observed that higher radiation doses and younger age at first exposure to radiation during Chornobyl cleanup work were associated with significantly shorter survival. Latent period was not associated with bone marrow radiation dose, stage of disease, chemotherapy treatment or any other clinical characteristics. However, we estimated that smoking and higher frequency of visits to the doctor were significantly associated with a shorter latent period.
The issue of radiation-related risks of CLL has been controversial for many years [21]. While earlier studies of atomic bomb (A-bomb) survivors from Japan reported no increase in radiation risks [22], a recent report based on12 cases of CLL identified in a cohort of about 113,000 survivors from 1950 to 2001 (3% of 371 cases of all leukemia) and using a simple age and gender baseline model, reported a significant linear radiation dose-response [5]. It now seems clear that the major reason for the previous negative findings with regards to radiogenicity of CLL in the A-bomb survivors was, most likely, due to very low incidence of CLL in the Japanese population (2–3% of all cases of leukemia [23,24]) compared to about 40% in Caucasian populations, based on registry data [25].
The evidence on radiation-related risks of CLL from incidence studies of Caucasian populations exposed to low doses of ionizing radiation continues to be mixed, with some showing no increase in risks [3,9,10,26] while others reporting a dose-related association [6–8,27]. Finch and Linet [28] have noted that over a quarter of all CLL cases may be asymptomatic for many years, and that survival is significantly longer compared to other types of leukemia. In addition, several recent publications[29,30] have suggested that mortality studies based on death certificates are not very reliable and have been shown to underestimate CLL occurrence by as much as 38% [31]. Thus, mortality data would underestimate, possibly substantially, the occurrence of CLL. Other problems with the detection of the possible radiation induction of CLL include short follow-up and small sample size [32]. For example, the first analysis of incidence data from the Techa River cohort reported a negative radiation risk estimate for CLL based on 1953–2005 follow-up with 22 CLL cases [26], while the second analysis based on 1953–2007 follow-up with 27 CLL cases showed non-significantly increased risks (ERR/Gy=0.01, 95% CI: <0, 1.2) [27]. We note that in the past, serious concerns have been raised about studies of Russian cleanup workers based on official doses and unverified diagnoses from the Chernobyl Registry, thus negative findings from this study should be treated with caution [33].
We were particularly interested in examining the effects of radiation exposures and the age at time of first exposure on the latent period on the basis of two previous reports of apparent alterations in the clinical course of leukemia due to induction by radiation or a chemical [34,35]. An early report concerning children with acute leukemia from the study of A-bomb survivors in Hiroshima and Nagasaki indicated that younger age at time of exposure was associated with a shorter latent period prior to the appearance of clinical disease [34]. The median age at diagnosis in our study was 57 years compared to the median age at diagnosis in the US of 72 years [36]. A recent study of 195 adult veterans who were exposed to Agent Orange and developed CLL showed no differences between the exposed and non-exposed for Rai staging, lymphocyte doubling time, cytogenetic changes or survival [35]. However, the Agent Orange exposed veterans were both significantly younger at time of diagnosis and the latent period was significantly shorter than it was for the non-exposed veterans. In our study, the median latent period was 14 years and both univariate and multivariate analyses showed no association of latent period with bone marrow radiation doses but a significant association with age at first exposure.
Our study provides important insights into the relationship between radiation exposure and survival of CLL cases from among radiation-exposed Chornobyl cleanup workers. Earlier studies of the cleanup workers reported that exposed to radiation had a more aggressive clinical course compared to those non- or little exposed [37]. The authors speculated that aggressive behavior could be explained by alterations in immunoglobulin variable heavy chain gene configuration [38]. In contrast to these studies, we had individual bone marrow doses for all CLL cases. Our analyses showed a significant dose-related increase in the hazard of dying. Whether this impaired survival is related to radiation exposure, per se, or other radiation co-morbidities or some other factors is not clear at this time. Higher median bone marrow doses were also associated with higher average frequency of visits to the doctor prior to diagnosis (Table 2). The overall infrequency of visits prior to the diagnosis of CLL (27.8% never visited a doctor prior to diagnosis and 36.7% visited no more than once per two years) may explain why the disease was quite advanced at the time of diagnosis of CLL for many of the workers (50% being diagnosed at stage two or higher, Table 4). This argues against any possible screening bias. The majority of CLL cases received chemotherapy and many were started on chemotherapy within a few days of disease diagnosis.
