Abstract
Natural killer (NK) cells are potent innate cytotoxic lymphocytes for the destruction of infected and transformed cells. Although they were originally considered to be ready-made assassins after their hematopoietic development, it has recently become clear that their activity is regulated by mechanisms such as repertoire composition, licensing, priming, and adaptive memory-like differentiation. Some of these mechanisms are influenced by infectious disease agents, including herpesviruses. In this review, we will compare expansion, stimulation, and effector functions of NK cell populations after infections with β- and γ 1-herpesviruses because, though closely related, these pathogens seem to drive completely opposite NK cell responses. The discussed findings suggest that different NK cell subsets expand and perform protective functions during infectious diseases and might be used diagnostically to predict resistance to the causative pathogens as well as treat them by adoptive transfer of the respective populations.
Keywords: natural killer cells, protection, effector, subsets, herpes simplex virus
Introduction
Natural killer (NK) cells are innate lymphocytes that have been initially identified by their ability to kill tumor or infected cells without prior activation 1– 3. Their target cell recognition is composed of signals that they receive from germ-line encoded activating and inhibitory receptors, and the net outcome of these interactions leads to recognizing or passing over respective cellular targets 4. A surplus of activating signals can be achieved either by loss of inhibition, named “missing-self” recognition, or by upregulation of stimulation, named “altered-self” recognition 5. Inhibitory NK cell receptors recognize primarily classical and non-classical major histocompatibility complex (MHC) class I molecules, and thereby “missing-self” recognition counterbalances cytotoxic CD8 + T-cell recognition restricted by pathogen- or tumor-induced loss of MHC class I 6. While CD94/NKG2A recognizes signal peptides of other MHC class I molecules on non-classical human HLA-E or mouse H2-Qa1 molecules for its inhibition, killer immunoglobulin-like receptors (KIRs) in humans and Ly49 molecules in mice can distinguish polymorphic classical MHC class I molecules. In contrast, activating signal recognition is less well understood and more diverse but requires—with the exception of dominant triggering by opsonizing antibody binding to CD16 on NK cells—the stimulation of at least two activating receptors to unleash NK cell responses 4. These are often referred to as main activating receptors, like NKG2D, or the natural cytotoxicity receptors (NCRs) NKp30, NKp46, and NKp44 plus co-receptors that need to be engaged, including signaling lymphocyte-activating molecules (SLAMs) 2B4 and NTB-A, as well as DNAX accessory molecule-1 (DNAM-1). Their ligands are diverse, including MHC class I-like molecules for NKG2D, B7 family members like B7-H6 for NKp30, CD48 for 2B4, and poliovirus receptor (PVR) or Nectin-2 for DNAM-1. Some of these ligands are upregulated upon cellular stress, like uncontrolled proliferation and associated DNA damage responses 7. Thus, NK cells can integrate a diversity of clues from the surface of transformed and infected cells for their recognition.
Apart from this multitude of receptors, NK cell activity is regulated by at least four additional mechanisms, namely the NK cell repertoire, NK cell activation by cytokines or priming, adaptive or memory-like NK cell differentiation, and NK cell licensing. The NK cell repertoire is composed of up to 30,000 subpopulations 8. These subpopulations differ among each other with respect to inhibitory and activating receptor expression. Inhibitory receptor distribution is determined mainly by the respective genotype of the individual 8– 11. For example, KIRs are highly polymorphic and NK cell subsets with no, one, or multiple KIRs exist in any given individual. In contrast, activating NK cell receptor expression differs between monozygotic twins and therefore seems to be regulated by environmental factors 8. This variability amounts to up to 30,000 different NK cell subsets in the NK cell repertoire of any given human individual 8. Furthermore, cytokines—mainly interleukin-2 (IL-2), IL-12, IL-15, IL-18, and type I interferon—augment NK cell function and activate NK cells in secondary lymphoid tissues 12– 14. This activation is often mediated by dendritic cells (DCs). Furthermore, the NK cell compartment contains, to a variable degree, adaptive or memory-like NK cells. These hyper-reactive and long-lived NK cells have been described after certain, mostly viral antigen encounters and are able to mount stronger protective responses upon re-encounter of the same pathogen and sometimes even antigen 15, 16. These adaptive NK cells will be discussed in more detail in the context of persistent infections with β-herpesviruses below. Finally, NK cell reactivity is also adjusted to its environment by a process called licensing 9, 17, 18. Licensing describes a process by which NK cells, which carry inhibitory receptors that are engaged by healthy cells, are more reactive during “missing-self” recognition. It has been suggested that in the absence of an inhibitory signal the continuous stimulation of activating receptors leads to disarming of NK cells, attenuating their activity. Thus, multiple NK cell receptors as well as the composition of the NK cell compartment with licensed, cytokine-activated, adaptive, and different receptor-expressing cells contribute to the reactivity of these innate cytotoxic lymphocytes. All of these regulatory mechanisms contribute to their role during herpesvirus infections.
