Abstract
Although earlier models of brain circuitry controlling wake-sleep focused on monaminergic and cholinergic arousal systems, recent evidence indicates that these play mainly a modulatory role, and that the backbone of the wake-sleep regulatory system depends upon fast neurotransmitters, such as glutmate and GABA. We review here recent advances in understanding the role these systems play in controlling sleep and wakefulness.
The first hypothesis about neuronal circuitry supporting wake-sleep came from the observations of the Baron Constantin von Economo [1] in patients with the viral illness, encephalitis lethargica in the 1920’s. He proposed a wake-promoting influence from the brainstem that kept the forebrain awake, and a sleep-promoting influence from the anterior hypothalamus that opposed waking drive during sleep. The wake-promoting system was later equated with inputs from the upper brainstem reticular formation to the thalamus.
In the latter decades of the 20th century, this hypothesis was refined. The wake-promoting influence was thought to arise mainly from monoaminergic neurons (noradrenergic locus coeruleus, serotonergic raphe nuclei, histaminergic tuberomammillary nucleus) and cholinergic neurons (in the pedunculopontine and laterodorsal tegmental nuclei) in the upper brainstem [2]. This influence was found both to innervate the thalamus (where it promoted transfer of information to the cerebral cortex) and to pass through the more ventrally situated hypothalamus (where it relayed information to and picked up axons from peptidergic orexin/hypocretin arousal neurons) and the basal forebrain (once more being augmented by cholinergic neurons) to project to the entire cerebral cortex. A sleep-promoting cell group was meanwhile identified in the ventrolateral preoptic (VLPO) and median preoptic nuclei, which provide GABAergic innervation of the entire wake-promoting system, thus allowing them to inhibit arousal during sleep [3;4]. A number of reviews of these cell groups and their interactions in regulating wake-sleep cycles have appeared in recent years [5;6], and they will not be the focus of this review.
Over the last 10 years, a number of new lines of investigation have indicated that this “standard model” of wake-sleep circuitry has serious deficiencies. For example, destroying neurons in the individual components of the ascending arousal system, including cholinergic neurons of the basal forebrain [7], orexin or histaminergic neurons in the hypothalamus [8;9], or locus coeruleus noradrenergic [10] or pedunculopontine tegmental cholinergic neurons in the pons [11;12], had little effect on the daily amount of wake-sleep in rodents. Even destroying several of these sites in the same animal (basal forebrain cholinergic, orexin, and locus coeruleus neurons) had no clear effect on the amount of wake-sleep [13]. In addition, large lesions destroying virtually the entire thalamus in rats was shown to have little effect on the amount of wake-sleep, or even the EEG pattern during wakefulness [7].
In contrast to the “standard model,” a new paradigm of wake-sleep circuitry has emerged in the last few years that is based on our understanding of circuits using fast neurotransmitters (glutamate and GABA), rather than modulatory neurotransmitters (monoamines, acetylcholine, peptides). The ability to detect, map, and manipulate these fast neurotransmitter circuits relies on their use of specific vesicular transporters to load them into synaptic vesicles [14–16]. This is important because these same amino acids that are used as neurotransmitters are used in protein biosynthesis and the molecules that synthesize or degrade them are ubiquitous in the nervous system, whereas the ability to load these amino acid transmitters into synaptic vesicles is now recognized as nearly synonymous with using them for synaptic neurotransmission. Thus, neurons that contain the vesicular GABA transporter (Vgat) reliably release GABA (or glycine, as the same vesicular transporter handles both) and hence mark neurons as inhibitory, whereas many neurons that contain glutamic acid decarboxylase, the enzyme that synthesizes GABA, but not Vgat may not use GABAergic neurotransmission. The situation is similar for synaptic release of glutamate, which requires vesicular transporters (Vgluts), although there are three different Vglut genes. Most glutamatergic CNS neurons appear to contain only one Vglut, and very few of these also contain Vgat (although there are exceptions, see section on supramammillary neurons below). Deleting the Vglut or Vgat from a neuron has been shown to eliminate its ability to use the cognate molecules for synaptic transmission [15;16]. Mice that express Cre recombinase under the promoters for Vglut or Vgat genes can be used to manipulate the neurons with optogenetic or chemogenetic approaches, or to trace their connections selectively.
