Abstract
Background
Accurate optical characterisation and removal of small adenomas (<10 mm) at colonoscopy would allow hyperplastic polyps to be left in situ and surveillance intervals to be determined without the need for histopathology. Although accurate in specialist practice the performance of narrow band imaging (NBI), colonoscopy in routine clinical practice is poorly understood.
Methods
NBI-assisted optical diagnosis was compared with reference standard histopathological findings in a prospective, blinded study, which recruited adults undergoing routine colonoscopy in six general hospitals in the UK. Participating colonoscopists (N=28) were trained using the NBI International Colorectal Endoscopic (NICE) classification (relating to colour, vessel structure and surface pattern). By comparing the optical and histological findings in patients with only small polyps, test sensitivity was determined at the patient level using two thresholds: presence of adenoma and need for surveillance. Accuracy of identifying adenomatous polyps <10 mm was compared at the polyp level using hierarchical models, allowing determinants of accuracy to be explored.
Findings
Of 1688 patients recruited, 722 (42.8%) had polyps <10 mm with 567 (78.5%) having only polyps <10 mm. Test sensitivity (presence of adenoma, N=499 patients) by NBI optical diagnosis was 83.4% (95% CI 79.6% to 86.9%), significantly less than the 95% sensitivity (p<0.001) this study was powered to detect. Test sensitivity (need for surveillance) was 73.0% (95% CI 66.5% to 79.9%). Analysed at the polyp level, test sensitivity (presence of adenoma, N=1620 polyps) was 76.1% (95% CI 72.8% to 79.1%). In fully adjusted analyses, test sensitivity was 99.4% (95% CI 98.2% to 99.8%) if two or more NICE adenoma characteristics were identified. Neither colonoscopist expertise, confidence in diagnosis nor use of high definition colonoscopy independently improved test accuracy.
Interpretation
This large multicentre study demonstrates that NBI optical diagnosis cannot currently be recommended for application in routine clinical practice. Further work is required to evaluate whether variation in test accuracy is related to polyp characteristics or colonoscopist training.
Trial registration number
The study was registered with clinicaltrials.gov (NCT01603927).
Keywords: ENDOSCOPY, COLONOSCOPY, COLORECTAL ADENOMAS, COLONIC NEOPLASMS
Significance of this study.
What is already known on this subject?
A review of the available literature evaluating the accuracy of narrow band imaging (NBI)–assisted optical diagnosis compared with histological assessment and recommendations from national bodies suggested that optical diagnosis could replace histology for diminutive polyps. Additionally, exploratory work has suggested a short learning curve for NBI-assisted optical diagnosis, making it an attractive option that could be applied widely into clinical practice, if minimal training was required. Notably, the majority of studies were performed by experts in the field of optical diagnosis or in academic centres with limited data from non-expert centres suggesting that the accuracy may not be consistently reproducible in non-expert hands. Confirming whether NBI-assisted optical diagnosis can reproducibly achieve the required level of accuracy is one of the most pressing questions within the field of GI endoscopy. It is essential to establish its accuracy before recommending its use in routine clinical practice.
What are the new findings?
This is the largest multicentre diagnostic study in this field. The study demonstrates that NBI-assisted optical diagnosis cannot currently be recommended for routine use outside of expert centres. The accuracy, both at polyp and patient level, was substantially below recommended levels. Importantly, polyp level analyses identified that accuracy was acceptable when two or more of the features of the NBI International Colorectal Endoscopic (NICE) classification system were positively identified. Possible explanations are that not all polyps exhibit NICE characteristics or that colonoscopists vary in their ability to identify these characteristics.
How might it impact on clinical practice in the foreseeable future?
The results of this study confirm that optical diagnosis cannot be recommended for use in routine clinical practice. Further research is required to understand what factors influence the reported variation in the accuracy of NBI-assisted optical diagnosis in this study. This research should focus on polyp and colonoscopist characteristics and training methods.