Protection from herpesvirus infections by natural killer cells
Herpesviruses are double-stranded enveloped DNA viruses that establish persistent infections 19. They are exquisitely adapted to their host species and its immune control. Indeed, the first description of a primary immunodeficiency in NK cells characterized a patient who had uncontrolled herpesvirus infections 20, 21. Herpesviruses fall into the groups of neurotrophic α-herpesviruses, at least in part myelotrophic β-herpesviruses, and lymphotrophic γ-herpesviruses. The paradigmatic representatives of these three subclasses in humans are the herpes simplex virus (HSV), the human cytomegalovirus (HCMV), and the Epstein-Barr virus (EBV), respectively. Although the basic composition of these viruses is very similar, the influence on the NK cell compartment and their restriction by it during infection are very different. With respect to phenotype, recurrent HSV2 infection does not change the NK cell composition 22. In contrast, β-herpesvirus infection with HCMV has become the paradigm of human adaptive NK cell differentiation with an accumulation of terminally differentiated NK cells 23. Finally, the γ-herpesvirus EBV expands early differentiated NK cells during primary infection 24. In addition to the phenotypic differences in the NK cell responses to different herpesviruses, their dependency on NK cell-mediated immune control differs significantly. Even though in the above-mentioned GATA2-deficient patient 20, 21 recurrent α-herpesvirus infections were observed, the protection from HSV infection by NK cells in mouse models is still controversial and might depend on the site of infection as well as the investigated mouse strain 25– 27. In contrast, immune control of β-herpesvirus infection (HCMV) was also compromised in the original NK cell-deficient indicator patient and HCMV is used as the paradigmatic viral infection to investigate protective NK cell responses in mice 28. Finally, NK cells control lytic replication by the human γ-herpesvirus EBV and protect mice with human immune system components from enhanced tumor formation by this virus 29. Along these lines, individuals with MCM4 mutations and diminished NK cell compartments suffer from uncontrolled EBV infection 30. In contrast, the mouse γ 2-herpesvirus MHV-68 is not affected by NK cell depletion during its infection in mice 31. Therefore, in the following sections, we will discuss cytomegalovirus and EBV as the two extremes for the remodeling of the NK cell compartments, although both of these viruses are controlled by NK cells.
Natural killer cell phenotypes during herpesvirus infections
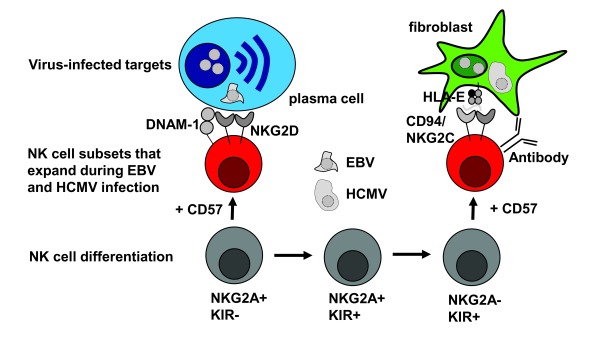
The remodeling of the NK cell phenotype by herpesvirus infections has been best described for human NK cells 23, 24. Human NK cells are thought to originate from the bone marrow as CD56 brightCD16 − cells with homing markers (CCR7 and CD62L) for secondary lymphoid tissues 32, 33. There, they can acquire cytotoxic function and KIRs upon activation by DC-derived cytokines 14. It is thought that during this differentiation ( Figure 1), they acquire more and more KIRs and eventually lose expression of the inhibitory CD94/NKG2A receptor 34. CD94/NKG2A expression can be reacquired in inflammatory environments through IL-12-dependent induction 35. However, all of these intermediate NK cell populations can differentiate from proliferating to CD57-positive senescent NK cells and thereby arrest in their differentiation 34.