In the remainder of this review, we will examine briefly the evidence for the role of fast neurotransmitters in specific wake-sleep circuits, where we have evidence that they are necessary to maintain a normal amount and relationship of sleep and wake.
Basal forebrain arousal system
The basal forebrain (BF) contains three different sets of neurons that project directly to the cerebral cortex: cholinergic, GABAergic, and glutamatergic[17;18]. Juxtacellular recordings found that the cholinergic neurons were most active in wake and REM sleep, and that about 36% of GABAergic neurons had a similar activity pattern and the activity of these cells correlated with cortical gamma oscillations, usually attributed to parvalbumin GABA neurons in the BF. Other BF GABAergic neurons fired most rapidly during sleep. About half of non-GABA, non-cholinergic cells (presumably mostly glutamatergic) fired most rapidly during wake, but almost as many fired mainly during sleep [19]. However, it is difficult from this approach to determine which of these neurons might drive wakefulness. The cholinergic neurons produce the p75 nerve growth factor receptor, and can be selectively killed using IgG192-saporin, a monoclonal antibody against that receptor linked to the cellular toxin saporin. Lesions of these neurons alone, however, have only minimal effects on cortical EEG (more slow wave power), and none on amount of wake-sleep [7;20]. Lesions of the non-cholinergic neurons in the BF, using orexin-saporin, had similar lack of effect on the amount of wake-sleep or the EEG [7]. However, large combined lesions of all cell types in the BF using higher doses of orexin-saporin, caused a permanent high voltage, slow wave state associated with behavioral unresponsiveness (although the animals retained the righting reflex and showed some disorganized EMG activity). A recent study using optogenetic activation of the three classes of BF neurons found that all three promoted wakefulness [21]. Activation of the parvalbumin-containing GABA cells in the BF increased high frequency cortical EEG, and inhibition of them reduced high frequency cortical EEG power [22]. However, chemogentic activation of glutamatergic neurons had little effect on wake-sleep or EEG; stimulation of cholinergic neurons reduced slow waves in the EEG but did not change the amount of wake-sleep; and activation of GABAergic neurons strongly promoted wake and the associated faster EEG rhythms [23;24]. Thus the BF arousal system, in particular the GABAergic parvalbumin neurons, are critical for driving wakefulness and the associated high frequency EEG rhythms (Fig. 1). The cholinergic neurons in the BF appear to play a less prominent role when the parvalbumin GABA neurons are intact, but they can compensate for loss of the GABA/parvalbumin neurons [23]. Other BF GABAergic neurons, e.g., those containing somatostatin, appear to act as inhibitory interneurons, which reduce the level of arousal [21].
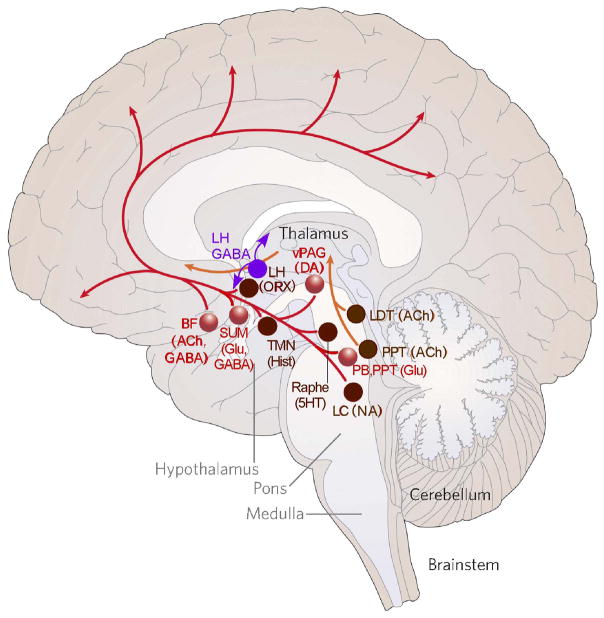
Figure 1.
