Summary
Background
The antibody–drug conjugate trastuzumab emtansine is indicated for the treatment of patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane. Approval of this drug was based on progression-free survival and interim overall survival data from the phase 3 EMILIA study. In this report, we present a descriptive analysis of the final overall survival data from that trial.
Methods
EMILIA was a randomised, international, open-label, phase 3 study of men and women aged 18 years or older with HER2-positive unresectable, locally advanced or metastatic breast cancer previously treated with trastuzumab and a taxane. Enrolled patients were randomly assigned (1:1) via a hierarchical, dynamic randomisation scheme and an interactive voice response system to trastuzumab emtansine (3·6 mg/kg intravenously every 3 weeks) or control (capecitabine 1000 mg/m2 self-administered orally twice daily on days 1–14 on each 21-day cycle, plus lapatinib 1250 mg orally once daily on days 1–21). Randomisation was stratified by world region (USA vs western Europe vs or other), number of previous chemotherapy regimens for unresectable, locally advanced, or metastatic disease (0 or 1 vs >1), and disease involvement (visceral vs non-visceral). The coprimary efficacy endpoints were progression-free survival (per independent review committee assessment) and overall survival. Efficacy was analysed in the intention-to-treat population; safety was analysed in all patients who received at least one dose of study treatment, with patients analysed according to the treatment actually received. On May 30, 2012, the study protocol was amended to allow crossover from control to trastuzumab emtansine after the second interim overall survival analysis crossed the prespecified overall survival efficacy boundary. This study is registered with ClinicalTrials.gov, number NCT00829166.
Findings
Between Feb 23, 2009, and Oct 13, 2011, 991 eligible patients were enrolled and randomly assigned to either trastuzumab emtansine (n=495) or capecitabine and lapatinib (control; n=496). In this final descriptive analysis, median overall survival was longer with trastuzumab emtansine than with control (29·9 months [95% CI 26·3–34·1] vs 25·9 months [95% CI 22·7–28·3]; hazard ratio 0·75 [95% CI 0·64–0·88]). 136 (27%) of 496 patients crossed over from control to trastuzumab emtansine after the second interim overall survival analysis (median follow-up duration 24·1 months [IQR 19·5–26·1]). Of those patients originally randomly assigned to trastuzumab emtansine, 254 (51%) of 495 received capecitabine and 241 [49%] of 495 received lapatinib (separately or in combination) after study drug discontinuation. In the safety population (488 patients treated with capecitabine plus lapatinib, 490 patients treated with trastuzumab emtansine), fewer grade 3 or worse adverse events occurred with trastuzumab emtansine (233 [48%] of 490) than with capecitabine plus lapatinib control treatment (291 [60%] of 488). In the control group, the most frequently reported grade 3 or worse adverse events were diarrhoea (103 [21%] of 488 patients) followed by palmar– plantar erythrodysaesthesia syndrome (87 [18%]), and vomiting (24 [5%]). The safety profile of trastuzumab emtansine was similar to that reported previously; the most frequently reported grade 3 or worse adverse events in the trastuzumab emtansine group were thrombocytopenia (70 [14%] of 490), increased aspartate aminotransferase levels (22 [5%]), and anaemia (19 [4%]). Nine patients died from adverse events; five of these deaths were judged to be related to treatment (two in the control group [coronary artery disease and multiorgan failure] and three in the trastuzumab emtansine group [metabolic encephalopathy, neutropenic sepsis, and acute myeloid leukaemia]).
Interpretation
This descriptive analysis of final overall survival in the EMILIA trial shows that trastuzumab emtansine improved overall survival in patients with previously treated HER2-positive metastatic breast cancer even in the presence of crossover treatment. The safety profile was similar to that reported in previous analyses, reaffirming trastuzumab emtansine as an efficacious and tolerable treatment in this patient population.
Funding
F Hoffmann-La Roche/Genentech.
Introduction
Amplification of the HER2 gene or HER2 receptor overexpression occurs in around 20% of all breast cancers and has been associated with biologically aggressive disease and shortened overall survival.1–3 Trastuzumab plus chemotherapy,4,5 as well as the combined use of trastuzumab plus pertuzumab and chemotherapy,6,7 have improved progression-free survival and overall survival in the first-line metastatic breast cancer setting; however, few therapies have shown significant benefit in the second or later lines of therapy after use of these agents.8 Trastuzumab emtansine is an antibody–drug conjugate that is approved in many countries worldwide for the treatment of HER2-positive metastatic breast cancer in patients who previously received trastuzumab and a taxane (separately or in combination) and who have received previous therapy for metastatic breast cancer or have developed disease recurrence within 6 months of completing adjuvant therapy. Trastuzumab emtansine is composed of the humanised monoclonal antibody trastuzumab stably linked to the cytotoxic microtubule inhibitor DM1.9
Approval of trastuzumab emtansine was based on the phase 3 EMILIA study, which showed that trastuzumab emtansine significantly improved progression-free survival and overall survival compared with capecitabine plus lapatinib in patients with HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane.10 Duration of follow-up at the time of the primary analysis was 12·4 months (IQR 6·9–20·2) for the control group and 12·9 months (IQR 7·7–21·1) for the trastuzumab emtansine group.
In the primary progression-free survival analysis of EMILIA, median progression-free survival was 9·6 months in the trastuzumab emtansine group and 6·4 months in the capecitabine plus lapatinib group (hazard ratio [HR] 0·65 [95% CI 0·55–0·77]; p<0·0001). Fewer grade 3 or worse adverse events were reported for trastuzumab emtansine versus capecitabine plus lapatinib (41% [200/490 patients] vs 57% [278/488 patients]). The median duration of follow-up at the second interim analysis was 18·6 months (IQR 12·6–26·6) for control and 19·1 months (IQR 13·7–27·8) for trastuzumab emtansine. At the second (confirmatory) interim overall survival analysis of EMILIA, the prespecified efficacy boundary for overall survival (HR<0·727 or p<0·0037) was crossed, with a median overall survival of 30·9 months in the trastuzumab emtansine group and 25·1 months in the capecitabine plus lapatinib group (HR 0·68 [95% CI 0·55–0·85]; p<0·001).10 On May 30, 2012, the study protocol was amended to allow crossover from control to trastuzumab emtansine, after the improved overall survival observed with trastuzumab emtansine was confirmed during the second interim overall survival analysis. Patients randomly assigned to the control group (capecitabine plus lapatinib) were allowed to cross over to trastuzumab emtansine if they still met the study’s original eligibility criteria. In this descriptive analysis, we aimed to assess final overall survival outcomes from EMILIA.