Introduction
Colorectal cancer (CRC) is a leading cause of morbidity and mortality in the Western world.1
Most CRCs develop from adenomas in a well-described adenoma–carcinoma genetic sequence.2 Colonoscopy with polypectomy interrupts this sequence, reducing the rate of subsequent CRC and associated mortality by 40–60%.3 4 Consequently, national bowel cancer screening programmes (BCSPs) have been developed, with over 14 million screening colonoscopies performed annually in the USA alone.5 Improved training, technology and awareness of colonoscopic quality have led to increased polyp detection rates. Over 90% of polyps detected at colonoscopy are small (6–9 mm) or diminutive (≤5 mm), with the latter forming the majority.6 7 Cancer risk or advanced features (villous elements or high-grade dysplasia) particularly in diminutive polyps is low.8 The incidence of dysplastic serrated polyps, thought to be precursors of cancer via an alternative pathway, is lower still, 0.3–0.5%.6 7 9
Approximately half of small polyps are non-neoplastic with the majority of these being hyperplastic6 7; therefore, many polypectomies are performed unnecessarily, increasing procedure-related risks such as bleeding and perforation. Currently, even diminutive polyps are resected and examined histologically. The number of adenomas detected is one of the best determinants of long-term risk of advanced neoplasia and informs surveillance decision-making. Diagnosing small polyps by optical diagnosis would allow recto-sigmoid hyperplastic polyps, with no malignant potential, to be diagnosed and left in situ and small adenomas to be resected and discarded without histopathology. Additionally, a positive diagnosis could be made for small polyps not retrieved or unsuitable for histological analysis.10 Optical diagnosis would enable immediate determination of surveillance intervals, with associated time and cost savings.
Traditional white light technology, used at routine colonoscopy, is not accurate enough for optical diagnosis to replace routine histopathological assessment. However, a number of image-enhancing, user-friendly technologies have been developed. NBI (Olympus, Japan) has been the most widely studied. It is a ‘blue light’ optical imaging modality operated by a button on the colonoscope that, by enhancing mucosal detail and vascular structures, allows assessment of microvascular density.11 Neoplastic tissue is characterised by increased angiogenesis making adenomas appear darker using NBI.12 The learning curve to accurately assess microvascularity appears to be short,13 making it an attractive and practical option for optical diagnosis.
A large meta-analysis of 56 studies using NBI for optical diagnosis found overall sensitivity to be 91.0% (95% CI 88.6–93.0%), specificity 85.6% (95% CI 81.3–89.0%) and negative predictive value of 82.5% (95% CI 75.4–87.9%).14 Another systematic review and meta-analysis of optical diagnosis for diminutive polyps suggested that accuracy was higher in academic centres and when performed by experienced endoscopists; however, only 3 of 20 NBI studies were undertaken in non-academic settings.15
Detect Inspect Characterise Resect and Discard (DISCARD) 2 was designed to determine whether clinical management based on NBI-assisted optical diagnosis is accurate in routine clinical practice outside academic centres.
Methods
Study design
A UK multicentre, prospective, blinded study comparing surveillance intervals determined by NBI-assisted optical diagnosis and histological assessment in patients referred for colonoscopy.
Hypotheses
NBI-assisted optical diagnosis correctly characterises small colonic polyps as adenomas or hyperplastic, allowing assignment of surveillance intervals with 95% sensitivity compared with histological assessment.
Patients
Adult patients referred for non-emergency colonoscopy (symptomatic referrals and Faecal Occult Blood positive (FOBT) BCSP referrals) between July 2012 and February 2014 were invited to participate and written informed consent obtained. All patients entered Phase 1 of the study, undergoing colonoscopy following standard clinical practice. Patients found to have one or more polyps <10 mm in size entered Phase 2 of the study. Patients with known IBD (UC or Crohn's disease), polyposis syndromes, pregnancy or lack of capacity to give informed consent were excluded.
Setting
Six NHS hospitals in the North of England participated, with a maximum of five recruiting colonoscopists per site. The UK NHS BCSP offers colonoscopy to patients between 60 and 74 years of age with evidence of faecal occult blood, with colonoscopy performed by accredited screening colonoscopists. BCSP colonoscopists may represent a particularly specialised population of endoscopists: to provide generalisable results a maximum of two BCSP colonoscopists were allowed per site.
Training
Colonoscopists underwent training and assessment on the use of NBI in polyp characterisation using a previously validated NBI training module, including the use of the NICE classification16 (table 1). Colonoscopists had to achieve 90% accuracy for optical diagnosis in the post-training test, with two attempts allowed.12 All procedures were performed using Olympus equipment (Olympus Lucera or Elite processors and 240 or 260 series endoscopes).
Table 1.
NICE classification
| Polyp classification using NBI |
||
|---|---|---|
| Colour | (S) Same or lighter than the background mucosa | (B) Browner relative to background mucosa |
| Vessels | (N) None or isolated lacy vessels | (T) Thick brown vessels surrounding white structures* |
| Surface pattern | (D) Dark or white spots of uniform size or homogeneous absence of pattern | (O) Oval, tubular or branched white structures* surrounded by brown vessels |
| Most likely pathology | Hyperplastic | Adenoma |
*These structures may represent the pits and the epithelium of the crypt opening.
