Abstract
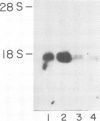
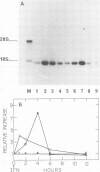
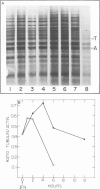
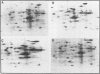
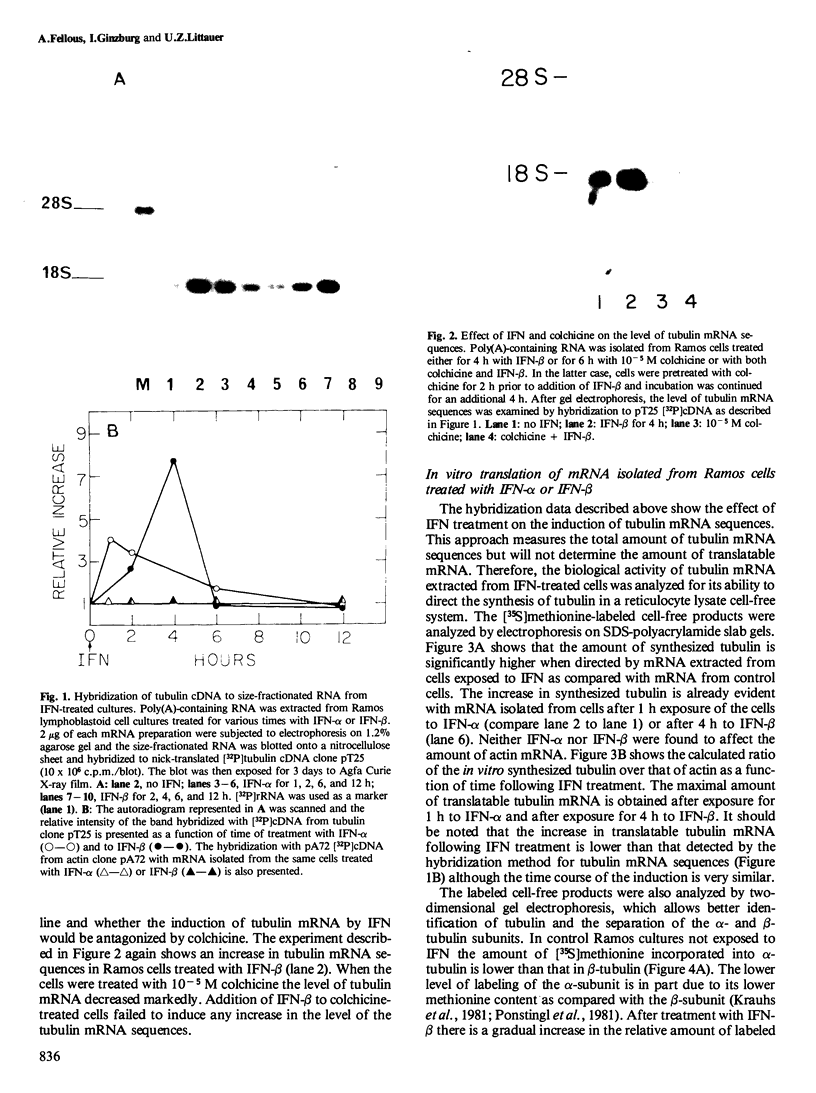
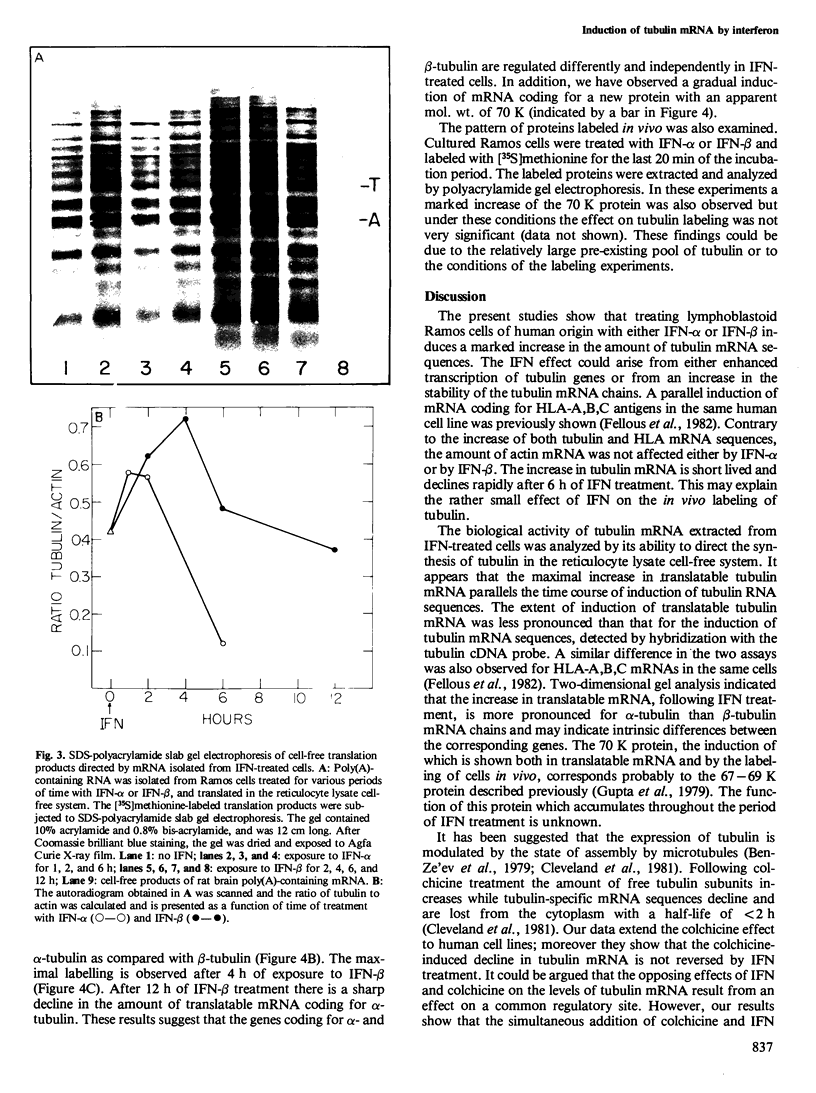
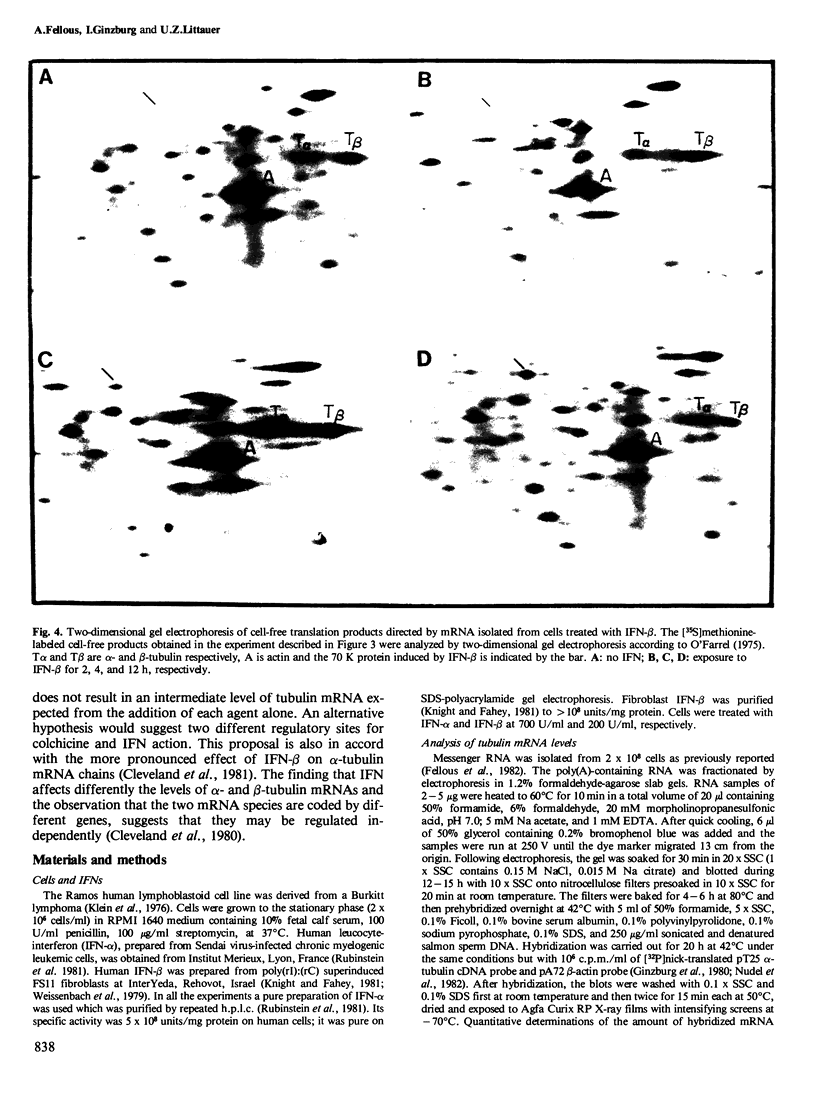
Blot hybridization with labeled tubulin cDNA showed that treatment of Ramos cells, a human cell line of lymphoblastoid origin, with either alpha or beta interferon (IFN) induced a marked increase in the amount of tubulin mRNA sequences. The level of tubulin mRNA sequences increased rapidly after exposure of cells to IFN-alpha and reached a maximum after 1 h of treatment, which was four times the control level. Treatment with IFN-beta induced a maximal increase after 4 h; the amount of tubulin mRNA sequences was seven times higher than the control level. The mRNA extracted from IFN-treated and nontreated cells was translated in vitro in a reticulocyte lysate cell-free system containing [35S]methionine. Electrophoretic analysis of the labeled cell-free products showed an increase in the amount of translatable tubulin mRNA that parallels the time course of induction of tubulin mRNA sequences. Two-dimensional gel electrophoresis of the labeled protein products directed by mRNA indicates that IFN caused a more pronounced increase in the level of alpha-tubulin than beta-tubulin mRNA. Treatment with colchicine, which disrupts the cell microtubules, caused a marked decrease in the tubulin mRNA content. Concomitant treatment of the cells with colchicine and IFN abolished the interferon-dependent induction of tubulin mRNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Bourgeade M. F., Rousset S., Paulin D., Chany C. Reorganization of the cytoskeleton by interferon in MSV-transformed cells. J Interferon Res. 1981 Feb;1(2):323–332. doi: 10.1089/jir.1981.1.323. [DOI] [PubMed] [Google Scholar]
