Abstract
Introduction
Corticosteroid injection is a recommended treatment option for carpal tunnel syndrome (CTS), before considering surgery. Nevertheless, the role of injections remains controversial since there is only strong evidence for short-term benefits. The purpose of this study was to determine the re-intervention rate and to identify prognostic indicators for subsequent treatment after a corticosteroid injection for CTS.
Methods
This study evaluated residents of Olmsted County, treated with a corticosteroid injection for CTS between 2001 and 2010. Failure of treatment was the primary outcome of interest. Two definitions for failure were examined: 1) patient receiving subsequent procedural intervention and 2) patient undergoing carpal tunnel release. Survival was estimated using Kaplan-Meier methods and association of covariates with increased failure was modeled using Cox proportional hazards regression.
Results
There were a total of 774 affected hands in 595 patients. The median follow-up period was 7.4 years. Re-intervention was performed in 68% of cases, of which 63% resulted in eventual surgery. Injectate volume was significant for the outcome of any retreatment (hazard ratio (HR) 0.879[0.804–0.96]) and surgery (HR 0.906[0.827–0.99]). Rheumatoid arthritis was also significant in both models, with HR 0.627[0.404–0.97] for any retreatment and HR 0.493[0.292–0.83] for surgery.
Conclusions
In this cohort, 32% of the patients did not receive subsequent treatment after a single injection, which indicates that there is a therapeutic role for corticosteroid injections in the treatment of CTS. Further research is necessary to identify those patients who will benefit from an injection, in order to provide more individually tailored treatment.
Introduction
Carpal tunnel syndrome (CTS) is a common yet disabling condition, with an annual incidence of 2–5% for women and 1–3% for men (1–3). Splinting, local steroid injection and carpal tunnel release (CTR) are all recommended treatment options for this condition (4, 5). Most guidelines suggest trying local steroid injection or splinting before considering surgery and several studies have shown that local steroid injections are effective in providing at least short-term symptom relief (4, 5). However, the role of steroid injections in the treatment of CTS remains controversial, since there is strong evidence only for benefits of steroid injections in the short term (6, 7).
Two recent systematic reviews on the effectiveness of steroid injections concluded that the effects appear to be time limited and there is limited evidence on the long-term effectiveness (6, 7). Reported rates of subsequent treatment, either reinjection or surgery, vary from 10%–81% at one year following initial injection (8–13). Jenkins et al. reported that 33% (N=272/824) of the patients with mild to moderate CTS who underwent a local corticosteroid injection required surgery at 5 years follow-up (13). This indicates that there is a subgroup of patients who will achieve lasting symptom improvement from an injection.
It would be important to identify the likelihood of long-term benefit from a local steroid injection. For those unlikely to have long-term benefit, surgery would be a more appropriate option, as it would hasten the resolution of symptoms, and avoid the discomfort or potential complications of an injection. For those likely to have long-term benefit, injection therapy would prevent unnecessary surgery and reduce health care costs. Previous studies have investigated predictors for subsequent treatment of CTS after initial injection. A diagnosis of diabetes mellitus, higher pre-injection score on the Boston Carpal Tunnel Questionnaire (BCTQ), longer duration of symptoms and a more severe electrodiagnostic study (EDS) result have all been suggested as risk factors for poor clinical outcome following a local corticosteroid injection (11–15). However, these results are not consistent across different studies.
The purpose of this study was to determine the long-term rate of re-intervention (additional injection or surgery) after a single corticosteroid injection in the management of CTS and to identify prognostic indicators for subsequent treatment in a population-based cohort.
Methods
Data collection
This retrospective study is based on data from residents of Olmsted County, MN, USA, treated with a corticosteroid injection for CTS between 2001 and 2010. Patients were identified using the resources of the Rochester Epidemiology Project (REP) medical records linkage-system (16). The REP organizes demographic data, diagnostic codes and surgical procedure codes in electronic indexes that can be searched. Patients’ residency status is also checked. Multiple medical records for the same individual are linked within and across institutions to create a comprehensive record, regardless of where a county resident is seen (17). Participating institutions and providers include not only those within Olmsted County but also those in the surrounding region. Studies have shown that the database includes nearly all care provided to nearly all (i.e., >90%) County residents. Our selection was based on a Current Procedural Terminology (CPT) code for diagnosis of carpal tunnel syndrome (ICD-9 354.0) and carpal tunnel injection (CPT 20526). The REP list of patients with diagnosis of CTS and a procedure of carpal tunnel injection within the specified time frame was retrieved using the computerized indexes and the consolidated records of these patients were reviewed by three physicians (SE, AB, TS) to verify the diagnosis. Information on the diagnosis was abstracted from the medical charts and cases of possible or unlikely CTS (based on previously described criteria (18)) were excluded from this study. In addition, detailed record abstraction was used to collect data on comorbidities, EDS severity and volume and dose of injectate. Patients were followed through their medical record until 2014. The last day of follow-up was defined as the most recent day the patient had visited a REP health care provider, or December 31, 2014 if the patient visited a REP health care provider after 2014.