NBI, narrow band imaging; NICE, NBI International Colorectal Endoscopic.
NBI-assisted optical assessment
During colonoscopy, polyps <10 mm were evaluated with both white light and NBI. Polyp site, size (measured using an instrument of known size), morphology (Paris Classification)17 and resection method were recorded. Using NBI and NICE classification (table 1), colonoscopists documented polyp colour, microvessel type and surface pattern, and classified each as adenoma, hyperplastic, cancer or other. Colonoscopists also recorded their diagnostic confidence as high or low. High confidence indicated hypothetically that the colonoscopist would have discarded the polyp without histological assessment, while low confidence indicated sending the polyp for histology. Confidence was considered during polyp level analysis only. Where all polyps identified in a patient were <10 mm, a surveillance interval (using the British Society for Gastroenterology (BSG) guidelines) based on optical diagnosis was assigned and recorded.18 All polyps were resected by snare polypectomy or by excision biopsy removal and sent for histological assessment. However, if multiple rectal hyperplastic polyps were found, endoscopists were not required to remove all and removal or sampling was done for the first five only. Colonoscopists were given feedback on their optical diagnosis accuracy after every 30 polyps assessed and were informed how well their optical diagnosis correlated with histopathology at per polyp level. No additional training was given during the study period. Endoscopists were not required to differentiate sessile serrated polyps (SSPs).
Histology
Histological assessment using standard H&E staining was performed. Histopathologists classified specimens according to WHO guidelines, blinded to endoscopic images and assessments. A subset from each centre was reviewed by an external specialist GI pathologist. Histological results were returned to lead investigators who, blinded to colonoscopic findings, assigned surveillance intervals per patient providing the reference standard for surveillance. All retrieved polyps <10 mm were characterised by NBI-assisted optical diagnosis and histology, with histological findings providing the reference standard for assessing optical diagnosis accuracy. All polyps <10 mm were included in polyp-level assessment whether patients had larger polyps or not.
Outcome measures
Patient-level test sensitivity was assessed at two thresholds comparing optical diagnosis with the histology reference standard:
Presence of an adenoma (including high-risk, intermediate-risk and low-risk findings).
Need for surveillance (where some low-risk patients are judged not to require surveillance).
Additionally, three further definitions of test accuracy were assessed:
Exact surveillance interval (precise agreement of surveillance interval by optical diagnosis and histology).
Conservative matching (correctly identifying or overspecifying need for surveillance)
Error rate (false-positive or false-negative diagnosis of adenoma)
In addition to patient-level analyses, factors influencing the accuracy of diagnosis were explored at the polyp level, including patient, organisational, colonoscopist and polyp variables.
Adverse events
Although there are no known complications of NBI-optical diagnosis, patients were monitored for procedure-related side effects and complications. Adverse events were recorded for 30 days post-procedure in phase II patients.
Sample size
The study was designed to estimate the test sensitivity of 95% (with 95% CI ±2.5%), based on 290 patients with at least one adenoma (<10 mm) but only small or diminutive polyps requiring determination of a surveillance interval. Using data from DISCARD and audit,19 an initial phase I sample size of 2500 was estimated based on 20% of patients having only small or diminutive polyps (500); 70% of small polyps anticipated to be adenomas (350) and up to 15% of patients having incomplete histology.20 An interim review indicated that significantly more than 20% of patients had polyps; therefore, the sample size was revised to 1400 phase I patients. Exceeding target recruitment with 1700 patients ensured eligibility criteria for the primary outcome were achieved.
Statistical analyses
Test performance was estimated using proportions with CIs (Clopper–Pearson) using STATA IC V.13.1 StataCorp. Exploration of variables at polyp level was performed using xtlogit, where proportions were estimated from models using reported ORs. Modelling provided a hierarchical structure of polyp within patient; population average estimates were used (to prevent overweighting by patients with more numerous polyps) and reported using robust SEs.
Ethical approval
The study was given a favourable ethical opinion by UK National Research Ethics Committee North East-Newcastle and North Tyneside. Approval was gained from the NHS BCSP Research Committee. A study steering committee provided study oversight. The study was registered with clinicaltrials.gov (NCT01603927). Study reporting followed the STARD statement (http://www.stard-statement.org).