Our results need to be considered in the light of several strengths and limitations. The results of the physical examination and clinical laboratory observations were recorded for each case at the time the diagnosis of leukemia was established [12] and greatly enhanced the quality of the incidence study. It is possible that some leukemia cases were missed, particularly those with shorter latent periods. However, all cleanup workers were registered in the Chornobyl State Registry and received pension benefits, so it is unlikely that we missed a large number of cases. Certain environmental factors and even local or national personal customs could have confounded our results. For example, a number of studies of farming populations now strongly suggest that pesticides and/or herbicides play a likely role in the etiology of CLL [39,40]. However, in our study, occupational exposures to pesticides, solvents and benzene were not independent risk factors of CLL [13,41].
Conclusion
Analysis of 79 cases of CLL identified over 20 years of follow-up of a large cohort of Chornobyl cleanup workers from Ukraine showed statistically significant association between younger age at first exposure and shorter latent period and shorter survival. Latent period was not associated with bone marrow radiation doses or any clinical characteristics but was significantly shorter among smokers and those with higher frequency of visits to the doctor prior to diagnosis. Higher bone marrow radiation doses and younger age as well as more advance disease stage at diagnosis were significantly associated with shorter survival. An increase in the risk of death with increasing bone marrow radiation dose requires further investigation to exclude effects of chance and unmeasured risk factors. Future studies are necessary with more cases in order to improve the statistical power of these analyses and to determine their significance.
Acknowledgments
Funding: Funding for this study was provided by the National Cancer Institute (contract OH 96-C-N030 to the RCRM, contract NO1-CP-21178 to S.F., R.F.R. and L.B.Z; NCI grant CA 132918 to L.B.Z.; and the intramural Research Program of the Division of Cancer Epidemiology and Genetics to A.B., M.H. and M.P.L.) Radiation dose reconstruction was partially supported by the Intra-Agency Agreement between the U.S. National Institute of Allergy and Infectious Diseases and the National Cancer Institute (NIAID agreement #Y2-Al-5077 and NCI agreement #Y3-CO-5117.) At the earlier stages of the study, the U.S. Department of Energy (contract HHSN 2004 55796C), the Nuclear Regulatory Commission, and the French Institute for Radiological Protection and Nuclear Safety contributed additional funding.
The investigators in this study are deeply indebted to Gilbert W. Beebe, who was the principal person responsible for the initiation and binational creation of the leukemia incidence study in the Chornobyl cleanup workers, which has made possible the clinical study of CLL. We also are very appreciative of the contributions of Geoffrey R. Howe who was principally responsible for the detailed design of the leukemia incidence study and for his insistence that CLL be included as one of the types of leukemia in the study. Another extremely valuable study participant was Victor Klimenko, whose assistance in the design of the leukemia incidence study, and in working closely with the regional hematologists in Ukraine for the conduct of the study were essential. Thanks also are extended to the members of the Leukemia Advisory Group (F. L. Wong, chair, H. Checkoway, K. Eckerman, B. Chabner, and most recently B. Cheson). We are grateful to members of the International Hematology Panel (B. Bain, UK; S. Gaidukova and D. Gluzman, Ukraine; P. McPhedran and L-A. Peterson, USA) for their hard work and valuable contributions.
Footnotes
Author contributions
S.C.F., R.F.R. and L.B.Z. contributed to the design of this clinical study, data acquisition, interpretation of results and preparation of the manuscript. I.D., N.G., N.B., T.L., V.B., A.R., V.C., and D.B. contributed to data acquisition and interpretation of results. A.B., M.H., and M.P.L. contributed to data acquisition, interpretation of the results and preparation of the manuscript. L.B.Z. analyzed the data.
Declarations
All authors have no relevant conflicts of interest.
