Abstract
The biomarker potential of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) for the in vivo characterization of preclinical stages in Alzheimer’s disease has not yet been explored. We measured GABA, glutamate + glutamine (Glx), and N-acetyl-aspartate (NAA) levels by single-voxel MEGA-PRESS magnetic resonance spectroscopy in the posterior cingulate cortex of 21 elderly subjects and 15 patients with amnestic mild cognitive impairment. Participants underwent Pittsburgh Compound B positron emission tomography, apolipoprotein E (APOE) genotyping, and neuropsychological examination. GABA, Glx, and NAA levels were significantly lower in patients. NAA was lower in Pittsburgh Compound B-positive subjects and APOE ε4 allele carriers. GABA, Glx, and NAA levels were positively correlated to CERAD word learning scores. Reductions in GABA, Glx, and NAA levels may serve as metabolic biomarkers for cognitive impairment in amnestic mild cognitive impairment. Because GABA and Glx do not seem to reflect amyloid β deposition or APOE genotype, they are less likely biomarker candidates for preclinical Alzheimer’s disease.
Keywords: Mild cognitive impairment, Alzheimer, Dementia, Magnetic resonance spectroscopy, MRS, GABA, Glx, Glutamate, Beta-amyloid, PiB, Posterior cingulate cortex, APOE
1. Introduction
Current diagnostic criteria for research define preclinical and prodromal stages of Alzheimer’s disease (AD) based on biomarkers (Albert et al., 2011; Dubois et al., 2007, 2010; Sperling et al., 2011). Established AD biomarkers are either measures of amyloid-β (Aβ), for example, Pittsburgh Compound B positron emission tomography (PiB-PET) or markers of neuronal injury (e.g., cerebral glucose hypometabolism and atrophy) (Sperling et al., 2011). Additional biomarkers are needed that more closely reflect functional brain impairment in early AD stages and are suitable to demonstrate functional recovery after successful treatment. Proton magnetic resonance spectroscopy (MRS) is a noninvasive imaging technique and allows in vivo, local measurement of several cerebral metabolites in a single scan and has been applied to study a wide range of central nervous system disorders both in animal models and humans (Duarte et al., 2012; Zhu and Barker, 2010). Although it is currently not part of the diagnostic routine, there is accumulating evidence that MRS detects pathologic changes not only at the stage of AD dementia but also in mild cognitive impairment (MCI) (Graff-Radford and Kantarci, 2013; Kantarci, 2013; Tumati et al., 2013). Furthermore, MRS was found to improve prognostic accuracy for conversion from MCI to dementia (Modrego et al., 2005) and even from cognitive health to MCI (Kantarci et al., 2013). Previous MRS studies in the field have primarily focused on abundant metabolites such as N-acetyl-aspartate (NAA), choline (Cho), myo-inositol (mI), and creatine (Cr) that are surrogate markers for neuronal integrity, membrane integrity, gliosis, and energy metabolism, respectively (Duarte et al., 2012; Graff-Radford and Kantarci, 2013; Kantarci, 2013; Tumati et al., 2013). Most consistently among these, the NAA to Cr ratio was found to be lower in MCI and AD as compared with healthy control subjects (HCS) (Graff-Radford and Kantarci, 2013; Tumati et al., 2013). Furthermore, concentrations of the primary excitatory neurotransmitter, glutamate (Glu), were also shown to be lower in posterior cingulate cortex (PCC) of AD patients, either when measured alone or as a combined measure of glutamate + glutamine (Glx) (Antuono et al., 2001; Fayed et al., 2011; Rupsingh et al., 2011). However, previous MRS studies found no difference in Glu or Glx levels between MCI and HCS (Fayed et al., 2011; Friedman et al., 2013; Rupsingh et al., 2011). Recently, the combination of Cho to Cr, Glx to Cr, and NAA to Cr ratios in the PCC (and precuneus) was successfully used to differentiate carriers from noncarriers of a fully penetrant, familial early-onset AD (presenilin-1 gene) mutation at different disease stages (Londono et al., 2013). The levels of the primary inhibitory neurotransmitter in the brain, γ-aminobutyric acid (GABA), have been studied less frequently, mainly because reliable GABA-MRS protocols have only recently become available (Mullins et al., 2014). Alterations in GABAergic neurotransmission are associated with cognitive symptoms of neuropsychiatric disorders such as schizophrenia (Gonzalez-Burgos et al., 2011) and autism spectrum disorder (Coghlan et al., 2012), and GABA shows changes during memory and visual tasks (Michels et al., 2012). With aging, GABA levels seem to decline in the human brain (Gao et al., 2013). However, the preclinical and pathologic evidence for alterations in GABAergic neurotransmission in AD is conflicting (Lanctôt et al., 2004). Strikingly, treatment of HCS and MCI patients with an analog of human growth hormone-releasing hormone led to an increase in GABA in all studied brain regions and was accompanied by improved cognition (Friedman et al., 2013). Moreover, the most important genetic risk factor for late-onset AD, apolipoprotein E genotype (APOE), may exert at least part of its effect through impairment of GABAergic interneurons (Huang and Mucke, 2012). The effect of Aβ deposition (measured by PiB-PET) on GABA and Glx as markers of brain function has not been studied yet. In the only combined MRS and/or PiB-PET study to date, higher cortical PiB uptake was associated with elevated mI to Cr and Cho to Cr ratios in cognitively normal older adults (Kantarci et al., 2011). In this cross-sectional study, we use MRS to investigate the levels of GABA (and Glx and NAA) in the PCC of HCS and patients with amnestic MCI (aMCI). Because altered GABAergic neurotransmission is a common finding in cognitive disorders, we hypothesized that PCC GABA levels are decreased in aMCI. Additionally, we hypothesized that PCC GABA levels may constitute a biomarker for preclinical AD and should therefore be related to local or global Aβ deposition or APOE genotype.
