Abstract
Purpose
we examined the role of consolidation radiotherapy for patients with DLBCL treated at institutions of the National Comprehensive Cancer Network during the Ritixumab era.
Patients and Methods
We analyzed failure-free survival (FFS) and overall survival (OS) in terms of patient and treatment’s characteristics. Potential associations were investigated with univariate and multivariable survival analysis and matched pair analysis.
Results
Of 841 patients, most (710 [84%]) had received 6–8 cycles of R-CHOP, and 294 (35%) had received consolidation RT. Failure occurred in 181 patients, 126 (70%) who did not receive RT and 55 (30%) who did. At 5 years, both OS and FFS rates were better for patients who received RT than for those who did not (OS 91% vs. 83%, p=0.01; FFS 83% vs. 76%, p=0.05). Matched-pair analysis (217 pairs, matched by age, stage, IPI score, B symptoms, disease bulk, and response to chemotherapy) showed that receipt of RT improved OS and FFS for patients with stage III/IV disease (hazard ratios [HRs] 0.53 [p=0.07] and 0.77 [p=0.34]), but too few events took place among those with stage I/II disease for meaningful comparison (HR for OS=0.94 [p=0.89]; for FFS=1.81 [p=0.15]). Multivariate analysis suggested that IPI score and response to chemotherapy had the greatest influence on outcome.
Conclusion
There is a trend of higher OS and FFS rates in patients who received consolidation RT after R-CHOP, especially in stage III/IV, but the difference did not reach statistical significance.
Keywords: Radiation, consolidation, RCHOP, early stage
INTRODUCTION
Over the past two decades, recognition of the complexity of diffuse large B cell lymphoma (DLBCL) has led to considerable changes in the recommended treatment, as reflected in guidelines from the U.S. National Comprehensive Cancer Network (NCCN). A variety of pathologic, laboratory, and cytogenetic factors are now used to predict individual patients’ response to therapy and subsequent clinical outcome (1–5). In addition, response-adapted therapy based on changes in 18F-fluorodeoxyglucose uptake on positron emission tomography (FDG-PET) is being introduced into the NCCN guidelines as findings on individually tailored chemotherapy become mature (6–11). The introduction of rituximab to CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy became standard therapy in the early 2000s based on available scientific evidence (12). On the other hand, whether radiation therapy (RT) has a role in the treatment of DLBCL continues to be the subject of an ongoing controversy(13), partly reflecting and extrapolating on the late side effects that developed in patients treated for Hodgkin’s several decades ago, where both radiation techniques and fields were rudimentary compared to the technology available today. Add to this a conflicting evidence coming from both randomized and single-institution studies (4, 10, 14–20). In view of these developments and technological advances in the planning and delivery of RT, we chose to examine the effects of using modern-day RT techniques for DLBCL at NCCN institutions on outcome.
For this analysis, we used the NCCN outcomes database, which includes comprehensive, prospectively collected data on clinical characteristics, treatment factors, and outcomes for patients being treated for non-Hodgkin lymphoma. We sought to determine if RT influenced, failure-free survival (FFS), and overall survival (OS) in patients with newly diagnosed DLBCL of any stage who had been treated with R-CHOP chemotherapy.
PATIENTS AND METHODS
Patients
We identified patients with newly diagnosed DLBCL presenting at one of seven member institutions of the NCCN from January 1, 2001 through December 31, 2008. The 841 patients in this analysis had a confirmed diagnosis of DLBCL that had been treated with R-CHOP, with or without consolidation RT (defined as RT received within 90 days after completion of chemotherapy). Patients were excluded who had experienced disease relapse or progression during first-line therapy (including RT) or those whose therapy was protocol-directed. The following characteristics were retrieved: age at diagnosis, sex, race, Ann Arbor disease stage, Revised International Prognostic Index (IPI) score based on rituximab-era data 15, presence of B symptoms and bulky disease (defined as ≥10 cm in maximum dimension), site of disease (nodal vs. extranodal, please note that patients who had any extranodal site were grouped as extranodal), type and number of cycles of chemotherapy, response to chemotherapy, type of imaging used to assess response, use of radiation, site of failure (in relation to radiation fields), and disease and vital status at last follow-up, with cause of death if applicable. The 5-year FFS and OS rates were calculated as described below.
Pathology specimens (excisional and core-needle biopsy samples) had been handled at each NCCN institution; no central review was done.
RT had been recommended at the discretion of the treating medical oncologist; the radiation fields used were involved-field. Unfortunately the radiation dose was not provided so we could not perform a dose response analysis.