References
- 1.Zablotska LB, Bazyka D, Lubin JH, Gudzenko N, Little MP, Hatch M, et al. Radiation and the risk of chronic lymphocytic and other leukemias among Chornobyl cleanup workers. Environ Health Perspect. 2013;121:59–65. doi: 10.1289/ehp.1204996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesminiene A, Evrard AS, Ivanov VK, Malakhova IV, Kurtinaitis J, Stengrevics A, et al. Risk of hematological malignancies among Chernobyl liquidators. Radiat Res. 2008;170:721–735. doi: 10.1667/RR1231.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivanov VK, Tsyb AF, Khait SE, Kashcheev VV, Chekin SY, Maksioutov MA, et al. Leukemia incidence in the Russian cohort of Chernobyl emergency workers. Radiat Environ Biophys. 2012;51:143–149. doi: 10.1007/s00411-011-0400-y. [DOI] [PubMed] [Google Scholar]
- 4.National Research Council (NRC) BEIR VII Phase 2. Washington, DC: NRC, National Academies Press; 2006. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health risks from exposure to low levels of ionizing radiation. [PubMed] [Google Scholar]
- 5.Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, et al. The Incidence of Leukemia, Lymphoma and Multiple Myeloma among Atomic Bomb Survivors: 1950–2001. Radiat Res. 2013;179:361–382. doi: 10.1667/RR2892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohner M, Gellissen J, Marsh JW, Gregoratto D. Occupational and diagnostic exposure to ionizing radiation and leukemia risk among German uranium miners. Health Phys. 2010;99:314–321. doi: 10.1097/HP.0b013e3181cd6536. [DOI] [PubMed] [Google Scholar]
- 7.Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect. 2006;114:818–822. doi: 10.1289/ehp.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zablotska LB, Lane RS, Frost SE, Thompson PA. Leukemia, lymphoma and multiple myeloma mortality (1950–1999) and incidence (1969–1999) in the Eldorado uranium workers cohort. Environ Res. 2014;130C:43–50. doi: 10.1016/j.envres.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muirhead CR, O'Hagan JA, Haylock RG, Phillipson MA, Willcock T, Berridge GL, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009;100:206–212. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillies M, Haylock R. The cancer mortality and incidence experience of workers at British Nuclear Fuels plc, 1946–2005. J Radiol Prot. 2014;34:595–623. doi: 10.1088/0952-4746/34/3/595. [DOI] [PubMed] [Google Scholar]
- 11.Bizzozero OJ, Jr, Johnson KG, Ciocco A, Kawasaki S, Toyoda S. Radiation-related leukemia in Hiroshima and Nagasaki 1946–1964. II. Ann Intern Med. 1967;66:522–530. doi: 10.7326/0003-4819-66-3-522. [DOI] [PubMed] [Google Scholar]
- 12.Romanenko A, Bebeshko V, Hatch M, Bazyka D, Finch S, Dyagil I, et al. The Ukrainian-American study of leukemia and related disorders among Chornobyl cleanup workers from Ukraine: I. Study methods. Radiat Res. 2008;170:691–697. doi: 10.1667/RR1402.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romanenko A, Finch S, Hatch M, Lubin JH, Bebeshko VG, Bazyka DA, et al. The Ukrainian-American study of leukemia and related disorders among Chornobyl cleanup workers from Ukraine: III. Radiation risks. Radiat Res. 2008;170:711–720. doi: 10.1667/RR1404.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fedorenko ZP, Gaisenko AV, Gulak L, Gorokh YL, Ryzhov AY, Sumkina OV, et al. Cancer incidence in Ukraine, 2009–2010. Vol. 12 Kyiv, Ukraine: Institute of Oncology of Academy of Medical Sciences of Ukraine; 2011. [Google Scholar]
- 15.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 16.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 17.Kryuchkov V, Chumak V, Maceika E, Anspaugh LR, Cardis E, Bakhanova E, et al. RADRUE method for reconstruction of external photon doses for Chernobyl liquidators in epidemiological studies. Health Phys. 2009;97:275–298. doi: 10.1097/HP.0b013e3181ac9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox DR, Oakes D. Analysis of Survival Data. New York: Chapman & Hall; 1984. [Google Scholar]
- 19.Akaike H. Likelihood of A Model and Information Criteria. Journal of Econometrics. 1981;16:3–14. [Google Scholar]
- 20.SAS Institute Inc. SAS Version 9.3 for Windows. Cary, NC: 2012. [Google Scholar]
- 21.Linet MS, Schubauer-Berigan MK, Weisenburger DD, Richardson DB, Landgren O, Blair A, et al. Chronic lymphocytic leukaemia: an overview of aetiology in light of recent developments in classification and pathogenesis. Br J Haematol. 2007;139:672–686. doi: 10.1111/j.1365-2141.2007.06847.x. [DOI] [PubMed] [Google Scholar]
- 22.Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat Res. 1994;137:S68–97. [PubMed] [Google Scholar]