Figure 1. Differentiation and stimulation of human natural killer (NK) cells during Epstein-Barr virus (EBV) and human cytomegalovirus (HCMV) infection.
Human NK cells differentiate with acquisition of killer immunoglobulin-like receptor (KIR) expression and lose NKG2A expression upon terminal differentiation. Expression of the senescence marker CD57 removes NK cell subpopulations from this differentiation. Lytically EBV-replicating plasma cells are preferentially recognized by early differentiated NK cells via their NKG2D and DNAX accessory molecule-1 (DNAM-1) receptors, while HCMV-infected cells expand terminally differentiated NK cells via CD94/NKG2C stimulation by HCMV peptide-presenting human leukocyte antigen (HLA)-E molecules. HCMV-infected cells are targeted by these terminally differentiated NK cells after antibody opsonization.
During persistent HCMV infection of healthy virus carriers, after HCMV reactivation in bone marrow transplant patients, in HCMV-infected children, and in HCMV-infected individuals with asymptomatic co-infections 23, 36– 38, terminally differentiated NK cells (phenotypically CD56 dimCD16 +KIR +LIR1 +NKG2C +CD57 +Bcl-2 +NKG2A -NKp46 lowNKp30 lowCD161 lowCD7 lowTim-3 lowPZLF lowSyk lowEat-2 lowDAB2 lowHelios lowFcεR1γ lowCD2 high) with many epigenetically silenced gene loci accumulate ( Figure 1) 39– 42. These terminally differentiated NK cells might be generated by two synergistic mechanisms. The activating CD94/NKG2C receptor on this NK cell subset can engage HCMV peptide-presenting HLA-E molecules on infected fibroblasts ( Figure 1) 43– 46. However, these expand the above-characterized NKG2C + NK cell subset usually only in the presence of monokines like IL-15 and IL-12, which have been shown to be provided by bystander monocytes 43, 44. In fact, NKG2C-dependent HLA-E recognition might not be strictly required for the expansion of terminally differentiated NK cells during HCMV infection because the 4% of the human population that lack NKG2C expand NK cells of similar phenotype during chronic HCMV infection 41, 47. Instead, cytokines might be sufficient for the expansion of the respective NK cells, and the accumulation of this NK cell subset seems to be further augmented by co-infections with hantavirus, chikungunya virus, HIV, and hepatitis virus 36, 48– 52, which might enhance NK cell-differentiating cytokine expression. It is tempting to speculate that HCMV infection of the myeloid lineage favors the production of NK cell-differentiating cytokines that lead to the accumulation of NKG2C + NK cells.
B lymphotropic EBV also causes NK cell expansion during primary infection 53– 56. In particular, CD56 dimCD16 +/−KIR −CD57 - NK cells proliferated during acute infectious mononucleosis (IM) ( Figure 1), and the frequency of this proliferating NK cell subset correlated with viral loads 24. The expansion of these early differentiated NK cells persists for at least 6 months 24, 57, 58, but they stop proliferating during this time period and acquire the senescence marker CD57 24, 58. Interestingly, newborns carry a high frequency of these early differentiated KIR - NK cells, which are progressively lost during the first decade of life 24. This differentiation might be induced by other pathogens and HCMV could contribute significantly to this loss of early differentiated NK cells. Thus, the β-herpesvirus HCMV and the γ-herpesvirus EBV drive the expansion of completely different NK cell phenotypes, and it remains to be seen whether one virus thereby influences the immune control of the other.
