Abstract
Objectives
To investigate the association between serum potassium, mortality, and kidney outcomes in the general population and whether potassium-altering medications modify these associations.
Patients and Methods
We studied 15,539 adults in the Atherosclerosis Risk in Communities (ARIC) study. Cox proportional hazard regression was used to investigate the association of serum potassium at baseline (1987–1989), evaluated categorically (hypokalemia, <3.5 mmol/L; normokalemia, ≥3.5 and < 5.5 mmol/L; hyperkalemia, ≥5.5 mmol/L) and continuously using linear spline terms (knots at 3.5 and 5.5 mmol/L), with mortality, sudden cardiac death (SCD), incident chronic kidney disease (CKD), and end-stage renal disease (ESRD). The end date of follow up for all outcomes was December 31, 2012. We also evaluated whether classes of potassium-altering medications modified the association between serum potassium and adverse outcomes.
Results
Overall, 2.7% of the participants had hypokalemia and 2.1% had hyperkalemia. In a fully adjusted model, hyperkalemia was significantly associated with mortality (HR: 1.24; 95% CI: 1.04–1.49) but not SCD, CKD, or ESRD. Hypokalemia as a categorical variable was not associated with any outcome; however, associations of hypokalemia with all-cause mortality and kidney outcomes were observed among those who were not taking potassium-wasting diuretics (all P for interaction <.001).
Conclusions
Higher values of serum potassium were associated with higher risk of mortality in the general population. Lower levels of potassium were associated with adverse kidney outcomes and mortality among participants not taking potassium-wasting diuretics.
Introduction
Potassium is crucial for physiological function, such as preserving intracellular fluid volume and maintaining nerve and muscle function. In normal physiology, serum potassium levels are maintained in the range of 3.5–5.5 mmol/L by both renal and extra-renal mechanisms.[1] The kidney in particular closely regulates potassium secretion, reabsorption and excretion.[2] Deviations of serum potassium from the normal range could indicate early kidney dysfunction not otherwise captured by standard biomarkers, and may herald subsequent adverse events.
Hypokalemia and hyperkalemia have been associated with chronic kidney disease (CKD) progression, end-stage renal disease (ESRD), and mortality in populations with CKD and heart failure.[3][4][5][6] However, little is known about whether abnormal potassium values are associated with mortality and incident kidney outcomes in the general population. In addition, serum potassium levels are heavily influenced by medications. For example, potassium-wasting diuretics can cause hypokalemia, while renin-angiotensin-aldosterone system inhibitors, β-blockers, and potassium-sparing diuretics can cause hyperkalemia.[7][8][9][10][11] Few studies have taken into account the role of these medications in the association between serum potassium and adverse outcomes.
In response to these uncertainties, we investigated the association between serum potassium and adverse outcomes, including all-cause mortality, sudden cardiac death (SCD), incident CKD, and ESRD, using data from the population-based Atherosclerosis Risk in Communities (ARIC) Study. To investigate whether deviations in serum potassium in the setting of medication use had different impact than deviations from other causes, we tested the interaction of serum potassium with the use of certain classes of potassium-influencing medications and adverse outcomes.
Methods
Data Source and Study Participants
The ARIC Study is a prospective epidemiologic study conducted in four U.S. communities. The baseline visit of the ARIC Study was in 1987–1989, when 15,792 participants aged 44–66 were recruited and received an extensive baseline examination. Four subsequent visits were held (1990–1992, 1993–1995, 1996–1998 and, 2011–2013), and follow-up took place yearly by telephone interview to gather information on participants’ health status.[12][13] Research protocols of the ARIC study were approved by the institutional review board at each participating institution, and all participants provided informed consent.
All ARIC participants attending visit 1 with measured serum potassium and baseline covariates were included in the study. In evaluating the risk of incident CKD, we excluded 206 participants with an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 at baseline; in evaluating the risk of ESRD, we excluded 14 participants with prevalent ESRD at baseline.
Measurement of serum potassium and other baseline covariates
The primary exposure was serum potassium concentration. Blood samples drawn at the baseline visit were aliquoted, centrifuged and stored at −70°C.[12] Potassium concentration was measured by an ion selective electrode using the DACOS Analyzer plus LYTES Option on the undiluted serum. The coefficient of variation of this measurement was 1.0%.[14]
Medical history and medication use were recorded at the baseline clinic interview.[12] Baseline serum creatinine was measured by the modified kinetic Jaffé method and calibrated to IDMS standards.[15][16] eGFR was calculated using the CKD-EPI creatinine equation[17]. Diabetes was defined based on historical definitions as the presence of any of the following conditions: random blood glucose ≥200 mg/dL, fasting blood glucose ≥140 mg/dL, self-report of a physician diagnosis of diabetes and medication use for diabetes or high blood glucose in past two weeks. Hypertension was defined as baseline diastolic blood pressure ≥90mmHg, systolic blood pressure ≥140mmHg or any medication use in the past two weeks for high blood pressure. History of cardiovascular disease was defined as presence of heart failure, prevalent coronary heart disease or angina. Potassium and total calorie intake was measured using a modified version of the 61-item food frequency questionnaire developed by Willett et al.[18][19] Potassium/calorie intake ratio was defined as potassium intake (mg) divided by total calories intake (kcal).