Results
Patients
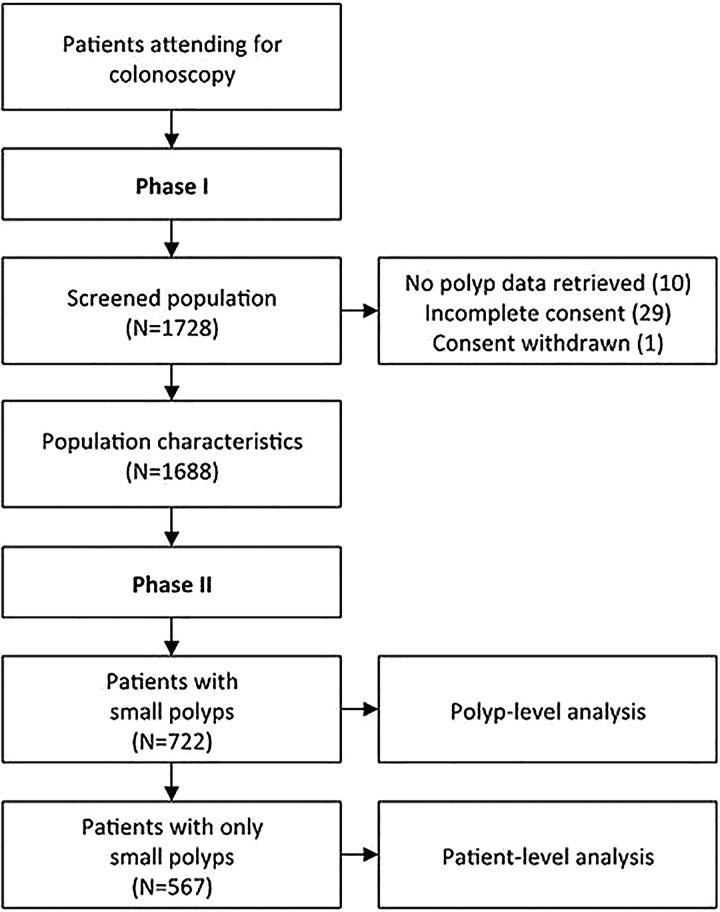
Between July 2012 and February 2014, 1688 patients referred for colonoscopy were recruited into phase I across the six participating hospitals: 722 patients (42.8%) had small or diminutive polyps with 567 (78.5%) having only polyps <10 mm (figure 1). Mean patient age was 64.3 years (IQR 55.0 to 70.2) and 53.1% were male. Patients were colonoscoped using high definition (HD, 22%) or standard definition (SD, 78%) imaging. Table 2 reports factors associated with higher polyp detection levels at the patient level. The only comorbidity significantly more common in patients with polyps was diabetes mellitus (20.3% vs 10.4%, p<0.001).
Figure 1.
Patient flowchart.
Table 2.
Screened cohort characteristics
| Patients with no polyps |
Patients with small polyps |
Patients with other findings |
Total patients |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N=883 | Per cent | N=567 | Per cent | N=238 | Per cent | N=1688 | Per cent | p Value* | |
| Polyps | |||||||||
| No polyps | 883 | 100.0 | 0 | 0.0 | 0 | 0.0 | 883 | 52.3 | <0.001 |
| Only small polyps (<10 mm) | 0 | 0.0 | 567 | 100.0 | 0 | 0.0 | 567 | 33.6 | |
| Small and large polyps | 0 | 0.0 | 0 | 0.0 | 150 | 63.0 | 150 | 8.9 | |
| Only large polyps | 0 | 0.0 | 0 | 0.0 | 58 | 24.4 | 58 | 3.4 | |
| Polyps and other findings | 0 | 0.0 | 0 | 0.0 | 16 | 6.7 | 16 | 0.9 | |
| No polyps, cancer suspected | 0 | 0.0 | 0 | 0.0 | 14 | 5.9 | 14 | 0.8 | |
| Incomplete colonoscopy | 54 | 6.1 | 1 | 0.2 | 9 | 3.8 | 64 | 3.8 | <0.001 |
| Discomfort-tortuosity related | 32 | 59.3 | 0 | 0.0 | 7 | 77.8 | 39 | 60.9 | 0.011 |
| Preparation-related | 21 | 38.9 | 0 | 0.0 | 1 | 11.1 | 22 | 34.4 | |
| Other | 1 | 1.9 | 1 | 100.0 | 1 | 11.1 | 3 | 4.7 | |
| Site | |||||||||
| County Durham and Darlington | 260 | 29.4 | 164 | 28.9 | 61 | 25.6 | 485 | 28.7 | <0.001 |
| North Cumbria | 38 | 4.3 | 28 | 4.9 | 9 | 3.8 | 75 | 4.4 | |
| North Tees and Hartlepool | 166 | 18.8 | 156 | 27.5 | 88 | 37.0 | 410 | 24.3 | |
| Northumbria | 70 | 7.9 | 37 | 6.5 | 24 | 10.1 | 131 | 7.8 | |
| South Tees | 193 | 21.9 | 65 | 11.5 | 20 | 8.4 | 278 | 16.5 | |
| South Tyneside | 156 | 17.7 | 117 | 20.6 | 36 | 15.1 | 309 | 18.3 | |
| Age (years); median, IQR | 62.2 | 49.8–68.3 | 65.9 | 60.0–70.4 | 66.7 | 62.3–72.4 | 64.3 | 55.0–70.2 | <0.001 |
| Gender | |||||||||
| Female | 488 | 55.3 | 218 | 38.4 | 86 | 36.1 | 792 | 46.9 | <0.001 |
| Male | 394 | 44.7 | 349 | 61.6 | 152 | 63.9 | 895 | 53.1 | |