Effector functions of natural killer cells during herpesvirus infections
Indeed, this early differentiated NK cell phenotype in children correlates with a higher frequency of asymptomatic primary EBV infection, whereas delay of initial infection with this γ-herpesvirus leads, increasingly with age, to a higher likelihood of having immunopathologic symptoms of lymphadenopathy, fever and fatigue, which are caused by massive CD8 + T-cell expansion and are collectively called IM 59. Indeed, African children often suffer from the same high viral loads as patients with IM, but the CD8 + T-cell expansion of the former is less pronounced and thus no disease is experienced 60. It is tempting to speculate that despite high viral loads early differentiated innate lymphocytes, including NK cells, primarily deal with the infection, curbing CD8 + T-cell lymphocytosis. Indeed, such a protective function of NK cells during primary EBV infection was recently documented 29. In mice with reconstituted human immune system components, early differentiated NK cells predominate 61. Infection with EBV led to the expansion of these NK cells, starting 3 weeks after infection 29. This time point coincides with the time point at which lytic replication of EBV can be detected in this in vivo model, as judged by comparing wild-type with lytic replication-deficient BZLF1 - EBV 62. Depletion of NK cells with an antibody directed against NKp46 leads to elevated viral loads, starting at 3 weeks after infection 29. Viral load is elevated 10-fold in total blood and 100-fold in the serum 29. Only lytic EBV infection is affected because viral load of BZLF1 - EBV did not increase upon NK cell depletion 29. In good agreement with these findings, NK cells primarily recognize lytically EBV-infected targets 24, 63 and preferentially the early differentiated KIR - NK cells degranulate 24. This recognition has been suggested to be mediated by NKG2D and DNAM-1 ( Figure 1) 63. Interestingly, patients with deficiency in a magnesium transporter (MAGT1), resulting in diminished surface expression of NKG2D on NK and T cells, suffer from EBV-associated lymphoproliferations 64. In the absence of NK cells, EBV-infected mice with reconstituted human immune system components develop mostly monoclonal lymphoproliferations as well as CD8 + T-cell lymphocytosis, splenomegaly, and cytokinemia, which are hallmarks of IM 29. These studies suggest that NK cells—in particular, early differentiated KIR - NK cells—restrict lytic EBV replication and could explain the risk of adolescents for IM.
In contrast, the function of the terminally differentiated NKG2C + NK cells during HCMV infection is less clear. During mouse cytomegalovirus (MCMV) infection of C57BL/6 mice, Ly49H + NK cells preferentially expand and directly bind with their Ly49H receptor to the MCMV m157 protein on the surface of infected cells 65, 66. NK cells are indeed required for efficient immune control of MCMV infection 67, 68, and Ly49H + antigen-experienced NK cells control MCMV infection better than other subsets 15. Even though NKG2C + and NKG2C - human NK cells might represent the counterparts of the recently described Ly49H + and Ly49H - mouse NK cells, which acquire their adaptive functional superiority by either receptor- or cytokine-mediated stimulation, respectively 69, it has remained difficult to demonstrate a protective function for the NK cell expansions during HCMV infection. Although these terminally differentiated NKG2C + NK cells more readily produce cytokines in response to HCMV-infected cells 70, 71 and their frequency correlates with protection from HCMV disease after kidney transplantation 72, they seem to clear infected targets only after antibody-mediated opsonization by antibody-dependent cellular cytotoxicity ( Figure 1) 73, 74. This would argue for a protective role of these accumulating NK cells rather late during the infection, when HCMV-specific antibodies have already formed. Indeed, during hantavirus co-infection, the enhanced functionality of these NKG2C + NK cells was suggested to cause immunopathology by promoting vascular leakage via uninfected endothelial cell killing 75. Thus, KIR -, NKG2C +, and Ly49H + NK cell subpopulations expand and persist for several months during EBV, HCMV, and MCMV infection, but although protection of the respective NK cell subset during EBV and MCMV infection has been demonstrated, this remains less clear for HCMV infection.