Outcomes
The primary outcome was mortality, ascertained by death certificates obtained monthly from state vital statistics files and county health departments. [20] The secondary outcomes included SCD, defined as definite or possible arrhythmic death adjudicated by a panel of physicians who reviewed all fatal coronary heart disease events,[21][22][23][24] and two incident kidney events: CKD, defined as baseline eGFR ≥60 ml/min/1.73m2 and at least one follow-up eGFR <60 mL/min/1.73m2 with a 25% drop in eGFR, or a CKD-related hospitalization (eTable 1); and ESRD, defined as patients on dialysis or with a kidney transplant, determined through linkage to the US Renal Data System. To identify the cause of out-of-hospital deaths, next of kin and other informants, certifying and family physicians, and coroners or medical examiners were contacted by letter and/or telephone interview.[20] Hospitalizations are determined by self-report and active surveillance of community hospital discharge lists.[25][26] The end date of follow up for all outcomes was December 31, 2012.
Statistical Analysis
Baseline serum potassium concentration was evaluated as a categorical variable (hypokalemia: <3.5 mmol/L, normokalemia: ≥3.5 and <5.5 mmol/L, and hyperkalemia: ≥5.5 mmol/ L) and as a continuous variable using linear spline terms with two knots at 3.5 and 5.5 mmol/L. These cut-offs were based on evaluation of the distribution of potassium levels in the study population and review of the existing literature.[27][28][29]
We tested for trends in potential confounders by baseline potassium levels by regressing each variable on the ordinal category of potassium. Kaplan-Meier curves were used to assess cumulative survival during follow-up and the log-rank test was used to test differences in survival by potassium group. We used Cox proportional hazards regression to evaluate the association between serum potassium and outcomes. An unadjusted model included only serum potassium concentrations; subsequent models adjusted for age, sex, race-study center (Model 1), Model 1 + health characteristics including eGFR (treated as spline terms with two knots at 60 and 90 ml/min/1.73 m2), diabetes, hypertension and history of cardiovascular disease (Model 2), and Model 2 + medication use, including angiotensin-converting-enzyme (ACE) inhibitors, potassium-wasting diuretics (loop diuretics, thiazide and thiazide-like diuretics), potassium sparing diuretics (aldosterone antagonists, triamterene and amiloride), β blockers and nonsteroidal anti-inflammatory drugs (NSAIDs) (Model 3). Of note, angiotensin receptor blockers came to the market in 1995, so no participant used this class of medications at baseline.
To evaluate whether the potassium-adverse outcome association differed among users of potassium-altering medications, we included a product term between drug class and potassium level in Model 3. A priori we specified three drug classes that might exhibit effect modification: potassium-wasting diuretics, potassium-sparing diuretics, and ACE inhibitors.
In sensitivity analyses, we evaluated risk-relationships using serum potassium levels measured at visit 2 as the exposure. Since potassium-wasting diuretics can cause hypomagnesemia, increased cholesterol, and increased uric acid levels[30][31][32][33][34][35], we evaluated levels by diuretic use and analyzed the potassium-adverse outcome association when adjusting for baseline magnesium, uric acid, and cholesterol (non-HDL-C) level. We tested the effect of additional adjustment for potassium supplementation and potassium/calorie intake ratio. We evaluated the relationship between corrected Q-T interval, potassium levels, and outcomes. To further address the possibility of confounding by indication (participants using potassium-wasting diuretics might be different from other participants), we performed propensity-matched analysis, matching 1:1 without replacement on the propensity to use potassium-wasting diuretics, using covariates from Model 3 (N=2,960 in each arm). Finally, we conducted competing risk analyses for SCD, incident CKD, and ESRD by including mortality (non-SCD in the SCD analysis) as a competing event.[36]
An α-level of 0.05 was used to determine statistical significance, and all statistical tests were two-tailed. Data analysis was performed using Stata/IC 14.0 for Windows.
Results
Study population and baseline characteristics
The study population included 15,539 adults aged 44–66 years old (Table 1). The mean serum potassium level of the participants was 4.4±0.5 mmol/L; mean eGFR was 102.4±15.8 ml/min/1.73 m2. Overall, 2.7% of the participants had hypokalemia and 2.1% had hyperkalemia. At baseline, 19.1% of the population used potassium-wasting diuretics; 6.8% used potassium-sparing diuretics, and 3.4% used ACE-inhibitors. Most participants (79.9%) with hypokalemia were taking potassium-wasting diuretics. Participants with potassium levels <3.5 mmol/L were more likely to be female, of black race, have lower potassium/calorie intake ratio, higher eGFR, diabetes, hypertension, a history of CVD, and use potassium-wasting diuretics, potassium-sparing diuretics, β-blockers, and potassium supplementation (P for all trends <.001). Hyperkalemia was associated with shorter corrected QT duration; hypokalemia was associated with longer corrected QT duration (eFigure 1).