A diagram summarizing the fast neurotransmitter systems that appear to play the largest role in promoting wakefulness, based on results of lesions and opto- and chemogenetic excitation and inhibition. Note that the monoaminergic, cholinergic, and peptidergic neurons in the brainstem and hypothalamus, which were prominent in earlier models, are here shown in brown as they appear to play a modulatory role, but lesions in these locations have little effect on wake-sleep amounts. The backbone of the arousal system in this model, shown in red, is the glutamatergic input from the parabrachial nucleus (PB) and pedunculopontine tegmental nucleus (PPT) to the basal forebrain, and the GABAergic and cholinergic neurons in the basal forebrain (BF) that diffusely innervate the cerebral cortex. Lesions at these sites cause complete loss of consciousness, whereas lesions of supramammillary (SUM) glutamatergic neurons or dopaminergic (DA) neurons in the ventral periaqueductal gray matter (vPAG) near the dorsal raphe nucleus cause about a 20% loss of wake time. In addition, two populations of GABAergic neurons in the lateral hypothalamus (LH), shown in purple, have recently been proposed to promote wakefulnes by inhibiting sleep promoting neurons in the thalamus and preoptic area. Abbreviations: 5HT, serotonin; Ach, acetylcholine; Hist, histamine; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus NA, noradrenlaine; ORX, orexin; TMN, tuberomammillary nucleus Modified with permission from [2].
Parabrachial and pedunculopontine glutamatergic arousal system
Retrograde tracers from the BF have consistently identified one brainstem site of input that is not part of the classical monoaminergic ascending arousal system: glutamatergic neurons in the parabrachial and pedunculopontine nucleus [7]. Large lesions of this area with orexin-saporin caused a permanent high voltage, slow wave EEG, similar to the large BF lesions, with no behavioral arousal and no EMG evidence of arousal, although the righting reflex was intact. This system probably consists of several separate wake-promoting cell populations, which are active under different conditions. Juxtacellular recordings from pedunculopontine neurons have found that nearly all cholinergic neurons in this region, as well as many glutamatergic and GABAergic neurons, are most active during wake and REM sleep [25], although some of the latter neurons were maximally active during either wake or REM, but not both. Neurons in the external lateral parabrachial nucleus (PBel), for example, are known to be activated in a variety of situations signaling noxious visceral stimuli [26–28]. These include pain, gastric stretch, as well as hypoxia or hypercarbia. They provide heavy innervation to the lateral hypothalamus, central nucleus of the amygdala, and BF [29;30]. Either deleting Vglut2 from these neurons or selectively killing them with a Cre-dependent diphtheria toxin A chain resulted in greatly diminished awakening to a CO2 stimulus during sleep, but had minimal effects on baseline wake-sleep [26]. Deletions of Vglut2 from the medial PB caused modest (about 20%) reductions in baseline wakefulness and increased delta power during NREM sleep. Chemogenetic activation of the pedunculopontine glutamatergic (possibly including an unknown number of parabrachial neurons) has been shown to cause profound wakefulness, and inhibition to produce increased NREM sleep [31]. Activation of cholinergic pedunculopontine neurons mainly reduced EEG slow waves during NREM sleep, and activation of GABAergic neurons mainly reduced REM sleep, but neither had major effects on the amount of wake-sleep.
Supramammillary glutamatergic and GABAergic arousal system
Another population of neurons that projects extensively to the BF and cerebral cortex is found in the supramammillary area [32]. Although these neurons were identified more than 30 years ago, recent studies have demonstrated that most of these neurons contain glutamate, and that some also contain GABA [33;34]. Supramammillary neurons that project to the dentate gyrus of the hippocampus may release both neurotransmitters, and are thought to be active during REM sleep [34]. However, the cortically projecting neurons are mainly just glutamatergic, and chemogenetic activation of them strongly promotes waking [35]. Deletion of Vglut2 from these neurons causes small (15–20%) reductions in total wake time.