Patients were included if they had a diagnosis of primary CTS, no previous injection for CTS or carpal tunnel release in that hand, received a therapeutic corticosteroid injection for CTS, were at least 18 years old, had at least one day of follow-up and had provided research authorization. Patients diagnosed with pregnancy-related CTS and observations missing injectate volume or steroid dose were excluded from the analysis. Ultrasound-guided injections were excluded from analysis, since they were the subject of a different report and also because literature suggests that they have a different failure rate compared to blind injections (19, 20). Study data were managed using Research Electronic Data Capture (REDCap).
The risk factors examined were age, gender, total injectate volume (combined steroid and anesthetic volume), effective dose of steroid, history of diabetes mellitus, diagnosis of peripheral neuropathy or cervical radiculopathy, diagnosis of rheumatoid arthritis and EDS severity at time of injection. Dose of steroid was standardized to equivalent effective dose of Triamcinolone, which had the highest use in the cohort and was converted using Table 3 in Leversee et al. (21). The severity of CTS was assessed using available EDS data. EDS severity was classified in the following categories: normal, mild, moderate and severe based on the classification of Stevens (22). Subjects without information, which allowed EDS severity to be assessed, were assigned to a fifth EDS category: untested.
Outcome measurements
Failure of treatment was the primary outcome of interest. Two definitions for failure of treatment were examined: 1) failure defined as any subsequent procedural intervention (i.e. corticosteroid injection or CTR) and 2) failure defined as patient receiving eventual CTR on the injected hand no matter how many injections.
Statistical analysis
Summary statistics for demographics and clinical characteristics are shown as N (%), mean ± standard deviation (SD) or median [interquartile range (IQR)]. Subjects without events were censored at the earlier of the date of last follow-up present in the medical record or Dec. 31, 2014. The Kaplan-Meier method was used to estimate median failure time for both definitions of failure. Cox proportional hazard models with robust variance estimators were fit to test for covariates’ associations with increased risk of treatment failure. Robust variance estimators were used to adjust for correlated outcomes between hands of the same patient. Model assumptions, such as proportional hazards, were assessed. Stratification was performed to adjust for variables failing the proportional hazard assumptions. Hazard ratios (HR) and 95% confidence intervals (CI) are reported. P-values less than 0.05 were considered significant. Statistical analyses were performed using R (version 3.1.2; Vienna, Austria); survival analyses used the survival package (version 2.39–4)(23–25).
Comparison to surgery without previous injection cohort
In order to give an overview of the characteristics of the full cohort of patients treated for CTS, the group of patients who went directly to surgery was also examined. This allowed us to compare the patient and disease specific characteristics of the patients who received an injection to patients who proceeded directly to surgery within the specified time window and within the same population. Patients that proceeded directly to surgery were selected using the same resource as the injection cohort with selection based on a CPT code for diagnosis of carpal tunnel syndrome (ICD-9 354.0) and open carpal tunnel release (64721) or endoscopic carpal tunnel release (29848).
The following were criteria for the inclusion: diagnosis of primary CTS, no previous injection or surgery for CTS in that hand, no acute CTS, at least 18 years old and provided research authorization. Patients who received a therapeutic injection on either hand, including bilateral cases where only one hand was treated with injection, were excluded from this subset. Characteristics of interest were age, gender, EDS severity, history of diabetes mellitus, diagnosis of peripheral neuropathy or cervical radiculopathy, and diagnosis of rheumatoid arthritis. Under the assumption that distributions would be similar to the injection cohort, this group was randomly sampled to allow estimation of EDS severity proportions with a precision of ± 5%. Chi-Square or Wilcoxon rank-sum tests were used to compare baseline characteristics between groups.