Table 1.
| Serum Potassium Concentration | |||||
|---|---|---|---|---|---|
|
|
|||||
| All participants (N=15539) |
<3.5 mmol/L (N=413) |
≥3.5 and <5.5 mmol/L (N=14805) |
≥5.5 mmol/L (N=321) |
P for trend | |
| % | 100% | 2.7% | 95.3% | 2.1% | |
| Age | 54.2 ± 5.8 | 54.6 ± 5.7 | 54.2 ± 5.8 | 54.5 ± 5.6 | .62 |
| Female | 8560 (55.1%) | 296 (71.7%) | 8104 (54.7%) | 160 (49.8%) | <.001 |
| Black | 4076 (26.2%) | 261 (63.2%) | 3796 (25.6%) | 19 (5.9%) | <.001 |
| eGFR (ml/min/1.73 m2) | 102.4 ± 15.8 | 106.2 ± 22.4 | 102.5 ± 15.5 | 94.9 ± 17.4 | <.001 |
| Diabetes | 1524 (9.8%) | 74 (18.0%) | 1424 (9.6%) | 26 (8.1%) | <.001 |
| Hypertension | 5380(34.8%) | 377(91.3%) | 4931(33.5%) | 72(22.6%) | <.001 |
| History of CVD | 1883 (12.1%) | 97 (23.5%) | 1754 (11.8%) | 32 (10.0%) | <.001 |
| Corrected QT duration (ms) | 416.4 ± 19.6 | 428.9 ± 29.8 | 416.1 ± 19.1 | 413.9 ± 21.7 | <.001 |
| ACE Inhibitor | 523 (3.4%) | 16 (3.9%) | 494 (3.3%) | 13 (4.0%) | .98 |
| K+ sparing diuretic | 1056 (6.8%) | 59 (14.3%) | 985 (6.7%) | 12 (3.7%) | <.001 |
| Potassium wasting diuretic | 2971 (19.1%) | 330 (79.9%) | 2623 (17.7%) | 18 (5.6%) | <.001 |
| β Blocker | 1638 (10.5%) | 80 (19.4%) | 1518 (10.3%) | 40 (12.5%) | <.001 |
| NSAID | 2789 (17.9%) | 75 (18.2%) | 2653 (17.9%) | 61 (19.0%) | .81 |
| Potassium supplementation | 819 (5.3%) | 121 (29.3%) | 689 (4.7%) | 9 (2.8%) | <.001 |
| Potassium/calorie ratio (mg/kcal) | 1.71 ± 0.43 | 1.64 ± 0.45 | 1.71 ± 0.43 | 1.84 ± 0.44 | <.001 |
For continuous variables: Mean ± SD; for dichotomous variables: N (%)
ACE: angiotensin-converting-enzyme; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; NSAID: nonsteroidal anti-inflammatory drugs
Serum potassium and all-cause mortality
There were 5,548 deaths over a median follow-up of 23.5 years, with 199, 5221 and 128 among participants with baseline hypokalemia, normokalemia and hyperkalemia, respectively. As categorical variables, both hypokalemia and hyperkalemia were associated with death in unadjusted analysis (reference: normokalemia) (Figure 1). The associations between hypokalemia and mortality were attenuated with adjustment for demographic characteristics (Model 1, HR: 1.28; 95% CI: 1.11–1.48, P=.001) and health characteristics (Model 2, HR: 1.01; 95% CI: 0.87–1.17, P=.92). Additional adjustment for medication use had little influence on this association. In contrast, the association between hyperkalemia and mortality persisted in all models (Model 1, HR: 1.33; 95% CI: 1.11–1.59, P=.002; Model 2, HR: 1.25; 95% CI: 1.04–1.50, P=.02; Model 3, HR: 1.24; 95% CI: 1.04–1.49, P=.02) (Table 2).
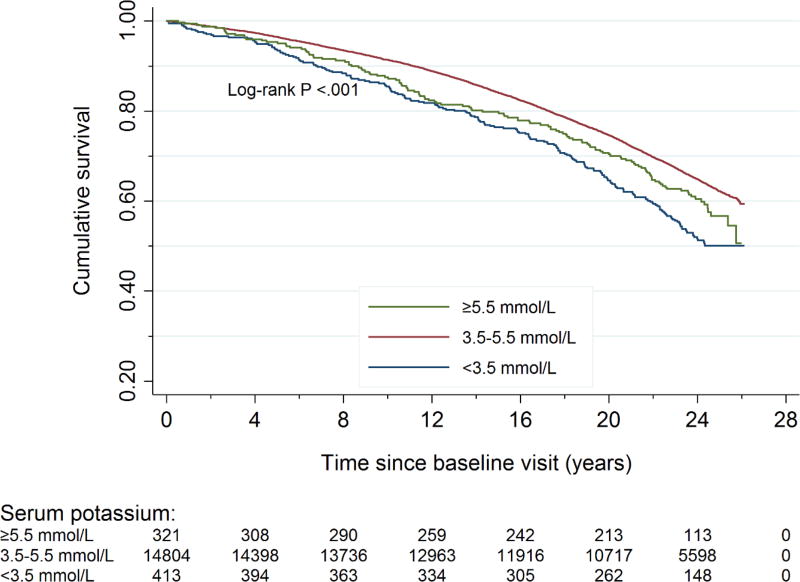
Figure 1. Cumulative survival by baseline serum potassium concentrationa.
aThere were 5,548 deaths over a median follow-up of 23.5 years. There were 199 deaths among participants with baseline hypokalemia, 5221 deaths among participants with baseline normokalemia, and 128 deaths among participants with baseline hyperkalemia. The cumulative survival was significantly different among three serum potassium groups.
Table 2.