| Ethnicity | |||||||||
| White—British | 866 | 99.3 | 560 | 98.8 | 235 | 99.6 | 1661 | 99.2 | 0.218 |
| Asian or Asian British | 5 | 0.6 | 2 | 0.4 | 0 | 0.0 | 7 | 0.4 | |
| Black or Black British | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.1 | |
| Chinese or other ethnic groups | 1 | 0.1 | 1 | 0.2 | 1 | 0.4 | 3 | 0.2 | |
| White—other | 0 | 0.0 | 3 | 0.5 | 0 | 0.0 | 3 | 0.2 | |
| Smoking | |||||||||
| Current smoker | 142 | 16.3 | 122 | 21.5 | 40 | 16.9 | 304 | 18.2 | <0.001 |
| Previous smoker | 321 | 36.9 | 250 | 44.1 | 113 | 47.7 | 684 | 40.9 | |
| Never smoked | 407 | 46.8 | 195 | 34.4 | 84 | 35.4 | 686 | 41.0 | |
| Alcohol use | 522 | 60.1 | 365 | 64.5 | 166 | 69.7 | 1053 | 63.0 | 0.016 |
| Units/week (users); median, IQR | 9.0 | 4.0–16.0 | 14.0 | 6.0–23.5 | 14.0 | 6.0–27.0 | 11.0 | 5.0–20.0 | <0.001 |
| Taking any regular medication | 720 | 81.5 | 499 | 88.0 | 198 | 83.5 | 1417 | 84.0 | 0.004 |
| Taking an NSAID | 81 | 11.2 | 38 | 7.6 | 11 | 5.5 | 130 | 9.1 | 0.018 |
| Taking a statin | 245 | 33.8 | 260 | 52.1 | 114 | 56.7 | 619 | 43.4 | <0.001 |
| Taking aspirin | 138 | 19.1 | 122 | 24.4 | 50 | 25.0 | 310 | 21.8 | 0.040 |
| Primary reason for colonoscopy | |||||||||
| BCSP | 162 | 18.3 | 190 | 33.5 | 124 | 52.1 | 476 | 28.2 | <0.001 |
| Change in bowel habit | 276 | 31.3 | 132 | 23.3 | 37 | 15.5 | 445 | 26.4 | <0.001 |
| Surveillance procedure | 122 | 13.8 | 137 | 24.2 | 34 | 14.3 | 293 | 17.4 | <0.001 |
| Per-rectal bleeding | 130 | 14.7 | 59 | 10.4 | 28 | 11.8 | 217 | 12.9 | 0.050 |
| Iron-deficiency anaemia | 122 | 13.8 | 60 | 10.6 | 13 | 5.5 | 195 | 11.6 | 0.001 |
| Other | 122 | 13.8 | 19 | 3.4 | 22 | 9.2 | 163 | 9.7 | <0.001 |
| Abdominal pain | 93 | 10.5 | 32 | 5.6 | 8 | 3.4 | 133 | 7.9 | <0.001 |
| Weight loss | 34 | 3.9 | 23 | 4.1 | 5 | 2.1 | 62 | 3.7 | 0.389 |
| Family history | 30 | 3.4 | 25 | 4.4 | 5 | 2.1 | 60 | 3.6 | 0.274 |
| Abnormal imaging | 9 | 1.0 | 2 | 0.4 | 2 | 0.8 | 13 | 0.8 | 0.396 |
| Previous colonoscopy, within 10y | 336 | 38.1 | 272 | 48.0 | 79 | 33.3 | 687 | 40.7 | <0.001 |
| Previous colorectal cancer (CRC) | 32 | 3.6 | 16 | 2.8 | 3 | 1.3 | 51 | 3.0 | 0.161 |
| Previous CRC (years); median, IQR | 5.5 | 3.9–6.8 | 4.8 | 1.6–10.2 | 10.7 | 4.8–63.8 | 5.5 | 3.3–9.6 | 0.441 |
| Family history of CRC† | 181 | 20.5 | 120 | 21.2 | 40 | 16.8 | 341 | 20.2 | 0.352 |
*Three-way Fisher’s exact test for counts, three-way Kruskal–Wallis test for continuous measures.