Results
Patient selection and baseline characteristics
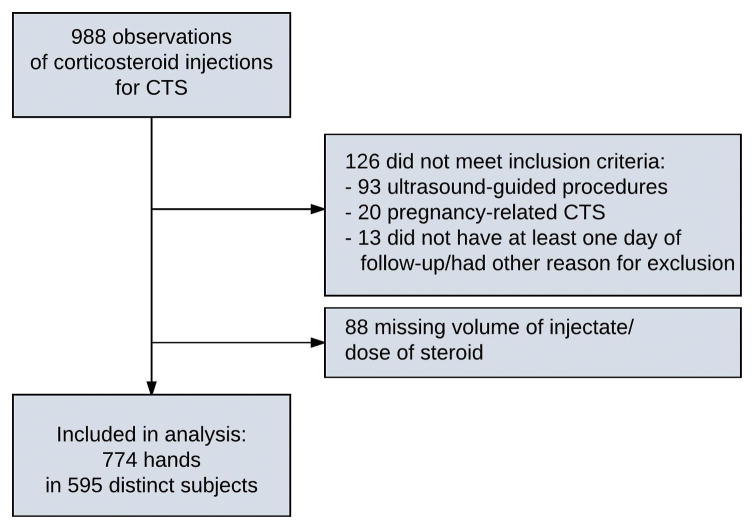
A total of 1144 observations within the specified time window were identified. Of these, a total of 988 subjects had a primary diagnosis of CTS. Subjects who had pregnancy-related CTS (N=20), had US-guided procedures (N=93), were missing injectate volume or dose of steroid (N=88), and subjects who did not have at least 1 day of follow-up or had another indication for exclusion (N=13) were also removed. After exclusions, there were a total of 774 affected hands in 595 distinct individuals (Figure 1).
Figure 1.
Subject selection flowchart.
Descriptive statistics are displayed in Table 1 and indicated whether they are reported at the subject or hand level. The cohort was 30.4% males, with 8.74% having a diagnosis of diabetes mellitus, 7.73% having peripheral neuropathy or cervical radiculopathy, and 5.38% having rheumatoid arthritis. The mean (SD) age at injection was 51 years (13.5). The injections used an average injectate volume of 3.7 mL (1.16) and an average steroid dose of 39.9 mg (22.3). For EDS severity, 14.5% were not tested, 8.3% were classified as normal, 29.6% as mild, 41.2% as moderate, and 6.5% were classified as severe. The median follow-up period was 7.3 years (minimum 7 days, maximum 12.6 years).
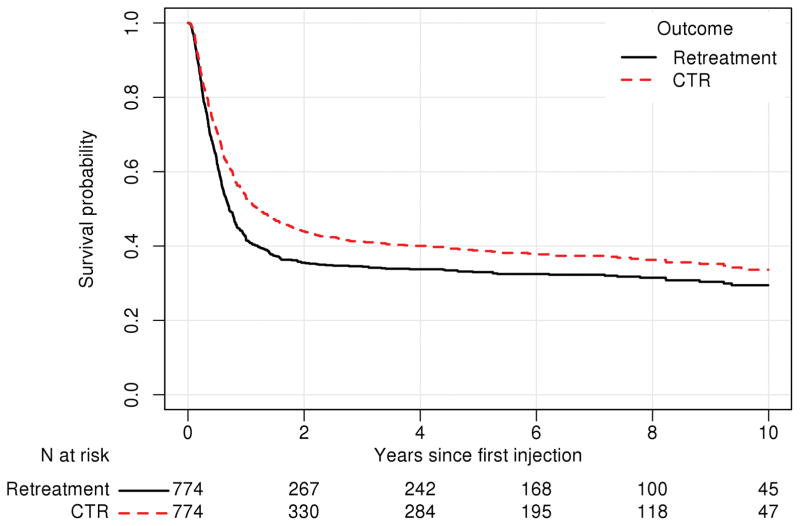
Overall, re-intervention (injection or CTR) was performed in 67.8% of cases (N=525/774), with eventual CTR in 62.7% of cases (N=485). Median (IQR) time to failure was 259 days (121-N/A) for any retreatment and 446 days (147-N/A) for CTR (Figure 2). Estimates of the 75th percentile for time to failure are not available as that proportion of failure was not observed. There were 131 subjects (N=159 hands) who received a second injection for treatment of CTS. Of these, 85 subjects (N=100 hands, 62.9%) eventually were treated with CTR.
Figure 2.
Kaplan-Meier curve for survival (i.e. no subsequent intervention received) in the injection cohort. Subsequent interventions were classified as 1) retreatment (i.e. either a second corticosteroid injection or carpal tunnel release (CTR)) or 2) CTR only. The table underneath the figure represents the number remaining at risk (i.e. who are still being followed and have not yet experienced the event of interest) at baseline, 2, 4, 6, 8, and 10 years after the initial injection for both the outcome of any retreatment and for CTR.