- Chandrabose K., Cuatrecasas P., Pottathil R. Changes in fatty acyl chains of phospholipids induced by interferon in mouse sarcoma S-180 cells. Biochem Biophys Res Commun. 1981 Feb 12;98(3):661–668. doi: 10.1016/0006-291x(81)91165-7. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Jay F. T., Friedman R. M. Physical, morphological, and biochemical alterations in the membrane of AKR mouse cells after interferon treatment. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1859–1863. doi: 10.1073/pnas.75.4.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg I., de Baetselier A., Walker M. D., Behar L., Lehrach H., Frischauf A. M., Littauer U. Z. Brain tubulin and actin cDNA sequences: isolation of recombinant plasmids. Nucleic Acids Res. 1980 Aug 25;8(16):3553–3564. doi: 10.1093/nar/8.16.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. L., Rubin B. Y., Holmes S. L. Interferon action: induction of specific proteins in mouse and human cells by homologous interferons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4817–4821. doi: 10.1073/pnas.76.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Hovanessian A. G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977 Aug 11;268(5620):540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shulman L., Schmidt A., Chernajovsky Y., Fradin A., Revel M. Kinetics of the induction of three translation-regulatory enzymes by interferon. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3208–3212. doi: 10.1073/pnas.76.7.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Zilberstein A., Schmidt A., Shulman L., Revel M. The interferon-induced protein kinase PK-i from mouse L cells. J Biol Chem. 1979 Oct 10;254(19):9846–9853. [PubMed] [Google Scholar]