Lateral hypothalamic arousal and sleep systems
The lateral hypothalamic area contains two important peptidergic neuronal systems. Orexin (hypocretin) neurons, which also are glutamatergic, fire most rapidly during active wakefulness, and activating these neurons drives wakefulness [36;37]. Knocking out orexin from these neurons cause narcolepsy; although this does not affect the amount of total wake or sleep, it reduces the consolidation of both states and causes more REM sleep to occur during the dark phase, when rodents are normally awake [38]. Although the orexin neurons also contain glutamate, killing them by causing expression of ataxin-3 under the orexin promoter, has no more effect on wake-sleep than deleting the orexin gene [39]. A second peptidergic system, the melanin-concentrating hormone (MCH) neurons, also contains Vglut2 [40]. Some of these neurons also contain glutamic acid decarboxylase, but apparently not Vgat and they fire maximally during REM sleep [41]. Studies suggest that they may release either glutamate or GABA, at different targets [40;42]. Recent optogenetic studies activating the MCH neurons indicate that they promote primarily REM sleep, although they may have some effects on promoting NREM under some circumstances too [42–44]. It is not known whether release of MCH, glutamate, GABA or some other transmitter underlies REM promotion. Two populations of GABAergic neurons in the lateral hypothalamus that also cause wake have recently been described [45;46]. Optogenetic activation of a population that is apparently in the dorsal lateral hypothalamus or zona incerta is thought to cause wakefulness by means of projections to the reticular nucleus of the thalamus, whereas chemogenetic activation of a GABAergic group in the ventral lateral hypothalamus causes wake, presumably by inhibiting the VLPO.
Preoptic sleep-promoting neurons
Sleep-active neurons in the VLPO and median preoptic nuclei were identified as GABAergic [3;4], although most of these in the ventrolateral preoptic nucleus also contain the peptide galanin. Because lesions in the ventrolateral preoptic area cause loss of up to 40% of total sleep in rats and mice, these neurons have been considered to be sleep-promoting. Recent opto- and chemogenetic studies have found that activation of these neurons does indeed induce profound increases in sleep [47]. However, it is not known whether this response is due to GABA, galanin, or to release of some other transmitter.
Parafacial zone GABAergic sleep-promoting neurons
Although literature going back to the 1960’s reported a “synchronizing” influence over the EEG coming from the lower brainstem, there was no clear idea of how this ascending sleep-promoting circuit was organized, nor was it clear if this influence promoted sleep. Anaclet and colleagues were able to identify such a pathway originating from GABAergic neurons in the parafacial zone, a region of reticular formation just ventrolateral to the genu of the facial nerve [48]. They found that these neurons innervated and presumably inhibited the wake-promoting parabrachial nucleus, and that their sleep-promoting activity depended on this pathway.
Sublaterodorsal glutamatergic REM sleep generator
During the sleep bout, the brain transitions from a slow wave state to one with a faster, low voltage EEG and loss of muscle tone. This state is associated with Rapid Eye Movements, thus giving it the name of REM sleep. The REM state appears to be generated by a population of glutamatergic neurons in the region just ventral to the sublaterodorsal nucleus in rodents, or the locus coeruleus in cats [49;50]. Thus, in rodents where these neurons have received closer study, they are called the sublaterodorsal nucleus, but in cats or humans, what is presumably the same population is often referred to as the subcoeruleus region [51]. Sublaterodorsal neurons presumably cause motor atonia due to activation of inhibitory interneurons in both the medulla and the spinal cord. Their mechanism for causing EEG desynchronization is not known, although there are several nearby cell groups (parabrachial nucleus, pedunculopontine and laterodorsal tegmental nucleus) which contain REM active neurons and which innervate the forebrain [49;52]. The activation of eye movements is presumed to be due to short projections to the paramedian pontine reticular formation, but this has yet to be tested. The descending projections come primarily from the ventral part of the sublaterodorsal nucleus. Deleting the Vglut2 gene in this area suppresses REM sleep, whereas selective chemogenetic activation of SLD Vglut2 neurons potently drives REM sleep [53] [54].
Ventrolateral periaqueductal gray matter REM inhibition
A main control over the sublaterodorsal REM generator apparatus is provided by inhibitory, largely GABAergic neurons in the nearby ventrolateral periaqueductal gray matter, at the level where the cerebral aqueduct begins to open into the fourth ventricle. Some of these neurons also spill out into the laterally adjacent pontine tegmentum. Lesions of these neurons releases excess REM sleep [49] [52].