Prognostic factors
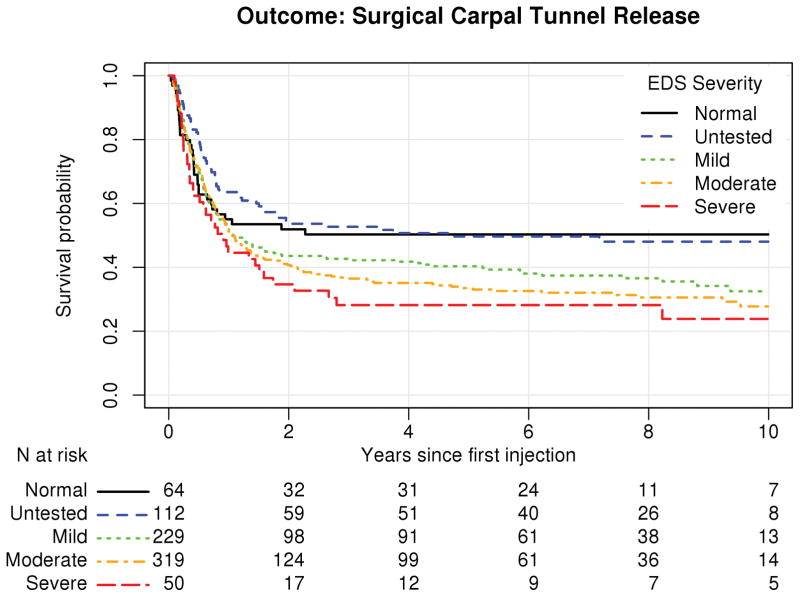
Results for Cox proportional hazard models are displayed in Tables 2 and 3 for the outcomes of any re-intervention and carpal tunnel release respectively. EDS severity failed proportional hazard assumptions and was used as a stratifying variable. Figures 3 and 4 show the survival curves stratified by EDS severity. While we are not able to formally test within the context of the model between strata, these curves indicate that patients with more severe EDS results were more likely to experience injection failure. Higher injectate volume was found to be significantly associated with a decreased likelihood of injection failure for the outcome of any retreatment (HR 0.879 [0.804–0.96], p=0.004) and carpal tunnel release (HR 0.906 [0.827–0.99], p=0.034). Rheumatoid arthritis was found to be significantly associated with a decreased likelihood of injection failure in both models, with HR 0.627 [0.404–0.97] (p=0.037) for any retreatment and HR 0.493 [0.292–0.83] (p=0.008) for carpal tunnel release. The effective dose of steroid was not significantly associated with either outcome measurement (any subsequent treatment: HR 0.998 [0.994, 1.00], p=0.510 and carpal tunnel release: HR 0.996 [0.991, 1.00], p=0.105).
Table 2.
Demographics and clinical characteristics and their association with re-intervention (either second corticosteroid injection or carpal tunnel release) in patients with carpal tunnel syndrome after initial treatment with corticosteroid injection in a Cox proportional hazards model. Risk factors, model parameter estimates (Beta) and standard errors (SE), hazard ratios, 95% confidence intervals (CI), and model p-values are presented. Electrodiagnostic study (EDS) severity was used as a stratifying variable.
| Risk factor | Beta (SE) | Hazard Ratio [95% CI] | p |
|---|---|---|---|
| Age(/10 years) | −0.026 (0.036) | 0.975 [0.909, 1.05] | 0.471 |
| Gender (male) | −0.155 (0.111) | 0.856 [0.689, 1.06] | 0.162 |
| Diabetes mellitus | −0.127 (0.191) | 0.881 [0.606, 1.28] | 0.508 |
| Rheumatoid arthritis | −0.467 (0.224) | 0.627 [0.404, 0.972] | 0.037 |
| Peripheral neuropathy or Cervical radiculopathy | −0.082 (0.179) | 0.921 [0.649, 1.31] | 0.647 |
| Injectate volume | −0.129 (0.045) | 0.879 [0.804, 0.960] | 0.004 |
| Effective dose of steroid | −0.002 (0.002) | 0.998 [0.994, 1.00] | 0.510 |
Table 3.