Association between baseline serum potassium (treated as categorical variables) and adverse outcomesa
| Events | Unadjusted | Model 1b | Model 2c | Model 3d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| HR | 95% CI | P values | HR | 95% CI | P values | HR | 95% CI | P values | HR | 95% CI | P values | ||
| Mortality | |||||||||||||
| Hypokalemia | 199 | 1.48 | [1.29–1.71] | <.001 | 1.28 | [1.11–1.48] | .001 | 1.01 | [0.87–1.17] | .92 | 1.03 | [0.89–1.20] | .66 |
| Normokalemia | 5221 | Ref. | Ref. | Ref. | Ref. | ||||||||
| Hyperkalemia | 128 | 1.21 | [1.01–1.44] | .04 | 1.33 | [1.11–1.59] | .002 | 1.25 | [1.04–1.50] | .02 | 1.24 | [1.04–1.49] | .02 |
| SCD | |||||||||||||
| Hypokalemia | 32 | 2.50 | [1.75–3.58] | <.001 | 1.93 | [1.34–2.77] | <.001 | 1.28 | [0.88–1.85] | .20 | 1.38 | [0.94–2.03] | .10 |
| Normokalemia | 491 | Ref. | Ref. | Ref. | Ref. | ||||||||
| Hyperkalemia | 11 | 1.09 | [0.60–1.98] | .78 | 1.47 | [0.80–2.69] | .22 | 1.30 | [0.70–2.42] | .41 | 1.25 | [0.67–2.32] | .48 |
| CKD | |||||||||||||
| Hypokalemia | 128 | 1.43 | [1.20–1.71] | <.001 | 1.25 | [1.05–1.50] | .01 | 0.90 | [0.76–1.08] | .28 | 0.93 | [0.77–1.12] | .42 |
| Normokalemia | 3632 | Ref. | Ref. | Ref. | Ref. | ||||||||
| Hyperkalemia | 75 | 1.04 | [0.83–1.31] | .72 | 1.18 | [0.94–1.49] | .16 | 1.15 | [0.91–1.45] | .24 | 1.13 | [0.90–1.43] | .30 |
| ESRD | |||||||||||||
| Hypokalemia | 22 | 2.54 | [1.65–3.92] | <.001 | 1.52 | [0.98–2.35] | .06 | 0.82 | [0.52–1.29] | .39 | 0.82 | [0.52–1.30] | .40 |
| Normokalemia | 341 | Ref. | Ref. | Ref. | Ref. | ||||||||
| Hyperkalemia | 9 | 1.28 | [0.66–2.48] | .47 | 2.14 | [1.08–4.23] | .03 | 0.61 | [0.28–1.30] | .20 | 0.57 | [0.26–1.24] | .15 |
SCD: sudden cardiac death; CKD: chronic kidney disease; ESRD: end-stage renal disease; HR: hazard ratio
Model 1: Adjusted for age, sex, race and study center
Model 2: Additionally adjusted for eGFR, diabetes, hypertension and history of CVD
Model 3: Additionally adjusted for ACE inhibitors, potassium sparing diuretics, potassium-wasting diuretics, β blockers and NSAIDs
When potassium was treated as a continuous variable, there was a nonlinear relationship between potassium levels and mortality (eTable 2). In unadjusted analysis, the hazard ratio for a 1 mmol/L increase in serum potassium was 0.39 (95%CI: 0.25–0.59, P<.001), 0.96 (95%CI: 0.90–1.02, P=.16) and 1.94 (95%CI: 1.01–3.71, P=.05) for participants with hypokalemia, normokalemia and hyperkalemia, respectively. Associations were attenuated and not significant in the fully adjusted model except within the range of normokalemia (HR: 1.11; 95%CI: 1.04–1.19, P=.002).
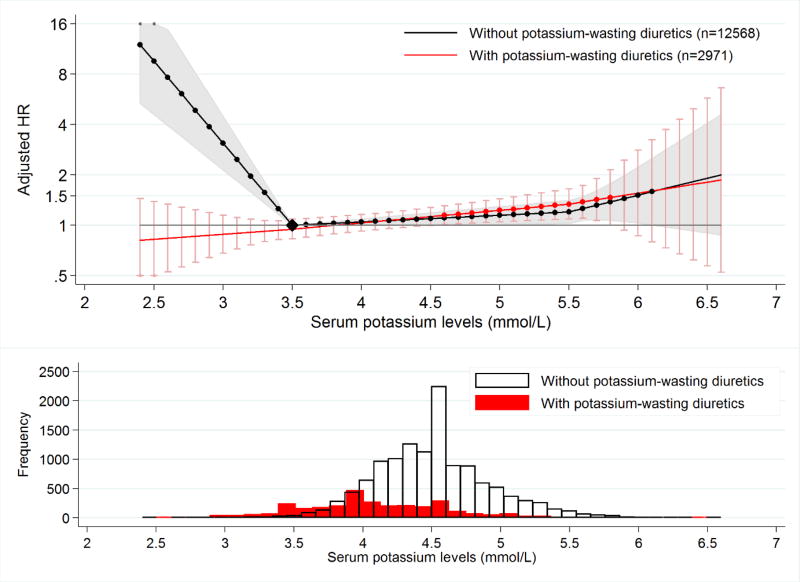
Among the three tested classes of medications that influence potassium levels, only one demonstrated significant effect modification of potassium level and mortality. The association between potassium and mortality was U-shaped in persons not taking potassium-wasting diuretics but flat in participants taking potassium-wasting diuretics (Figure 2). For example, hypokalemia was associated with death in persons not taking potassium-wasting diuretics (HR: 1.74 95%CI: 1.31–2.30, P<.001) but not in participants taking potassium-wasting diuretics (HR: 0.90; 95%CI: 0.75–1.06, P=.21; P for interaction: <.001) (Table 3). No significant interaction with medication was detected for hyperkalemia, indicating that hyperkalemia was associated with higher risk of death regardless of potassium-wasting diuretic use.