†A: Two or more first-degree relatives or one first-degree relative <45 years old; B: one first-degree relative >45 years old; C: one or more second-degree or third-degree relative(s); D: none. BCSP, Bowel Cancer Screening Programme; NSAID, non-steroidal anti-inflammatory drug.
Patient-level analysis
From the phase I cohort, 722 patients (assessed by 28 colonoscopists) had at least one polyp <10 mm and entered phase II. Of these, 567 had only small or diminutive polyps permitting patient-level analysis for surveillance interval. A surveillance interval determined by optical diagnosis was unavailable for 3.7% (incomplete data), and surveillance interval determined by histology was unavailable for 11.1% (non-retrieval of polyps or incomplete histology assessment). Comparison of surveillance interval was possible in 499 patients. Of these 499 patients, 452 patients (90.6%) had only diminutive polyps.
Using the threshold of the presence of one or more adenomas (including all high-risk, intermediate-risk and low-risk patients), test sensitivity of optical diagnosis was 83.4% (95% CI 79.6% to 86.9%) (tables 3 and 4). Test sensitivity (correctly identifying need for surveillance vs no surveillance) was 73.0% (95% CI 66.5% to 79.9%). Both measures were considerably lower than the 95% requirement (p<0.001) set by the study team. When considering exact or conservative matching, test accuracy was 67.9% (64.1% to 71.9%) and 87.6% (84.6% to 90.4%). In post hoc analyses, when considering only diminutive polyps (<6 mm) test sensitivity of optical diagnosis for detecting adenoma was 83.7% (95% CI 79.5% to 87.4%) and for surveillance was 74.2% (95% CI 66.8% to 80.8%).
Table 3.
Test performance: need for patient surveillance
| Histology interval (reference standard) |
||||||
|---|---|---|---|---|---|---|
| High risk: 1 year |
Intermediate risk: 3 years | Low risk: 5 years |
Low risk: no surveillance | No adenoma: no surveillance | Total | |
| NBI colonoscopy interval | ||||||
| High risk: 1 year | (a) 9 | 4 | 0 | (b′) 3 | (b) 2 | 18 |
| Intermediate risk: 3 years | 3 | 30 | 12 | 10 | 2 | 57 |
| Low risk: 5 years | 2 | 13 | 46 | 52 | 13 | 126 |
| Low risk: no surveillance | (c′) 0 | 4 | 9 | (d′) 100 | 19 | 132 |
| No adenoma: no surveillance | (c) 0 | 4 | 27 | 28 | (d) 107 | 166 |
| Total | 14 | 55 | 94 | 193 | 143 | (N) 499 |
Adenoma present (solid line partitions)
Test sensitivity=sum of cells in box (a)/sum of cells in boxes (a)+(c)=297/356=83.4%.
Test specificity=sum of cells in box (d)/sum of cells in boxes (d)+(b)=107/143=74.8%.
Error rate=(b+c)/N=95/499=19.0%.
Surveillance required (dashed line partitions)
Test sensitivity=sum of cells in box (a)/sum of cells in boxes (a)+(c′)=119/163=73.0%.
Test specificity=sum of cells in box (d′)/sum of cells in boxes (d′)+(b′)=254/336=75.6%.
Exact matching=sum of cells with matching surveillance interval / N=339/499=67.9%.
Conservative matching=sum of cells with matching or over surveillance/N=437/499=87.6%.
Error rate=(b′+c′)/N=126/499=25.2%.
NBI, narrow band imaging.
Table 4.
Test performance: summary findings
| NBI colonoscopy vs histology (reference) | Estimate (%) | 95% CI |
|---|---|---|
| Adenoma (yes/no)* | ||
| Sensitivity | 83.4 | 79.6% to 86.9% |
| Specificity | 74.8 | 67.6% to 81.1% |
| PPV | 89.2 | 85.9% to 92.4% |
| NPV | 64.5 | 57.3% to 71.8% |
| Surveillance (yes/no)* | ||
| Sensitivity | 73.0 | 66.5% to 79.9% |
| Specificity | 75.6 | 70.9% to 80.1% |
| PPV | 59.2 | 52.3% to 66.0% |
| NPV | 85.2 | 81.0% to 89.1% |
| Exact match | 67.9 | 64.1% to 71.9% |
| Conservative match | 87.6 | 84.6% to 90.4% |
*For explanation see table 3.
