Abstract
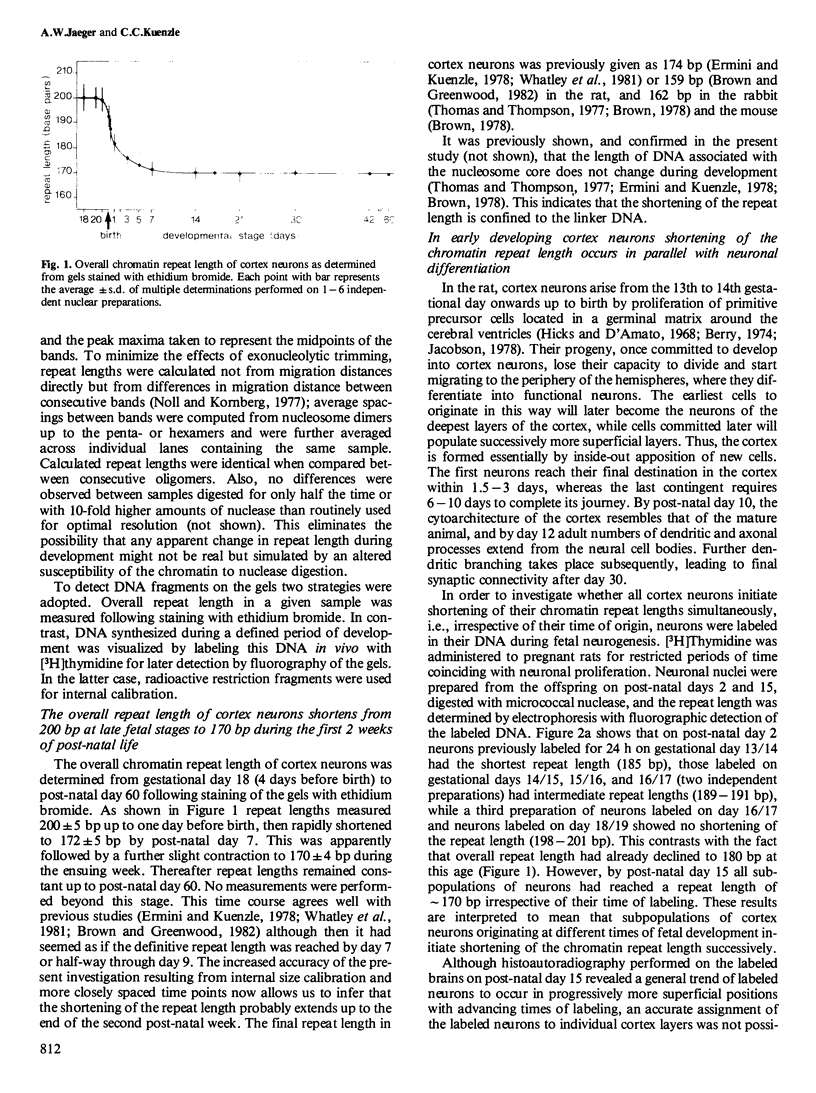
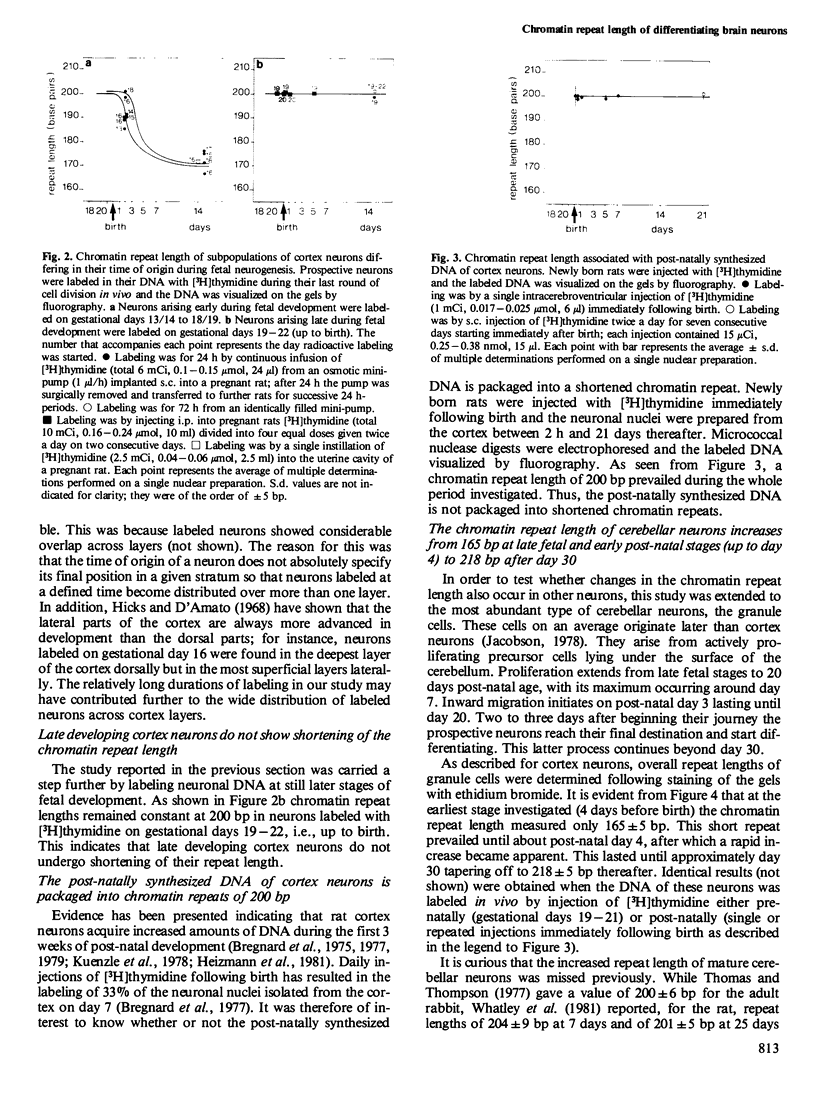
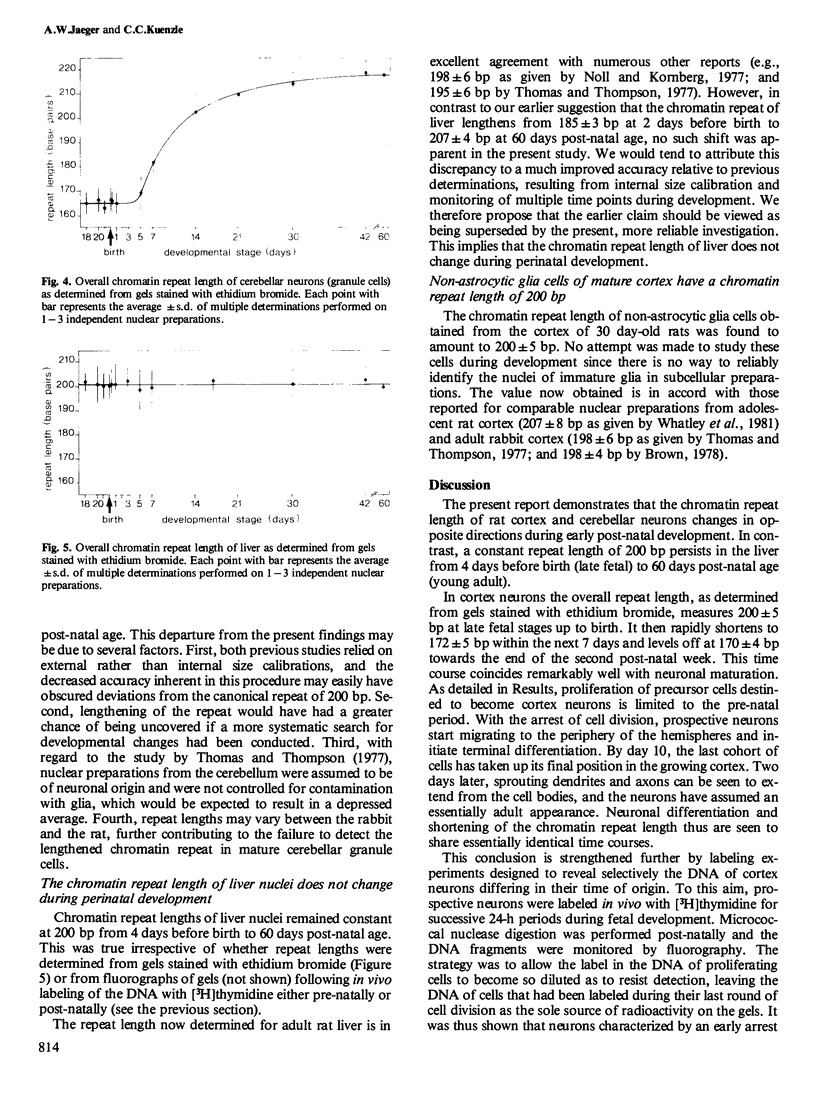
Chromatin repeat lengths in neuronal, glial, and liver nuclei of the rat were determined by micrococcal nuclease digestion followed by gel electrophoresis. The repeat length of cortex neurons decreased from 200 base pairs (bp) before birth to 170 bp at 14 days and all subsequent stages. Administration of [3H]thymidine to pregnant rats during the period of fetal neurogenesis allowed neurons differing in their time of origin to be labeled individually. This revealed that the shortening of the chromatin repeat length affected only neurons generated early during development, i.e., between gestational days 13/14 and 18/19, whereas neurons continuing to proliferate beyond gestational day 19 and up to birth (day 22) did not undergo shortening of their repeat length. In contrast to the cortex neurons, cerebellar neurons (granule cells) underwent lengthening of the repeat length from 165 bp at fetal and early post-natal stages (up to day 4) to 218 bp after day 30. Thus, in both cortex and cerebellar neurons the changes occurred temporally coincident with major developmental processes. No changes were detected in liver nuclei during the same period. Non-astrocytic glia cells of the adult cortex had 200 bp repeats.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Glover C. V., Bowen J. K., Gorovsky M. A. Histone variants specific to the transcriptionally active, amitotically dividing macronucleus of the unicellular eucaryote, Tetrahymena thermophila. Cell. 1980 Jul;20(3):609–617. doi: 10.1016/0092-8674(80)90307-4. [DOI] [PubMed] [Google Scholar]
- Arceci R. J., Gross P. R. Histone variants and chromatin structure during sea urchin development. Dev Biol. 1980 Nov;80(1):186–209. doi: 10.1016/0012-1606(80)90508-4. [DOI] [PubMed] [Google Scholar]