Methods
Study design and participants
EMILIA was a randomised, international, open-label study of patients with HER2-positive unresectable, locally advanced, or metastatic breast cancer previously treated with trastuzumab and a taxane.10 Eligible patients had centrally confirmed (Targos Molecular Pathology GmbH, Kassel, Germany), HER2-positive disease (immuno-histochemistry [IHC] analysis score of 3+ or fluorescence in-situ hybridisation amplification ratio ≥2·0, or both) and progression during or after their most recent treatment for locally advanced or metastatic disease or within 6 months after treatment for early-stage disease. Eligible men and women were aged 18 years or older and had measurable disease per modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.011 or evaluable, but non-measurable disease; a left ventricular ejection fraction of 50% or more; and an Eastern Cooperative Oncology Group performance status score of 0 or 1. Patients also had to have adequate organ function in the 30 days before being randomly assigned (absolute neutrophil count >1500 cells/µL; platelet count >100 000 platelets/µL; haemoglobin level >9·0 g/dL; albumin concentration ≥2·5 g/dL; total bilirubin ≤1·5 × the upper limit of normal [ULN]; aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels ≤2·5 × ULN [patients with bone metastases could have had alkaline phosphatase levels ≤5 × ULN]; creatinine clearance >50 mL/min per the Cockroft-Gault formula; and an international normalised ratio and activated partial thromboplastin time <1·5 × ULN [unless on therapeutic coagulation]). There was no requirement in the eligibility or exclusion criteria specifically relating to minimum life expectancy. Patients were excluded if they had grade 3 or worse peripheral neuropathy, symptomatic CNS metastases or treatment for these metastases within 2 months before randomisation, or had been previously treated with trastuzumab emtansine, lapatinib, or capecitabine. Patients were also excluded if they had received hormonal therapy in the 7 days before being randomly assigned or any non-hormonal anticancer drug or biological drug or investigational treatment in the 21 days before randomisation.
The study was done in accordance with International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent, and the study was approved by the relevant institutional review board or independent ethics committee at each participating site. This protocol amendment was approved by all Institutional Review Boards and Ethics Committees before it could be implemented at the individual sites.
Randomisation and masking
Patients were randomly assigned (1:1) to trastuzumab emtansine or capecitabine plus lapatinib (control group) via a hierarchical, dynamic randomisation assignment procedure and an interactive voice-response system. Stratification factors were world region (USA vs western Europe vs other), number of previous chemotherapy regimens for unresectable, locally advanced, or metastatic disease (0 or 1 vs >1), and disease involvement (visceral vs non-visceral). The study was open label. An independent review committee masked to treatment assignment assessed tumour responses through review of all available radiographic and other tumour assessment data at the time of the final progression-free survival analysis.
Procedures
Patients in the control group self-administered capecitabine (1000 mg/m2 orally twice daily [total daily dose 2000 mg/m2] on days 1–14 of each 21-day treatment cycle) plus lapatinib (1250 mg orally once daily on days 1–21). Patients recorded their doses in a diary. Dose delays, reductions, and discontinuations due to adverse events were protocol defined.10 For capecitabine, the first dose reduction was 75% of the total daily dose, and the second dose reduction was 50% of that dose.10 For lapatinib, the first dose reduction was to 1000 mg daily.10 Patients could continue lapatinib if capecitabine was discontinued and vice versa. If treatment with both capecitabine and lapatinib was delayed for more than 42 consecutive days, both drugs were discontinued. Other reasons for discontinuation of both drugs were disease progression (assessed by the investigator), unmanageable toxicity, or early study termination by the sponsor. Patients randomly assigned to trastuzumab emtansine treatment received 3·6 mg/kg intravenously every 3 weeks. The first dose of trastuzumab emtansine was administered as a 90-min infusion and, if well tolerated, subsequent doses were administered over 30 min, with at least a 30-min observation period after infusion. Dose reductions were permitted if adverse events occurred and the maximum duration of dose delays was 42 days.10 The first dose reduction was to 3·0 mg/kg and the second to 2·4 mg/kg; if any further dose reductions were required, treatment was discontinued. Dose escalation was not allowed after a dose reduction. If a safety event did not resolve to grade 1 or baseline status within 42 days after a dose, study treatment was discontinued. All patients were assessed for safety before each treatment cycle. Laboratory testing, including complete blood counts with platelet counts and three part differential serum chemistries (blood urea nitrogen, creatinine, total bilirubin, lactate dehydrogenase, aspartate amino transferase, alanine aminotransferase, and alkaline phosphatase), was done weekly during cycles 1–4 and within 96 h before day 1 of all subsequent treatment cycles. Patients received study treatment until disease progression (as assessed by the investigator) or unmanageable toxic effects.
Study investigators and an independent review committee did tumour assessments at baseline and every 6 weeks thereafter until investigator-assessed disease progression. Until the primary progression-free survival analysis, progression was assessed according to modified RECIST, version 1.0. After the primary analysis of progression-free survival, investigators were instructed to perform tumour assessment per their usual clinical practice, and use of the modified RECIST criteria was recommended.11 An additional tumour assessment was required 6 weeks after disease progression. The investigator had the right to discontinue a patient from study therapy for any medical condition with the potential to jeopardise patient safety, for non-compliance (eg, missed doses or visits), if the patient became pregnant, or if the investigator determined that it was in the patient’s best interest. Patients were allowed to voluntarily withdraw from the study at any time. Patients were required to be withdrawn from study therapy if they had disease progression (defined by modified RECIST11) or unacceptable toxic effects.
All patients were followed up for survival in the clinic or by phone until death. On May 30, 2012, the study protocol was amended to allow crossover. Crossover from the control group to trastuzumab emtansine group was allowed after the efficacy boundary for overall survival was crossed. All patients in the control group were eligible to crossover to treatment with trastuzumab emtansine as long as they met the original study eligibility criteria for treatment with trastuzumab emtansine. Because a substantial amount of time could have passed since an individual was originally screened for study eligibility, all patients in the control group were rescreened before switching to trastuzumab emtansine treatment to ensure safety (ie, to confirm that they were eligible to receive crossover therapy). Patients were followed up until this descriptive analysis of final overall survival. Patients originally assigned to trastuzumab emtansine were not allowed to crossover to receive control as study treatment. If patients progressed, they could receive the control regimen as post-progression therapy; however, we did not do a sensitivity analysis of this scenario.
An independent data monitoring committee and a cardiac review committee monitored safety. Adverse events were continuously monitored and graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.12 The incidence of grade 3 or worse cardiac dysfunction was assessed as a composite term because at the time of protocol development cardiac-related adverse events were of interest due to the trastuzumab component of trastuzumab emtansine. The composite term included acute pulmonary oedema, cardiac asthma, cardiac failure (acute, chronic, congestive, or high output), decreased cardiac output, cardiogenic shock, cardio myopathy, cardiopulmonary failure, cardiorenal syndrome, cardiotoxicity, cor pulmonale (acute or chronic), diastolic or systolic dysfunction, decreased ejection fraction, hepatic congestion, hepatojugular reflux, ventricular dysfunction (left or right), ventricular failure (acute, chronic, left, or right), low cardiac output syndrome, obstructive shock, oedema due to cardiac disease, pulmonary oedema, and decreased stroke volume. Similarly, the incidence of grade 3 or worse haemorrhage was specifically investigated because it is an adverse event that has been of concern in patients treated with trastutumab emtansine. Therefore, grade 3 or higher haemorrhage was investigated as a composite term of preferred terms such as epistaxis, rectal haemorrhage, petechiae, menorrhagia, metrorrhagia, haemorrhage, gastrointestinal haemorrhage, peptic ulcer haemorrhage, and subdural haemorrhage. Patients were followed up for adverse events until 30 days after study treatment discontinuation. For any adverse events that occurred, investigators were advised to follow up all unresolved adverse events until the events resolved or stabilised.
Outcomes
The coprimary efficacy endpoints in EMILIA were progression-free survival (assessed by the independent review committee) and overall survival. The safety endpoint included an assessment of incidence, nature, and severity of adverse events. Progression-free survival was defined as the time from randomisation to progression or death from any cause. Progression was assessed according to modified RECIST, version 1.0.11 Overall survival was defined as the time from randomisation to death from any cause and analysed at three prespecified timepoints (at the time of the primary progression-free survival analysis, when approximately 316 deaths had occurred [ie, 50% of the 632 deaths for the final overall survival analysis], and when 632 deaths had occurred). Patients for whom death was not reported before data cutoff were censored for overall survival at the last known date they were alive. Patients with no post-baseline information were censored at the date of randomisation plus 1 day. The secondary endpoints of this study were investigator-assessed progression-free survival, the proportion of patients with an objective response (complete and partial responses), the proportion of patients with clinical benefit (defined as complete response, partial response, or stable disease at 6 months post-randomisation), duration of response, time to treatment failure (time from randomisation to discontinuation of treatment for any reason), and time to symptom progression. These results have already been presented elsewhere.10
Statistical analysis
The selected sample size of EMILIA ensured that the final overall survival analysis was appropriately powered. To detect an overall survival HR of 0·80, an increase in median overall survival from 17·2 months in the control group to 21·5 months in the trastuzumab emtansine group, approximately 632 deaths were required to achieve 80% power at the two-sided 5% α level. A total of 980 patients were required for enrolment. Sample size was estimated by EAST software.
Information on primary analyses and secondary endpoints have already been published.10 At the first interim analysis of overall survival, which was done at the time of the primary progression-free survival analysis (data cutoff Jan 14, 2012), 223 deaths had occurred, which did not cross the prespecified efficacy boundary (HR<0·617 or p<0·0003) for overall survival (Lan DeMets α spending function with an O’Brien-Fleming boundary).10 At the second interim overall survival analysis (data cutoff July 31, 2012), 331 deaths had occurred. This analysis crossed the prespecified efficacy boundary for overall survival and therefore statistically confirmed the improvement in overall survival for trastuzumab emtansine versus control. The final overall survival analysis, which is the focus of the present report, was planned to occur after 632 patients had died. Because the efficacy boundary for overall survival was crossed at the time of the second interim overall survival analysis, this final analysis of overall survival is descriptive.
Overall survival was assessed in the intention-to-treat population, which comprised all patients who were randomly assigned to a treatment group. The Kaplan-Meier method was used to estimate median overall survival. A Cox proportional-hazards model was used to calculate HRs (stratified by randomisation factors) and associated 95% CIs. For the second (confirmatory) interim overall survival analysis and the descriptive analysis of final overall survival, overall survival was assessed in prespecified clinically relevant patient subgroups defined by the presence or absence of visceral disease, age, world region, and race; an unstratified Cox proportional-hazards model was used to estimate HRs and associated 95% CIs. With the same methods, a post-hoc sensitivity analysis was done in which patients randomly assigned to the control group were censored at the time of crossing over to trastuzumab emtansine. The safety population included all patients who received at least one dose of study treatment, with patients analysed according to the treatment actually received. Adverse events were summarised cumulatively for the descriptive analysis of final overall survival. SAS (version 9.2) was used for the statistical analyses.
This study is registered with ClinicalTrials.gov, number NCT00829166.
Role of the funding source
The EMILIA study was designed by the funder in collaboration with the study steering committee. Data were collected by the funder and analysed in collaboration with the authors. VD prepared the initial draft of the manuscript with support from a medical writer, who was paid by the funder. All authors were involved in data analysis and interpretation and contributed to subsequent drafts of the report. The report was also reviewed by the funder. The corresponding author had full access to all the study data and had final responsibility for the decision to submit this report for publication.
Results
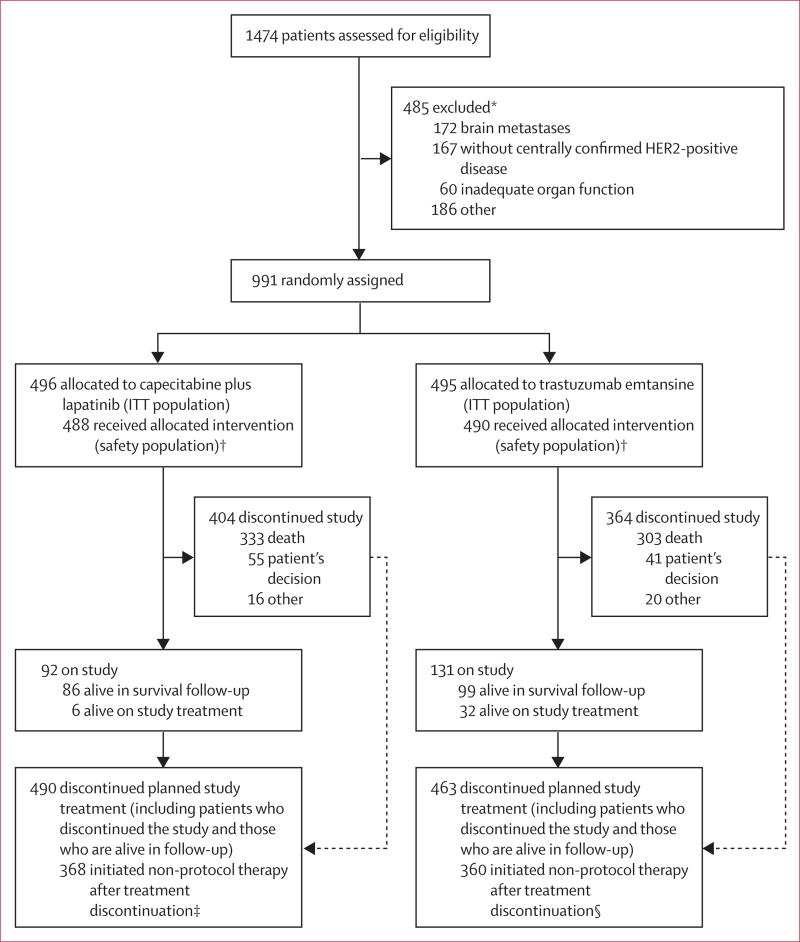
Between Feb 23, 2009, and Oct 13, 2011, 1474 patients were assessed for eligibility; of these, 483 were ineligible, most often due to the presence of untreated, symptomatic or recently treated brain metastases or because HER2 status was not confirmed as positive at the central facility. A total of 991 patients from 213 centres in 26 countries (appendix pp 1–8) were enrolled and randomly assigned to either capecitabine and lapatinib (control group; n=496) or trastuzumab emtansine (n=495; figure 1). As reported previously, patient baseline characteristics were well balanced between treatment groups (table 1).10
Figure 1. Trial profile.
ITT=intention to treat.*Patients might have had multiple reasons for ineligibility. †Eight patients in the lapatinib plus capecitabine group and five patients in the trastuzumab emtansine group did not receive study treatment; reasons for why these patients did not receive study treatment are not available. ‡Non-protocol therapy included: capecitabine (n=53), lapatinib (n=82), trastuzumab (n=235), pertuzumab (n=12), and trastuzumab emtansine (n=7); patients could have received more than one of the treatments listed because data were collected on individual drugs, not complete regimens. §Non-protocol therapy included: capecitabine (n=254), lapatinib (n=241), trastuzumab (n=177), pertuzumab (n=21), and trastuzumab emtansine (n=11); patients could have received more than one of the treatments listed.
Table 1.
Baseline characteristics
| Capecitabine plus lapatinib (n=496) |
Trastuzumab emtansine (n=495) |
|
|---|---|---|
| Age (years) | 53 (24–83) | 53 (25–84) |
|
| ||
| Race* | ||
| White | 374 (75%) | 358 (72%) |
| Asian | 86 (17%) | 94 (19%) |
| Black | 21 (4%) | 29 (6%) |
| Other | 10 (2%) | 7 (1%) |
| Not available | 5 (1%) | 7 (1%) |
|
| ||
| World region | ||
| USA | 136 (27%) | 134 (27%) |
| Western Europe | 160 (32%) | 157 (32%) |
| Asia | 76 (15%) | 82 (17%) |
| Other | 124 (25%) | 122 (25%) |
|
| ||
| ECOG performance status† | ||
| 0 | 312 (63%) | 299 (60%) |
| 1 | 176 (35%) | 194 (39%) |
| Not available | 8 (2%) | 2 (<1%) |
|
| ||
| Site of disease involvement | ||
| Visceral | 335 (68%) | 334 (67%) |
| Non-visceral | 161 (32%) | 161 (33%) |
|
| ||
| Hormone receptor status | ||
| Oestrogen receptor positive, progesterone receptor positive, or both | 263 (53%) | 282 (57%) |
| Oestrogen receptor negative and progesterone receptor negative | 224 (45%) | 202 (41%) |
| Unknown | 9 (2%) | 11 (2%) |
|
| ||
| Previous systemic therapy‡ | ||
| Anthracycline | 302 (61%) | 303 (61%) |
| Other chemotherapy | 382 (77%) | 385 (78%) |
| Biological drug other than trastuzumab or pertuzumab | 21 (4%) | 13 (3%) |
| Endocrine therapy | 204 (41%) | 205 (41%) |
|
| ||
| Number of previous chemotherapy regimens for locally advanced or metastatic disease | ||
| 0 or 1 | 305 (61%) | 304 (61%) |
| >1 | 191 (39%) | 191 (39%) |
|
| ||
| Previous trastuzumab treatment‡ | ||
| For metastatic or early-stage breast cancer | 419 (84%) | 417 (84%) |
| For early-stage breast cancer only | 77 (16%) | 78 (16%) |
Data are median (range) or n (%). Reproduced from Verma S et al10 with permission from Massachusetts Medical Society, copyright 2012. ECOG=Eastern Cooperative Oncology Group.
Race was self-reported.
An ECOG performance status of 0 indicates that the patient is asymptomatic, and a status of 1 indicates that the patient is restricted in strenuous activity but ambulatory and able to do light work.
The study protocol specified that previous treatment with a taxane and trastuzumab was required for enrolment.
The cutoff date for this descriptive analysis of final overall survival was Dec 31, 2014. Median duration of follow-up was 41·9 months (IQR 34·6–50·7) in the control group and 47·8 months (41·9–55·5) in the trastuzumab emtansine group. At the time of the final overall survival analysis, 333 (67%) of 496 patients originally randomly assigned to the control group and 303 (61%) of the 495 patients originally randomly assigned to trastuzumab emtansine had died. Excluding deaths that occurred after crossover to trastuzumab emtansine, 278 patients who were initially randomly assigned to the control group died (273 due to disease progression; five due to adverse events). Of the 303 patients who received trastuzumab emtansine who died, 292 deaths were due to disease progression, four were due to adverse events, and seven were due to events that occurred more than 30 days after study treatment and were thus not reportable as adverse events.
In this descriptive analysis of final overall survival, the median duration of treatment was shorter in the control group than in the trastuzumab emtansine group (capecitabine 5·3 months [range 0·0–60·5]; lapatinib 5·5 months [0·0–62·4]; trastuzumab emtansine 7·6 months [0·0–63·5]). A total of 136 (27%) of 496 patients in the control group crossed over from capecitabine plus lapatinib to trastuzumab emtansine after the second (confirmatory) interim overall survival analysis and subsequent protocol amendment. The number of patients screened but not eligible for crossover is not available. The median duration between the discontinuation of control treatment and crossover to trastuzumab emtansine was 51·2 weeks (IQR 15·4–87·2). The median duration of follow-up in the subset of patients who crossed over to trastuzumab emtansine was 24·1 months (IQR 19·5–26·1). The median duration of treatment with trastuzumab emtansine in this subset of patients was 6·3 months (range 0·0–26·9).
Among those patients who were randomly assigned to trastuzumab emtansine but who discontinued study treatment, most discontinued because of disease progression (363 [74%] of 490). Of the 495 patients in this group, 360 (73%) initiated non-protocol therapy after treatment discontinuation, of whom 254 (51%) received capecitabine and 241 patients (49%) received lapatinib. Data for therapy use after study treatment discontinuation were collected for individual drugs, not complete regimens; thus, capecitabine and lapatinib could have been received in combination with each other or other drugs. In the control group, 482 patients discontinued capecitabine and 482 discontinued lapatinib, of whom 376 (77%) discontinued lapatinib and 366 (75%) discontinued capecitabine because of disease progression. 368 (75%) of the 496 patients in this group initiated non-protocol therapy after treatment discontinuation (figure 1).
Of patients originally assigned to the lapatinib plus capecitabine group, 136 crossed over to treatment with trastuzumab emtansine. Of those patients, 69 had discontinued the study and 67 were alive at the data cutoff (five of whom were still on treatment and 62 were in survival follow-up).
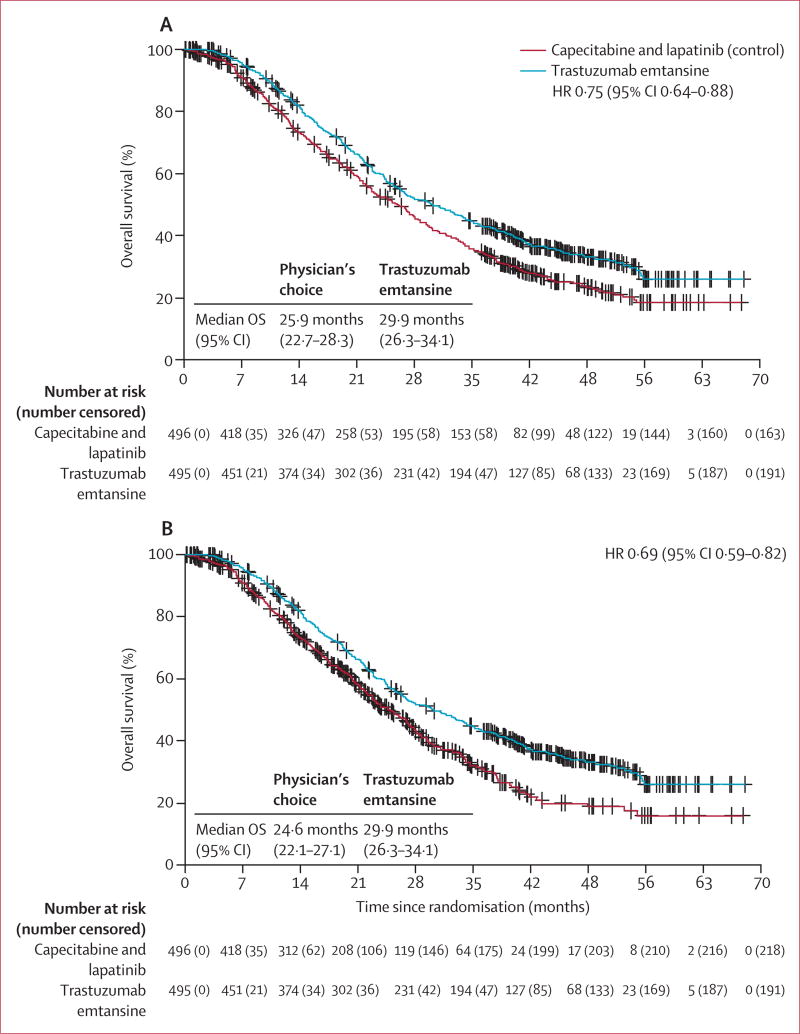
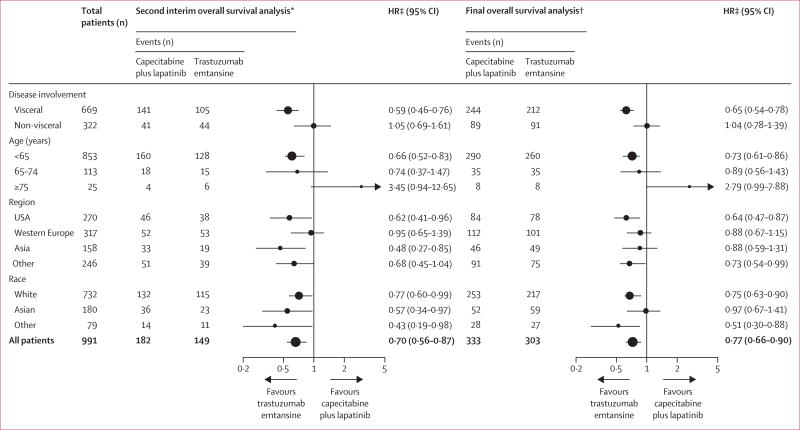
In this descriptive analysis of final overall survival, the overall survival benefit reported in the second interim analysis in favour of trastuzumab emtansine was maintained through the final overall survival analysis (figure 2A). Median overall survival in the intention-to-treat population was 25·9 months (95% CI 22·74–28·32) in the control group compared with 29·9 months (95% CI 26·32–34·10) in the trastuzumab emtansine group (stratified HR 0·75 [95% CI 0·64–0·88]; table 2, figure 2A). These results were consistent with a post-hoc sensitivity analysis in which patients were censored at the time of crossover to trastuzumab emtansine (figure 2B). Median overall survival in the control group censored at crossover was 24·6 months (95% CI 22·7–27·1) compared with 29·9 months (26·3–34·1) in the trastuzumab emtansine group (stratified HR 0·69 [95% CI 0·59–0·82]; figure 2B, table 2). In a prespecified subgroup analysis, the overall survival benefit was observed in most clinically relevant subgroups for both the second (confirmatory) interim analysis and the descriptive analysis of final overall survival (figure 3).
Figure 2. Overall survival.
Tick marks on the curve represent censored patients. (A) Descriptive analysis of final overall survival in the intention-to-treat population. (B) Sensitivity analysis for overall survival in which patients were censored at the time of crossover from capecitabine plus lapatinib to trastuzumab emtansine. OS=overall survival.
Table 2.
Summary of overall survival analyses in the EMILIA study
| Capecitabine plus lapatinib (n=496) |
Trastuzumab emtansine (n=495) |
Stratified HR (95% CI) |
p value | Stopping boundary | |||
|---|---|---|---|---|---|---|---|
| Events, n (%) |
Median overall survival, months (95% CI) |
Events, n (%) |
Median overall survival, months (95% CI) |
||||
| First interim analysis*10 | 129 (26%) | 23·3 (20·9-NR) | 94 (19%) | NR (26·3-NR) | 0·62 (0·48–0·81) | p=0·0005 | p<0·0003 or HR<0·617 |
|
| |||||||
| Second (confirmatory) interim analysis†10 | 182 (37%) | 25·1 (22·7–28·0) | 149 (30%) | 30·9 (26·8–343) | 0·68 (0·55–0·85) | p=0·0006 | p<0·0037 or HR<0·727 |
|
| |||||||
| Final analysis† | 333 (67%) | 25·9 (22·7–28·3) | 303 (61%) | 29·9 (263–34·1) | 0·75 (0·64–0·88) | Descriptive only | Boundary met at second interim analysis |
|
| |||||||
| Sensitivity analysis with crossover patients censored‡ | 278 (56%) | 24·6 (22·1–27·1) | 303 (61%) | 29·9 (26·3–34·1) | 0·69 (0·59–0·82) | Descriptive only | Boundary met at second interim analysis |
HR=hazard ratio. NR=not reached. HRs and 95% CIs were estimated using a Cox proportional-hazards model stratified by world region, number of previous chemotherapeutic regimens for locally advanced or metastatic disease, and presence or absence of visceral disease.
Data cutoff Jan 14, 2012.
Data cutoff July 31, 2012.
Data cutoff Dec 31, 2014.
Figure 3. Overall survival in clinically relevant subgroups at the time of the second (confirmatory) interim overall survival analysis and at the descriptive analysis of final overall survival.
Error bars represent 95% CIs. HR=hazard ratio. *Data cutoff July 31, 2012. †Data cutoff Dec 31, 2014. ‡Hazard ratios are from unstratified analyses.
The safety analysis population comprised all patients who received at least one dose of the study treatment: 488 patients in the capecitabine plus lapatinib group and 490 patients in the trastuzumab emtansine group. The overall incidence and distribution of adverse events in the safety population noted at the final overall survival analysis are numerically similar in type to those reported at previous analyses of this trial.10 The proportion of patients with a grade 3 or worse adverse event was 291 (60%) of 488 patients in the control group and 233 (48%) of 490 patients in the trastuzumab emtansine group. As a result of adverse events, 53 (11%) of 488 patients discontinued capecitabine, 42 (9%) of 488 patients discontinued lapatinib, and 49 (10%) of 490 patients discontinued trastuzumab emtansine. Patients in the trastuzumab emtansine group had fewer adverse events leading to dose reductions (91 [19%] of 490 patients) than those in the control group (205 [42%] of 488 patients treated with capecitabine and 98 [20%] of 488 patients treated with lapatinib). Diarrhoea was the most frequently reported grade 3 or worse adverse event in the control group (103 [21%] of 488 patients) followed by palmar–plantar erythrodysaesthesia syndrome (87 patients [18%]) and vomiting (24 patients [5%]; table 3). The incidence and type of grade 3 or worse adverse events reported in the trastuzumab emtansine group were generally consistent between the second (confirmatory) interim overall survival analysis10 (table 3; appendix pp 9–22). By the time of this descriptive analysis of final overall survival, thrombocytopenia was the most frequently reported grade 3 or worse adverse event in patients given trastuzumab emtansine (70 [14%] of 490 patients), followed by increased aspartate aminotransferase (22 [5%]), and anaemia (19 [4%]). The incidence of grade 3 or worse haemorrhage (investigated as a composite term) in patients treated with trastuzumab emtansine was 12 (2%) of 490 patients compared with four (1%) of 488 patients in the control group for the descriptive analysis of final overall survival. The incidence of grade 3 or worse cardiac dysfunction (investigated as a composite term) was very low in both the control group (three patients [<1%]) and trastuzumab emtansine group (one patient [<1%]).
Table 3.
All adverse events
| Capecitabine plus lapatinib (n=488)
|
Trastuzumab emtansine (n=490)
|
|||||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Diarrhoea | 286 (59%) | 100 (21%) | 3 (1%) | 115 (24%) | 9 (2%) | 0 |
|
| ||||||
| Nausea | 212 (43%) | 12 (3%) | 1 (<1%) | 198 (40%) | 4 (1%) | 0 |
|
| ||||||
| Palmar–plantar erythrodysaesthesia syndrome | 204 (42%) | 86 (18%) | 1 (<1%) | 7 (1%) | 0 | 0 |
|
| ||||||
| Fatigue | 128 (26%) | 16 (3%) | 1 (<1%) | 169 (34%) | 11 (2%) | 1 (<1%) |
|
| ||||||
| Rash | 127 (26%) | 7 (1%) | 1 (<1%) | 64 (13%) | 0 | 0 |
|
| ||||||
| Vomiting | 126 (26%) | 23 (5%) | 1 (<1%) | 97 (20%) | 5 (1%) | 0 |
|
| ||||||
| Decreased appetite | 112 (23%) | 5 (1%) | 0 | 103 (21%) | 2 (<1%) | 0 |
|
| ||||||
| Mucosal inflammation | 82 (17%) | 9 (2%) | 2 (<1%) | 32 (7%) | 1 (<1%) | 0 |
|
| ||||||
| Asthenia | 79 (16%) | 8 (2%) | 1 (<1%) | 88 (18%) | 4 (1%) | 0 |
|
| ||||||
| Headache | 73 (15%) | 4 (1%) | 0 | 143 (29%) | 4 (1%) | 0 |
|
| ||||||
| Stomatitis | 69 (14%) | 2 (<1%) | 0 | 20 (4%) | 0 | 0 |
|
| ||||||
| Cough | 67 (14%) | 1 (<1%) | 0 | 99 (20%) | 1 (<1%) | 0 |
|
| ||||||
| Back pain | 61 (13%) | 2 (<1%) | 0 | 75 (15%) | 4 (1%) | 0 |
|
| ||||||
| Constipation | 60 (12%) | 0 | 0 | 137 (28%) | 2 (<1%) | 0 |
|
| ||||||
| Pain in extremity | 57 (12%) | 5 (1%) | 0 | 69 (14%) | 2 (<1%) | 0 |
|
| ||||||
| Dyspepsia | 55 (11%) | 2 (<1%) | 0 | 51 (10%) | 0 | 0 |
|
| ||||||
| Dry skin | 54 (11%) | 1 (<1%) | 0 | 17 (3%) | 0 | 0 |
|
| ||||||
| Paronychia | 53 (11%) | 6 (1%) | 0 | 2 (<1%) | 0 | 0 |
|
| ||||||
| Dizziness | 52 (11%) | 1 (<1%) | 0 | 61 (12%) | 2 (<1%) | 0 |
|
| ||||||
| Arthralgia | 47 (10%) | 0 | 0 | 99 (20%) | 3 (1%) | 0 |
|
| ||||||
| Increased AST | 46 (9%) | 7 (1%) | 0 | 101 (21%) | 22 (4%) | 0 |
|
| ||||||
| Insomnia | 45 (9%) | 1 (<1%) | 0 | 67 (14%) | 3 (1%) | 0 |
|
| ||||||
| Epistaxis | 44 (9%) | 0 | 0 | 120 (25%) | 2 (<1%) | 0 |
|
| ||||||
| Upper abdominal pain | 44 (9%) | 1 (<1%) | 0 | 58 (12%) | 2 (<1%) | 0 |
|
| ||||||
| Pyrexia | 43 (9%) | 2 (<1%) | 0 | 99 (20%) | 1 (<1%) | 0 |
|
| ||||||
| Nasopharyngitis | 41 (8%) | 0 | 0 | 49 (10%) | 0 | 0 |
|
| ||||||
| Upper respiratory tract infection | 40 (8%) | 0 | 0 | 57 (12%) | 0 | 0 |
|
| ||||||
| Dyspnoea | 39 (8%) | 1 (<1%) | 1 (<1%) | 59 (12%) | 4 (1%) | 0 |
|
| ||||||
| Increased ALT | 39 (8%) | 9 (2%) | 0 | 78 (16%) | 15 (3%) | 0 |
|
| ||||||
| Anaemia | 30 (6%) | 11 (2%) | 0 | 49 (10%) | 18 (4%) | 1 (<1%) |
|
| ||||||
| Peripheral neuropathy | 29 (6%) | 1 (0·2%) | 0 | 50 (10%) | 9 (2%) | 0 |
|
| ||||||
| Dry mouth | 25 (5%) | 1 (<1%) | 0 | 85 (17%) | 0 | 0 |
|
| ||||||
| Hypokalaemia | 23 (5%) | 21 (4%) | 1 (<1%) | 37 (8%) | 11 (2%) | 0 (0%) |
|
| ||||||
| Neutropenia | 23 (5%) | 16 (3%) | 5 (1%) | 26 (5%) | 9 (2%) | 2 (<1%) |
|
| ||||||
| Urinary tract infection | 21 (4%) | 0 | 0 | 50 (10%) | 3 (1%) | 0 |
|
| ||||||
| Myalgia | 20 (4%) | 0 | 0 | 67 (14%) | 3 (1%) | 0 |
|
| ||||||
| Thrombocytopenia | 12 (3%) | 0 | 2 (<1%) | 80 (16%) | 56 (11%) | 14 (3%) |
Data are n (%). This table shows all grade 1–2 adverse events that occurred in at least 10% of patients in either group and all grade 3 and 4 events that occurred in at least 2% of patients in either group. A full table of all adverse events is provided in appendix pp 9–22. Five patients died from adverse events in the capecitabine plus lapatinib (control) group (one each from coronary artery disease, multiorgan failure, coma, hydrocephalus, and acute respiratory distress syndrome); of these, coronary artery disease and multiorgan failure were judged to be related to capecitabine plus lapatinib. Four patients died from adverse events in the trastuzumab emtansine group (one each from metabolic encephalopathy, neutropenic sepsis, pneumonia, and acute myeloid leukaemia); of these, metabolic encephalopathy, neutropenic sepsis, and acute myeloid leukaemia were judged to be related to trastuzumab emtansine. AST=aspartate aminotransferase. ALT=alanine aminotransferase.
A total of 99 (20%) of 488 patients who received capecitabine plus lapatinib and 91 (19%) of 490 patients who received trastuzumab emtansine had at least one serious adverse event. The body systems in which serious adverse events were reported in at least 2% of patients in either treatment group were gastrointestinal disorders (31 [6%] of 488 in the trastuzumab emtansine group vs 16 [3%] of 490 in the control group); infections and infestations (15 [3%] vs 26 [5%]); respiratory, thoracic, and mediastinal disorders (16 [3%] vs seven [1%]); general disorders and disorders of the administration site (nine [2%] vs 15 [3%]); and nervous system disorders (ten [2%] vs nine [2%]).
Of the 136 patients who crossed over from capecitabine plus lapatinib to trastuzumab emtansine, 18 (13%) patients had dose reductions due to adverse events and 14 (10%) patients discontinued treatment due to adverse events. In total, 41 (30%) of 136 patients who crossed over to trastuzumab emtansine had a grade 3 or worse adverse event. The most common grade 3 or worse adverse events in this population were thrombocytopenia (six patients [4%]), anaemia (four patients [3%]), and asthenia (four patients [3%]).
Overall, adverse events led to five deaths in the control group (coronary artery disease, multiorgan failure, coma, hydrocephalus, and acute respiratory distress syndrome), and to four deaths in the trastuzumab emtansine group (metabolic encephalopathy, neutropenic sepsis, pneumonia, and acute myeloid leukaemia). In the control group, two of these deaths (coronary artery disease and multiorgan failure) were considered by study investigators to be related to capecitabine plus lapatinib. Three of the adverse events that resulted in death in the trastuzumab emtansine group had occurred by the second (confirmatory) interim overall survival analysis. The fourth occurred by the descriptive analysis of final overall survival analysis. Metabolic encephalopathy, neutropenic sepsis, and acute myeloid leukaemia were considered by study investigators to be related to trastuzumab emtansine.
Discussion
This descriptive analysis of final overall survival in the phase 3 EMILIA study shows an overall survival benefit for trastuzumab emtansine treatment compared with the control regimen of capecitabine plus lapatinib in patients with HER2-positive locally advanced or metastatic breast cancer previously treated with trastuzumab and a taxane. Although the trial was not designed to formally assess the effect of immediate crossover treatment, the overall survival benefit of trastuzumab emtansine was noted despite the fact that 27% of patients originally randomly assigned to the control group had crossed over to trastuzumab emtansine treatment, and more than 50% of patients randomly assigned to trastuzumab emtansine received treatment with either capecitabine or lapatinib after discontinuation of trastuzumab emtansine. With regard to the latter, only the individual drugs prescribed, not the actual regimens used, were captured after discontinuation of study drug; therefore, one limitation of this analysis is that the number of patients treated with trastuzumab emtansine who ultimately received combination treatment with capecitabine plus lapatinib is unknown. In total, 182 (37%) of 496 patients randomly assigned to the control group of EMILIA died before the second (confirmatory) interim overall survival analysis10 and thus were not candidates for post-progression treatment with trastuzumab emtansine.
In patients who crossed over from the capecitabine plus lapatinib control group to trastuzumab emtansine, the median time between the discontinuation of control treatment and crossover to trastuzumab emtansine was 51·2 weeks; whether the time between discontinuation of control treatment and initiation of trastuzumab emtansine had any effect on the duration of trastuzumab emtansine crossover treatment or the overall survival outcomes remains unknown. However, one hypothesis is that an overall survival advantage for trastuzumab emtansine would have been recorded even if immediate crossover had been protocol specified, given that the overall survival curves separate close to the time of median progression-free survival in the capecitabine plus lapatinib treatment group (ie, at around 6·4 months). The risk of rapid progression and inability to receive trastuzumab emtansine in later treatment lines might diminish the number of patients who can receive trastuzumab emtansine after treatment with capecitabine plus lapatinib; this contingent would, in turn, affect overall survival. Of note, the protocol of the separate phase 3 TH3RESA trial13,14—which compared trastuzumab emtansine with treatment of physician’s choice in patients previously treated with both trastuzumab and lapatinib—was amended during recruitment, after the results of the second (confirmatory) interim overall survival of EMILIA, to allow patients to receive crossover treatment immediately post-progression. In TH3RESA,14 a statistically significant increase (>6 months) in median overall survival for trastuzumab emtansine compared with treatment of physician’s choice was reported, despite 47% of patients treated with control receiving trastuzumab emtansine after progression.
Since the completion of EMILIA, the HER2-targeted monoclonal antibody pertuzumab has been approved— in combination with trastuzumab and a taxane—for the first-line treatment of HER2-positive metastatic breast cancer.15 Although EMILIA did not exclude patients who had received previous pertuzumab treatment, the drug was available only as an investigational therapy at the time of study enrolment. Therefore, only a small number of patients in EMILIA are suspected to have received previous pertuzumab. Even so, a limitation of the EMILIA study is that it does not provide evidence regarding the efficacy of trastuzumab emtansine after a patient has been treated with dual HER2 blockade. However, the phase 3 THE3RESA study enrolled patients with HER2-positive advanced breast cancer previously treated with both trastuzumab and lapatinib (advanced setting) and a taxane (any setting).13 At the time of the final overall survival analysis, trastuzumab emtansine was associated with significantly improved overall survival compared with treatment of physician’s choice (median overall survival 22·7 months (95% CI 19·4–27·5) vs 15·8 months (13·5–18·7); HR 0·68 [95% CI 0·54–0·85]; p=0·0007), despite 93 (47%) of 198 patients receiving trastuzumab emtansine after treatment of physician’s choice.14 The TH3RESA study results showed that trastuzumab emtansine is effective in patients previously treated with more than one mechanism of HER2 blockade. The overall survival benefits noted with trastuzumab emtansine in both EMILIA and TH3RESA confirm the role of trastuzumab emtansine in the treatment of recurrent metastatic breast cancer. Moreover, trastuzumab emtansine monotherapy was shown to confer clinically relevant benefits in a real-world study16 of patients previously treated with trastuzumab and pertuzumab. Additional data for the use of trastuzumab emtansine in patients previously treated with HER2-targeted therapy plus chemotherapy will be obtained from the phase 3b KAMILLA study (NCT01702571).
Long-term follow-up allows for the capture of potentially late-emerging safety issues attributable to study drug. At a median follow-up of 47·8 (IQR 41·9–55·5) months at the time of final overall survival analysis, the safety profile of trastuzumab emtansine was similar to that described in the first analysis.10 Median drug exposure was longer in the trastuzumab emtansine group than in the control group; despite this finding, trastuzumab emtansine seemed to have a favourable safety profile. There were numerically fewer grade 3 or worse adverse events with trastuzumab emtansine compared with the control group and numerically fewer adverse events leading to dose reductions. The most frequently occurring adverse events associated with trastuzumab emtansine were laboratory based (eg, thrombocytopenia and elevated transaminases), whereas the most common adverse events associated with capecitabine and lapatinib tended to be symptomatic (eg, diarrhoea).17 Results from this safety analysis were consistent with those noted for the second (confirmatory) interim analysis, with no notable increases in high-grade (grade ≥3) adverse events despite a longer duration of treatment in the final analysis than in previous reports. The safety profile reported in patients who crossed over from the control group to trastuzumab emtansine was also consistent with previous reports.10
Together with the second (confirmatory) interim analysis of overall survival in EMILIA, the descriptive results from this final overall survival analysis show that trastuzumab emtansine improves overall survival compared with capecitabine and lapatinib in this patient population. Coupled with the survival benefit reported in the TH3RESA study, the EMILIA study data reaffirm that trastuzumab emtansine is an efficacious and tolerable treatment for patients with previously treated HER2-positive metastatic breast cancer.18,19
Supplementary Material
Research in context.
Evidence before this study
The primary progression-free survival (Jan 14, 2012) and second interim overall survival (July 31, 2012) analyses of the phase 3 EMILIA study provided the basis for the approval of trastuzumab emtansine for the treatment of HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane (separately or in combination). At the time when the results from the primary analysis of EMILIA were reported, the study was ongoing, as the final analysis for overall survival was planned to occur after 632 patients had died. We searched PubMed for clinical trials (in the English language only) assessing therapeutic interventions for patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane (separately or in combination) published since the publication of initial results from EMILIA (Jan 14, 2012) through March 1, 2016. Search terms included, “trastuzumab”, “HER2-positive”, “taxane”, and (“advanced breast cancer” or “metastatic breast cancer”). To identify reports that were most relevant to the present topic, the search was further limited to phase 3 studies reporting on overall survival in the abstract. Of 112 hits, two relevant articles were identified: one for the primary report of EMILIA and one for the primary report of the TH3RESA study.
Added value of this study
Our findings confirm the survival benefit of trastuzumab emtansine compared with a treatment regimen consisting of capecitabine plus lapatinib in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane. This survival benefit was reported even though 27% of patients had crossed over from capecitabine plus lapatinib to trastuzumab emtansine. Consistent with previous reports, trastuzumab emtansine was associated with a favourable safety profile.
Implications of all the available evidence
Together with the second (confirmatory) interim overall survival analysis of the EMILIA study, the descriptive results from this final overall survival analysis show that trastuzumab emtansine improves survival, despite the fact that a substantial number of patients crossed over from the control group to the trastuzumab emtansine group. Coupled with the survival benefit seen in the TH3RESA study, these EMILIA study data reaffirm that trastuzumab emtansine is an efficacious and tolerable treatment for patients with previously treated HER2-positive metastatic breast cancer. At present, trastuzumab and pertuzumab plus taxane is the recommended first-line treatment regimen for patients with HER2-positive metastatic breast cancer. Additional data are needed on the clinical activity of trastuzumab emtansine in patients previously exposed to both trastuzumab and pertuzumab.
Acknowledgments
Support for third-party writing assistance for this report was provided by Meredith Kalish of CodonMedical, an Ashfield Company (part of UDG Healthcare plc), and was funded by F Hoffmann-La Roche.
Footnotes
Contributors
VD, DM, SV, MW, JB, IEK, KB, and LG contributed to the design of the EMILIA study, data collection, and data analysis and interpretation. MP contributed to the design of the EMILIA study and to data analysis and interpretation. SH, JX, and MG contributed to data analysis and interpretation. All authors were involved in the writing or review of the report and approved the final version.
Declaration of interests
VD has served as a consultant for F Hoffmann-La Roche/Genentech, Novartis/GlaxoSmithKline, Abbvie, and Pfizer. DM has served as a consultant for F Hoffmann-La Roche/Genentech. MP has served as a consultant for Genentech. His institution has received research support from Genentech. MW has served as a consultant for F Hoffmann-La Roche, Novartis, AstraZeneca, Janssen, Lilly, and Bristol-Myers Squibb. IEK’s institution has received research support from Genentech. KB has served as a consultant for F Hoffmann-La Roche/Genentech, Novartis, Puma, Amgen, AstraZeneca, Celgene, Eli Lilly, Eisai, GE Healthcare, Hospira, Pfizer, Rockwell, Spectrum, Incyte, Sandoz, Janssen, and Bayer. KB has also received research support from Genentech, Novartis, Pfizer, and Celgene. SH is an employee of CRO KOEHLER-eClinical, which was contracted to work on behalf of F Hoffmann-La Roche. JX and MG are salaried employees of Genentech and own stock in F Hoffmann-La Roche. LG has received consulting fees from F Hoffmann-La Roche/Genentech, GlaxoSmithKline, Novartis, Pfizer, Boehringer Ingelheim, Celgene, Tahio, Onkaido, and Tiziana. SV and JB declare no competing interests.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 3.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 Study Group. J Clin Oncol. 2005;23:4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 6.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–71. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 9.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 10.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12. [accessed July 15, 2016];Common Terminology Criteria for Adverse Events version 3.0 (CTCAE) http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 13.Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–99. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 14.Krop IE, Kim S-B, Gonzales Martin A, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised phase 3 study. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30313-3. published online May 16. http://dx.doi.org/10.1016/S1470-2045(17)30313-3. [DOI] [PubMed]
- 15.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzimitrowicz H, Berger M, Vargo C, et al. T-DM1 activity in metastatic human epidermal growth factor receptor 2-positive breast cancers that received prior therapy with trastuzumab and pertuzumab. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.3624. published online June 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welslau M, Diéras V, Sohn JH, et al. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. Cancer. 2014;120:642–51. doi: 10.1002/cncr.28465. [DOI] [PubMed] [Google Scholar]
- 18.Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Ann Oncol. 2014;25:1871–88. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. [accessed July 15, 2016];Breast Cancer. 2016 https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.