NBI, narrow band imaging; NPV, negative predictive value; PPV, positive predictive value.
Polyp-level analysis
The accuracy of NBI-assisted optical diagnosis was explored using polyp-level data comparing optical and histological diagnoses (adenoma vs non-adenoma). In total, 722 patients provided data on 1620 retrieved polyps, with individual patients providing between 1 and 27 polyps (mean 2.2). Of 1620 polyps retrieved, 1580 were characterised by optical diagnosis and 1540 by histology (table 5). A description of polyp characteristics at the polyp level determined by optical diagnosis is shown in table 6. Of these polyps, 73.7% were diminutive and 26.3% were small. Of 1014 adenomas identified by histology, the grade of dysplasia was 1 (0.1%) cancer, 3 (0.3%) high-grade dysplasia, 1005 (99.1%) low-grade dysplasia and 5 (0.5%) unreported dysplasia grade. The cancerous polyp was a 5 mm Is lesion found in the sigmoid colon and removed by cold snare polypectomy; the optical diagnosis was given with high confidence as adenoma. A villous component was found in 49 (4.8%) adenomas and 964 (95%) were non-villous (status not recorded in 1). Three polyps were histologically reported as SSPs.
Table 5.
Polyps: optical and histological determination
| Histology diagnosis (reference standard) |
|||||
|---|---|---|---|---|---|
| Hyperplastic | Adenoma | Other | Not possible | Total | |
| NBI colonoscopy | |||||
| Hyperplastic | 305 | 210 | 75 | 29 | 619 |
| Adenoma | 82 | 772 | 45 | 40 | 939 |
| Other | 7 | 8 | 6 | 1 | 22 |
| Not possible | 6 | 24 | 0 | 10 | 40 |
| Total | 400 | 1014 | 126 | 80 | 1620 |
NBI, narrow band imaging.
Table 6.
Characterisation of polyps retrieved, by histological determination
| Adenoma* |
Hyperplastic* |
Other/not determined* |
Total |
|||||
|---|---|---|---|---|---|---|---|---|
| N=1014 | Per cent | N=400 | Per cent | N=206 | Per cent | N=1620 | Per cent | |
| Polyp size | ||||||||
| Diminutive (≤5 mm) | 706 | 69.6 | 315 | 78.8 | 173 | 84.0 | 1194 | 73.7 |
| Polyp site | ||||||||
| Ascending colon | 188 | 18.5 | 19 | 4.8 | 24 | 11.7 | 231 | 14.3 |
| Caecum | 119 | 11.7 | 18 | 4.5 | 34 | 16.5 | 171 | 10.6 |
| Descending colon | 99 | 9.8 | 22 | 5.5 | 21 | 10.2 | 142 | 8.8 |
| Distal transverse colon | 97 | 9.6 | 20 | 5.0 | 17 | 8.3 | 134 | 8.3 |
| Hepatic flexure | 73 | 7.2 | 19 | 4.8 | 12 | 5.8 | 104 | 6.4 |
| Proximal transverse colon | 71 | 7.0 | 15 | 3.8 | 6 | 2.9 | 92 | 5.7 |
| Rectum | 94 | 9.3 | 146 | 36.5 | 38 | 18.4 | 278 | 17.2 |
| Sigmoid | 204 | 20.1 | 130 | 32.5 | 41 | 19.9 | 375 | 23.1 |
| Sigmoid descending | 8 | 0.8 | 4 | 1.0 | 2 | 1.0 | 14 | 0.9 |
| Splenic flexure | 61 | 6.0 | 5 | 1.3 | 8 | 3.9 | 74 | 4.6 |
| Not recorded | 0 | 0.0 | 2 | 0.5 | 3 | 1.5 | 5 | 0.3 |
| Polyp shape | ||||||||
| Ip | 40 | 3.9 | 9 | 2.3 | 4 | 1.9 | 53 | 3.3 |
| Ips | 117 | 11.5 | 27 | 6.8 | 12 | 5.8 | 156 | 9.6 |
| Is | 478 | 47.1 | 208 | 52.0 | 101 | 49.0 | 787 | 48.6 |
| IIa | 349 | 34.4 | 149 | 37.3 | 83 | 40.3 | 581 | 35.9 |
| IIa/c | 2 | 0.2 | 0 | 0.0 | 0 | 0.0 | 2 | 0.1 |
| IIb | 20 | 2.0 | 3 | 0.8 | 5 | 2.4 | 28 | 1.7 |
| Not recorded | 8 | 0.8 | 4 | 1.0 | 1 | 0.5 | 13 | 0.8 |
| Polyp resection | ||||||||
| Cold biopsy | 171 | 16.9 | 168 | 42.0 | 69 | 33.5 | 408 | 25.2 |
| Cold snare | 540 | 53.3 | 146 | 36.5 | 89 | 43.2 | 775 | 47.8 |