Conclusions
The extent of the complexity of the human NK cell compartment with up to 30,000 distinct subpopulations has only recently been appreciated 8. As discussed above, certain pathogens, exemplified in this review by the human herpesviruses HCMV and EBV, seem to drive expansions of distinct NK cell subsets, which then persist at elevated frequencies for months 23, 24. The protective features of these expanded NK cell populations are beginning to emerge 29, 74, as are how their expansion can be stimulated 44. Thus, it might be possible not only to use the NK cell phenotype as a predictor of immune control against these specific pathogens but also to adoptively transfer or stimulate these NK cell subsets in patients with diminished immune control of the respective viruses, starting with HCMV and EBV. However, in order to narrow the NK cell phenotype that might be required for clinical benefit, the receptor interactions and effector functions that mediate protection need to be better defined in the future.
Abbreviations
DC, dendritic cell; DNAM-1, DNAX accessory molecule-1; EBV, Epstein-Barr virus; HCMV, human cytomegalovirus; HSV, herpes simplex virus; IL, interleukin; IM, infectious mononucleosis; KIR, killer immunoglobulin-like receptor; MCMV, mouse cytomegalovirus; MHC, major histocompatibility complex; NK, natural killer.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Lewis Lanier, Department of Microbiology and Immunology, University of California San Francisco, San Francisco, CA, USA; Parker Institute for Cancer Immunotherapy, San Francisco, CA, USA
Andre Boonstra, Department of Gastroenterology and Hepatology, Erasmus MC, University Medical Center, Rotterdam, Netherlands
Funding Statement
Research in our laboratory is supported by Cancer Research Switzerland (KFS-3234-08-2013), Worldwide Cancer Research (14-1033), SPARKS (15UOZ01), KFSP MS and KFSP HHLD of the University of Zurich, the Sobek Foundation, the Swiss Vaccine Research Institute, and the Swiss National Science Foundation (310030_162560 and CRSII3_160708) (to CM) and Cancer Research Zurich (to OC).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Herberman RB, Nunn ME, Lavrin DH: Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16(2):216–29. [DOI] [PubMed] [Google Scholar]
- 2. Trinchieri G, Santoli D: Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978;147(5):1314–33. 10.1084/jem.147.5.1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiessling R, Klein E, Wigzell H: “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–7. 10.1002/eji.1830050208 [DOI] [PubMed] [Google Scholar]
- 4. Long EO, Kim HS, Liu D, et al. : Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–58. 10.1146/annurev-immunol-020711-075005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moretta A, Bottino C, Vitale M, et al. : Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. 10.1146/annurev.immunol.19.1.197 [DOI] [PubMed] [Google Scholar]
- 6. Ljunggren HG, Kärre K: In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237–44. 10.1016/0167-5699(90)90097-S [DOI] [PubMed] [Google Scholar]
- 7. Iannello A, Thompson TW, Ardolino M, et al. : Immunosurveillance and immunotherapy of tumors by innate immune cells. Curr Opin Immunol. 2016;38:52–8. 10.1016/j.coi.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horowitz A, Strauss-Albee DM, Leipold M, et al. : Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5(208):208ra145. 10.1126/scitranslmed.3006702 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Landtwing V, Raykova A, Pezzino G, et al. : Cognate HLA absence in trans diminishes human NK cell education. J Clin Invest. 2016;126(10):3772–82. 10.1172/JCI86923 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Rettman P, Willem C, David G, et al. : New insights on the natural killer cell repertoire from a thorough analysis of cord blood cells. J Leukoc Biol. 2016;100(3):471–9. 10.1189/jlb.1HI0116-036R [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Horowitz A, Djaoud Z, Nemat-Gorgani N, et al. : Class I HLA haplotypes form two schools that educate NK cells in different ways. Sci Immunol. 2016;1(3): pii: eaag1672. 10.1126/sciimmunol.aag1672 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Chijioke O, Münz C: Interactions of human myeloid cells with natural killer cell subsets in vitro and in vivo. J Biomed Biotechnol. 2011;2011: 251679. 