Demographics and clinical characteristics and their association with subsequent carpal tunnel release in patients with carpal tunnel syndrome after initial treatment with corticosteroid injection in a Cox proportional hazards model. Risk factors, model parameter estimates (Beta) and standard errors (SE), hazard ratios, 95% confidence intervals (CIs), and model p-values are presented. Electrodiagnostic study (EDS) severity was used as a stratifying variable.
| Risk factor | Beta (SE) | Hazard Ratio [95% CI] | p |
|---|---|---|---|
| Age(/10 years) | −0.036 (0.037) | 0.965 [0.897, 1.04] | 0.336 |
| Gender (male) | −0.177 (0.115) | 0.837 [0.668, 1.05] | 0.124 |
| Diabetes mellitus | −0.078 (0.194) | 0.925 [0.632, 1.35] | 0.687 |
| Rheumatoid arthritis | −0.708 (0.266) | 0.493 [0.292, 0.830] | 0.008 |
| Peripheral neuropathy or Cervical radiculopathy | −0.011 (0.190) | 0.989 [0.682, 1.44] | 0.955 |
| Injectate volume | −0.098 (0.046) | 0.906 [0.827, 0.993] | 0.034 |
| Effective dose of steroid | −0.004 (0.002) | 0.996 [0.991, 1.00] | 0.105 |
Figure 3.
Survival curve for injection cohort stratified by electrodiagnostic study (EDS) severity based on estimates from a Cox proportional hazards model. The table underneath the figure represents the number remaining at risk (i.e. who are still being followed and have not yet experienced the event of interest) at baseline, 2, 4, 6, 8, and 10 years after the initial injection for the outcome of any retreatment (re-injection or carpal tunnel release).
Figure 4.
Survival curve for injection cohort stratified by electrodiagnostic study (EDS) severity based on estimates from a Cox proportional hazards model. The table underneath the figure represents the number remaining at risk (i.e. who are still being followed and have not yet experienced the event of interest (carpal tunnel release)) at baseline, 2, 4, 6, 8, and 10 years after the initial injection within each stratum.
Injection versus Surgical cohort
There were 931 unique subjects who received carpal tunnel surgery without previously receiving an injection in 2001–2010; 300 were randomly selected to represent this group. Of the 300 subjects in the direct to surgery sample, 104 had surgery on both hands (total N=404 hands), 35.3% were male, 15.7% had diabetes mellitus, 2.0% had rheumatoid arthritis, and 8.33% had peripheral neuropathy or cervical radiculopathy. They were on average 55.0 (SD 14) years of age and 9.65%, 3.47%, 16.3%, 44.8%, 25.7% had EDS severities of untested, normal, mild, moderate, and severe respectively. Compared to the injection cohort, they were older (p<0.001), had a lower proportion of patients with rheumatoid arthritis (p=0.028), a higher proportion of diabetics (p=0.003), and more severe EDS results (p<0.001). Table 4 shows the demographics and clinical characteristics of the surgical sample and comparison to characteristics of the injection cohort.
Table 4.
Demographics and clinical characteristics comparing subjects who went directly to surgery (i.e. carpal tunnel release without previous corticosteroid injection: surgical sample) and the injection cohort. *Hand-level characteristics
| Surgical sample Subject level N= 300 Hand level N= 404* |
Injection Cohort Subject level N=595 Hand level N= 774* |
p | |
|---|---|---|---|
| Characteristic | |||
| Demographics | 55 (14) | 50.6 (13.5) | <0.001 |
| Age at intervention, year, mean (SD)* | |||
| Gender (Male) (N/%) | 106 (35.3%) | 181 (30.4%) | 0.158 |
| EDS severity (N/%)* | <0.001 | ||
| Normal | 14 (3.47%) | 64 (8.27%) | |
| Mild | 66 (16.3%) | 229 (29.6%) | |
| Moderate | 181 (44.8%) | 319 (41.2%) | |
| Severe | 104 (25.7%) | 50 (6.46%) | |
| Unknown | 39 (9.65%) | 112 (14.5%) | |
| Comorbidity (N/%) | |||
| Diabetes mellitus | 47 (15.7%) | 52 (8.74%) | 0.003 |
| Rheumatoid arthritis | 6 (2%) | 32 (5.38%) | 0.028 |
| Peripheral neuropathy/Cervical radiculopathy | 25 (8.33%) | 46 (7.73%) | 0.854 |
Discussion
In this population-based cohort, with median follow-up of 7.4 years, 32% of the subjects did not receive subsequent treatment after a single steroid injection. The presence of rheumatoid arthritis and a higher volume of injectate were associated with decreased likelihood of subsequent treatment.