- 23.Finch SC, Hoshino T, Itoga T, Ichimaru M, Ingram RH., Jr Chronic lymphocytic leukemia in Hiroshima and Nagasaki, Japan. Blood. 1969;33:79–86. [PubMed] [Google Scholar]
- 24.Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, et al. Cancer incidence and incidence rates in Japan in 2007: a study of 21 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2013;43:328–336. doi: 10.1093/jjco/hys233. [DOI] [PubMed] [Google Scholar]
- 25.Dores GM, Anderson WF, Curtis RE, Landgren O, Ostroumova E, Bluhm EC, et al. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol. 2007;139:809–819. doi: 10.1111/j.1365-2141.2007.06856.x. [DOI] [PubMed] [Google Scholar]
- 26.Krestinina L, Preston DL, Davis FG, Epifanova S, Ostroumova E, Ron E, et al. Leukemia incidence among people exposed to chronic radiation from the contaminated Techa River, 1953–2005. Radiat Environ Biophys. 2010;49:195–201. doi: 10.1007/s00411-009-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krestinina LY, Davis FG, Schonfeld S, Preston DL, Degteva M, Epifanova S, et al. Leukaemia incidence in the Techa River Cohort: 1953–2007. Br J Cancer. 2013;109:2886–2893. doi: 10.1038/bjc.2013.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finch SC, Linet MS. Chronic leukaemias. Baillieres Clin Haematol. 1992;5:27–56. doi: 10.1016/s0950-3536(11)80034-x. [DOI] [PubMed] [Google Scholar]
- 29.Richardson DB, Wing S, Schroeder J, Schmitz-Feuerhake I, Hoffmann W. Ionizing radiation and chronic lymphocytic leukemia. Environ Health Perspect. 2005;113:1–5. doi: 10.1289/ehp.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schubauer-Berigan MK, Daniels RD, Fleming DA, Markey AM, Couch JR, Ahrenholz SH, et al. Chronic lymphocytic leukaemia and radiation: findings among workers at five US nuclear facilities and a review of the recent literature. Br J Haematol. 2007;139:799–808. doi: 10.1111/j.1365-2141.2007.06843.x. [DOI] [PubMed] [Google Scholar]
- 31.Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer. 2001;92:1325–1330. doi: 10.1002/1097-0142(20010901)92:5<1325::aid-cncr1454>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Silver SR, Hiratzka SL, Schubauer-Berigan MK, Daniels RD. Chronic lymphocytic leukemia radiogenicity: a systematic review. Cancer Causes Control. 2007;18:1077–1093. doi: 10.1007/s10552-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 33.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Annex D: Health Effects Due to Radiation from the Chernobyl Accident. New York: United Nations; 2011. 2008 Report to the General Assembly with Scientific Annexes. Volume II. [Google Scholar]
- 34.Ichimaru M, Ishimaru T, Belsky JL. Incidence of leukemia in atomic bomb survivors belonging to a fixed cohort in Hiroshima and Nagasaki, 1950–71. Radiation dose, years after exposure, age at exposure, and type of leukemia. J Radiat Res. 1978;19:262–282. doi: 10.1269/jrr.19.262. [DOI] [PubMed] [Google Scholar]
- 35.Baumann Kreuziger LM, Tarchand G, Morrison VA. The impact of Agent Orange exposure on presentation and prognosis of patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2014;55:63–66. doi: 10.3109/10428194.2013.794267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; Bethesda, MD: 2014. [April 15, 2015]. Available from: http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted April 2014. [Google Scholar]
- 37.Gluzman D, Imamura N, Sklyarenko L, Nadgornaya V, Zavelevich M, Machilo V. Patterns of hematological malignancies in Chernobyl clean-up workers (1996–2005) Exp Oncol. 2006;28:60–63. [PubMed] [Google Scholar]
- 38.Abramenko I, Bilous N, Chumak A, Davidova E, Kryachok I, Martina Z, et al. Chronic lymphocytic leukemia patients exposed to ionizing radiation due to the Chernobyl NPP accident--with focus on immunoglobulin heavy chain gene analysis. Leuk Res. 2008;32:535–545. doi: 10.1016/j.leukres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Brown LM, Blair A, Gibson R, Everett GD, Cantor KP, Schuman LM, et al. Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Res. 1990;50:6585–6591. [PubMed] [Google Scholar]
- 40.Lee E, Burnett CA, Lalich N, Cameron LL, Sestito JP. Proportionate mortality of crop and livestock farmers in the United States, 1984–1993. Am J Ind Med. 2002;42:410–420. doi: 10.1002/ajim.10131. [DOI] [PubMed] [Google Scholar]
- 41.Gudzenko N, Hatch M, Bazyka D, Dyagil I, Reiss RF, Brenner A, et al. Non-radiation risk factors for leukemia: A case-control study among chornobyl cleanup workers in Ukraine. Environ Res. 2015;142:72–76. doi: 10.1016/j.envres.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]