Figure 2. Adjusted hazard ratio of mortality by potassium-wasting diuretics usea,b.
aReference point (black diamond): The hazard of death among people not taking potassium-wasting diuretics and with baseline serum potassium at 3.5 mmol/L Dots on the line denote that the corresponding hazard ratios are significant different from 1. Shading and error bars correspond to 95% confidence interval for the HR.
bHR: Hazard ratio
Table 3.
Association between baseline serum potassium and adverse outcomes by potassium-wasting diuretics use based on Model 3a
| Events | Without potassium-wasting diuretics (N=12568) |
With potassium-wasting diuretics (N=2971) |
P for interaction |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | 95% CI | P values | HR | 95% CI | P values | |||
| Mortality | ||||||||
| Hypokalemia | 199 | 1.74 | [1.31–2.30] | <.001 | 0.90 | [0.75–1.06] | .21 | <.001 |
| Normokalemia | 5221 | Ref. | Ref. | |||||
| Hyperkalemia | 128 | 1.23 | [1.01–1.48] | .04 | 1.42 | [0.82–2.47] | .21 | .62 |
| SCD | ||||||||
| Hypokalemia | 32 | 2.38 | [1.25–4.54] | .008 | 1.12 | [0.71–1.78] | .63 | .06 |
| Normokalemia | 491 | Ref. | Ref. | |||||
| Hyperkalemia | 11 | 1.34 | [0.69–2.62] | .39 | 0.92 | [0.21–4.11] | .92 | .65 |
| CKD | ||||||||
| Hypokalemia | 128 | 1.65 | [1.16–2.35] | .006 | 0.80 | [0.65–0.99] | .04 | <.001 |
| Normokalemia | 3632 | Ref. | Ref. | |||||
| Hyperkalemia | 75 | 1.09 | [0.85–1.39] | .51 | 1.99 | [0.93–4.22] | .07 | .14 |
| ESRD | ||||||||
| Hypokalemia | 22 | 2.63 | [1.32–5.23] | .006 | 0.50 | [0.28–0.91] | .02 | <.001 |
| Normokalemia | 341 | Ref. | Ref. | |||||
| Hyperkalemia | 9 | 0.99 | [0.36–2.70] | .98 | 0.33 | [0.11–0.96] | .04 | .15 |
SCD: sudden cardiac death; CKD: chronic kidney disease; ESRD: end-stage renal disease; HR: hazard ratio
Serum potassium and SCD
The number of SCD cases among participants with baseline hypokalemia, normokalemia and hyperkalemia were 32, 491, and 11, respectively. In the unadjusted model, only hypokalemia was associated with SCD and this association disappeared in model 2 and model 3. (Table 2). However, in continuous analysis, both lower levels of potassium <3.5 mmol/L and higher levels of potassium ≥5.5 mmol/L were associated with SCD in the fully adjusted analysis (eTable 2). No effect modification by medication class was found in the association between serum potassium and SCD.
Serum potassium and kidney outcomes
The number of incident CKD cases among participants with baseline hypokalemia, normokalemia and hyperkalemia were 128, 3,632, and 75, respectively; the number of incident ESRD cases among participants in these three categories were 22, 341, and 9. There were few consistent relationships between categories of hypokalemia and hyperkalemia and kidney outcomes (Table 2). In unadjusted analysis, hypokalemia was associated with incident CKD and ESRD; however, associations did not persist in the adjusted models.
Potassium-wasting diuretic use modified the relationship between hypokalemia and the kidney outcomes. In participants not taking potassium-wasting diuretics, hypokalemia was significantly associated with incident CKD and ESRD (HR for CKD: 1.65; 95% CI: 1.16–2.35, P=.006; HR for ESRD: 2.63; 95% CI: 1.32–5.23, P=.006) (Table 3). However, in participants taking potassium-wasting diuretics, hypokalemia was associated with a decreased risk of kidney outcomes (HR for CKD: 0.80; 95% CI: 0.65–0.99, P=.04; P for interaction: <.001 (eFigure 2); HR for ESRD: 0.50; 95% CI: 0.28–0.91, P=.02; P for interaction <.001 (eFigure 3)). In addition, when we treated potassium level as a continuous variable, we observed that higher potassium levels were associated with CKD only in participants taking potassium-wasting diuretics (eFigure 2). We did not observe significant effect modification by potassium-sparing diuretic or ACE-inhibitor use.