- Bregnard A., Knüsel A., Kuenzle C. C. Are all the neuronal nuclei polyploid? Histochemistry. 1975;43(1):59–61. doi: 10.1007/BF00490154. [DOI] [PubMed] [Google Scholar]
- Bregnard A., Kuenzle C. C., Ruch F. Cytophotometric and autoradiographic evidence for post-natal DNA synthesis in neurons of the rat cerebral cortex. Exp Cell Res. 1977 Jun;107(1):151–157. doi: 10.1016/0014-4827(77)90396-2. [DOI] [PubMed] [Google Scholar]
- Bregnard A., Ruch F., Lutz H., Kuenzle C. C. Histones and DNA increase synchronously in neurons during early postnatal development of the rat forebrain cortex. Histochemistry. 1979 Jul 11;61(3):271–279. doi: 10.1007/BF00508448. [DOI] [PubMed] [Google Scholar]
- Brown I. R. Postnatal appearance of short DNA repeat length in neurons of the cerebral cortex. Biochem Biophys Res Commun. 1978 Sep 29;84(2):285–292. doi: 10.1016/0006-291x(78)90168-7. [DOI] [PubMed] [Google Scholar]
- Ermini M., Kuenzle C. C. The chromatin repeat length of cortical neurons shortens during early posnatal development. FEBS Lett. 1978 Jun 1;90(1):167–172. doi: 10.1016/0014-5793(78)80322-6. [DOI] [PubMed] [Google Scholar]
- Heizmann C. W., Arnold E. M., Kuenzle C. C. Fluctuations of non-histone chromosomal proteins in differentiating brain cortex and cerebellar neurons. J Biol Chem. 1980 Dec 10;255(23):11504–11511. [PubMed] [Google Scholar]
- Heizmann C. W., Hobi R., Winkler G. C., Kuenzle C. C. The extra DNA of brain cortex neurons is qualitatively indistinguishable from other somatic DNA. Exp Cell Res. 1981 Oct;135(2):331–339. doi: 10.1016/0014-4827(81)90168-3. [DOI] [PubMed] [Google Scholar]
- Hicks S. P., D'Amato C. J. Cell migrations to the isocortex in the rat. Anat Rec. 1968 Mar;160(3):619–634. doi: 10.1002/ar.1091600311. [DOI] [PubMed] [Google Scholar]