10.1155/2011/251679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucas M, Schachterle W, Oberle K, et al. : Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26(4):503–17. 10.1016/j.immuni.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Ferlazzo G, Pack M, Thomas D, et al. : Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101(47):16606–11. 10.1073/pnas.0407522101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun JC, Beilke JN, Lanier LL: Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–61. 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Paust S, Gill HS, Wang BZ, et al. : Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11(12):1127–35. 10.1038/ni.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Goodridge JP, Önfelt B, Malmberg KJ: Newtonian cell interactions shape natural killer cell education. Immunol Rev. 2015;267(1):197–213. 10.1111/imr.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Boudreau JE, Liu XR, Zhao Z, et al. : Cell-Extrinsic MHC Class I Molecule Engagement Augments Human NK Cell Education Programmed by Cell-Intrinsic MHC Class I. Immunity. 2016;45(2):280–91. 10.1016/j.immuni.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Roizman B, Whitley RJ: An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol. 2013;67:355–74. 10.1146/annurev-micro-092412-155654 [DOI] [PubMed] [Google Scholar]
- 20. Biron CA, Byron KS, Sullivan JL: Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–5. 10.1056/NEJM198906293202605 [DOI] [PubMed] [Google Scholar]
- 21. Hsu AP, Sampaio EP, Khan J, et al. : Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118(10):2653–5. 10.1182/blood-2011-05-356352 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Björkström NK, Svensson A, Malmberg KJ, et al. : Characterization of natural killer cell phenotype and function during recurrent human HSV-2 infection. PLoS One. 2011;6(11):e27664. 10.1371/journal.pone.0027664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gumá M, Angulo A, Vilches C, et al. : Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–71. 10.1182/blood-2004-05-2058 [DOI] [PubMed] [Google Scholar]
- 24. Azzi T, Lünemann A, Murer A, et al. : Role for early-differentiated natural killer cells in infectious mononucleosis. Blood. 2014;124(16):2533–43. 10.1182/blood-2014-01-553024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Habu S, Akamatsu K, Tamaoki N, et al. : In vivo significance of NK cell on resistance against virus (HSV-1) infections in mice. J Immunol. 1984;133(5):2743–7. [PubMed] [Google Scholar]
- 26. Pereira RA, Scalzo A, Simmons A: Cutting edge: a NK complex-linked locus governs acute versus latent herpes simplex virus infection of neurons. J Immunol. 2001;166(10):5869–73. 10.4049/jimmunol.166.10.5869 [DOI] [PubMed] [Google Scholar]
- 27. Bukowski JF, Welsh RM: The role of natural killer cells and interferon in resistance to acute infection of mice with herpes simplex virus type 1. J Immunol. 1986;136(9):3481–5. [PubMed] [Google Scholar]
- 28. Min-Oo G, Kamimura Y, Hendricks DW, et al. : Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34(6):251–8. 10.1016/j.it.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chijioke O, Müller A, Feederle R, et al. : Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013;5(6):1489–98. 10.1016/j.celrep.2013.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Gineau L, Cognet C, Kara N, et al. : Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122(3):821–32. 10.1172/JCI61014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Usherwood EJ, Meadows SK, Crist SG, et al. : Control of murine gammaherpesvirus infection is independent of NK cells. Eur J Immunol. 2005;35(10):2956–61. 10.1002/eji.200526245 [DOI] [PubMed] [Google Scholar]
- 32. Ferlazzo G, Münz C: NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172(3):1333–9. 10.4049/jimmunol.172.3.1333 [DOI] [PubMed] [Google Scholar]
- 33. Ferlazzo G, Thomas D, Lin SL, et al. : The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172(3):1455–62. 10.4049/jimmunol.172.3.1455 [DOI] [PubMed] [Google Scholar]
- 34. Bjorkstrom NK, Riese P, Heuts F, et al. : Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(4):3853–64. 10.1182/blood-2010-04-281675 [DOI] [PubMed] [Google Scholar]
- 35. Saez-Borderias A, Romo N, Magri G, et al. : IL-12-dependent inducible expression of the CD94/NKG2A inhibitory receptor regulates CD94/NKG2C + NK cell function. J Immunol. 2009;182(2):829–36. 10.4049/jimmunol.182.2.829 [DOI] [PubMed] [Google Scholar]