Sensitivity analyses
In sensitivity analyses using serum potassium levels at visit 2 as the exposure, results were largely qualitatively similar but attenuated (eTable 3). We observed that participants using potassium-wasting diuretics had lower magnesium levels and higher uric acid and non-HDL-C levels; however, additional adjustment for these variables did not change the results. Similarly, incorporating potassium supplementation, and the potassium/caloric intake ratio in our fully adjusted models had very little difference in results (eTable 4). While corrected QT duration was itself a predictor of subsequent mortality, including it in Model 3 did not significantly change the observed associations. In the propensity score-matched analyses, associations were of similar magnitude with maintained interactions; however, some of the associations were no longer statistically significant, likely due to the smaller sample size (eTable 5). There were no significant differences in the overall associations by competing risk analyses (eTable 6).
Discussion
In our population-based study of over 15,000 participants, we found that nearly 5% of the population had abnormal serum potassium values. Both hypokalemia and hyperkalemia were associated with mortality in unadjusted and demographic-adjusted models, and hyperkalemia continued to be associated with mortality after full adjustment. In addition, abnormal values of potassium were associated with SCD in continuous analysis. We also observed an interesting interaction, whereby associations between hypokalemia and adverse outcomes were strongest among participants not using potassium-wasting diuretics.
Serum potassium and mortality
Few studies have evaluated the association between potassium levels and mortality in the general population. A study of 2,836 NHANES participants found that high-normal serum potassium levels (4.5–5.4 mmol/L) were significantly associated with all-cause mortality.[27] Another study of 7,636 men found that potassium >5.2 mmol/L was associated with a nearly two-fold risk of death among smokers.[37] Although we used slightly different cut-points for serum potassium, our results were consistent, adding to these efforts by investigating the modifying effect of potassium-altering medications, by specifically evaluating SCD as cause of death, and by extending follow-up to a median of 23.5 years.
Interactions with medications – mortality
One previous observational study investigated whether potassium-influencing medications altered the association between serum potassium and mortality in the general population. Fang et al found that high serum potassium associated with cardiovascular mortality in patients using diuretics (all classes), but not among those not using diuretics.[27] We also saw differences in the shape of the association between potassium and mortality by potassium-wasting diuretic use, but our results suggested effect modification at low levels of potassium. We note there were few participants with hyperkalemia taking potassium-wasting diuretics, limiting power. Heterogeneity in risk associations between potassium and mortality was also seen in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), where the associations were weakest among persons in the diuretic arm.[28] Certainly, stronger associations among participants not taking diuretics may suggest that hypokalemia in such patients represents an underlying disease process such as chronic illness or hyperaldosteronism rather than a causal risk factor for mortality.
Interactions with medications – kidney outcomes
Our results are consistent with a previous study investigating the relationship between potassium levels and kidney outcomes. Among 1,001 healthy Japanese participants not taking diuretics, serum potassium levels <4 mmol/L were significantly associated with CKD.[38] We observed an a similar association among participants not using potassium-wasting diuretics. Interestingly, the reverse was seen in participants taking potassium-wasting diuretics, where hypokalemia was associated with decreased risk of CKD. A similar pattern held for mortality and ESRD, possibly suggesting that the health condition that caused hypokalemia (such as gastrointestinal disease or hyperaldosteronism) may be the source of the increased risk, rather than hypokalemia itself. Unfortunately, we have little data on this health conditions at baseline.
Potential mechanism of action
Potassium is highly regulated in the collecting duct of the nephron. The association between abnormal values of potassium and adverse outcomes may be explained by underlying disruption in kidney function, or by downstream effects of potassium itself. Both hypo- and hyperkalemia have been associated with arrhythmias,[39][40][41][42][43] which can cause sudden cardiac death. Potassium levels affect the resting membrane potential of cardiomyocytes and the activity of potassium channels important for the cardiac electrical cycle, with hypokalemia inducing hyperpolarization, faster electrical conduction, and delays in ventricular repolarization, and hyperkalemia resulting in slower electrical conduction.[44][45] Prolonged hypokalemia is also thought to induce interstitial fibrosis and tubular atrophy in the kidney.[46][47]
Clinical implications
We demonstrate that abnormal values of potassium are relatively common in the general population. Although our study suggests that both hypokalemia and hyperkalemia may lead to adverse outcomes, our data are observational and cannot demonstrate causality. If abnormal potassium itself causes adverse outcomes – a plausible scenario, particularly for the development of arrhythmias – then clinical treatment may be beneficial. Indeed, correction of abnormal values of potassium is often prescribed, either by supplementation, changes in diet, or potassium-altering medications.[29][48][49][50][51] Whether any of these options improves outcomes is yet unknown and requires a randomized controlled trial.
Limitations
Our study has a number of limitations. First, potassium was measured once at baseline. Outcomes could occur up to 25 years later. Thus, we report not on the immediate impact of abnormal values of potassium as reported by Einhorn et al [52] but the long-term impact, which could be mediated by other events, such as the development of fibrosis. On the other hand, our prospective cohort was large, allowing for investigation of multiple outcomes, and provided clear temporality. Furthermore, in sensitivity analysis using potassium levels at visit 2 as the exposure, we obtained similar results. Second, residual confounding is a concern in all observational studies. However, adjusting for potassium intake, potassium supplementation, and magnesium, cholesterol, and uric acid levels did not change the association. Third, the number of SCD and ESRD cases was limited, limiting power to test effect modification by medication use.