- Horgen P. A., Silver J. C. Chromatin in eukaryotic microbes. Annu Rev Microbiol. 1978;32:249–284. doi: 10.1146/annurev.mi.32.100178.001341. [DOI] [PubMed] [Google Scholar]
- Humphries S. E., Young D., Carroll D. Chromatin structure of the 5S ribonucleic acid genes of Xenopus laevis. Biochemistry. 1979 Jul 24;18(15):3223–3231. doi: 10.1021/bi00582a006. [DOI] [PubMed] [Google Scholar]
- Klug A., Rhodes D., Smith J., Finch J. T., Thomas J. O. A low resolution structure for the histone core of the nucleosome. Nature. 1980 Oct 9;287(5782):509–516. doi: 10.1038/287509a0. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Kuenzle C. C., Bregnard A., Hübscher U., Ruch F. Extra DNA in forebrain cortical neurons. Exp Cell Res. 1978 Apr;113(1):151–160. doi: 10.1016/0014-4827(78)90095-2. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Miki B. L., Neelin J. M. DNA repeat lengths of erythrocyte chromatins differing in content of histones H1 and H5. Nucleic Acids Res. 1980 Feb 11;8(3):529–542. doi: 10.1093/nar/8.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. R. A comparison of the structure of chicken erythrocyte and chicken liver chromatin. Cell. 1976 Dec;9(4 Pt 1):627–632. doi: 10.1016/0092-8674(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Booth G. M. Improved methods for the fluorographic detection of weak beta-emitting radioisotopes in Agarose and acrylamide gel electrophoresis media. J Biochem Biophys Methods. 1981 Jun;4(5-6):339–346. doi: 10.1016/0165-022x(81)90074-9. [DOI] [PubMed] [Google Scholar]