Most guidelines suggest a course of non-operative treatment in patients diagnosed with CTS, however there remains controversy about the role of steroid injections. Some physicians consider corticosteroid injections to be a diagnostic tool only (4, 5). It has been suggested that regional variation in the use of surgery for many conditions is mainly a result of physician beliefs about the indications for surgery (18, 26). This may explain the regional variation in rates of carpal tunnel release (27). Our result, that approximately a third of patients did not receive subsequent treatment in the long term suggests a role for corticosteroid injections in the treatment of CTS. A local corticosteroid injection is less invasive and less expensive compared to surgery and does not require time off from work. Although corticosteroids injections do come with risks and potential adverse events, as median nerve injury or infection may occur (28), the low morbidity and low cost of a steroid injection make it an excellent form of initial treatment in some CTS patients (29–31). We believe that this approach is especially relevant since the natural history of CTS remains unknown, and some patients experience spontaneous improvement in their symptoms, with reported rates of 33% to 40% experiencing some improvement, depending on EDS severity (32–35).
The rate of subsequent treatment found in this study correlates with previous studies, which mostly looked at shorter term follow up. Meys et al. followed 113 patients who received an injection for CTS and found that 67% had surgery within one year (12). Jenkins et al. found that 33% of the patients receiving a local corticosteroid injection underwent carpal tunnel release within 5 years post initial treatment (13). The proportion is lower than in our study, but their result was based on patients with mild to moderately severe CTS only. Berger et al. found that 75% underwent surgery after a single injection, a slightly higher proportion than our result (10). In that prospective study patients were offered a re-injection or surgery when there was minor or no relief of symptoms at follow-up, which might have resulted in a higher proportion of injection failure. A randomized placebo-controlled trial on the effect of steroid dose showed a surgery rate of 73% – 81% at one year follow-up (8). The choice to proceed to surgery was solely made by the patient, however; the study design might make it challenging to extrapolate the failure rate to a clinical setting, because the participation in an RCT and chance of receiving a placebo injection may heighten vigilance of patients, thereby increasing the chance of residual symptoms (36). Our retrospective study was free from this type of bias.
The proportion of patients undergoing surgery after the second injection was similar to the proportion proceeding to surgery after one injection. Ashworth and Bland assessed the effectiveness of second corticosteroid injections for carpal tunnel syndrome in 229 patients (37). They found that the change in Boston Symptom Severity Scale and Functional Status Scale was not significantly different between first and second injections and concluded that second injections appear to be at least as effective as first injections. To the best of our knowledge, the maximum number of injections that an individual might benefit from is unknown (10).
Several risk factors for recurrence after a single steroid injection have previously been identified. However, the negative association between a diagnosis of rheumatoid arthritis and subsequent treatment found in this study has not been previously described. Rheumatoid arthritis associated CTS might respond better to an injection due to the underlying pathophysiology; an inflammatory condition versus non-inflammatory fibrosis in idiopathic CTS (38). However, we cannot rule out that rheumatoid arthritis was a confounder in our cohort. Rheumatologists might be less likely to refer patients to a hand surgeon for a carpal tunnel release. The effect of volume of injection has not been studied in depth. A Cochrane review by Marshall et al. stated that no particular dosage or type of medication provided a superior outcome for the treatment of CTS (6). Our results that a larger volume of injection is associated with lower risk of injection failure could be related to greater fluid distribution or greater contact area with the soft tissues within the carpal tunnel (39). The finding that patients with more severe EDS results are more likely to experience injection failure has already been documented (14).
In contrast to previous studies, we did not find a diagnosis of diabetes mellitus to be a predictor for subsequent treatment (11, 13, 15). However, there was a significantly smaller proportion of patients with diabetes mellitus in the injection cohort compared to the surgical sample. Thus, patients with diabetes mellitus might have had more severe CTS and were therefore more likely to proceed to surgery directly or their physicians may have been more likely to operate on CTS patients with diabetes because it has already been well-described that they are less likely to benefit from an injection.
Our study has some limitations. First, this study was retrospective in design and lacked a control group. Ideally, there should have been a control group of patients without treatment, since some patients improve spontaneously (34, 35). Second, our outcome measure where failure of injection is defined as receiving subsequent treatment may not adequately capture clinically relevant failures, where patients have ongoing symptoms of CTS but elect for some other reason not to receive subsequent treatment. Third, there was variability in techniques of corticosteroid injection that were used. Literature suggests that some techniques are more effective than others and this might have affected the results (39, 40). Fourth, although the study is based on a large number of patients, we have to take into account an exclusion of about 7% of potential cases because the patients had not authorized the use of their medical records for research (16). Finally, despite the comprehensive nature of the REP medical record linkage system, it is possible that some patients received treatment from non-REP providers, for example while traveling. Despite these limitations, the strength of this study is the comparison with patients who proceeded directly to surgery which describes the characteristics of the full population-based cohort of patients treated for CTS.