Conclusion
In the general population, abnormal values of potassium were associated with all-cause mortality. There was effect modification by potassium-wasting diuretic use for many of the outcomes, with stronger associations in participants not taking this class of medications. While in clinical practice, hypokalemia can be corrected by increasing potassium intake and hyperkalemia can be alleviated by dietary potassium restriction, discontinuation of hyperkalemia-inducing medications and use of potassium-lowering medications,[29] future studies are needed to assess whether treatment of potassium levels outside of the normal range might improve outcomes.
Supplementary Material
Acknowledgments
The study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript. The authors declare that they have no competing financial interests; however, Dr. Coresh and Dr. Grams received grants from the US National Kidney Foundation (NKF funding sources include Relypsa, AbbVie and Amgen).
Dr. Grams is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K08DK092287). Dr. McAdams-DeMarco is supported by NIH grant K01AG043501-01A1, the Carl W. Gottschalk Research Scholar Award from the American Society of Nephrology. Dr. Chang receives support from the National Institute of Diabetes and Digestive and Kidney Diseases Grant (NIDDK; 1K23DK106515-01).
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
Abbreviations
- ACE
angiotensin-converting-enzyme
- ARIC study
Atherosclerosis Risk in Communities study
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- HR
hazard ratio
- SCD
sudden cardiac death
References
- 1.Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377–84. doi: 10.1007/s00467-010-1699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giebisch G, Hebert SC, Wang WH. New aspects of renal potassium transport. Pflugers Arch. 2003;446(3):289–97. doi: 10.1007/s00424-003-1029-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang HH, Hung CC, Hwang DY, et al. Hypokalemia, its contributing factors and renal outcomes in patients with chronic kidney disease. PLoS ONE. 2013;8(7):e67140. doi: 10.1371/journal.pone.0067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes J, Kalantar-zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012;120(1):c8–16. doi: 10.1159/000329511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed A, Zannad F, Love TE, et al. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28(11):1334–43. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J, Brunelli SM, Jensen DE, Yang A. Association between Serum Potassium and Outcomes in Patients with Reduced Kidney Function. Clin J Am Soc Nephrol. 2016;11(1):90–100. doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tannen RL. Diuretic-induced hypokalemia. Kidney Int. 1985;28(6):988–1000. doi: 10.1038/ki.1985.229. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10–24. [PubMed] [Google Scholar]
- 9.Maddirala S, Khan A, Vincent A, Lau K. Effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on serum potassium levels and renal function in ambulatory outpatients: risk factors analysis. Am J Med Sci. 2008;336(4):330–5. doi: 10.1097/MAJ.0b013e3181836ac7. [DOI] [PubMed] [Google Scholar]
- 10.Arrizabalaga P, Montoliu J, Martinez vea A, Andreu L, López pedret J, Revert L. Increase in serum potassium caused by beta-2 adrenergic blockade in terminal renal failure: absence of mediation by insulin or aldosterone. Proc Eur Dial Transplant Assoc. 1983;20:572–6. [PubMed] [Google Scholar]
- 11.Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10(11):653–62. doi: 10.1038/nrneph.2014.168. [DOI] [PubMed] [Google Scholar]
- 12.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute website. [Accessed February 12]; http://www.nhlbi.nih.gov/research/resources/obesity/population/aric.htm.
- 14.National Heart, Lung and Blood Institute Atherosclerosis Risk in Communities ARIC Study. Operations Manual No. 10: Clinical Chemistry Determinations. Vol. 45. Bethesda, MD: National Heart, Lung and Blood Institute; 1987. p. 67. [Google Scholar]
- 15.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 16.Coresh J, Astor BC, Mcquillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39(5):920–9. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–99. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee R, Yeh HC, Shafi T, et al. Serum and dietary potassium and risk of incident type 2 diabetes mellitus: The Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2010;170(19):1745–51. doi: 10.1001/archinternmed.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49(2):223–33. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen LY, Sotoodehnia N, Bůžková P, et al. Atrial fibrillation and the risk of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med. 2013;173(1):29–35. doi: 10.1001/2013.jamainternmed.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soliman EZ, Prineas RJ, Case LD, et al. Electrocardiographic and clinical predictors separating atherosclerotic sudden cardiac death from incident coronary heart disease. Heart. 2011;97(19):1597–601. doi: 10.1136/hrt.2010.215871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;160(3):464–70. doi: 10.1016/j.ahj.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson KA, Viera AJ, Soliman EZ, Crow RS, Rosamond WD. Long-term prognosis associated with J-point elevation in a large middle-aged biracial cohort: the ARIC study. Eur Heart J. 2011;32(24):3098–106. doi: 10.1093/eurheartj/ehr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grams ME, Rebholz CM, Mcmahon B, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64(2):214–21. doi: 10.1053/j.ajkd.