- Klein G., Zeuthen J., Terasaki P., Billing R., Honig R., Jondal M., Westman A., Clements G. Inducibility of the Epstein-Barr virus (EBV) cycle and surface marker properties of EBV-negative lymphoma lines and their in vitro EBV-converted sublines. Int J Cancer. 1976 Nov 15;18(5):639–652. doi: 10.1002/ijc.2910180513. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Fahey D. Human fibroblast interferon. An improved purification. J Biol Chem. 1981 Apr 25;256(8):3609–3611. [PubMed] [Google Scholar]
- Kohn L. D., Friedman R. M., Holmes J. M., Lee G. Use of thyrotropin and cholera toxin to probe the mechanism by which interferon initiates its antiviral activity. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3695–3699. doi: 10.1073/pnas.73.10.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonai P., Steinman L. Physiological regulation of antigen binding to T cells: role of a soluble macrophage factor and of interferon. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5662–5666. doi: 10.1073/pnas.74.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel U., Katcoff D., Zakut R., Shani M., Carmon Y., Finer M., Czosnek H., Ginsburg I., Yaffe D. Isolation and characterization of rat skeletal muscle and cytoplasmic actin genes. Proc Natl Acad Sci U S A. 1982 May;79(9):2763–2767. doi: 10.1073/pnas.79.9.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Wang E., Tamm I. Interferon effects on microfilament organization, cellular fibronectin distribution, and cell motility in human fibroblasts. J Cell Biol. 1980 Apr;85(1):9–17. doi: 10.1083/jcb.85.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- Revel M. Interferon-induced translational regulation. Tex Rep Biol Med. 1977;35:212–220. [PubMed] [Google Scholar]
- Revel M., Kimchi A., Shulman L., Fradin A., Shuster R., Yakobson E., Chernajovsky Y., Schmidt A., Shure A., Bendori R. Role of interferon-induced enzymes in the antiviral and antimitogenic effects of interferon. Ann N Y Acad Sci. 1980;350:459–472. doi: 10.1111/j.1749-6632.1980.tb20649.x. [DOI] [PubMed] [Google Scholar]
- Rubinstein M., Levy W. P., Moschera J. A., Lai C. Y., Hershberg R. D., Bartlett R. T., Pestka S. Human leukocyte interferon: isolation and characterization of several molecular forms. Arch Biochem Biophys. 1981 Aug;210(1):307–318. doi: 10.1016/0003-9861(81)90194-6. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Chernajovsky Y., Shulman L., Federman P., Berissi H., Revel M. An interferon-induced phosphodiesterase degrading (2'-5') oligoisoadenylate and the C-C-A terminus of tRNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4788–4792. doi: 10.1073/pnas.76.10.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Zilberstein A., Shulman L., Federman P., Berissi H., Revel M. Interferon action: isolation of nuclease F, a translation inhibitor activated by interferon-induced (2'-5') oligo-isoadenylate. FEBS Lett. 1978 Nov 15;95(2):257–264. doi: 10.1016/0014-5793(78)81006-0. [DOI] [PubMed] [Google Scholar]
- Shulman L., Revel M. Interferon-dependent induction of mRNA activity for (2'-5')oligo-isoadenylate synthetase. Nature. 1980 Nov 6;288(5786):98–100. doi: 10.1038/288098a0. [DOI] [PubMed] [Google Scholar]
- Tovey M., Brouty-Boyé D., Gresser I. Early effect of interferon on mouse leukemia cells cultivated in a chemostat. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2265–2269. doi: 10.1073/pnas.72.6.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Zeevi M., Landau T., Revel M. Identification of the translation products of human fibroblast interferon mRNA in reticulocyte lysates. Eur J Biochem. 1979 Jul;98(1):1–8. doi: 10.1111/j.1432-1033.1979.tb13153.x. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]