- 36. Gumá M, Cabrera C, Erkizia I, et al. : Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006;194(1):38–41. 10.1086/504719 [DOI] [PubMed] [Google Scholar]
- 37. Monsiváis-Urenda A, Noyola-Cherpitel D, Hernandez-Salinas A, et al. : Influence of human cytomegalovirus infection on the NK cell receptor repertoire in children. Eur J Immunol. 2010;40(5):1418–27. 10.1002/eji.200939898 [DOI] [PubMed] [Google Scholar]
- 38. Della Chiesa M, Falco M, Bertaina A, et al. : Human cytomegalovirus infection promotes rapid maturation of NK cells expressing activating killer Ig-like receptor in patients transplanted with NKG2C -/- umbilical cord blood. J Immunol. 2014;192(4):1471–9. 10.4049/jimmunol.1302053 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Lee J, Zhang T, Hwang I, et al. : Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42(3):431–42. 10.1016/j.immuni.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Schlums H, Cichocki F, Tesi B, et al. : Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–56. 10.1016/j.immuni.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Liu LL, Landskron J, Ask EH, et al. : Critical Role of CD2 Co-stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep. 2016;15(5):1088–99. 10.1016/j.celrep.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Rölle A, Brodin P: Immune Adaptation to Environmental Influence: The Case of NK Cells and HCMV. Trends Immunol. 2016;37(3):233–43. 10.1016/j.it.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 43. Gumá M, Budt M, Sáez A, et al. : Expansion of CD94/NKG2C + NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107(9):3624–31. 10.1182/blood-2005-09-3682 [DOI] [PubMed] [Google Scholar]
- 44. Rölle A, Pollmann J, Ewen EM, et al. : IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C + NK cell expansion. J Clin Invest. 2014;124(12):5305–16. 10.1172/JCI77440 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Tomasec P, Braud VM, Rickards C, et al. : Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287(5455):1031–1033. 10.1126/science.287.5455.1031 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Millo E, Pietra G, Armirotti A, et al. : Purification and HPLC-MS analysis of a naturally processed HCMV-derived peptide isolated from the HEK-293T/HLA-E+/Ul40+ cell transfectants and presented at the cell surface in the context of HLA-E. J Immunol Methods. 2007;322(1–2):128–36. 10.1016/j.jim.2007.01.018 [DOI] [PubMed] [Google Scholar]
- 47. Muntasell A, Pupuleku A, Cisneros E, et al. : Relationship of NKG2C Copy Number with the Distribution of Distinct Cytomegalovirus-Induced Adaptive NK Cell Subsets. J Immunol. 2016;196(9):3818–27. 10.4049/jimmunol.1502438 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Björkström NK, Lindgren T, Stoltz M, et al. : Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. 10.1084/jem.20100762 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Petitdemange C, Becquart P, Wauquier N, et al. : Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011;7(9):e1002268. 10.1371/journal.ppat.1002268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mavilio D, Lombardo G, Benjamin J, et al. : Characterization of CD56 -/CD16 + natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102(8):2886–91. 10.1073/pnas.0409872102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oliviero B, Varchetta S, Paudice E, et al. : Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137(3):1151–60, 1160.e1–7. 10.1053/j.gastro.2009.05.047 [DOI] [PubMed] [Google Scholar]
- 52. Béziat V, Dalgard O, Asselah T, et al. : CMV drives clonal expansion of NKG2C + NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42(2):447–57. 10.1002/eji.201141826 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Tomkinson BE, Wagner DK, Nelson DL, et al. : Activated lymphocytes during acute Epstein-Barr virus infection. J Immunol. 1987;139(11):3802–7. [PubMed] [Google Scholar]