2. Methods
2.1. Participants
Participants were enrolled in this MRS substudy from 2 ongoing longitudinal studies on cognitive performance in elderly subjects (approved by the ethics committee of canton Zurich, Switzerland, file numbers E_22_2009 and E_64_2009). All participants gave written informed consent. Cognitive health was defined by a Mini Mental State Examination (MMSE) score ≥27 and a clinical and neuropsychological assessment not indicative of MCI or other cognitive disorders. Amnestic MCI was diagnosed according to the standard criteria (Winblad et al., 2004). Participants had to be of age ≥55 years. Exclusion criteria were somatic or psychiatric conditions or use of medication that would significantly affect cognition. Likewise, patients were excluded if they had contraindications for magnetic resonance (MR) or PET imaging or evidence of focal lesions in critical memory structures.
2.2. Clinical and neuropsychological assessment
All subjects underwent clinical assessment including clinical history, physical, and neurologic examination. For neuropsychological assessment, the CERAD-Plus test battery was applied (Morris et al., 1989; Welsh et al., 1992), including MMSE, letter fluency, verbal learning, recall and recognition, figure copy and recall, Boston naming test, trail making tests A and B, category, and letter fluency. Furthermore, the German version of the Rey Auditory Verbal Learning Test (VLMT), the Visual Paired Associates test from the Wechsler Memory Scale Revised, and a short version of the Stroop Test were applied. Raw scores were z-transformed based on test-specific normative data adjusted for age and gender. The mean period between the neuropsychological evaluation and MRS scanning was 8.6 months for HCS (range: 2–13 months) and 3.6 months for aMCI (range: 0–8 months).
2.3. Structural and functional imaging
MR scans were recorded on a 3T Philips Ingenia scanner (Philips Healthcare, Best, the Netherlands), equipped with a 16-channel head coil. MR-compatible headphones and cushions were used to minimize subjects’ head motion. A T1-weighted 3D-MPRAGE anatomic scan was recorded before the MRS scan (repetition time/echo time: 8.2 ms/3.8 ms, pixel resolution: 1 × 1 × 1 mm, field of view: 240 mm, and 170 slices). Gray matter (GM) and white matter (WM) differences were estimated and compared between groups (corrected for age, total intracranial volume, and ventricle size) by whole-brain voxel-based morphometry using SPM8 (Hutton et al., 2009).
2.4. MRS acquisition
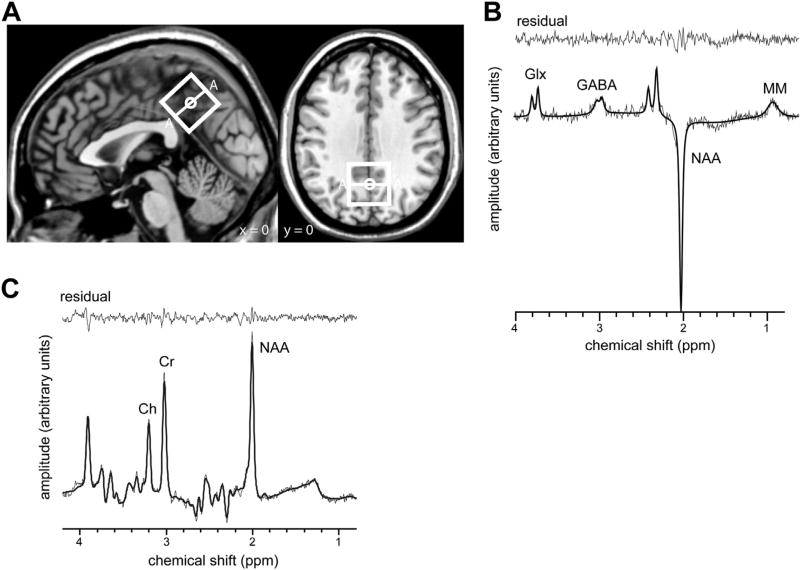
MRS was measured during an eyes-closed rest condition from a voxel (size 30 × 30 × 30 mm) placed at the PCC covering Brodmann area 23 and 31 (extending posteriorly into the precuneus (Brodmann area 7) and inferiorly into the retrosplenial cortex (Brodmann area 29 and 30) following the same localization recently described by Gao et al. (2013). The center of the voxel was defined on the T1-weighted image on the midline sagittal plane (Fig. 1A). To cover as much as possible of the PCC, the voxel was placed posterior and superior to the splenium of the corpus callosum, aligned with the shape of the corpus callosum and repositioned on the medial aspect of the axial plane. Second order B0 shimming was performed using the vendor-implemented projection-based B0 shimming method (Hock et al., 2013). GABA and Glx were measured using the MEGA-PRESS sequence (Edden and Barker, 2007; Puts and Edden, 2012). MEGA editing (a frequency-selective editing technique) was combined with the point-resolved spectroscopy sequence (PRESS), inner volume saturation localization (Henning et al., 2008), and interleaved “VAPOR” water suppression (Hock et al., 2013; Tkác et al.,1999). MEGA-PRESS with inner volume saturation reduces the deleterious effects of spatial variation in coupling evolution and increases sensitivity for edited single-voxel measurements of Glu and GABA (Edden and Barker, 2007). Ten blocks (number of spectral averages: 32/block) were recorded, resulting in 320 averages in total. Per block, the editing pulse was applied in an alternating manner either at 1.9 ppm or at 7.5 ppm. All blocks were retrospectively frequency aligned using the Cho and Cr as frequency references. The radio frequency carrier frequency was set to Cr. Parameters for the MRS sequence were repetition time/echo time: 1800 ms/68 ms, editing pulse duration: 16 ms, and total scanning time = 10:48 minutes.
Fig. 1.
MRS voxel position (A) and representative single-subject spectra (B and C). (A) The MRS voxel (indicated by white rectangles shown on sagittal and axial views) was placed in the posterior cingulate cortex, without including the splenium of the corpus callosum. (B) Representative 68 ms sub-spectrum (PRESS) with raw data (fine black line), fit (bold black line), and residual (fine black line on top). (C) The single-subject MEGA-PRESS spectrum shows peaks of Glx, GABA, NAA, and macromolecules (MM). Raw data are indicated by the fine black line, the spectral fit by the bold black line. The residual is shown on top (thin black line). MRS values are depicted in arbitrary units. Abbreviations: GABA, γ-aminobutyric acid; Glx, glutamate + glutamine; MRS, magnetic resonance spectroscopy; NAA, N-acetyl-aspartate; PRESS, point-resolved spectroscopy sequence.
2.5. MRS analysis
TARQUIN V4.2.5 software was used to estimate metabolite and neurotransmitter values (Wilson et al., 2011). TARQUIN yields comparable reliability in metabolite concentration estimates to the widely-used LCModel software (Mullins et al., 2014; Provencher, 1993; Wilson et al., 2011). Quantification reliability and spectral quality were assessed using Cramer-Rao lower bounds (CRLB) and signal-to-noise ratio. Metabolite data with CRLB >20% (for any given metabolite) and signal-to-noise ratio <3 were considered unreliable so that 2 participants were excluded from analysis. GABA and Glx were analyzed based on MEGA-edited spectra reconstructed from all averages (editing on and off blocks; 320 averages total) of the water-suppressed data, whereas all other metabolites (NAA, Cr, Cho, and mI) were quantified based on the average of the editing off blocks only (160 averages total). Data parameters for the fitting routine were sampling frequency: 2000 Hz, transmitter frequency: 127 MHz, data points: 1024, reference offset: 4.7, water cutoff: 45 Hz, start point for the fitting: 10, end point: 512, initial mu: 0.001, lamba: 0.2. The reference signal was set to H2O (= H2O scaled). For the MEGA-PRESS difference spectra, TARQUIN models the GABA peak as the sum of 2 single Gaussian peaks, and the coedited Glx peak as the sum of 4 Lorentzians, with frequency, line width, and amplitude as the fitted parameters. The fitted peak areas were summed to give a total estimate of GABA and Glx, respectively. No attempts were made to correct for possible coedited macromolecular resonances. The additionally recorded unsuppressed water data (2 per dynamic = 20 averages) served as an internal calibration reference to generate metabolite ratios to water for spectral normalization. Metabolite-to-H2O ratios were corrected for differences in GM, WM, and cerebrospinal fluid (CSF) voxel content influencing the measured H2O concentration. To that end, the T1-weighted 3D-MPRAGE images were segmented using SPM 8 (Hutton et al., 2009) and a custom written script implemented the procedure described in detail elsewhere (Gasparovic et al., 2006). The resulting metabolite-to-H2O ratios are presented in arbitrary units.
2.6. Pittsburgh compound B positron emission tomography
After antecubital injection of approximately 350 MBq of [11C]-PiB a dynamic scan was acquired over 70 minutes. Time activity curves were obtained from predefined voxel of interests of a maximum probability atlas (Hammers N30R83) (Gousias et al., 2008; Hammers et al., 2003) which were intersected with the individual GM masks. Uptake was calculated in cortical and cerebellar voxel of interests from frames 50 to 70 minutes using a volume-weighted averaging procedure. To standardize between patients, the cortical and/or cerebellar uptake ratio was calculated (henceforth, global PiB). The cutoff for dichotomizing subjects (PiB status) into PiB-positive (PiB+) and PiB negative (PiB−) was determined to be 1.265, following an established procedure (Vandenberghe et al., 2010). PCC-specific PiB (local PiB) was calculated from the averaged PET image using the GM and WM masks of the corresponding MRS voxel. Image processing was performed using PMOD PNEURO tool V3.4.
2.7. Genotyping
APOE genotyping was performed according to standard procedures (Hixson and Vernier, 1990). Participants were classified (APOE4-status) as either carriers (APOE4+) or noncarriers (APOE4−) of the ε4 allele.
2.8. Statistical analysis
Demographic variables were analyzed using χ2 test for categorial data (e.g., gender, PiB-positivity [yes/no], APOE [carrier/noncarrier]) or Mann-Whitney U for continuous variables. The amount of WM and CSF in the MRS voxel was compared between both groups using unpaired t tests. MRS group comparisons were performed using t statistics because MRS values were normal distributed (exact Kolmogorov-Smirnov test, p > 0.122, separately performed for all metabolites and grouping parameters). Likewise, differences in neuropsychological scores for PiB status and APOE4-status were explored by t tests (exact Kolmogorov-Smirnov test, p > 0.05). Between-metabolite and MRS-neuropsychology interactions were examined by partial correlation analysis (p < 0.05, corrected for age, gender, and education). The relationship between MRS levels and local and global PiB (not normal distributed) were tested using Spearman correlations (p < 0.05, uncorrected). Group differences for metabolites from unedited spectra (NAA, Cho, Cr, and mI) were compared by unpaired 2-tailed t tests (p < 0.05, uncorrected). No attempts were made to correct for multiple comparisons. Analyses were performed with SPSS (IBM, Version 22).
3. Results
3.1. Sample characteristics
The final sample consisted of 21 HCS and 15 patients with aMCI. Groups were matched for age, sex, and education. As expected, the MMSE score was higher in HCS than in aMCI. Detailed sample characteristics are listed in Table 1. None of the subjects changed in cognitive status (i.e., HCS to aMCI or aMCI to AD; or vice versa) in the time period between study inclusion, MRS examination, and neuropsychological testing.
Table 1.
Sample characteristics
| Group | p-value | ||||
|---|---|---|---|---|---|
|
|
|||||
| HCS (N = 21) | aMCI (N = 15) |
||||
|
|
|
||||
| Mean | SD | Mean | SD | ||
| Age (y) | 70.5 | 4.0 | 74.2 | 9.6 | 0.14 |
| Gender (male, n) | 14 | 11 | 0.67a | ||
| Education (y) | 14.7 | 3.1 | 14.8 | 4.0 | 0.78 |
| MMSE score (/30) | 29.7 | 0.6 | 28.6 | 1.2 | 0.001 |
| APOE ε4 carrier (n) | 4 | 5 | 0.33a | ||
| PiB(+) status (n) | 5 | 7 | 0.11a | ||
| Local PiB retention (in PCC) | 1.3 | 0.38 | 1.6 | 0.7 | 0.61 |
| Global cortical PiB retention | 1.2 | 0.21 | 1.5 | 0.4 | 0.12 |
Diagnostic groups did not differ for age, gender, education, APOE carrier status, PiB status, or local and global PiB uptake ratios but for MMSE score. PiB uptake ratios were calculated by dividing uptake in the posterior cingulate cortex (local) and the entire cerebral cortex (global cortical) by cerebellar PiB uptake as a reference. Group comparisons were either performed by Mann-Whitney U test or χ2 statistics depending on the scale of measure. Significant p-values are indicated in bold letters (p < 0.05).
Key: aMCI, amnestic mild cognitive impairment; APOE, apolipoprotein E; HCS, healthy control subjects; MMSE, Mini Mental State Examination; PCC, posterior cingulate cortex; PiB, Pittsburg compound B; SD, standard deviation.
Indicates χ2.
3.2. Magnetic resonance spectroscopy
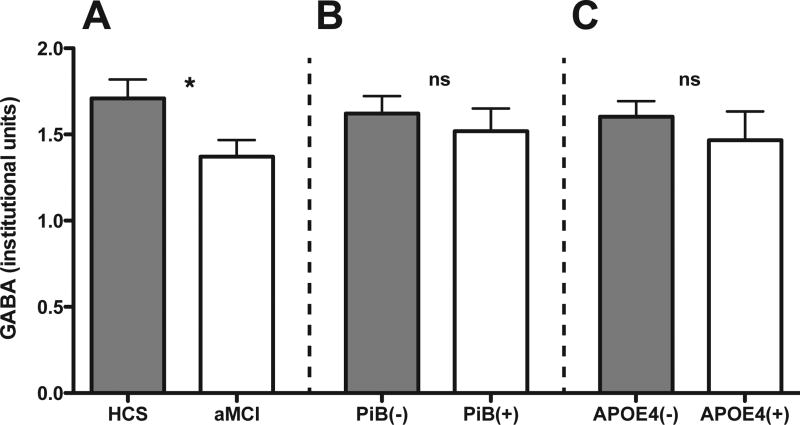
Voxel placement, the spectral fit for GABA and Glx (MEGA-PRESS) and a representative 68 ms spectrum (PRESS) are shown in Fig. 1. GM (p = 0.23), WM (p = 0.42), and CSF (p = 0.89) contents were not different between HCS and aMCI. The CRLB for GABA (HCS: mean 13.21 [range: 6.83–17.9] and aMCI: mean: 13.35 [range: 9.8–17.42]), and Glx (HCS: mean: 6.11 [range: 4.1–8.5] and aMCI: mean: 6.90 [range: 4.7–12.6]) were not different across both cohorts (GABA: p = 0.9 and Glx: p = 0.2). GABA levels and Glx were significantly reduced in aMCI (Table 2, Fig. 2). GABA was positively linked to Glx across all subjects (p = 0.002, r = 0.521) but not to NAA (p = 0.818). GABA and Glx were not correlated with age (p > 0.122). NAA was lower in aMCI (Table 2, Supplementary Fig. 2). All other examined metabolites (e.g., Cho, Cr, and mI) were not different across cohorts (p > 0.077). Across all participants, GABA and NAA but not Glx (p > 0.4) correlated with CERAD word learning score (p = 0.007, Spearman rho = 0.467 and p = 0.025, Spearman rho = 0.395, respectively; Supplementary Fig. 3).
Table 2.
Metabolite levels
| Group | p-value | ||||
|---|---|---|---|---|---|
|
|
|||||
| HCS (N = 21) | MCI (N = 15) | ||||
|
|
|
||||
| Mean | SD | Mean | SD | ||
| Metabolite levels | |||||
| GABA (AU) | 1.71 | 0.50 | 1.37 | 0.37 | 0.034 |
| Glx (AU) | 16.27 | 2.67 | 14.27 | 1.58 | 0.014 |
| NAA (AU) | 7.80 | 0.91 | 7.18 | 0.81 | 0.045 |
Group comparisons were performed by t test statistics.
GABA, Glx, and NAA levels were corrected for CSF, WM, and atrophy. Significant p-values are indicated in bold letters (p < 0.05).
Key: AU, arbitrary units; CSF, cerebrospinal fluid; GABA, γ-aminobutyric acid; Glx, glutamate + glutamine; HCS, healthy control subjects; MCI, mild cognitive impairment; NAA, N-acetyl-aspartate; SD, standard deviation; WM, white matter.
Fig. 2.
GABA levels grouped by diagnostic category (A), PiB status (B), and APOE carrier status (C). * p < 0.05; ns: not significant; t test. Abbreviations: APOE, apolipoprotein E; GABA, γ-aminobutyric acid; PiB, Pittsburgh compound B.
3.3. Pittsburgh compound B
In our sample, 12 subjects (33%) were PiB+ (5 HCS and 7 aMCI patients). As expected, local and global PiB were highly correlated across all subjects (p < 0.001, r = 0.831). Diagnostic groups were not different with respect to PiB-positivity, local, and global cortical PiB (Table 1). GABA and Glx were not different between PiB+ and PiB− subjects (p > 0. 553), whereas NAA was lower in PiB+ participants (p = 0.023, t test; Fig. 2, Supplementary Figs.1 and 2). Neither global (p > 0.114) nor local (p > 0.211) PiB were correlated with GABA, Glx, and NAA. Mean Stroop test scores were higher in PiB− (0.50 versus −0.16, p = 0.031).
3.4. Apolipoprotein E
In total, 9 participants (25%) were APOE ε4 carriers and diagnostic groups did not differ for APOE4-status (4 HCS and 5 MCI; Table 1). GABA and Glx levels were similar between APOE4+ and APOE4− (p > 0.47, Fig. 2 and Supplementary Fig. 1), whereas NAA was lower in APOE4+ (p = 0.016, Supplementary Fig. 2). Mean local (2.00 vs. 1.22) and global PiB (1.71 vs. 1.21) were higher in APOE4+ (p < 0.001). Mean CERAD recall scores were higher in APOE4− (0.13 vs. −0.83, p = 0.049).