Statistical Analysis
Definitions of response used in the NCCN database were as follows. Complete remission (including complete remission undetermined) was defined as the complete disappearance of all detectable clinical and radiographic evidence of disease and all disease-related symptoms if present before therapy, and normalization of biochemical abnormalities (LDH) attributable to non-Hodgkin lymphoma lasting at least 4 weeks. Partial remission was defined as <50% response as indicated by images obtained at the end of therapy. Bulky disease was ≥10 cm in maximum dimension. For the purposes of this study, FFS was defined as the time from completion of treatment to relapse; OS was defined as the time from time of diagnosis to date of last follow up or date of death. Baseline demographic and clinical characteristics were compared between subgroups with Chi-square tests (for categorical variables) or Wilcoxon rank-sum tests (for medians). Univariate logistic regression analysis was used to assess the potential factors that influenced the 5 year overall survival and freedom from progression. Factors with a p value ≤ 0.25 were tested in a multivariate model. Cox proportional hazards regression was used to build the multivariate model. All statistical analyses were done Stata/SE version 2011 (StataCorp, College Station, TX).
We also assessed FFS and OS by using matched cohort analysis as follows. Patients were paired according to whether they had or had not received RT and matched for known prognostic factors including age, sex, disease stage, IPI score, the presence of B symptoms or bulky disease, number of chemotherapy cycles delivered, and response to chemotherapy. Patients who had received RT were exactly matched (without placement) to R-CHOP-only patients to the third decimal place (thousandths) of the propensity score on a 1:1 basis. Cox proportional hazards regression was done with stratification on the propensity score (rounded to the tenth place) to account for the matching nature of the data.
RESULTS
Clinical characteristics are summarized in Table 1. Median follow-up time was 4.5 years (range 0.5–10.7 years) and median age at diagnosis was 57.1 years (range 17–90 years); 455 patients (54%) were male; 689 (82%) were Caucasian; 402 (48%) had stage I or II disease; 186 (22%) had an IPI score of 0 and 446 (53%) an IPI score of 1 or 2; 240 (29%) had B symptoms at presentation; 192 (23%) had bulky disease at presentation (including 88 patients with stage I/II disease); and 620 patients (74%) presented with nodal disease. Most. patients (710, or 84%) had had 6–8 cycles of R-CHOP (74% of those with stage I/II disease and 94% of those with stage III/IV) and 101 (12%) received intrathecal methotrexate; 293 (35%) received consolidation RT (of whom 217 had stage I/II disease [64 bulky] and 76 had stage III/IV disease [23 bulky]). In terms of disease response, 632 (75%) had a complete response. Most (73%) of the patients with bulky stage I/II disease had received RT as compared with 22% of patients with bulky stage III/IV disease.
Table 1.
Patient Characteristics
| Treatment Group | ||||
|---|---|---|---|---|
| Characteristic | All No. (%) | CHOP-R No. (%) | CHOP-R+RT No. (%) | P Value |
| 841 | 548 (65.2%) | 293 (34.8) | ||
|
| ||||
| Age at diagnosis, y | <0.0001 | |||
| Median (range) | 57.1 (17.9–90.7) | 58.3 (17.9–90.7) | 54.1 (18.1–89.4) | |
| Sex | ||||
| Male | 455 (54.1) | 286 (52.2) | 169 (57.7) | 0.13 |
| Female | 386 (45.9) | 262 (47.8) | 124 (42.3) | |
| Race/Ethnicity | 0.81 | |||