- Savić A., Richman P., Williamson P., Poccia D. Alterations in chromatin structure during early sea urchin embryogenesis. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3706–3710. doi: 10.1073/pnas.78.6.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R. A., Haye K. R., Litwack A. H., Phelps B. M. Nucleosome repeat lengths in the definitive erythroid series of the adult chicken. Biochim Biophys Acta. 1980 Feb 29;606(2):316–330. doi: 10.1016/0005-2787(80)90041-6. [DOI] [PubMed] [Google Scholar]
- Sperling L., Weiss M. C. Chromatin repeat length correlates with phenotypic expression in hepatoma cells, their dedifferentiated variants, and somatic hybrids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3412–3416. doi: 10.1073/pnas.77.6.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Thompson R. J. Variation in chromatin structure in two cell types from the same tissue: a short DNA repeat length in cerebral cortex neurons. Cell. 1977 Apr;10(4):633–640. doi: 10.1016/0092-8674(77)90096-4. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The nucleosome repeat length increases during erythropoiesis in the chick. Nucleic Acids Res. 1978 Apr;5(4):1179–1188. doi: 10.1093/nar/5.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley S. A., Hall C., Lim L. Chromatin organization in the rat hypothalamus during early development. Biochem J. 1981 Apr 15;196(1):115–119. doi: 10.1042/bj1960115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalenskaya I. A., Pospelov V. A., Zalensky A. O., Vorob'ev V. I. Nucleosomal structure of sea urchin and starfish sperm chromatin. Histone H2B is possibly involved in determining the length of linker DNA. Nucleic Acids Res. 1981 Feb 11;9(3):473–487. doi: 10.1093/nar/9.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zongza V., Mathias A. P. The variation with age of the structure of chromatin in three cell types from rat liver. Biochem J. 1979 May 1;179(2):291–298. doi: 10.1042/bj1790291. [DOI] [PMC free article] [PubMed] [Google Scholar]