3.5. Structural findings
There were no significant GM group-differences in the PCC (even at a cluster-corrected voxel threshold of p < 0.01). Further, PCC-specific GM, WM, and CSF content was not different between the 2 groups (all p > 0.23).
4. Discussion
In this cross-sectional MRS study, we compare in vivo levels of GABA, Glx, and NAA in the posterior cingulate cortex of HCS and age-, sex-, and education-matched aMCI. In particular, our study systematically investigates the relationship between these metabolites, cognitive performance, Aβ deposition, and APOE genotype, which are important parameters in AD. Amnestic MCI showed reduced GABA, Glx, and NAA levels. GABA and Glx were not associated with PiB status, global and local Aβ deposition, and APOE genotype. GABA and NAA were linked to CERAD word learning performance. NAA was lower in PiB+ and APOE4+.
4.1. Neurotransmitter alterations in aMCI
Previous studies have identified GABA as a metric closely related to cognitive performance in health (Boy et al., 2011; Edden et al., 2009; Michels et al., 2012; Muthukumaraswamy et al., 2009; Northoff et al., 2007; Sumner et al., 2010) and cognitive symptoms in neuropsychiatric disorders (Coghlan et al., 2012; Gonzalez-Burgos et al., 2011). Neurotransmitter levels correspond more closely to neuronal functioning than, for example, NAA, a surrogate marker of neuronal integrity and function, and may therefore be more responsive markers of treatment effects. Indeed, in the only other study investigating GABA levels in aMCI and HCS, treatment with growth hormone-releasing hormone increased GABA levels and improved cognitive function (Friedman et al., 2013). Contrary to our study, no GABA or Glu differences were found between HCS and aMCI before treatment, which may be because of different normalization procedures (normalization to Cr vs. H2O normalization) and the lack of correction for atrophy in the previous study. Mechanistically, the lower GABA levels could be caused either by a loss of GABAergic interneurons, a decrease in GABA synthesis, or alterations in astrocytic cycling of GABA, glutamate, and glutamine (Rae, 2014). A loss of GABAergic neurons would be expected to result in decreased NAA, as observed in the present study. However, GABA and NAA levels were uncorrelated in our sample, suggesting that the lower GABA levels are not driven by nonspecific neuronal loss.
4.2. GABA and Glx are not related to amyloid plaque deposition
The functional impact of Aβ plaque load remains the subject of intense discussion. In this context, our study demonstrates that PiB-PET measures of global and local (PCC-specific) Aβ deposition are not associated with changes in GABA and Glx levels—at least in our sample. In the only other combined MRS/PiB-PET study to date, global and local PCC PiB signal were correlated with mI and Cho, but not with NAA (Kantarci et al., 2011). Beyond MRS, functional MRI appears to be sensitive to Aβ deposition, because aberrant default network activity (Sperling et al., 2009) and decreased functional connectivity (Sheline et al., 2010) were reported in PiB(+) asymptomatic or minimally impaired individuals. These techniques therefore seem to reflect the underlying disease process better than GABA and Glx MRS, and thus are more suitable candidates as noninvasive biomarkers for preclinical AD. However, subsequent longitudinal studies may reveal the prognostic value of neurotransmitter levels beyond Aβ deposition. In contrast to PiB, GABA (and NAA) was linked to memory performance (Supplementary Fig. 3). The observed interaction between GABA and cognitive performance is in line with a recent study (Michels et al., 2012).
4.3. GABA and Glx are not related to APOE genotype
Although there is preclinical evidence for an impact of APOE genotype on GABAergic and glutamatergic neurotransmission (Andrews-Zwilling et al., 2010; Chen et al., 2010; Dumanis et al., 2013), we did not find a correlation between genotype and neurotransmitter levels in our sample. Similar to our findings, total tissue GABA and Glu content (as determined by MRS) did not differ between 1-year-old ApoE-e4 TR and ApoE-e3 TR mice (Dumanis et al., 2013). Our study therefore suggests that the known effects of APOE genotype on cognitive performance (Wishart et al., 2006; Wolk et al., 2010) may either be because of altered neurotransmitter levels in brain regions other than PCC or because of changes at receptor level.