- 54. Williams H, McAulay K, Macsween KF, et al. : The immune response to primary EBV infection: a role for natural killer cells. Br J Haematol. 2005;129(2):266–74. 10.1111/j.1365-2141.2005.05452.x [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Wallace DL, de Lara CM, et al. : In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–65. 10.1111/j.1365-2567.2007.02573.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Balfour HH, Jr, Odumade OA, Schmeling DO, et al. : Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J Infect Dis. 2013;207(1):80–8. 10.1093/infdis/jis646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dunmire SK, Grimm JM, Schmeling DO, et al. : The Incubation Period of Primary Epstein-Barr Virus Infection: Viral Dynamics and Immunologic Events. PLoS Pathog. 2015;11(12):e1005286. 10.1371/journal.ppat.1005286 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Hendricks DW, Balfour HH, Jr, Dunmire SK, et al. : Cutting edge: NKG2C hiCD57 + NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J Immunol. 2014;192(10):4492–6. 10.4049/jimmunol.1303211 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Luzuriaga K, Sullivan JL: Infectious mononucleosis. N Engl J Med. 2010;362(21):1993–2000. 10.1056/NEJMcp1001116 [DOI] [PubMed] [Google Scholar]
- 60. Jayasooriya S, de Silva TI, Njie-jobe J, et al. : Early virological and immunological events in asymptomatic Epstein-Barr virus infection in African children. PLoS Pathog. 2015;11(3):e1004746. 10.1371/journal.ppat.1004746 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Strowig T, Chijioke O, Carrega P, et al. : Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence. Blood. 2010;116(20):4158–67. 10.1182/blood-2010-02-270678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Antsiferova O, Muller A, Ramer PC, et al. : Adoptive transfer of EBV specific CD8 + T cell clones can transiently control EBV infection in humanized mice. PLoS Pathog. 2014;10(8):e1004333. 10.1371/journal.ppat.1004333 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Pappworth IY, Wang EC, Rowe M: The switch from latent to productive infection in Epstein-Barr virus-infected B cells is associated with sensitization to NK cell killing. J Virol. 2007;81(2):474–82. 10.1128/JVI.01777-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chaigne-Delalande B, Li FY, O'Connor GM, et al. : Mg 2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341(6142):186–91. 10.1126/science.1240094 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Arase H, Mocarski ES, Campbell AE, et al. : Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296(5571):1323–6. 10.1126/science.1070884 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Smith HR, Heusel JW, Mehta IK, et al. : Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A. 2002;99(13):8826–31. 10.1073/pnas.092258599 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Jonjić S, Pavić I, Polić B, et al. : Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med. 1994;179(5):1713–7. 10.1084/jem.179.5.1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Polić B, Hengel H, Krmpotić A, et al. : Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med. 1998;188(6):1047–54. 10.1084/jem.188.6.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nabekura T, Lanier LL: Tracking the fate of antigen-specific versus cytokine-activated natural killer cells after cytomegalovirus infection. J Exp Med. 2016;213(12):2745–58. 10.1084/jem.20160726 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Foley B, Cooley S, Verneris MR, et al. : Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C + natural killer cells with potent function. Blood. 2012;119(11):2665–74. 10.1182/blood-2011-10-386995 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Lopez-Vergès S, Milush JM, Schwartz BS, et al. : Expansion of a unique CD57 + NKG2C hi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108(36):14725–32. 10.1073/pnas.1110900108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Redondo-Pachón D, Crespo M, Yélamos J, et al. : Adaptive NKG2C + NK Cell Response and the Risk of Cytomegalovirus Infection in Kidney Transplant Recipients. J Immunol. 2017;198(1):94–101. 10.4049/jimmunol.1601236 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Zhang T, Scott JM, Hwang I, et al. : Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J Immunol. 2013;190(4):1402–6. 10.4049/jimmunol.1203034 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Costa-Garcia M, Vera A, Moraru M, et al. : Antibody-mediated response of NKG2C bright NK cells against human cytomegalovirus. J Immunol. 2015;194(6):2715–24. 10.4049/jimmunol.1402281 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Gupta S, Braun M, Tischler ND, et al. : Hantavirus-infection confers resistance to cytotoxic lymphocyte-mediated apoptosis. PLoS Pathog. 2013;9(3):e1003272. 10.1371/journal.ppat.1003272 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation