Abstract
Recent studies have posited a relationship between cannabis use and the biological stress system, but this critical relationship has not been evaluated during the ultra high-risk (UHR) period immediately preceding the onset of psychotic disorders. Salivary cortisol samples were collected on 46 UHR and 29 control adolescents; these individuals were assessed for current cannabis use with a urine panel and self-report. UHR participants where separated into two groups: Current Cannabis Use (UHR-CU) and No Current Cannabis Use (UHR-NC). Healthy Control participants (HC) were free of cannabis use. Consistent with the literature, results indicate UHR individuals showed elevated cortisol levels when compared to HC participants. Further, we also observed that UHR-CU participants exhibited elevated levels when compared to both the non-using UHR and HC groups. Findings suggest that cannabis use may interact with underlying biological vulnerability associated with the hypothalamic-pituitary-adrenal (HPA) axis system.
Keywords: Cortisol, Cannabis, HPA Axis, Prodrome, High-Risk, Schizophrenia
1. Introduction
The mean age of onset of psychotic disorders occurs during adolescence; a period that is characterized by significant changes to neurobiological systems (Walker et al., 2008) as well as exposure to substance use (Addington et al., 2014). Cannabis use is common during adolescence and the literature indicates that individuals who are at ultra high risk (UHR) for developing psychosis have higher rates of cannabis use than their peers (Addington et al., 2014). It has been suggested that cannabis use may play an important role in the etiology of psychosis (Moore et al., 2007), yet little is known about the relationship between cannabis use and neurobiological systems such as the hypothalamic-pituitary-adrenal (HPA) axis in psychosis development. In the general population, exposure to cannabis has been shown to affect the HPA axis as indicated by elevated baseline cortisol levels (King et al., 2011). Accumulating evidence suggests that the HPA axis is dysregulated in UHR youth (Aiello et al., 2012; Carol and Mittal, 2015; Walker et al., 2013); however, the relationship between cannabis use and HPA system activity has never been examined in a psychosis risk population. Understanding this relationship stands to inform the etiological conceptualization of psychosis risk and may refine efforts of early detection for adolescents exhibiting sub-threshold psychotic-like symptoms.
The HPA axis is one of the primary biological stress response systems that is activated by psychological and physiological stressors, and its activity is sensitive to a variety of psychoactive substances including cannabis (Ranganathan et al., 2009). The active ingredient in cannabis, delta-9-tetrahydrocannabinol (Δ-9-THC), binds to cannabinoid receptors (CB1) and activates the HPA axis, leading to increased levels of cortisol (Pagotto et al., 2006). Notably, the hippocampus participates in the glucocorticoid negative feedback system for de-escalating stress response of the HPA axis (Sapolsky et al., 1990), and it expresses a high density of cannabinoid receptors (Egertova and Elphick, 2000). HPA axis function is most commonly assessed through measuring cortisol levels and a variety of studies suggest that cannabis use may have different effects on the HPA axis depending on the type of cortisol measure or experimental manipulation that is utilized (Huizink et al., 2006; King et al., 2011; Ranganathan et al., 2009; van Leeuwen et al., 2011). Increased cortisol levels in response to cannabis exposure in humans have been demonstrated in both lab based intravenous administration of Δ-9-THC and in recreational cannabis users (D’Souza et al., 2004; King et al., 2011; Ranganathan et al., 2009). Specifically, intravenous administration of increasing Δ-9-THC levels has been shown to raise plasma cortisol levels in a dose-dependent manner in healthy participants when cortisol was measured before and after the exposure to cannabis (Ranganathan et al., 2009). It has also been reported that chronic active cannabis users have higher basal salivary cortisol levels than non-users (King et al., 2011). Additionally, cannabis use has been associated with a blunted cortisol awakening response (CAR (Huizink et al., 2006; Monteleone et al., 2014), and lower HPA axis stress-reactivity (van Leeuwen et al., 2011). HPA axis dysregulation can have detrimental effects on mental health (Goodyer et al., 2001) and is specifically associated with chronic psychosis and UHR individuals (Carol and Mittal, 2015; Corcoran et al., 2003; Karanikas and Garyfallos, 2015).
As noted above, UHR individuals use cannabis at higher rates and frequency than controls, and this pattern is persistent over time (Addington et al., 2014; Buchy et al., 2015). Additionally, UHR individuals start using cannabis at a younger age and are more likely to use alone during the day when compared with their peers (Buchy et al., 2015). Research indicates that UHR individuals have more perceptual disturbances and worse functioning during periods of increased cannabis use (Corcoran et al., 2008), and positive symptoms are associated with the frequency of cannabis use (Buchy et al., 2015). Therefore, the relationship between cannabis use and psychosis conversion has received increasing attention; however, results focusing on conversion as an outcome measure remain inconclusive. A review of studies examining UHR individuals indicates that two of ten studies have found a significant relationship between cannabis use and the transition to psychosis (Addington et al., 2014). Additionally, a recent meta-analysis suggests a dose-response relationship between cannabis use and transition to psychosis as cannabis abuse and dependence, but not lifetime use, was associated with psychosis conversion (Kraan et al., 2016). This relationship remains complex (Rentzsch et al., 2016), and more research evaluating other domains outside of conversion is needed to better understand if and how cannabis use contributes to the transition to psychosis (Buchy et al., 2015).
Given that cannabis use affects the HPA axis (King et al., 2011; Ranganathan et al., 2009), and the high rates of cannabis use among UHR individuals (Addington et al., 2014), the examination of the relationship between cannabis use and HPA axis functioning could help clarify the role of cannabis as a risk factor for psychosis. Interestingly, in a study of healthy individuals, increasing intravenous doses of Δ-9-THC not only significantly increased cortisol levels, but also produced transient positive and negative symptoms, perceptual alterations, euphoria, anxiety, and cognitive deficits (D’Souza et al., 2004). Although patients with schizophrenia have a flattened cortisol awakening response after cannabis exposure (Monteleone et al., 2014), this relationship has not been examined in UHR individuals. Understanding the relationship between cannabis use and basal cortisol levels during the psychosis risk period has the potential to impact treatment efforts as cannabis use and HPA axis functioning could be targeted with specialized therapy.
In the present study, we evaluated if basal cortisol levels during the first half of the day in a laboratory setting are different across participant groups of UHR youth with Current Cannabis Use (UHR-CU), UHR youth with no current cannabis use (UHR-NC), and healthy control participants with no current cannabis use (HC). In addition, the association between cannabis use frequency and symptomatology was also examined. Based on prior research observing that cannabis use increases cortisol levels (Ranganathan et al., 2009), we predicted that the UHR-CU would exhibit higher cortisol levels compared to UHR-NC. Finally, on the basis of studies reporting a relationship between cannabis use and psychosis risk symptoms (Buchy et al., 2015), we also predicted that greater frequency of cannabis use would be associated with greater symptomatology (i.e. higher positive, negative, and disorganized symptoms).
2. Methods
A total of 75 participants (46 UHR and 29 controls) were divided into three groups: UHR with Current Cannabis Use (UHR-CU), UHR with no current cannabis use (UHR-NC), and healthy control participants with no current cannabis use (HC). Cannabis use was defined by the presence of THC in a urine screen and also by self-reported use of cannabis in the past month; both assessment strategies were employed to capture a range of more immediate and then longer-term use, respectively. Further, the urine panel helped to address concerns about over or under reporting (Carol and Mittal, 2014) and the self-report measure provided detailed data about frequency that was not possible to determine with the urine panel alone.
2.1 Participants
Participants were recruited at the Adolescent Development and Preventative Treatment (ADAPT) research program (see Table 1 for demographic characteristics of this sample). Adolescent UHR and control participants (mean age=18.4) were recruited by a range of methods including Craigslist, e-mail postings, newspaper ads, bus ads, and community professional referrals. A community professional referral was not a requirement for UHR participants. Exclusion criteria included history of head injury, the presence of a neurological disorder, and lifetime substance dependence. The presence of an Axis I psychotic disorder (e.g. schizophrenia, schizoaffective disorder, schizophreniform) was an exclusion criterion for UHR participants. Other comorbid Axis I disorders were not exclusion criteria for UHR participants. Rates of current comorbid Axis I disorders in the UHR participants included 11(24%) mood disorders, 4(9%) PTSD, 18(39%) other anxiety disorders, and 4(9%) ADHD. Comorbid Axis I disorders are typical of UHR individuals and the present rates are comparable to other studies (Fusar-Poli et al., 2014). UHR individuals met criteria for a prodromal syndrome including: (a) recent onset or escalation of moderate levels of attenuated positive symptoms (a score of 3–5), and/or (b) a decline in global functioning over the last 12 months accompanying the presence of schizotypal personality disorder (SPD), and/or (c) a decline in global functioning over the last 12 months accompanying the presence of a first-degree relative with a psychotic disorder such as schizophrenia (Miller et al., 1999). Due to differences observed in basal cortisol levels in UHR individuals treated with medication and medication-free UHR individuals (Day et al., 2014; Sugranyes et al., 2012), current neuroleptic medication was also an exclusionary criterion. Meeting for an Axis I disorder, the presence of a psychotic disorder in a first-degree relative, a positive urine screen for tetrahydrocannabinol (THC), or self-reported cannabis use in the past month were exclusionary criteria for HC. The protocol and informed consent procedures were approved by the Institutional Review Board. The authors would also like to note that 31 out of the 75 participants included in the present study participated in a previous study at the ADAPT research program (Carol et al., 2016).
TABLE 1.
| A. Participant Demographics & Symptom Characteristics for Cannabis Use in the Past Week (Urine) | |||||
|---|---|---|---|---|---|
|
| |||||
| Group | 1. UHR-CU | 2. UHR-NU | 3. HC | p-value | Group diff. |
| Sex | |||||
| Male | 9 | 12 | 13 | ||
| Female | 8 | 14 | 16 | ||
| Total | 17 | 26 | 29 | p=.860 | 1=2=3 |
| Age | |||||
| Mean years (SD) | 19.59(0.87) | 18.46(1.92) | 17.34(2.82) | p=.005 | 3>2*;2>3*;2=3 |
| Years of Education | |||||
| Mean years (SD) | 12.71(1.60) | 12.48(2.01) | 11.69(2.69) | p=.255 | 1=2=3 |
| Symptoms mean (SD) | |||||
| SIPS Positive | 13.53(4.00) | 11.04(5.04) | 0.76(1.27) | p < .001 | 1=2;1>3*;2>3* |
| SIPS Negative | 8.24(5.82) | 9.54(6.72) | 0.34(0.67) | p < .001 | 1=2;1>3*;2>3* |
| SIPS Disorganized | 6.29(2.80) | 4.62(3.56) | 0.23(0.51) | p < .001 | 1=2;1>3*;2>3* |
| Depression | 16.41(13.37) | 16.41(13.37) | 4.56(6.17) | p < .001 | 1=2;1>3*;2>3* |
| Anxiety | 21.29(14.96) | 21.29(14.96) | 4.72(6.26) | p < .001 | 1=2;1>3*;2>3* |
| B. Participant Demographics & Symptom Characteristics for Cannabis Use in the Past Month (Self-report) | |||||
|---|---|---|---|---|---|
|
| |||||
| Group | 1. UHR-CU | 2. UHR-NU | 3. HC | p-value | Group diff. |
| Sex | |||||
| Male | 16 | 7 | 13 | ||
| Female | 12 | 11 | 16 | ||
| Total | 28 | 18 | 29 | p = .438 | 1=2=3 |
| Age | |||||
| Mean years (SD) | 19.38(1.11) | 18.38(2.01) | 17.34(2.82) | p = .007 | 1=2;1>3*;2>3* |
| Years of Education | |||||
| Mean years (SD) | 12.74(1.62) | 12.47(2.08) | 11.69(2.69) | p = .232 | 1=2=3 |
| Symptoms mean (SD) | |||||
| SIPS Positive | 12.07(5.10) | 12.56(4.25) | 0.76(1.27) | p < .001 | 1=2;1>3*;2>3* |
| SIPS Negative | 8.39(6.22) | 10.78(6.19) | 0.34(0.67) | p < .001 | 1=2;1>3*;2>3* |
| SIPS Disorganized | 5.56(3.41) | 5.17(3.54) | 0.23(0.51) | p < .001 | 1=2;1>3*;2>3* |
| Depression | 16.54(12.14) | 18.59(12.59) | 4.56(6.17) | p < .001 | 1=2;1>3*;2>3* |
| Anxiety | 21.29(12.62) | 24.24(13.39) | 4.72(6.26) | p < .001 | 1=2;1>3*;2>3* |
| Cannabis Use | |||||
| Mean frequency (SD) | 3(1.49) | 0(0.00) | 0(0.00) | p < .001 | 1>2*,>3*;2=3 |
Note: Cannabis use groups in Table 1. A are defined by a urine panel and reflect use in the past week and cannabis use groups in Table 1. B are defined by self-reported use in the past month (UHR with Current Cannabis Use = UHR-CU, UHR with no current cannabis use = UHR-NC, and healthy control participants with no current cannabis use = HC); p value represents group difference across the 3 use groups (Chi square for sex, ANCOVA for all other variables), post hoc t-test are reported as Group diff where *p<.05; Positive, negative, Disorganized symptoms reflect the mean of total sums from domains from the Structured Interview for Prodromal Syndromes (SIPS); The Depression mean reflects the total sum form the depression domain of the Beck Depression Inventory (BDI); The Anxiety mean reflects the total sum form the anxiety domain of the Beck Anxiety Inventory (BAI); Cannabis use frequency reflects the mean frequency of cannabis use in the past month from the AUS/DUS scale 0 = “no use” to 5 = “almost daily.”
2.2 Symptom Assessment
The Structured Interview for Prodromal Syndromes (SIPS; Miller et al., 1999) was administered to diagnose a prodromal syndrome. As noted, UHR participants in the present study met criteria for a prodromal or high-risk syndrome. The SIPS gauges several distinct categories of prodromal symptom domains including positive, negative, and disorganized dimensions. A sum score for each category is used as an indicator of the respective dimensions of symptomatology. The Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID-IV; First, 1995) was also administered to rule out formal psychosis in UHR participants and an Axis I disorder in the control group (noted exclusionary criterion). This measure has been demonstrated to have excellent inter-rater reliability in adolescent populations (Martin et al., 2000) and has been used in several previous studies focusing on adolescent populations with schizophrenia spectrum disorders (Howes et al., 2009). Training of interviewers (who were advanced doctoral students) was conducted over a 2-month period, and inter-rater reliabilities exceed the minimum study criterion of Kappa ≥ .80. The Beck Depression Inventory (BDI, Beck et al., 1996) and the Beck Anxiety Inventory (BAI) were used to measure self-reported depressive and anxiety symptoms.
2.3 Cannabis
Cannabis use was measured through the rapid drug screen of a urine sample and the Alcohol/Drug Use Scale (AUS/DUS; Drake et al., 1996). A urine sample was screened for the presence of tetrahydrocannabinol (THC cutoff 50 ng/mL) utilizing Instant Technologies iCup (Norfolk, VA). The rapid drug screen has detection times up to one month and is commonly used in drug research (McRae-Clark et al., 2013). A study examining concordance between self-report and on-site urine screening for cannabis (using the same 50 ng/ml cutoff as the present investigation) in adolescents meeting criteria for abuse/dependence observed good consistency between urine panel results and self reported use in the last seven days (up to 94%) but noted that for reported use past one week, agreement dropped considerably (Buchan et al., 2002). Therefore, a positive urine screen was interpreted as indicating cannabis use in the past week. Urine analysis was not available for 3 participants due to either a problem with the screening process or the participant being unwilling to provide a sample.
The AUS/DUS scale (Drake et al., 1996) was used to measure cannabis use and frequency in the past month according to participant report. This scale is among the most widely used in UHR programs (Buchy et al., 2015; Woods et al., 2009) and found to be highly reliable in psychosis populations (ICCs≥.93; Brunette et al., 2006). The scale has good convergent (Wusthoff et al., 2011) as well as face validity, directly asking “Please rate your use of cannabis in the past 1 month according to the following scale: 0 = “no use” to 5 = “almost daily.”
2.4 Cortisol
Three saliva samples were collected in the laboratory setting over the course of 2 hours (every 60 minutes) and all participants were assessed between 8:45am and 2pm. Based on our prior studies, saliva was collected utilizing a passive-drool method (Carol and Mittal, 2015; Mittal et al., 2007; Mittal and Walker, 2011). The participants were not exposed to a stressor; the cortisol level indexed represents the participant’s cortisol secretion in the context of the novelty of the assessment. Participants were provided with written and verbal dietary instructions to observe the evening before and the morning of sampling. Instructions allowed a light breakfast but instructed participants to refrain from caffeine, alcohol, and nonprescription medications, as well as brushing teeth within 30 min prior to sampling. Subjects were questioned to confirm their compliance with the instructions and it was not necessary to exclude any participants on this basis. The saliva samples (75 μl) were stored in a −20C freezer until ready for assay. The Salimetrics High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics, LLC, College Park, PA) was used and following gold standard procedures, samples were subjected to duplicate analyses and the average of all points was calculated to yield the raw cortisol value. Participants with missing samples, samples with an extremely low value (<.007μg/dL; sensitivity cut-off value recommended by Salimetrics), and samples collected after 2pm were excluded from the study to avoid confounds with diurnal cycle.
2.5 Statistical approach
Independent t-tests and chi-square tests were employed to examine differences between groups in respective continuous and categorical demographic variables. Based on an equation provided by Pruessner et al. (2003), mean cortisol values for each of the three sample points were used to calculate the total area under the curve with respect to ground (AUCg). This method was utilized to examine cortisol levels as the measure takes into account both sensitivity (the difference between the single measurements from each other) and intensity (the distance of these measures from ground) (Pruessner et al., 2003). One-way ANCOVAs controlling for age were employed to examine overall differences in cortisol levels across cannabis groups. Age is often controlled for in studies examining cortisol levels in adolescent and young adult populations to avoid any possible effect of age (Carol and Mittal, 2015; Mittal et al., 2007; Walker et al., 2013), and further, the UHR group was slightly older in this study. Separate ANCOVAs were run to examine the effect of cannabis across groups defined by the urine panel and self-reported cannabis use in the past month (UHR-CU, UHR-NC, HC). Post hoc independent t-tests were used to measure group differences in cortisol. Pearson correlations controlling for age were used to examine the relationship between the frequency of cannabis use and cortisol levels. Bivariate correlations were used to examine the relationship between cannabis use frequency and positive/negative/disorganized symptoms. Spearman’s Rho correlations were employed to examine the association between cannabis use as defined by the urine screen and the noted variables of interest.
3. Results
3.1 Demographic and clinical characteristics
As noted, a total of 75 (46 UHR and 29 control) adolescents participated in the study. UHR participants were older [t(44)=2.90, p=.006] and there were no significant differences in years of education [t(44)=1.57, p=.123] or sex [χ2(1)=0.19, p=.813] between the UHR and control groups. As expected, the UHR group showed significantly more positive [t(55)=15.59, p<.001], negative [t(47)=9.65, p<.001], disorganized [t(47)=9.93, p<.001], depression [t(65)=5.72, p<.001], and anxiety [t(65)=7.74, p<.001], symptoms when compared with healthy controls. Demographic and clinical characteristics specific to UHR-CU, UHR-NC, and HC can be viewed in Table 1.
Rates of current comorbid Axis I disorders in the UHR-CU group (for both urine and self-report) included mood disorders: 6(35%)-urine, 7(25%)-self-report; PTSD: 1(6%)-urine, 1(4%)-self-report; other anxiety disorders: 5(29%)-urine, 8(29%)-self-report; and ADHD: 1(6%)-urine, 2(7%)-self-report. Rates for the UHR-NC group included mood disorders: 5(19%)-urine, 4(22%)-self-report; PTSD: 3(12%)-urine, 4(22%)-self-report; other anxiety disorders: 9(35%)-urine, 7(39%)-self-report; and ADHD: 3(12%)-urine, 2(11%)-self-report.
3.2 Cannabis group differences in cortisol
Independent of cannabis use, the UHR group as a whole showed significantly elevated resting cortisol levels when compared with the HC adolescents, [t(73)=3.37, p=.001]. The UHR group was then divided into UHR-CU and UHR-NC to evaluate cannabis use.
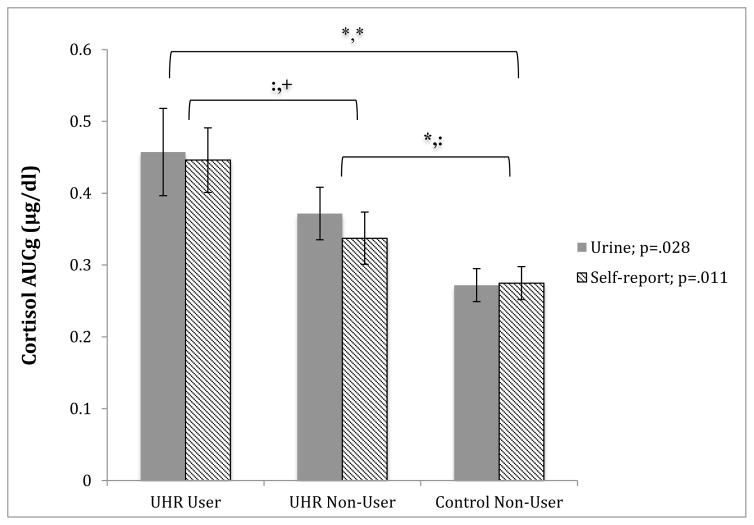
Based on the rapid drug screen of the urine sample, groups included 17 UHR-CU, and 26 UHR-NC and 29 HC participants screened negative. To examine overall differences in cortisol across these groups, a one-way ANCOVA with group (UHR-CU, UHR-NC, HC) as independent factors, cortisol as the dependent variable, and age as a covariate, was conducted. The analysis revealed a significant effect of cannabis use in the past week on cortisol after controlling for age [(F(2, 68)=3.77; p=.028)] (see Figure 1). Post hoc analysis showed no significant difference between UHR-CU and UHR-NC [t(41)=.796, p=.206]), and significant difference between UHR-NC and HC groups [t(53)=2.37, p=.022]), and UHR-CU and HC groups [t(21)=2.86, p=.010]).
Figure 1. Cannabis group differences in cortisol AUCg.
Group differences in cortisol levels for the overall sample. Cannabis use groups are defined by a urine screen (positive = use in the past week) and by self-report (positive = use in the past month); cortisol levels are presented as mean area under the curve with respect to ground (AUCg) for 3 samples of cortisol collected every 60 minutes over the course of 2 hours; vertical bars represent standard error of the mean; Urine and Self-report p value refers to the effect of cannabis use in the past week (urine) and the past month (self-report) on cortisol across groups controlling for age (ANCOVA); Significance of post hoc t-test indicate * p <.05, + p <.08,: p >.08, with urine reported first, followed by self-report.
The same analyses were conducted based on self-reported cannabis use in the past month. The AUS/DUS scale revealed 28 UHR-CU, 18 UHR-NC, and 29 HC. Taken together, the results were highly consistent with findings from the urine panel. One-way ANCOVA analysis indicated a significant effect of cannabis use in the past month on cortisol after controlling for age [(F(2,71)=4.79; p=.011)]. Post hoc analysis indicated a trend level difference between UHR-CU and UHR-NC [t(43)=1.88, p=.066]), no difference between UHR-NC and HC [t(45)=1.60, p=.117]), and significant difference between UHR-CU and HC [t(40)=3.45, p=.001]).
3.3 Associations between cannabis use frequency, cortisol, and symptoms
Analysis in the whole sample revealed a trend level association between the frequency of cannabis use in the past month (0 = “no use” to 5 = “almost daily”) and cortisol levels (r=.222, p=.057). There was no significant correlation in the UHR sample alone (r=.147; p=.336). Symptom associations were examined in the UHR sample. Cannabis use in the past week defined by the urine screen was not significantly associated with positive (rs=.23, p=.137) or (rs=−.08, p=.598) negative symptoms, and a significant associate with disorganized symptoms (rs=.31, p=.046) was observed. Self-reported frequency of cannabis use in the past month was also not related to positive (r=.09; p=.563), negative (r=−.20; p=.180), or disorganized symptoms (r=.10; p=.497).
4. Discussion
It is increasingly important to study cannabis use in UHR populations and examine specific factors that may contribute to HPA axis function and symptomatology, particularly in adolescence (Addington et al., 2014; Walker et al., 2008). To our knowledge, this is the first study to explore the relationship between cannabis use and basal cortisol in UHR adolescents. Consistent with previous research, our results show that UHR status alone, independent of cannabis use, is linked with elevated basal cortisol, when compared with HCs. Additionally, the results from the urine panel and self-report measure suggest that the use of cannabis in UHR individuals is associated with further elevations. Additionally, there was a trend level elevation when the UHR-NU group was compared to the HC group, and future studies are needed to determine if this is due to reduced power. While we cannot infer causality with this design (patients with higher levels of cortisol may be driven to seek out cannabis), taken together, the results highlight the potential that one mechanism by which cannabis may confer risk for psychosis is through an interaction with a HPA axis vulnerability.
As indicated, in examining the relationship between cannabis use, cortisol levels, and UHR status, results emerged indicating a significant effect of cannabis use on cortisol across groups as determined by both a urine screen and self-reported use. These findings are consistent with and inform the literature indicating that UHR individuals have higher basal cortisol levels compared to healthy controls in laboratory settings (Belvederi Murri et al., 2012; Carol and Mittal, 2015; Karanikas and Garyfallos, 2015). However, the root of stress hormone elevations in UHR individuals remains unclear as HPA dysregulation in this population has been linked to anxiety (Corcoran et al., 2012; Karanikas and Garyfallos, 2015), suspiciousness (Corcoran et al., 2012), stressful life events (Labad et al., 2014), and stress intolerance (Karanikas and Garyfallos, 2015; Pruessner et al., 2013). A better understanding of how cannabis use may be related to HPA dysfunction in UHR individuals could clarify inconsistencies as UHR individuals use cannabis at higher rates and frequency (Addington et al., 2014; Ksir and Hart, 2016), and the current results indicate that UHR individuals who use cannabis have higher cortisol levels. Future studies should also examine how cannabis use relates to other aspects of HPA axis function, as patients with schizophrenia experienced a flattened cortisol awakening response after cannabis exposure (Monteleone et al., 2014) and UHR individuals show blunted CAR and morning cortisol levels during the first hour after awakening in a home setting (Carol et al., 2016; Day et al., 2014).
The current study found that the frequency of cannabis use in the past month was positively associated with basal cortisol levels at a trend level of significance; therefore, results should be interpreted with caution. The direction of this trend result is consistent with prior work indicating that cannabis use is associated with higher cortisol levels in non-psychosis risk populations (D’Souza et al., 2004; King et al., 2011; Ranganathan et al., 2009). Additionally, chronic active cannabis users have higher basal salivary cortisol levels than non-users (King et al., 2011). Interestingly, there is also evidence indicating that chronic cannabis users show blunted cortisol reactivity (Ranganathan et al., 2009). Specifically, it has been demonstrated that intravenous administration of Δ-9-THC raises plasma cortisol levels in a dose-dependent manner, but frequent users show blunted increases relative to healthy controls (Ranganathan et al., 2009). As such, it has been theorized that chronic cannabis use could lead to the development of tolerance to the neuroendocrine effects of cannabis (Brown and Dobs, 2002; King et al., 2011; Ranganathan et al., 2009). Future longitudinal research specifically examining frequency of use in UHR individuals who are chronic daily users and lower levels of use is needed to better understand the possible implications of tolerance in this population. Additionally, the cannabis plant is immensely complex and can be bred to yield hundreds of strains, each with a unique cannabinoid profile (Lisdahl et al., 2014). Specifically THC and cannabidiol (CBD) are 2 cannabinoids that account for differences in plant varieties and a more nuanced understanding of the effects of these different cannabinoids in a UHR population could inform questions around tolerance (Hagerty et al., 2015). More longitudinal studies with large sample sizes, a greater range in usage rates, and more specificity on cannabinoid type are needed before definite conclusions can be made.
A significant relationship between cannabis use and positive and negative symptoms in our UHR group was not observed in the present study; however, a significant relationship with disorganized symptoms based on the urine panel was observed. This is inconsistent with previous research showing cannabis use was significantly associated with positive attenuated psychotic symptoms (Buchy et al., 2015). Additionally, longitudinal assessment has shown that UHR individuals with a baseline history of cannabis use experienced more perceptual disturbances and worse functioning during times of increased cannabis use (Corcoran et al., 2008), and UHR users have more basic symptoms compared to UHR youth who do not use cannabis (Korver et al., 2010). Interestingly, a study investigating self-reported reasons for cannabis use by UHR individuals yielded enhancement of mood and social motives as primary reasons for cannabis use, whereas motivation for symptom relief was rare (Gill et al., 2015). Using cannabis for mood and social enhancement is consistent with reported reasons for use in first episode psychosis (Pencer and Addington, 2008), and the author’s interpretations were that negative symptoms might drive cannabis use in UHR individuals (Gill et al., 2015). In the current study neuroleptic medication was an exclusion criterion for UHR individuals. Therefore, it is possible that our UHR group was less symptomatic compared to groups in previous studies, making it difficult to observe relationships with symptoms. Future studies are needed to better understand this relationship between cannabis use and symptomatology and possible causal influences.
This is the first study to examine the relationship between cannabis use and HPA axis dysfunction in UHR individuals and the current results add to the understanding of potential environmental and biological risk factors of psychosis (Pruessner et al., 2017; Walker et al., 2008). There is currently much debate and uncertainty around the causal role that cannabis use may play in the development of psychotic disorders (Buchy et al., 2015). A number of large longitudinal studies find that baseline cannabis use does not predict psychosis onset (Auther et al., 2012; Buchy et al., 2015; Buchy et al., 2014; Phillips et al., 2002); however, there is alternative evidence suggesting that cannabis use increases risk for psychotic outcomes (Moore et al., 2007). It is clear that early and heavy use of cannabis is more common in UHR individuals and the role and reasons for this use pattern is, at this point, uncertain (Ksir and Hart, 2016). A neural diathesis-stress model posits that an early biological vulnerability of the HPA system later interacts in the adolescent period with individual factors and environmental stressors, as well as normative and pathological neuroendocrine development, eventually leading to the onset of psychotic disorders such as schizophrenia (Pruessner et al., 2017; Walker et al., 2008). The present findings support this model and suggest that cannabis use could be an environmental stressor that interacts with underlying biological vulnerability of the HPA system in UHR individuals. However, future longitudinal studies are needed to better understand the causal relationship between these factors and to help the field understand how cannabis use is tied to the neuroendocrine abnormalities in part driving the disorder. This future work should specifically focus on understanding cannabis use and its potential links to etiology pathophysiology, compensatory strategies, disease consequences, or epiphenomenon (Appiah-Kusi et al., 2016; Hill and Tasker, 2012).
4.1 Limitations
The current approach has several strengths including use of both a urine screen and self-report to measure cannabis use, a conservative assessment of cortisol (i.e., controlling for time/diet/exercise/contraceptives), and exclusion of individuals treated with neuroleptic medications. However, there were also several limitations that should be noted. While we were able to recruit UHR individuals who use cannabis, a history of lifetime substance dependence was an exclusion criteria and this limited our ability to evaluate chronic use. Future studies with larger samples and more chronic cannabis use should be conducted to further investigate the possible impact of tolerance. It should also be noted that our sample size is small and the cohorts were not age matched. Although our sample size is comparable to other studies conducted in the UHR population (Corcoran et al., 2012; Pruessner et al., 2013) and we controlled for age in our analysis, future studies with larger samples, and more power to control for multiple comparisons, should be conducted before any definitive conclusions are made. Larger studies should also examine sex-differences in cannabis use and cortisol levels as sex-differences in morning cortisol samples collected in home samples in UHR youth and in patients with schizophrenia have been reported (Carol et al., 2016; Pruessner et al., 2008). Additionally, while the current methods used to collect and analyze cortisol levels are consistent with previous examinations (Mittal and Walker, 2011; Walker et al., 2013), future studies that collect more cortisol samples across the day, especially the cortisol awakening response (CAR), and over consecutive days are needed to better understand cannabis use and the 24-hour circadian cycle of the HPA axis in UHR youth.
An additional limitation of the study was the absence of a HC group who uses cannabis. Future studies that include a HC user group are needed before we can fully understand this phenomenon, to provide further insight on dose dependent relationships, and to more comprehensively evaluate an interaction between cannabis and psychosis vulnerability status. Similarly, the present study was only able to exclude individuals treated with neuroleptic medication and there was a small minority of UHR participants being treated naturalistically in the community with other psychotropic medications (UHR-CU=4 participants; UHR-NC=4 participants). Relatedly, the present study did not have exclusion criteria regarding potential endocrinological disorders, inflammatory conditions, or medications involving corticosteroid, which impact on HPA axis function. Therefore, it is important for future studies with larger sample sizes to evaluate the potential impact of a variety of psychotropic medications and these noted medical conditions and treatments. While causation could not be determined in the current study, this is the first step in evaluating the effect of cannabis use on the HPA axis in UHR individuals. Additionally, the design of current study does not allow for examination of UHR youth transition to psychosis rates. Future longitudinal studies, predicting change in cortisol over time on the basis of cannabis usage, are needed to determine if cannabis use moderates symptom and HPA dysfunction progression and the relationship to psychosis transition rates.
4.2 Conclusions
In conclusion, the results from the current study are the first to show UHR-CU have higher cortisol levels than UHR-NC and HC. A significant difference between UHR-CU and HC participants was present for both cannabis use in the past week based on the urine sample and cannabis use in the past month based on self-report. Higher frequency of cannabis use in the past month was related at a trend level to higher cortisol levels. Among UHR individuals, no relationships between frequency of cannabis use and positive, negative, or disorganized symptoms were observed. These novel results augment the field’s understanding of cannabis use as a risk factor of psychosis.
Highlights.
Group differences in cortisol levels among cannabis users/non-users were examined.
Groups included psychosis risk users, non-users, and healthy control non-users
Cannabis use was assessed with a urine panel and self-report.
Psychosis risk cannabis users had the highest salivary cortisol levels.
Footnotes
Disclosures: Dr. Mittal is a consultant for Takeda Pharmaceuticals. The authors have no potential conflicts of interests to report. This work was supported by the National Institutes of Health (RO1MH094650) and R21/R33MH103231 to V.A.M.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Case N, Saleem MM, Auther AM, Cornblatt BA, Cadenhead KS. Substance use in clinical high risk for psychosis: a review of the literature. Early Interv in Psychiatr. 2014;8:104–112. doi: 10.1111/eip.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello G, Horowitz M, Hepgul N, Pariante CM, Mondelli V. Stress abnormalities in individuals at risk for psychosis: a review of studies in subjects with familial risk or with “at risk” mental state. Psychoneuroendocr. 2012;37:1600–1613. doi: 10.1016/j.psyneuen.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Appiah-Kusi E, Leyden E, Parmar S, Mondelli V, McGuire P, Bhattacharyya S. Abnormalities in neuroendocrine stress response in psychosis: the role of endocannabinoids. Psychol Med. 2016;46:27–45. doi: 10.1017/S0033291715001786. [DOI] [PubMed] [Google Scholar]
- Auther AM, McLaughlin D, Carrion RE, Nagachandran P, Correll CU, Cornblatt BA. Prospective study of cannabis use in adolescents at clinical high risk for psychosis: impact on conversion to psychosis and functional outcome. Psychol Med. 2012;42:2485–2497. doi: 10.1017/S0033291712000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the BDI-II. 1996. [Google Scholar]
- Belvederi Murri M, Pariante CM, Dazzan P, Hepgul N, Papadopoulos AS, Zunszain P, Di Forti M, Murray RM, Mondelli V. Hypothalamic-pituitary-adrenal axis and clinical symptoms in first-episode psychosis. Psychoneuroendocr. 2012;37:629–644. doi: 10.1016/j.psyneuen.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Brown TT, Dobs AS. Endocrine effects of marijuana. J of Clin Pharmacol. 2002;42:90S–96S. doi: 10.1002/j.1552-4604.2002.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Brunette MF, Drake RE, Xie H, McHugo GJ, Green AI. Clozapine use and relapses of substance use disorder among patients with co-occurring schizophrenia and substance use disorders. Schizophr Bull. 2006;32:637–643. doi: 10.1093/schbul/sbl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan BJ, Dennis LM, Tims FM, Diamond GS. Cannabis use: consistency and validity of self-report, on-site urine testing and laboratory testing. Addict. 2002;97:98–108. doi: 10.1046/j.1360-0443.97.s01.1.x. [DOI] [PubMed] [Google Scholar]
- Buchy L, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Heinssen R, Bearden CE, Mathalon D, Addington J. Substance use in individuals at clinical high risk of psychosis. Psychol Med. 2015;45:2275–2284. doi: 10.1017/S0033291715000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchy L, Perkins D, Woods SW, Liu L, Addington J. Impact of substance use on conversion to psychosis in youth at clinical high risk of psychosis. Schizophr Res. 2014;156:277–280. doi: 10.1016/j.schres.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol EE, Mittal VA. Self-reported cannabis use is inconsistent with the results from drug-screening in youth at ultra high-risk for psychosis in Colorado. Schizophr Res. 2014;157:317–318. doi: 10.1016/j.schres.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol EE, Mittal VA. Resting cortisol level, self-concept, and putative familial environment in adolescents at ultra high-risk for psychotic disorders. Psychoneuroendocr. 2015;57:26–36. doi: 10.1016/j.psyneuen.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol EE, Spencer RL, Mittal VA. Sex differences in morning cortisol in youth at ultra-high-risk for psychosis. Psychoneuroendocr. 2016;72:87–93. doi: 10.1016/j.psyneuen.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Corcoran CM, Kimhy D, Stanford A, Khan S, Walsh J, Thompson J, Schobel S, Harkavy-Friedman J, Goetz R, Colibazzi T, Cressman V, Malaspina D. Temporal association of cannabis use with symptoms in individuals at clinical high risk for psychosis. Schizophr Res. 2008;106:286–293. doi: 10.1016/j.schres.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, Smith C, McLaughlin D, Auther A, Malaspina D, Cornblatt B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr Res. 2012;135:170–174. doi: 10.1016/j.schres.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacol: off publ of the Am Coll of Neuropsychopharmacol. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Day FL, Valmaggia LR, Mondelli V, Papadopoulos A, Papadopoulos I, Pariante CM, McGuire P. Blunted cortisol awakening response in people at ultra high risk of developing psychosis. Schizophr Res. 2014;158:25–31. doi: 10.1016/j.schres.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Drake R, Mueser KT, McHuge GJ. Clinical Rating Scales: Alcohol Use Scale (AUS), Drug use Scale (DUS), and Substance Abuse Treatment Scale (SATS) In: Sederer L, Dickey B, editors. Outcomes Assessment in Clinical Practice. Williams and Wilkins; Baltimnore: 1996. [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. The J of Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I), Patient Edition. American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull. 2014;40:120–131. doi: 10.1093/schbul/sbs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Poe L, Azimov N, Ben-David S, Vadhan NP, Girgis R, Moore H, Cressman V, Corcoran CM. Reasons for cannabis use among youths at ultra high risk for psychosis. Early Interv in Psychiatr. 2015;9:207–210. doi: 10.1111/eip.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. The Br J of Psychiatr: the J of Ment Sci. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Hagerty SL, Williams SL, Mittal VA, Hutchison KE. The cannabis conundrum: Thinking outside the THC box. J of Clin Pharmacol. 2015;55:839–841. doi: 10.1002/jcph.511. [DOI] [PubMed] [Google Scholar]
- Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neurosci. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated Striatal Dopamine Function Linked to Prodromal Signs of Schizophrenia. Arch of Gen Psychiatr. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Ferdinand RF, Ormel J, Verhulst FC. Hypothalamic–pituitary–adrenal axis activity and early onset of cannabis use. Addict. 2006;101:1581–1588. doi: 10.1111/j.1360-0443.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- Karanikas E, Antoniadis D, Garyfallos GD. The role of cortisol in first episode of psychosis: a systematic review. Curr Psychiatr Rep. 2014;16:503. doi: 10.1007/s11920-014-0503-7. [DOI] [PubMed] [Google Scholar]
- Karanikas E, Garyfallos G. The role of cortisol in At Risk for Psychosis mental state and psychopathological correlates: a systematic review. Psychiatr and Clin Neurosci. 2015;69:268–282. doi: 10.1111/pcn.12259. [DOI] [PubMed] [Google Scholar]
- King GR, Ernst T, Deng W, Stenger A, Gonzales RM, Nakama H, Chang L. Altered brain activation during visuomotor integration in chronic active cannabis users: relationship to cortisol levels. The J of Neurosci: the Off J of the Soc for Neurosci. 2011;31:17923–17931. doi: 10.1523/JNEUROSCI.4148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korver N, Nieman DH, Becker HE, van de Fliert JR, Dingemans PH, de Haan L, Spiering M, Schmitz N, Linszen DH. Symptomatology and neuropsychological functioning in cannabis using subjects at ultra-high risk for developing psychosis and healthy controls. The Aust and N Z J of Psychiatr. 2010;44:230–236. doi: 10.3109/00048670903487118. [DOI] [PubMed] [Google Scholar]
- Kraan T, Velthorst E, Koenders L, Zwaart K, Ising H, van den Berg D, de Haan L, van der Gaag M. Cannabis use and transition to psychosis in individuals at ultra-high risk: review and meta-analysis. Psychol Med. 2016;46:673–681. doi: 10.1017/S0033291715002329. [DOI] [PubMed] [Google Scholar]
- Ksir C, Hart CL. Cannabis and Psychosis: a Critical Overview of the Relationship. Curr Psychiatr Rep. 2016;18:12. doi: 10.1007/s11920-015-0657-y. [DOI] [PubMed] [Google Scholar]
- Labad J, Stojanovic-Perez A, Montalvo I, Sole M, Cabezas A, Ortega L, Moreno I, Vilella E, Martorell L, Reynolds RM, Gutierrez-Zotes A. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: Roles for cortisol, prolactin and albumin. J of psychiatr Res. 2014;60C:163–169. doi: 10.1016/j.jpsychires.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, Shollenbarger S. Considering Cannabis: The Effects of Regular Cannabis Use on Neurocognition in Adolescents and Young Adults. Curr Addict Rep. 2014;1:144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Pollock NK, Bukstein OG, Lynch KG. Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug Alcohol Depen. 2000;59:173–176. doi: 10.1016/s0376-8716(99)00119-2. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MM, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacol. 2013;228:623–631. doi: 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. The Psychiatr Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: Minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry. 2007;61:1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Walker EF. Minor physical anomalies and vulnerability in prodromal youth. Schizophr Res. 2011;129:116–121. doi: 10.1016/j.schres.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Di Filippo C, Fabrazzo M, Milano W, Martiadis V, Corrivetti G, Monteleone AM, Maj M. Flattened cortisol awakening response in chronic patients with schizophrenia onset after cannabis exposure. Psychiatr Res. 2014;215:263–267. doi: 10.1016/j.psychres.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. The Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- Pencer A, Addington J. Reasons for using substances in adolescents with and without psychosis. Early Interv in Psychiatr. 2008;2:42–44. doi: 10.1111/j.1751-7893.2007.00055.x. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Curry C, Yung AR, Pan Yuen H, Adlard S, Mcgorry PD. Cannabis use is not associated with the development of psychosis in an ‘ultra’high-risk group. Australian and New Zealand Journal of Psychiatry. 2002;36:800–806. doi: 10.1046/j.1440-1614.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocr. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Bechard-Evans L, Boekestyn L, Iyer SN, Pruessner JC, Malla AK. Attenuated cortisol response to acute psychosocial stress in individuals at ultra-high risk for psychosis. Schizophr Res. 2013;146:79–86. doi: 10.1016/j.schres.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Boekestyn L, Bechard-Evans L, Abadi S, Vracotas N, Joober R, Pruessner JC, Malla AK. Sex differences in the cortisol response to awakening in recent onset psychosis. Psychoneuroendocrinology. 2008;33:1151–1154. doi: 10.1016/j.psyneuen.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci and Biobehav Rev. 2017;73:191–218. doi: 10.1016/j.neubiorev.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, Braley G, Pittman B, Cooper T, Perry E, Krystal J, D’Souza DC. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacol. 2009;203:737–744. doi: 10.1007/s00213-008-1422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch J, Koller K, Kronenberg G. Letter to the Editor: Disentangling cause and effect in the relationship between cannabis and psychosis: are we there yet? Psychol Med. 2016:1–2. doi: 10.1017/S0033291716001434. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Armanini MP, Packan DR, Sutton SW, Plotsky PM. Glucocorticoid feedback inhibition of adrenocorticotropic hormone secretagogue release. Relationship to corticosteroid receptor occupancy in various limbic sites. Neuroendocr. 1990;51:328–336. doi: 10.1159/000125357. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Thompson JL, Corcoran CM. HPA-axis function, symptoms, and medication exposure in youths at clinical high risk for psychosis. J of Psychiatr Res. 2012;46:1389–1393. doi: 10.1016/j.jpsychires.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen AP, Creemers HE, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC. Hypothalamic-pituitary-adrenal axis reactivity to social stress and adolescent cannabis use: the TRAILS study. Addict. 2011;106:1484–1492. doi: 10.1111/j.1360-0443.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Ann Rev of Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH, Perkins DO, Seidman LJ, Tsuang MT, Cannon TD, McGlashan TH, Woods SW. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatr. 2013;74:410–417. doi: 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, McGlashan TH. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wusthoff LE, Waal H, Ruud T, Roislien J, Grawe RW. Identifying co-occurring substance use disorders in community mental health centres. Tailored approaches are needed. Nordic J of Psychiatr. 2011;65:58–64. doi: 10.3109/08039488.2010.489954. [DOI] [PubMed] [Google Scholar]