Medullary cell groups that promote REM sleep
A recent study reported a cell group in the ventral medullary tegmentum, whose optogenetic stimulation caused REM sleep [55]. These neurons were maximally active during REM sleep, but showed some activity during waking as well, particularly during eating and grooming. Because the injections in the ventral medulla were large, it was not possible in these experiments to determine their exact location. However, previous work had identified GABAergic neurons in the ventrolateral medulla at this level that expressed cFos during REM sleep [56], so it is possible that these were the neurons that were being activated.
In summary, the landscape for cell groups that regulate wake-sleep has expanded dramatically over the last few years, as investigators have taken advantage of the newer methods that allow manipulation of cell groups that use fast neurotransmitters such as GABA and glutamate. The backbone of the systems that promote arousal and REM sleep appear to employ primarily these two fast neurotransmitters, whereas NREM sleep-promoting cell groups and those that inhibit REM generally have been found to use GABA as a neurotransmitter to inhibit arousal and REM-promoting systems. These fast neurotransmitter systems may interact with the classical monoaminergic modulatory systems in a variety of ways, including presynaptic modulation of transmitter release as well as modulating the activity of target neurons (e.g., in the cerebral cortex). Hence, absence of a modulatory system may have minimal effects on baseline wake-sleep, but may result in failure of the wake-sleep system to adjust to unusual conditions (e.g., a novel stimulus that requires wakefulness during the wrong circadian phase) [10]. The challenge for the next decade will be to uncover how these newly identified components work together and with the modulatory systems to regulate sleep and wakefulness.
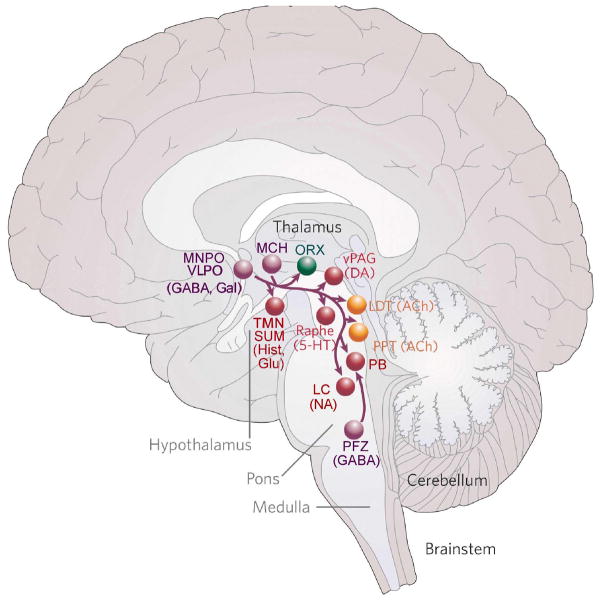
Figure 2.
A diagram summarizing the fast neurotransmitter systems that contribute to sleep promotion (shown in purple). Ventrolateral preoptic (VLPO) and median preoptic (MnPO) GABAergic neurons send axons to most components of the arousal system (shown in red, yellow, and green), and are thought to inhibit them in a coordinated fashion. Parafacial zone (PFZ) GABAergic neurons in the medulla induce sleep mainly by inhibiting the parabrachial glutamatergic arousal neurons. Melanin-concentrating hormone (MCH) neurons in the lateral hypothalamus contain both GABA and glutamate, and may be able to release them at different terminal sites. They innervate neurons in the brainstem that control REM sleep (see text for details). Other abbreviations as in Fig. 1. Modified with permission from [2].
Highlights.
Glutamatergic neurons in the parabrachial nucleus provide main ascending arousal influence from the brainstem.
GABAergic neurons in the brainstem provide the main ascending arousal influence from the forebrain.
GABAergic neurons in the preoptic area and parafacial zone inhibit the arousal system to cause sleep.
Acknowledgments
The authors thank USPHS grants NS085477, NS073613, and NS092652 for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259. [Google Scholar]
- 2.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 3.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. This review provides a more detailed description of the circuit basis by which the brain regulates transitions between sleep and wake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. A comprehensive review that provides a more detailed description of the circuit basis by which the brain regulates sleep and wake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. An experimental assessment of the structural basis by which the brain regulates and maintains behavioral and electrocortical arousal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerashchenko D, Chou TC, Blanco-Centurion CA, Saper CB, Shiromani PJ. Effects of lesions of the histaminergic tuberomammillary nucleus on spontaneous sleep in rats. Sleep. 2004;27:1275–1281. doi: 10.1093/sleep/27.7.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, Lu J. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30:14543–14551. doi: 10.1523/JNEUROSCI.3037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shouse MN, Siegel JM. Pontine regultaion of REM sllep components in cats--integrity of the penduculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571:50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrovic J, Ciric J, Lazic K, Kalauzi A, Saponjic J. Lesion of the pedunculopontine tegmental nucleus in rat augments cortical activation and disturbs sleep/wake state transitions structure. Exp Neurol. 2013;247:562–71. doi: 10.1016/j.expneurol.2013.02.007. Epub;%2013 Feb 26.:562–571. [DOI] [PubMed] [Google Scholar]
- 13.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–14048. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blakely RD, Edwards RH. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harb Perspect Biol. 2012;4:a005595. doi: 10.1101/cshperspect.a005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. A pioneering paper that first demonstrated that removing a vesicular transporter can cause neurons to lose the ability to package that neurotransmitter for synaptic release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- 18.Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected] J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- 19.Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–11840. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang WC, Weissbourd B, Sakai N, Luo L, Nishino S, Dan Y. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18:1641–1647. doi: 10.1038/nn.4143. An optogenetic investigation of the role of different classes of basal forebrain neurons in driving wakefulness vs. sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, Basheer R, Brown RE, McCarley RW. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci US A. 2015;112:3535–3540. doi: 10.1073/pnas.1413625112. The first optogenetic investigation of the role of parvalbumin-expressing basal forebrain neurons in regulating the cortical EEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E, Fuller PM. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6:8744. doi: 10.1038/ncomms9744.:8744. A chemogenetic analysis of the role of basal forebrain neurons in driving wakefulness and fast cortical rhythms associated with cognition which complements ref. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Yin D, Wang TX, Guo W, Dong H, Xu Q, Luo YJ, Cherasse Y, Lazarus M, Qiu ZL, Lu J, Qu WM, Huang ZL. Basal Forebrain Cholinergic Neurons Primarily Contribute to Inhibition of Electroencephalogram Delta Activity, Rather Than Inducing Behavioral Wakefulness in Mice. Neuropsychopharmacology. 2016;41:2133–2146. doi: 10.1038/npp.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708–4727. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013;33:7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. Demonstration of a specific circuit from the parabrachial nucleus that wakes up the brain during sleep apnea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial CGRP neurons control meal termination. Cell Metab. 2016;XX:YY–ZZ. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernard JF, Huang GF, Besson JM. The parabrachial area: Electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol. 1994;71:1646–1660. doi: 10.1152/jn.1994.71.5.1646. [DOI] [PubMed] [Google Scholar]
- 29.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Brain Res Rev. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 30.Bernard JF, Carroue J, Besson JM. Efferent projections from the external parabrachial area to the forebrain--a phaseolus-vulgaris leucoagglutinin study in the rat. Neurosci Lett. 1991;122:257–260. doi: 10.1016/0304-3940(91)90872-q. [DOI] [PubMed] [Google Scholar]
- 31.Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E, Scammell TE. Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice. J Neurosci. 2017;37:1352–1366. doi: 10.1523/JNEUROSCI.1405-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Hypothalamocortical projections. J Comp Neurol. 1985;237:21–46. doi: 10.1002/cne.902370103. [DOI] [PubMed] [Google Scholar]
- 33.Soussi R, Zhang N, Tahtakran S, Houser CR, Esclapez M. Heterogeneity of the supramammillary-hippocampal pathways: evidence for a unique GABAergic neurotransmitter phenotype and regional differences. Eur J Neurosci. 2010;32:771–785. doi: 10.1111/j.1460-9568.2010.07329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Renouard L, Billwiller F, Ogawa K, Clement O, Camargo N, Abdelkarim M, Gay N, Scote-Blachon C, Toure R, Libourel PA, Ravassard P, Salvert D, Peyron C, Claustrat B, Leger L, Salin P, Malleret G, Fort P, Luppi PH. The supramammillary nucleus and the claustrum activate the cortex during REM sleep. Sci Adv. 2015;1:e1400177. doi: 10.1126/sciadv.1400177. A paper exploring the role of the supramammillary nucleus neurons in causing the activation of the dentate gyrus during REM sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen NP, Saper CB, Fuller PM. Activation of Nos1 neurons in the caudal hypothalamus produces prolonged wakefulness. Society for Neuroscience Abstracts. 2016;42:254.07. [Google Scholar]
- 36.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de LL. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong YM, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 39.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 40.Chee MJ, Arrigoni E, Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 2015;35:3644–3651. doi: 10.1523/JNEUROSCI.4187-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D, Adamantidis AR. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka KF, Hasuwa H, Kilduff TS, Terao A, Yamanaka A. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci. 2014;34:6896–6909. doi: 10.1523/JNEUROSCI.5344-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konadhode RR, Pelluru D, Blanco-Centurion C, Zayachkivsky A, Liu M, Uhde T, Glen WB, Jr, van den Pol AN, Mulholland PJ, Shiromani PJ. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 2013;%19;33:10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Venner A, Anaclet C, Broadhurst RY, Saper CB, Fuller PM. A Novel Population of Wake-Promoting GABAergic Neurons in the Ventral Lateral Hypothalamus. Curr Biol. 2016;26:2137–2143. doi: 10.1016/j.cub.2016.05.078. Chemo- and optogenetic identification of GABA neurons in the lateral hypothalamus that promote wake by inhibiting the VLPO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Herrera CG, Cadavieco MC, Jego S, Ponomarenko A, Korotkova T, Adamantidis A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat Neurosci. 2016;19:290–298. doi: 10.1038/nn.4209. Optogenetic demonstration of GABAergic neurons in the lateral hypothalamus that promote wake by inhibiting the reticular nucleus of the thalamus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroeger D, Ferrari LL, Arrigoni E, Scammell TE, Saper CB, Vetrivelan R. Optogenetic activation of VLPO galaninergic neurons promotes sleep in mice. Society for Neuroscience Abstracts. 2016;41:816.13. [Google Scholar]
- 48*.Anaclet C, Ferrari L, Arrigoni E, Bass CE, Saper CB, Lu J, Fuller PM. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci. 2014;17:1217–1224. doi: 10.1038/nn.3789. The first paper identifying the parafacial zone as a key site for promoting sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 50.Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100:271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, Benarroch EE, Ahlskog JE, Smith GE, Caselli RC, Tippman-Peikert M, Olson EJ, Lin SC, Young T, Wszolek Z, Schenck CH, Mahowald MW, Castillo PR, Del TK, Braak H. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 52.Luppi PH, Clement O, Sapin E, Peyron C, Gervasoni D, Leger L, Fort P. Brainstem mechanisms of paradoxical (REM) sleep generation. Pflugers Arch. 2012;463:43–52. doi: 10.1007/s00424-011-1054-y. [DOI] [PubMed] [Google Scholar]
- 53.Anaclet C, Fuller PM. Differential regulation of wake, slow wave sleep, and REM sleep by pontomedullary GABAergic, glutamatergic, and phox2b-expressing neurons. Society for Neuroscience Abstracts. 2016;41:816.15. [Google Scholar]
- 54.Krenzer M, Anaclet C, Vetrivelan R, Wang N, Vong L, Lowell BB, Fuller PM, Lu J. Brainstem and spinal cord circuitry regulating REM sleep and muscle atonia. PLoS ONE. 2011;6:e24998. doi: 10.1371/journal.pone.0024998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Weber F, Chung S, Beier KT, Xu M, Luo L, Dan Y. Control of REM sleep by ventral medulla GABAergic neurons. Nature. 2015;526:435–438. doi: 10.1038/nature14979. The first study to demonstrate an executive role for medullary circuitry in REM control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sapin E, Lapray D, Berod A, Goutagny R, Leger L, Ravassard P, Clement O, Hanriot L, Fort P, Luppi PH. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS ONE. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]