4.4. Limitations
Because the present study is cross-sectional, no conclusion regarding the prognostic value of reduced GABA or Glx levels for Aβ deposition or conversion to AD dementia can be drawn. In addition, we did not examine brain regions other than the PCC. Yet, PCC has been studied extensively by MRS (Graff-Radford and Kantarci, 2013; Tumati et al., 2013) and shows high Aβ plaque deposition (Price et al., 2005). PCC ante mortem NAA to Cr, NAA to mI, and mI to Cr ratios furthermore correlate with the likelihood of AD-pathology at autopsy (Kantarci et al., 2008). Subcortical structures (e.g., hippocampus) are challenging for edited MRS and have therefore not been widely studied by MEGA-PRESS spectroscopy to date. The voxel size was relatively large but standard for GABA-MRS studies (Puts and Edden, 2012) and identical in size to that of a recent study examining GABA in the PCC of elderly HCS (Gao et al., 2013). In addition, MRS values were corrected for individual GM, WM, and CSF content and thus for differences in brain atrophy. No attempt was made to isolate Glu from Glx. Signals from Glu are difficult to separate from the analogous peaks arising from glutamine (Gln), so the observed reduction seen for Glx could either result from reduced Glu or Gln or both. Other sequences, such as J-resolved PRESS in combination with ProFit 2.0 (Fuchs et al., 2013) or Carr-Purcell PRESS (Hancu, 2009), might be used in future high-field MRS studies to isolate Glu from Gln and simultaneously correct for macromolecule content. Finally, because of the exploratory nature of our study, no attempts were made to correct for multiple comparisons. Thus, larger follow-up studies will be needed to confirm our results.
5. Conclusion
We show that local inhibitory (GABA) and excitatory (Glx) neurotransmitters as well as NAA are reduced in amnestic MCI. GABA and Glx levels are unrelated to Aβ deposition and APOE genotype, and thus may only reflect impaired cognitive function but not the preclinical stage of AD.
Supplementary Material
Acknowledgments
The authors thank all subjects for their participation in this study. They furthermore thank Isabella Blum, Esmeralda Gruber, and Faith Sieber (study nurses); Diana Bundschuh, Wiebke Buck, and Ioannis Giapitzakis for technical assistance; Sabine Spoerri, Stefan Kluge, and Stefan Doppler for data management, and all study physicians and neuropsychologists for help with data acquisition. This study was made possible by Swiss National Science Foundation grants 33CM30_124111, 33CM30_140335 and 320030_125378. R.A.E. Edden receives support from National Institutes of Health grant R01 EB016089.
Footnotes
Disclosure statement
Christoph Hock is a cofounder and board member of Neurimmune Holding AG, Schlieren. The other authors have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging.2014.07.030.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer‘s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antuono PG, Jones JL, Wang Y, Li SJ. Decreased glutamate + glutamine in Alzheimer’s disease detected in vivo with 1H-MRS at 0.5 T. Neurology. 2001;56:737–742. doi: 10.1212/wnl.56.6.737. [DOI] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, Lawrence AD, Singh KD, Husain M, Sumner P. Dorsolateral prefrontal γ-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol. Psychiatry. 2011;70:866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc. Natl. Acad. Sci. U.S.A. 2010;107:12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci. Biobehavioral Rev. 2012;36:2044–2055. doi: 10.1016/j.neubiorev.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JMN, Lei H, Mlynárik V, Gruetter R. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. Neuroimage. 2012;61:342–362. doi: 10.1016/j.neuroimage.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- Dumanis SB, DiBattista AM, Miessau M, Moussa CEH, Rebeck GW. APOE genotype affects the pre-synaptic compartment of glutamatergic nerve terminals. J. Neurochem. 2013;124:4–14. doi: 10.1111/j.1471-4159.2012.07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn. Reson. Med. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- Edden RAE, Muthukumaraswamy SD, Freeman TCA, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed N, Modrego PJ, Rojas-Salinas G, Aguilar K. Brain glutamate levels are decreased in Alzheimer’s disease: a magnetic resonance spectroscopy study. Am. J. Alzheimer’s Dis. Other Demen. 2011;26:450–456. doi: 10.1177/1533317511421780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SD, Baker LD, Borson S, Jensen JE, Barsness SM, Craft S, Merriam GR, Otto RK, Novotny EJ, Vitiello MV. Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging. JAMA Neurol. 2013;70:883–890. doi: 10.1001/jamaneurol.2013.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Boesiger P, Schulte RF, Henning A. ProFit revisited. Magn. Reson. Med. 2013 doi: 10.1002/mrm.24703. http://dx.doi.org/10.1002/mrm.24703 [Epub ahead of print] [DOI] [PubMed]
- Gao F, Edden RAE, Li M, Puts NAJ, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006;55:1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:1–24. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousias IS, Rueckert D, Heckemann RA, Dyet LE, Boardman JP, Edwards AD, Hammers A. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. Neuroimage. 2008;40:672–684. doi: 10.1016/j.neuroimage.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Graff-Radford J, Kantarci K. Magnetic resonance spectroscopy in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2013;9:687–696. doi: 10.2147/NDT.S35440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A, Koepp MJ, Richardson MP, Hurlemann R, Brooks DJ, Duncan JS. Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI. A PET study of 44 patients. Brain. 2003;126:1300–1318. doi: 10.1093/brain/awg138. [DOI] [PubMed] [Google Scholar]
- Hancu I. Optimized glutamate detection at 3T. J. Magn. Reson. Imaging. 2009;30:1155–1162. doi: 10.1002/jmri.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning A, Schär M, Schulte RF, Wilm B, Pruessmann KP, Boesiger P. SELOVS: brain MRSI localization based on highly selective T1- and B1- insensitive outer-volume suppression at 3T. Magn. Reson. Med. 2008;59:40–51. doi: 10.1002/mrm.21374. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Hock A, Fuchs A, Boesiger P, Kollias SS, Henning A. Electrocardiogram-triggered, higher order, projection-based B0 shimming allows for fast and reproducible shim convergence in spinal cord 1H MRS. NMR Biomed. 2013;26:329–335. doi: 10.1002/nbm.2852. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Knopman DS, Dickson DW, Parisi JE, Whitwell JL, Weigand SD, Josephs KA, Boeve BF, Petersen RC, Jack CR. Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 2008;248:210–220. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Lowe V, Przybelski SA, Senjem ML, Weigand SD, Ivnik RJ, Roberts R, Geda YE, Boeve BF, Knopman DS, Petersen RC, Jack CR. Magnetic resonance spectroscopy, amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77:951–958. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K. Proton MRS in mild cognitive impairment. J. Magn. Reson. Imaging. 2013;37:770–777. doi: 10.1002/jmri.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Weigand SD, Przybelski SA, Preboske GM, Pankratz VS, Vemuri P, Senjem ML, Murphy MC, Gunter JL, Machulda MM, Ivnik RJ, Roberts RO, Boeve BF, Rocca WA, Knopman DS, Petersen RC, Jack CR. MRI and MRS predictors of mild cognitive impairment in a population-based sample. Neurology. 2013;81:126–133. doi: 10.1212/WNL.0b013e31829a3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctôt KL, Herrmann N, Mazzotta P, Khan LR, Ingber N. GABAergic function in Alzheimer’s disease: evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia. Can J. Psychiatry. 2004;49:439–453. doi: 10.1177/070674370404900705. [DOI] [PubMed] [Google Scholar]
- Londono AC, Castellanos FX, Arbelaez A, Ruiz A, Aguirre-Acevedo DC, Richardson AM, Easteal S, Lidbury BA, Arcos-Burgos M, Lopera F. H-MRS framework predicts the onset of Alzheimer’s disease symptoms in PSEN1 mutation carriers. Alzheimer’s Dement. 2013 doi: 10.1016/j.jalz.2013.08.282. http://dx.doi.org/10.1016/j.jalz.2013.08.282 [Epub ahead of print] [DOI] [PubMed]
- Michels L, Martin E, Klaver P, Edden R, Zelaya F, Lythgoe DJ, Lüchinger R, Brandeis D, O’Gorman RL. Frontal GABA levels change during working memory. PLoS One. 2012;7:e31933. doi: 10.1371/journal.pone.0031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrego PJ, Fayed N, Pina MA. Conversion from mild cognitive impairment to probable Alzheimer’s disease predicted by brain magnetic resonance spectroscopy. Am. J. Psychiatry. 2005;162:667–675. doi: 10.1176/appi.ajp.162.4.667. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer“ s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O’Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Cardiff Symposium on MRS of GABA. Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat. Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res. 2014;39:1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol. Aging. 2011;32:802–810. doi: 10.1016/j.neurobiolaging.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Edden RAE, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat. Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Tumati S, Martens S, Alemán A. Magnetic resonance spectroscopy in mild cognitive impairment: systematic review and meta-analysis. Neurosci. Biobehavioral Rev. 2013;37(10 Pt 2):2571–2586. doi: 10.1016/j.neubiorev.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, Hasselbalch S, Law I, Andersen A, Korner A, Minthon L, Garraux G, Nelissen N, Bormans G, Buckley C, Owenius R, Thurfjell L, Farrar G, Brooks DJ. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann. Neurol. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer’s disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer’s Disease. Arch. Neurol. 1992;49:448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- Wilson M, Reynolds G, Kauppinen RA, Arvanitis TN, Peet AC. A constrained least-squares approach to the automated quantitation of in vivo 1H magnetic resonance spectroscopy data. Magn. Reson. Med. 2011;65:1–12. doi: 10.1002/mrm.22579. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, Mamourian AC, Belloni AC, Rhodes DR, McAllister TW. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am. J. Psychiatry. 2006;163:1603–1610. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC Alzheimer’s Disease Neuroimaging Initiative. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Barker PB. Magnetic resonance neuroimaging. Vol. 711. Humana Press; Totowa, NJ: 2010. MR spectroscopy and spectroscopic imaging of the brain; pp. 203–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.