In conclusion, this study shows that a substantial proportion of the patients undergoing a steroid injection for CTS did not receive subsequent treatment, even after a lengthy follow up, ranging up to 12 years. Further research is necessary to identify those patients who will benefit in the long term from a corticosteroid injection, in order to provide more individualized treatment for patients with CTS.
Table 1.
Demographics and clinical characteristics of the injection cohort. *Hand-level characteristics
| Subject level (N=595) Hand level* (N=774) |
|
|---|---|
| Age at injection* Mean (SD) | 50.6 (13.5) |
| Gender (Male) (N/%) | 181 (30.4%) |
| Diabetes mellitus (N/%) | 52 (8.74%) |
| Peripheral neuropathy or cervical radiculopathy (N/%) | 46 (7.73%) |
| Rheumatoid arthritis (N/%) | 32 (5.38%) |
| EDS severity* | |
| Normal | 64 (8.27%) |
| Untested | 112 (14.5%) |
| Mild | 229 (29.6%) |
| Moderate | 319 (41.2%) |
| Severe | 50 (6.46%) |
| Injectate volume (mL)* Mean (SD) | 3.66 (1.16) |
| Effective steroid dose (mg)* Mean (SD) | 39.9 (22.3) |
Acknowledgments
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The project was additionally supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780, NIH/NIAMS Grant Number R01AR62613 and Mayo Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: No conflicts of interest to report.
References
- 1.Atroshi I, Englund M, Turkiewicz A, et al. Incidence of physician-diagnosed carpal tunnel syndrome in the general population. Archives of internal medicine. 2011;171:943–944. doi: 10.1001/archinternmed.2011.203. [DOI] [PubMed] [Google Scholar]
- 2.Gelfman R, Melton LJ, 3rd, Yawn BP, et al. Long-term trends in carpal tunnel syndrome. Neurology. 2009;72:33–41. doi: 10.1212/01.wnl.0000338533.88960.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. Journal of neurology, neurosurgery, and psychiatry. 2006;77:263–265. doi: 10.1136/jnnp.2005.066696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218–219. doi: 10.2106/JBJS.I.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huisstede BM, Friden J, Coert JH, et al. Carpal tunnel syndrome: hand surgeons, hand therapists, and physical medicine and rehabilitation physicians agree on a multidisciplinary treatment guideline-results from the European HANDGUIDE Study. Arch Phys Med Rehabil. 2014;95:2253–2263. doi: 10.1016/j.apmr.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. The Cochrane database of systematic reviews. 2007:CD001554. doi: 10.1002/14651858.CD001554.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments--a systematic review. Archives of physical medicine and rehabilitation. 2010;91:981–1004. doi: 10.1016/j.apmr.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309–317. doi: 10.7326/0003-4819-159-5-201309030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Graham RG, Hudson DA, Solomons M, et al. A prospective study to assess the outcome of steroid injections and wrist splinting for the treatment of carpal tunnel syndrome. Plast Reconstr Surg. 2004;113:550–556. doi: 10.1097/01.PRS.0000101055.76543.C7. [DOI] [PubMed] [Google Scholar]
- 10.Berger M, Vermeulen M, Koelman JH, et al. The long-term follow-up of treatment with corticosteroid injections in patients with carpal tunnel syndrome. When are multiple injections indicated? J Hand Surg Eur Vol. 2013;38:634–639. doi: 10.1177/1753193412469580. [DOI] [PubMed] [Google Scholar]
- 11.Blazar PE, Floyd WEt, Han CH, et al. Prognostic Indicators for Recurrent Symptoms After a Single Corticosteroid Injection for Carpal Tunnel Syndrome. J Bone Joint Surg Am. 2015;97:1563–1570. doi: 10.2106/JBJS.N.01162. [DOI] [PubMed] [Google Scholar]
- 12.Meys V, Thissen S, Rozeman S, et al. Prognostic factors in carpal tunnel syndrome treated with a corticosteroid injection. Muscle Nerve. 2011;44:763–768. doi: 10.1002/mus.22183. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand (N Y) 2012;7:151–156. doi: 10.1007/s11552-012-9390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser LH, Ngo Q, Groeneweg SJ, et al. Long term effect of local corticosteroid injection for carpal tunnel syndrome: a relation with electrodiagnostic severity. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2012;123:838–841. doi: 10.1016/j.clinph.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan SJ, Glickel SZ, Eaton RG. Predictive factors in the non-surgical treatment of carpal tunnel syndrome. J Hand Surg Br. 1990;15:106–108. doi: 10.1016/0266-7681_90_90061-8. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rempel D, Evanoff B, Amadio PC, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. Am J Public Health. 1998;88:1447–1451. doi: 10.2105/ajph.88.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ustun N, Tok F, Yagz AE, et al. Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: A single-blind randomized prospective study. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2013;92:999–1004. doi: 10.1097/PHM.0b013e31829b4d72. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Park Y, Park KD, et al. Effectiveness of ultrasound-guided carpal tunnel injection using in-plane ulnar approach: a prospective, randomized, single-blinded study. Medicine. 2014;93:e350. doi: 10.1097/MD.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leversee JH. Aspiration of joints and soft tissue injections. Primary care. 1986;13:579–599. [PubMed] [Google Scholar]
- 22.Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle & nerve. 1997;20:1477–1486. doi: 10.1002/(sici)1097-4598(199712)20:12<1477::aid-mus1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Therneau T. A Package for Survival Analysis in R. version 2.38. 2015. [Google Scholar]
- 24.Therneau TMaG, PM . Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 25.R Core Team. R: A language and environment for statistical computing. R foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- 26.Birkmeyer JD, Reames BN, McCulloch P, et al. Understanding of regional variation in the use of surgery. Lancet. 2013;382:1121–1129. doi: 10.1016/S0140-6736(13)61215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelfman R, Amadio PC. Trends in carpal tunnel release in the United States. J Hand Surg Am. 2013;38:210. doi: 10.1016/j.jhsa.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 28.McConnell JR, Bush DC. Intraneural steroid injection as a complication in the management of carpal tunnel syndrome. A report of three cases. Clin Orthop Relat Res. 1990:181–184. [PubMed] [Google Scholar]
- 29.Palmer DH, Hanrahan LP. Social and economic costs of carpal tunnel surgery. Instructional course lectures. 1995;44:167–172. [PubMed] [Google Scholar]
- 30.Karl JW, Gancarczyk SM, Strauch RJ. Complications of Carpal Tunnel Release. Orthop Clin North Am. 2016;47:425–433. doi: 10.1016/j.ocl.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62:352–356. doi: 10.3171/jns.1985.62.3.0352. [DOI] [PubMed] [Google Scholar]
- 32.Gong HS, Baek GH, Oh JH, et al. Factors affecting willingness to undergo carpal tunnel release. J Bone Joint Surg Am. 2009;91:2130–2136. doi: 10.2106/JBJS.H.01221. [DOI] [PubMed] [Google Scholar]
- 33.Pensy RA, Burke FD, Bradley MJ, et al. A 6-year outcome of patients who cancelled carpal tunnel surgery. J Hand Surg Eur Vol. 2011;36:642–647. doi: 10.1177/1753193411410155. [DOI] [PubMed] [Google Scholar]
- 34.Padua L, Padua R, Lo Monaco M, et al. Natural history of carpal tunnel syndrome according to the neurophysiological classification. Ital J Neurol Sci. 1998;19:357–361. doi: 10.1007/BF02341782. [DOI] [PubMed] [Google Scholar]
- 35.Resende LA, Tahara A, Fonseca RG, et al. The natural history of carpal tunnel syndrome. A study of 20 hands evaluated 4 to 9 years after initial diagnosis. Electromyogr Clin Neurophysiol. 2003;43:301–304. [PubMed] [Google Scholar]
- 36.Hrobjartsson A, Kaptchuk TJ, Miller FG. Placebo effect studies are susceptible to response bias and to other types of biases. J Clin Epidemiol. 2011;64:1223–1229. doi: 10.1016/j.jclinepi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashworth NL, Bland JD. Effectiveness of second corticosteroid injections for carpal tunnel syndrome. Muscle Nerve. 2013;48:122–126. doi: 10.1002/mus.23725. [DOI] [PubMed] [Google Scholar]
- 38.Ettema AM, Amadio PC, Zhao C, et al. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. The Journal of bone and joint surgery. American volume. 2004;86-A:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Ozturk K, Esenyel CZ, Sonmez M, et al. Comparison of carpal tunnel injection techniques: a cadaver study. Scandinavian journal of plastic and reconstructive surgery and hand surgery / Nordisk plastikkirurgisk forening [and] Nordisk klubb for handkirurgi. 2008;42:300–304. doi: 10.1080/02844310802401363. [DOI] [PubMed] [Google Scholar]
- 40.MacLennan A, Schimizzi A, Meier KM, et al. Comparison of needle position proximity to the median nerve in 2 carpal tunnel injection methods: a cadaveric study. J Hand Surg Am. 2009;34:875–879. doi: 10.1016/j.jhsa.2009.01.028. [DOI] [PubMed] [Google Scholar]