| Caucasian/non-Hispanic | 689 (81.9) | 447 (81.6) | 242 (82.6) | |
| Hispanic | 51 (6.1) | 31 (5.7) | 20 (6.8) | |
| African-American, non- Hispanic | 37 (4.4) | 27 (4.9) | 10 (3.4) | |
| Asian, PI, non-Hispanic | 38 (4.5) | 24 (4.4) | 14 (4.8) | |
| American Indian, non-Hispanic | 1 (0.2) | 1 (0.2) | 0 | |
| Other non-Hispanic | 5 (0.6) | 3 (0.55) | 2 (0.7) | |
| Unknown | 20 (2.4) | 15 (2.7) | 5 (1.7) | |
| Disease Stage at Diagnosis | <0.0001 | |||
| I | 218 (25.9) | 86 (15.7) | 132 (45.0) | |
| II | 184 (21.9) | 99 (18.1) | 85 (29.0) | |
| III | 137 (16.3) | 119 (21.7) | 18 (6.1) | |
| IV | 302 (35.9) | 244 (44.5) | 58 (19.8) | |
| IPI Score | <0.0001 | |||
| 0 | 186 (22.1) | 89 (16.2) | 97 (33.1) | |
| 1–2 | 446 (53.0) | 282 (51.5) | 164 (55.8) | |
| 3+ | 209 (24.8) | 177 (32.3) | 32 (10.1) | |
| No. of Chemotherapy Cycles | <0.0001 | |||
| <6 cycles | 131 (15.6) | 49 (8.9) | 82 (28.0) | |
| 6–8 cycles | 710 (84.4) | 499 (91.1) | 211 (72.0) | |
| Response to therapy | ||||
| CR | 633 (75.1) | 441 (80.4) | 192 (65.3) | <0.0001 |
| B Symptoms at Presentation | 0.03 | |||
| Unknown | 6 (0.7) | 4 (0.7) | 2 (0.7) | |
| No | 595 (70.7) | 371 (67.7) | 224 (76.4) | |
| Yes | 240 (28.5) | 173 (31.6) | 67 (22.9) | |
| Bulky Disease | 0.0005 | |||
| No | 649 (77.2) | 443 (80.8) | 206 (70.3) | |
| Yes | 192 (22.8) | 105 (19.2) | 87 (29.7) | |
| Nodal versus extranodal presentation | <0.0001 | |||
| Extranodal | 221 (26.3) | 109 (20.0) | 112 (38.2) | |
| Nodal | 620 (73.7) | 439 (80.1) | 181 (61.8) | |
Patients who received radiation and compared to those who did not, were significantly younger (54 versus 58 years; <0.0001), more likely to have stage I-II disease (74% versus 38%; p<0.0001), lower IPI score (89% versus 68%; p<0.0001), more likely to have bulky disease (30% versus 19%; p=0.0005), more likely to have extranodal disease (38% versus 20%; p<0.0001), and to receive abbreviated chemotherapy (28% versus 9%; p<0.0001). Response to treatment, evaluated within 2 months of therapy completion, was assessed with computed tomography (CT) in 219 patients (26%) (143 in R-CHOP-only and 76 in R-CHOP+RT groups); PET in 145 patients (17%) (97 in R-CHOP-only and 48 in R-CHOP+RT groups); and PET/CT in 327 patients (39%) (217 in R-CHOP-only and 110 in R-CHOP+RT groups). Most patients (633, or 75%) experienced complete remission after R-CHOP; 192 of those patients had received RT. Another 187 patients (22%) experienced partial remission, of whom 93 had received RT; 6 patients had stable disease; and 15 could not be evaluated. At the time of this analysis, 119 patients had died (88 in the R-CHOP-only and 31 in the R-CHOP+RT groups); notably, a majority (66, or 55%) had died of progressive disease (Table 2).
Table 2.
Causes of Death
| Treatment Group | |||
|---|---|---|---|
| Coded Cause of Death | All No. (%) | CHOP-R No. (%) | CHOP-R+RT No. (%) |
| All Deaths | 119 | 88 (73.9) | 31 (26.0) |
| Missing | 3 (2.5) | 2 (2.3) | 1 (3.2) |
| Unknown | 22 (18.5) | 17 (19.3) | 5 (16.1) |
| Other | 18 (15.1) | 14 (15.9) | 4 (12.9) |
| Progressive Disease | 66 (55.5) | 49 (55.7) | 17 (54.8) |
| Excessive Toxicity | 3 (2.5) | 2 (2.3) | 1 (3.2) |
| Secondary Malignancy | 6 (5.0) | 3 (3.4) | 3 (9.7) |
| Accidental Death | 1 (0.8) | 1 (0.8) | 0 |
Factors Contributing to OS and FFS
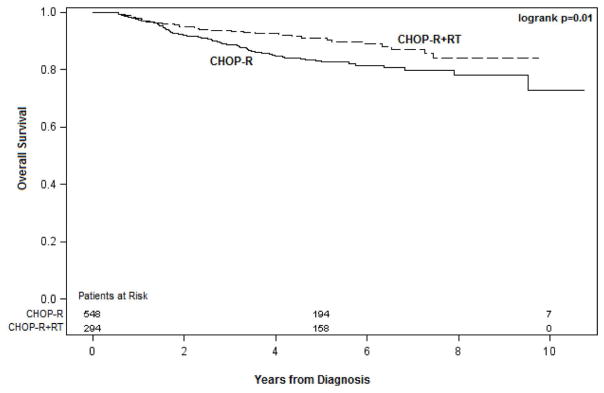
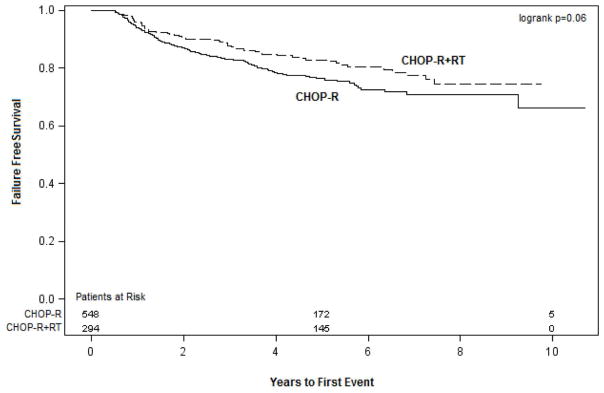
The 5-year OS and FFS rates significantly higher in patients who received RT (OS 91% and FFS 83%) than for those who did not (OS 83% [p=0.05] and FFS 76% [p=0.01]) (Figures. 1 and 2). Univariate analysis revealed that the following factors influenced both 5-year OS and FFS (Table 3): Age (≤ 60 years, OS 90% and FFS 83% vs. >60 years, OS 80% and FFS 73%, p=0.0006); disease stage at diagnosis (I/II, OS 93% and FFS 89% vs. III/IV, OS 79% and FFS 69%, p< 0.0001); IPI score (0, OS 98% and FFS 95% vs. 1–2, OS 87% and FFS 79% vs. ≥;3, OS 73% and FFS 64%, p<0.0001); B symptoms (no, OS 91% and FFS 74% vs. yes, OS 84% and FFS 67% , p<0.0001); and bulky disease (no, OS 88% and FFS 81% vs. yes, OS 77% OS and FFS 70%, p=0.0003). Having extranodal disease at presentation was associated with FFS (85% vs. 76% for nodal disease only, p=0.008), but not with OS. Finally, response to therapy was also associated with both OS and FFS (complete, 88% OS and 81% FFS vs. no complete response, 79% OS and 72% FFS, p=0.0003).
Figure 1.
The 5-year OS and FFS rates for patients who received RT versus those who did not.
Figure 2.
The 5-year OS and FFS rates for patients who received RT versus those who did not.
Table 3.
Univariate Analysis of Overall and Failure-Free Survival at 5 Years: All Patients
| 5-Year Overall Survival | 5-Year Failure-Free Survival | |||||
|---|---|---|---|---|---|---|
| Variable | Survival Estimate | 95% CI | Log-Rank P Value | Survival Estimate | 95% CI | Log-Rank P Value |
| Age at Diagnosis | ||||||
| ≤60 years | 0.90 | 0.87–0.92 | <0.0001 | 0.83 | 0.79–0.86 | 0.0006 |
| >60 years | 0.80 | 0.75–0.84 | 0.73 | 0.67–0.78 | ||
| Sex | ||||||
| Female | 0.86 | 0.82–0.90 | 0.71 | 0.80 | 0.75–0.84 | 0.59 |
| Male | 0.85 | 0.81–0.88 | 0.78 | 0.73–0.81 | ||
| Race | ||||||
| Other | 0.82 | 0.73–0.88 | 0.47 | 0.76 | 0.67–0.83 | 0.63 |
| Caucasian | 0.86 | 0.83–0.89 | 0.79 | 0.76–0.82 | ||
| Stage at Diagnosis | ||||||
| I/II | 0.93 | 0.89–0.95 | <0.0001 | 0.89 | 0.86–0.92 | <0.0001 |
| III/IV | 0.79 | 0.75–0.83 | 0.69 | 0.64–0.73 | ||
| IPI Score | ||||||
| 0 | 0.98 | 0.94–0.99 | <0.0001 | 0.95 | 0.90–0.98 | <0.0001 |
| 1–2 | 0.87 | 0.83–0.90 | 0.79 | 0.75–0.83 | ||
| 3+ | 0.73 | 0.66–0.79 | 0.64 | 0.56–0.70 | ||
| Chemotherapy | ||||||
| <6 cycles | 0.79 | 0.69–0.86 | 0.08 | 0.75 | 0.65–0.82 | 0.88 |
| 6–8 cycles | 0.87 | 0.84–0.89 | 0.79 | 0.76–0.82 | ||
| B Symptoms | ||||||
| No | 0.91 | 0.88–0.93 | <0.0001 | 0.84 | 0.80–0.87 | <0.0001 |
| Yes | 0.74 | 0.67–0.79 | 0.67 | 0.60–0.73 | ||
| Bulky Disease | ||||||
| No | 0.88 | 0.85–0.91 | <0.0001 | 0.81 | 0.78–0.84 | 0.0003 |
| Yes | 0.77 | 0.71–0.83 | 0.70 | 0.63–0.76 | ||
| Response to Therapy | ||||||
| Other | 0.79 | 0.73–0.84 | <0.0001 | 0.72 | 0.65–0.78 | 0.0003 |
| Complete | 0.88 | 0.85–0.90 | 0.81 | 0.77–0.84 | ||
| Lymph Node Involvement at Initial Site | ||||||
| No | 0.88 | 0.82–0.92 | 0.45 | 0.85 | 0.79–0.90 | 0.008 |
| Yes | 0.85 | 0.81–0.88 | 0.76 | 0.72–0.80 | ||
| Receipt of Radiotherapy | ||||||
| No | 0.83 | (0.79–0.86) | 0.01 | 0.76 | (0.72–0.80) | 0.05 |
| Yes | 0.91 | (0.87–0.94) | 0.83 | (0.78–0.87) | ||
In multivariate analyses, the use of RT was associated with a non-significant trend of lower risk of death (HR=0.67) and failure (HR=0.86). Response to therapy and IPI score had the greatest influences on both OS and FFS; patients with IPI>3 had HRs of 9.9 for death and 6.4 for failure (p<0.0001 for both), and those who achieved a complete remission had HRs of 0.50 for death (p=0.0009) and 0.57 for failure (p=0.001). The presence of B symptoms and bulky disease and receipt of <6 cycles of chemotherapy also influenced both OS and FFS (p=0.01), details in Table 5.
Table 5.
Multivariate Analyses for Overall and Failure-Free Survival by Number of Cycles of R-CHOP
| Patients Receiving <6 Cycles of R-CHOP (n=103) | Patients Receiving 6–8 cycles of R-CHOP (n=296) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall Survival | Failure-Free Survival | Overall Survival | Failure-Free Survival | |||||||||
| Variable | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value |
| Sex | 0.09 | 0.03 | --dropped-- | --dropped | ||||||||
| Female | 1.0 | 1.0 | ||||||||||
| Male | 0.32 | 0.08–1.19 | 0.22 | 0.06–0.84 | ||||||||
| Race | 0.02 | 0.005 | --dropped-- | --dropped-- | ||||||||
| Other | 1.0 | 1.0 | ||||||||||
| Caucasian | 0.21 | 0.06–0.74 | 0.17 | 0.05–0.59 | ||||||||
| IPI Score | 0.28 | 0.24 | 0.09 | 0.26 | ||||||||
| 0 | 1.0 | 1.00 | 1.0 | 1.0 | ||||||||
| 1–2 | 5.55 | 0.67–46.01 | 6.12 | 0.74–50.56 | 3.52 | 0.97–12.79 | 1.90 | 0.84–4.27 | ||||
| 3+ | NE | NE | 8.47 | 0.85–84.31 | 2.67 | 0.33–21.40 | ||||||
| B Symptoms | 0.52 | 0.98 | 0.93 | 0.82 | ||||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| Yes | 1.59 | 0.38–6.59 | 0.98 | 0.24–3.96 | 1.07 | 0.29–3.93 | 1.12 | 0.42–3.00 | ||||
| Bulky Disease | 0.50 | 0.07 | 0.58 | 0.73 | ||||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| Yes | 1.65 | 0.39–6.95 | 3.57 | 0.90–14.11 | 1.38 | 0.44–4.39 | 0.86 | 0.37–2.01 | ||||
| Response to Therapy | 0.50 | 0.97 | 0.55 | 1.0 | ||||||||
| Other | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| Complete | 0.63 | 0.16–2.42 | 0.97 | 0.26–3.58 | 1.5 | 0.40–5.58 | 1.0 | 0.41–2.42 | ||||
| Radiotherapy | 0.20 | 0.22 | 0.30 | 0.01 | ||||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| Yes | 0.42 | 0.11–1.59 | 0.44 | 0.12–1.64 | 1.85 | 0.58–5.95 | 3.08 | 1.25–7.58 | ||||
Abbreviations: R-CHOP, rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone; HR, hazard ratio; CI, confidence interval; IPI, International Prognostic Index; NE, could not be estimated
To further examine influences on FFS and OS, we undertook a matched-pair survival analysis using propensity scores to estimate the effect of RT and the observed covariates of age, disease stage, IPI score, number of chemotherapy cycles, B symptoms, bulky disease, and response to therapy on OS and FFS (Table 4). Of the 217 total matched pairs, 125 had stage I/II disease and 76 had stage III/IV disease. This analysis showed that use of RT seemed to reduce the risk of death (HR=0.76) and failure (HR=0.92) among all patients and in particular among patients with stage III/IV disease (HR= 0.53 for death and HR=0.77 for failure), but these effects were not statistically significant (Table 4). Surprisingly, the use of RT for patients with stage I/II disease seemed to be associated with a higher failure rate (HR=1.81), although the p value for this comparison was also not significant.
Table 4.
Matched-Pair Analyses for Overall and Failure-Free Survival by Disease Stage
| All Patients (217 matched pairs) | Stage I/II Patients (125 matched pairs) | Stage III/IV Patients (76 matched pairs) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Survival Estimate | 95% CI | Log-Rank P Value | Survival Estimate | 95% CI | Log-Rank P Value | Survival Estimate | 95% CI | Log-Rank P Value |
| Overall Survival | |||||||||
| Radiotherapy | |||||||||
| No | Baseline | 0.32 | Baseline | 0.89 | Baseline | 0.07 | |||
| Yes | 0.76 | 0.45–1.30 | 0.94 | 0.38–2.34 | 0.53 | 0.27–1.06 | |||
| Failure-Free Survival | |||||||||
| Radiotherapy | |||||||||
| No | Baseline | 0.69 | Baseline | 0.15 | Baseline | 0.34 | |||
| Yes | 0.92 | 0.60–1.40 | 1.81 | 0.81–4.02 | 0.77 | 0.46–1.32 | |||
The rather illogical finding of higher failure rates for patients given RT for stage I/II disease led us to perform the following subset analyses. The first of these subset analyses separated patients with stage I/II disease into one of two subgroups, those who had received abbreviated chemotherapy (<6 cycles of R-CHOP, n=103) and those who had received the full 6–8 cycles of R-CHOP (n=296). For patients who got <6 cycles of R-CHOP, receipt of RT (vs. no RT) was associated with a non-significant trend of higher OS (HR=0.4, p=0.19) and FFS (HR=0.44, p=0.24). On the other hand, patients who received RT after the standard full-course R-CHOP had worse OS (HR=2.06, p=0.22) and FFS (HR=3.27, p=0.01) than those who did not receive RT (Table 5). Other attempts to evaluate OS and FFS in terms of disease bulk and nodal-only versus extranodal presentation also showed no statistically significant associations (data not shown).
In the second and final subset analysis, we looked at sites of failure in relation to receipt or no receipt of RT (Table 6). In that comparison, we found that the total number of failure sites was 126 (70%) among patients who did not receive RT versus 55 (30%) among those who did receive RT; moreover, failure appeared at the same presenting disease site in 23% of those who did not receive RT versus only 8% in those who did receive RT. These findings suggest that RT was helpful for minimizing the number of failure sites and preventing local recurrence.
Table 6.
Sites of Failure versus Receipt of Radiation
| Treatment Group | |||
|---|---|---|---|
| Failure Site | All No. (%) | CHOP-R No. (%) | CHOP-R+RT No. (%) |
| No. of Failures | 181 (100) | 126 (69.6) | 55 (30.4) |
| Distant Site Only | 47 (26.0) | 31 (17.1) | 16 (8.8) |
| Row percent | 66.0 | 34.0 | |
| Column percent | 24.6 | 29.1 | |
| Same Site | 55 (30.4) | 41 (22.6) | 14 (7.7) |
| Row percent | 74.5 | 25.4 | |
| Column percent | 32.5 | 25.4 | |
| Same and Distant Site | 37 (20.4) | 24 (13.3) | 13 (7.2) |
| Row percent | 64.9 | 35.1 | |
| Column percent | 19.0 | 23.6 | |
| No Sites of Failure | 28 (15.5) | 20 (11.0) | 8 (4.4) |
| Row percent | 71.4 | 28.6 | |
| Column percent | 15.9 | 14.5 | |
| Unknown | 14 (7.7) | 10 (5.5) | 4 (2.2) |
| Row percent | 71.4 | 28.6 | |
| Column percent | 7.9 | 7.2 | |
Frequency missing = 660
DISCUSSION
Recent clinical reports indicate that RT may have merit for treatment of patients with DLBCL during the rituximab era. The published report of the RICOVER-60 trial (21), in which 166 elderly patients with DLBCL of all stages of disease were treated, compared the results of the best arm of immune-chemotherapy (6RCHOP+2R) plus 36 Gy to initial bulky sites (≥7.5 cm) to the results of a cohort treated without radiation. Although the radiation treatment decision was not randomized, those treated with radiation showed statistically significant improvements in OS (90% for RT group versus 65% for no RT; p=0.001) and event free survival (EFS) (80% for RT group versus 54% for no RT; p=0.001). The UNFOLDER trial conducted by the German High Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL), on the other hand, randomized 450 patients with DLBCL of all stages to receive either R-CHOP-14 versus R-CHOP-21, with a second randomization to radiation versus observation for patients with extra-nodal or bulky disease. These were patients with all stages of disease, similarly to the RICOVER trial. Final results have not yet been published. However, the RT randomization arms were closed when the second interim analysis showed a higher failure rate in the no-RT arm (data presented at the American Society of Hematology 2012).
Since rituximab became part of the standard of care for DLBCL, other trials have also addressed the role of radiation treatment for DLBCL of all stages, albeit indirectly or retrospectively. The MinT trial(17), evaluated the benefit of adding rituximab to CHOP for patients with stages II-IV or bulky stage I DLBCL. Rituximab minimized but did not eliminate the adverse prognostic effect of tumor bulk on outcome, thereby suggesting that RT could have merit for patients with bulky disease regardless of stage. A retrospective study from MD Anderson Cancer Center in which most patients had received 6 or more cycles of R-CHOP showed that the addition of radiation improved both OS and progression-free survival (PFS) across all disease stages: the 5-year OS and PFS rates were 90% and 91% for those who received radiation compared with 75% and 83% for those who did not (p<0.001). A matched-pair analysis based on disease stage and accounting for number of cycles of R-CHOP, radiation, IPI score, tumor response to therapy, and disease bulk confirmed the benefit of radiation, with longer OS and PFS times for those who received radiation (HRs 0.52 for OS and 0.45 for PFS) (20).
The present observational analysis of outcome includes a large cohort of patients with DLBCL of all stages, treated with RCHOP chemotherapy, with or without radiation. Radiation was predominantly given to patients with limited stage disease, or to those with extranodal, and/or bulky tumor sites. The decision for RT referral for treatment was at the discretion of the treating medical oncologist, which in turn represents an uncontrolled selection bias for the radiation treatment group. In addition, patients were treated across several institutions, and compliance of intended treatment might have been unaccounted. The use of radiation varied among the institutions, between 5% and 31%, a heterogeneity that also is inherently difficult to control.
Kaplan Meier survival analysis of the whole group of patients indicated overall improvements in both OS and FFS. Multivariate analysis, however, did not show FFS improvement, and there was no significant difference in outcomes by institution. Given the heterogeneity of factors in the patient cohort, however, we did a matched-pair analysis including 217 pairs of patients. In the matched pair analysis, there was no statistically different outcome for those who received radiation. A possible explanation for not having an overall positive benefit of RT in the matched cohorts is that these were predominantly patients with early stage disease. The number of failure events seen overall in the limited stage group was very low (with only 26 failures out 402 patients, or 6%). When we analyzed outcomes by subsets with the worst prognostic factors (high IPI score, bulky disease, less than complete response to chemotherapy), a benefit from RT was seen for those with stage III/IV disease, as well as those with stage I/II who had received abbreviated chemotherapy (< 6 cycles of chemotherapy).
The results of this analysis in general aligned with those observed in the previously noted studies that suggest RT may contribute to improved outcomes in certain subsets of patients with DLBCL. The challenge remains in defining the criteria by which the patients most likely to benefit can be identified. It is evident from practice patterns that, despite observed variations, medical and radiation oncologists are applying radiation treatment in combination with chemotherapy for DLBCL in selected patients. Radiation, however, is not without adverse effects, particularly when combined with chemotherapy. Hence, to obtain the most benefit with least side effects, it is essential to incorporate the technological innovations in the field of radiation oncology planning and delivery to substantially reduce the acute and long-term side effects of RT. In addition, to avoid unnecessary treatment and potential harm, it is critical to prospectively study and definitively identify the factors that define the unique subsets of patients for whom combined modality therapy would be most appropriate.
Acknowledgments
Research Support: This study was supported in part by Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center.
Footnotes
Presented at the 12th International Conference on Malignant Lymphoma, June 19–22, 2013, Palazzo dei Congressi, Lugano, Switzerland
Previous publication of this information: Presented in part 12th International conference on malignant lymphoma, Lugano, 2011.
Authorship Contributions
Conception and design: Bouthaina Dabaja and Alma Rodriguez
Gathering of data for the Study: All authors
Data analysis and interpretation: Bouthaina Dabaja, Alma Rodriguez, Ann M. Vanderplas, Allison L. Crosby-Thompson
Manuscript writing: Bouthaina Dabaja, Alma Rodriguez, Ann LaCasce, and Jonathan Friedberg.
Final approval of manuscript: All authors
Disclosures: All authors have no financial disclosures, Alma Rodriguez has research funding from Amgen, Pfizer, orthobiotec and Glaxo.
References
- 1.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000 Feb 3;403(6769):503–11. doi: 10.1038/35000501. Epub 2000/02/17. eng. [DOI] [PubMed] [Google Scholar]
- 2.Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, et al. Double-hit B-cell lymphomas. Blood. 2011 Feb 24;117(8):2319–31. doi: 10.1182/blood-2010-09-297879. Epub 2010/12/02. eng. [DOI] [PubMed] [Google Scholar]
- 3.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004 Jan 1;103(1):275–82. doi: 10.1182/blood-2003-05-1545. Epub 2003/09/25. eng. [DOI] [PubMed] [Google Scholar]
- 4.Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Jan 10;29(2):200–7. doi: 10.1200/JCO.2010.30.0368. Epub 2010/12/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, Banham AH, Leppa S. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod Pathol. 2009 Aug;22(8):1094–101. doi: 10.1038/modpathol.2009.73. Epub 2009/05/19. eng. [DOI] [PubMed] [Google Scholar]
- 6.Yang DH, Min JJ, Song HC, Jeong YY, Chung WK, Bae SY, et al. Prognostic significance of interim (1)(8)F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur J Cancer. 2011 Jun;47(9):1312–8. doi: 10.1016/j.ejca.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Itti E, Lin C, Dupuis J, Paone G, Capacchione D, Rahmouni A, et al. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-Cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med. 2009 Apr;50(4):527–33. doi: 10.2967/jnumed.108.057703. [DOI] [PubMed] [Google Scholar]
- 8.Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro-2-deoxy- D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005 Aug 15;106(4):1376–81. doi: 10.1182/blood-2005-01-0272. Epub 2005/04/30. eng. [DOI] [PubMed] [Google Scholar]
- 9.Mikhaeel NG, Hutchings M, Fields PA, O'Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high- grade non-Hodgkin lymphoma. Ann Oncol. 2005 Sep;16(9):1514–23. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- 10.Kostakoglu L, Goldsmith SJ, Leonard JP, Christos P, Furman RR, Atasever T, et al. FDG-PET after 1 cycle of therapy predicts outcome in diffuse large cell lymphoma and classic Hodgkin disease. Cancer. 2006 Dec 1;107(11):2678–87. doi: 10.1002/cncr.22276. [DOI] [PubMed] [Google Scholar]
- 11.Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, et al. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jan 10;30(2):184–90. doi: 10.1200/JCO.2011.38.2648. Epub 2011/12/14. eng. [DOI] [PubMed] [Google Scholar]
- 12.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002 Jan 24;346(4):235–42. doi: 10.1056/NEJMoa011795. Epub 2002/01/25. eng. [DOI] [PubMed] [Google Scholar]
- 13.Kahl BS. Bulky aggressive B-cell lymphoma: to radiate or not to radiate--that is the question. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Apr 10;32(11):1097–8. doi: 10.1200/JCO.2013.54.1730. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet C, Fillet G, Mounier N, Ganem G, Molina TJ, Thieblemont C, et al. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d'Etude des Lymphomes de l'Adulte. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Mar 1;25(7):787–92. doi: 10.1200/JCO.2006.07.0722. [DOI] [PubMed] [Google Scholar]
- 15.Horning SJ, Weller E, Kim K, Earle JD, O'Connell MJ, Habermann TM, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non- Hodgkin's lymphoma: Eastern Cooperative Oncology Group study 1484. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004 Aug 1;22(15):3032–8. doi: 10.1200/JCO.2004.06.088. Epub 2004/06/24. eng. [DOI] [PubMed] [Google Scholar]
- 16.Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998 Jul 2;339(1):21–6. doi: 10.1056/NEJM199807023390104. Epub 1998/07/02. eng. [DOI] [PubMed] [Google Scholar]
- 17.Pfreundschuh M, Ho AD, Cavallin-Stahl E, Wolf M, Pettengell R, Vasova I, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good- prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol. 2008 May;9(5):435–44. doi: 10.1016/S1470-2045(08)70078-0. [DOI] [PubMed] [Google Scholar]
- 18.Reyes F, Lepage E, Ganem G, Molina TJ, Brice P, Coiffier B, et al. ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005 Mar 24;352(12):1197–205. doi: 10.1056/NEJMoa042040. [DOI] [PubMed] [Google Scholar]
- 19.Marcheselli L, Marcheselli R, Bari A, Liardo EV, Morabito F, Baldini L, et al. Radiation therapy improves treatment outcome in patients with diffuse large B-cell lymphoma. Leukemia & lymphoma. 2011 Oct;52(10):1867–72. doi: 10.3109/10428194.2011.585526. [DOI] [PubMed] [Google Scholar]
- 20.Phan J, Mazloom A, Medeiros LJ, Zreik TG, Wogan C, Shihadeh F, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Sep 20;28(27):4170–6. doi: 10.1200/JCO.2009.27.3441. Epub 2010/08/18. eng. [DOI] [PubMed] [Google Scholar]
- 21.Held G, Murawski N, Ziepert M, Fleckenstein J, Poschel V, Zwick C, et al. Role of Radiotherapy to Bulky Disease in Elderly Patients With Aggressive B-Cell Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Feb 3; doi: 10.1200/JCO.2013.51.4505. Epub 2014/02/05. Eng. [DOI] [PubMed] [Google Scholar]
- 22.Bucci MK. CHOP Alone Compared to CHOP Plus Radiotherapy for Early Stage Aggressive Non-Hodgkin's Lymphomas: Update of the Southwest Oncology Group (SWOG) Randomized [Google Scholar]