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebholz CM, Coresh J, Ballew SH, et al. Kidney Failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) Study: Comparing Ascertainment of Treated and Untreated Kidney Failure in a Cohort Study. Am J Kidney Dis. 2015;66(2):231–9. doi: 10.1053/j.ajkd.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang J, Madhavan S, Cohen H, Alderman MH. Serum potassium and cardiovascular mortality. J Gen Intern Med. 2000;15(12):885–90. doi: 10.1046/j.1525-1497.2000.91021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alderman MH, Piller LB, Ford CE, et al. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension. 2012;59(5):926–33. doi: 10.1161/HYPERTENSIONAHA.111.180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovesdy CP. Management of Hyperkalemia: An Update for the Internist. Am J Med. 2015;128(12):1281–7. doi: 10.1016/j.amjmed.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 30.Lim P, Jacob E. Magnesium deficiency in patients on long-term diuretic therapy for heart failure. Br Med J. 1972;3(5827):620–2. doi: 10.1136/bmj.3.5827.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wester PO, Dyckner T. Diuretic treatment and magnesium losses. Acta Med Scand Suppl. 1981;647:145–52. doi: 10.1111/j.0954-6820.1981.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 32.Perez-stable E, Caralis PV. Thiazide-induced disturbances in carbohydrate, lipid, and potassium metabolism. Am Heart J. 1983;106(1 Pt 2):245–51. doi: 10.1016/0002-8703(83)90124-2. [DOI] [PubMed] [Google Scholar]
- 33.Grimm RH, Leon AS, Hunninghake DB, Lenz K, Hannan P, Blackburn H. Effects of thiazide diuretics on plasma lipids and lipoproteins in mildly hypertensive patients: a double-blind controlled trial. Ann Intern Med. 1981;94(1):7–11. doi: 10.7326/0003-4819-94-1-7. [DOI] [PubMed] [Google Scholar]
- 34.Bloomgarden ZT, Ginsberg-fellner F, Rayfield EJ, Bookman J, Brown WV. Elevated hemoglobin A1c and low-density lipoprotein cholesterol levels in thiazide-treated diabetic patients. Am J Med. 1984;77(5):823–7. doi: 10.1016/0002-9343(84)90518-7. [DOI] [PubMed] [Google Scholar]
- 35.Steele TH, Oppenheimer S. Factors affecting urate excretion following diuretic administration in man. Am J Med. 1969;47(4):564–74. doi: 10.1016/0002-9343(69)90187-9. [DOI] [PubMed] [Google Scholar]
- 36.Fine, Jason P, Robert J, Gray A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94(446):496–509. [Google Scholar]
- 37.Wannamethee SG, Lever AF, Shaper AG, Whincup PH. Serum potassium, cigarette smoking, and mortality in middle-aged men. Am J Epidemiol. 1997;145(7):598–606. doi: 10.1093/oxfordjournals.aje.a009156. [DOI] [PubMed] [Google Scholar]
- 38.Fukui M, Tanaka M, Toda H, et al. Low serum potassium concentration is a predictor of chronic kidney disease. Int J Clin Pract. 2014;68(6):700–4. doi: 10.1111/ijcp.12367. [DOI] [PubMed] [Google Scholar]
- 39.Patel RB, Tannenbaum S, Viana-tejedor A, et al. Serum potassium levels, cardiac arrhythmias, and mortality following non-ST-elevation myocardial infarction or unstable angina: insights from MERLIN-TIMI 36. Eur Heart J Acute Cardiovasc Care. 2015 doi: 10.1177/2048872615624241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hulting J. In-hospital ventricular fibrillation and its relation to serum potassium. Acta Med Scand Suppl. 1981;647:109–16. doi: 10.1111/j.0954-6820.1981.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 41.Solomon RJ, Cole AG. Importance of potassium in patients with acute myocardial infarction. Acta Med Scand Suppl. 1981;647:87–93. doi: 10.1111/j.0954-6820.1981.tb02643.x. [DOI] [PubMed] [Google Scholar]
- 42.Friedensohn A, Faibel HE, Bairey O, Goldbourt U, Schlesinger Z. Malignant arrhythmias in relation to values of serum potassium in patients with acute myocardial infarction. Int J Cardiol. 1991;32(3):331–8. doi: 10.1016/0167-5273(91)90295-z. [DOI] [PubMed] [Google Scholar]
- 43.Mattsson N, Sadjadieh G, Kumarathurai P, Nielsen OW, Køber L, Sajadieh A. Ambulatory cardiac arrhythmias in relation to mild hypokalaemia and prognosis in community dwelling middle-aged and elderly subjects. Europace. 2015 doi: 10.1093/europace/euv204. [DOI] [PubMed] [Google Scholar]
- 44.Podrid PJ. Potassium and ventricular arrhythmias. Am J Cardiol. 1990;65(10):33E–44E. doi: 10.1016/0002-9149(90)90250-5. [DOI] [PubMed] [Google Scholar]
- 45.Weisberg LS. Management of severe hyperkalemia. Crit Care Med. 2008;36(12):3246–51. doi: 10.1097/CCM.0b013e31818f222b. [DOI] [PubMed] [Google Scholar]
- 46.Menahem SA, Perry GJ, Dowling J, Thomson NM. Hypokalaemia-induced acute renal failure. Nephrol Dial Transplant. 1999;14(9):2216–8. doi: 10.1093/ndt/14.9.2216. [DOI] [PubMed] [Google Scholar]
- 47.Reungjui S, Roncal CA, Sato W, et al. Hypokalemic nephropathy is associated with impaired angiogenesis. J Am Soc Nephrol. 2008;19(1):125–34. doi: 10.1681/ASN.2007030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang AR, Sang Y, Leddy J, et al. Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large Health System. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.07363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–21. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 50.Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222–31. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 51.Lepage L, Dufour AC, Doiron J, et al. Randomized Clinical Trial of Sodium Polystyrene Sulfonate for the Treatment of Mild Hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136–42. doi: 10.2215/CJN.03640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–62. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.