| Endoscopic mucosal resection | 44 | 4.3 | 13 | 3.3 | 5 | 2.4 | 62 | 3.8 |
| Hot biopsy | 22 | 2.2 | 15 | 3.8 | 1 | 0.5 | 38 | 2.3 |
| Hot snare | 232 | 22.9 | 58 | 14.5 | 25 | 12.1 | 315 | 19.4 |
| Not recorded | 5 | 0.5 | 0 | 0.0 | 17 | 8.2 | 22 | 1.4 |
| Confidence in optical diagnosis | ||||||||
| High confidence | 769 | 75.8 | 340 | 85.0 | 157 | 76.2 | 1266 | 78.1 |
| NICE classification | ||||||||
| Vessels: T | 661 | 65.2 | 67 | 16.8 | 72 | 35.0 | 882 | 54.4 |
| Surface pattern: O | 696 | 68.6 | 85 | 21.3 | 76 | 36.9 | 800 | 49.4 |
| Colour: B | 726 | 71.6 | 82 | 20.5 | 74 | 35.9 | 857 | 52.9 |
| None of the above | 202 | 20.4 | 285 | 72.3 | 101 | 51.8 | 588 | 37.2 |
| One of the above | 92 | 9.3 | 40 | 10.2 | 23 | 11.8 | 155 | 9.8 |
| Two of the above | 101 | 10.2 | 13 | 3.3 | 14 | 7.2 | 128 | 8.1 |
| Three of the above | 595 | 60.1 | 56 | 14.2 | 57 | 29.2 | 708 | 44.8 |
*Data shown give proportions within groups and are unadjusted for hierarchy at the patient level.
NBI, narrow band imaging; NICE, NBI International Colorectal Endoscopic.
Determinants of test accuracy were explored on the subset of polyps graded as adenoma or hyperplastic by NBI and histology (1369/1620, 85% of cases, table 5). In an unadjusted hierarchical model, NBI provided test sensitivity of 76.1% (table 7, model (1)) similar to the patient-level analysis. A number of variables fitted this base model in simple adjusted regression analyses; however, only the presence of NICE polyp characteristics (p<0.001) and polyp size (p<0.05) fitted a fully adjusted multivariable model (table 7).
Table 7.
Hierarchical regression modelling of adenoma detection
| (1) Unadjusted model | ||
| Sensitivity | 76.1% | (72.8% to 79.1%) |
| Specificity | 77.5% | (71.0% to 82.8%) |
| Sensitivity (%) | (95% CI) | |
| (2) Model adjusted by polyp characteristics and combinations | ||
| None | 6.5 | (3.6% to 11.2%) |
| T | 60.0 | (31.5% to 83.0% |
| O | 68.0 | (44.5% to 85.0%) |
| B | 56.8 | (33.2% to 77.7%) |
| OB | 94.9 | (80.8% to 98.8%) |
| TO | 96.7 | (76.0% to 99.6%) |
| TB | 97.3 | (80.3% to 99.7%) |
| TOB | 99.9 | (97.8% to 100.0%) |
| (3) Model adjusted by number of polyp characteristics* | ||
| None | 6.5 | (3.6% to 11.4%) |
| 1 of TOB | 62.3 | (42.7% to 78.6%) |
| 2 of TOB | 96.3 | (88.5% to 98.9%) |
| 3 of TOB | 99.9 | (97.9% to 100.0%) |
Hierarchical regression models of polyps nested within patients (see Methods section).
Polyp categories:
None denotes a polyp without T, O or B observed.
T=Thick brown vessels surrounding white structures.
O=Oval, tubular or branched white structures surrounded by brown vessels.
B=Browner relative to background.
*Model 3 was rerun with two or three polyp characteristics combined giving a test sensitivity of 99.4% (95% CI 98.2% to 99.8%) when two or more characteristics were present.
NICE characteristics
When considering NICE polyp characteristics test sensitivity at polyp level was 99.9% (95% CI 97.8% to 100.0%), where all three characteristics suggestive of an adenoma were positively identified (T=Thick brown vessels surrounding white structures, B=Browner relative to background, O=Oval, tubular or branched white structures surrounded by brown vessels). If ≥2 characteristics were identified, then the sensitivity was 99.4% (95% CI 98.2% to 99.8%). Of 1369 polyps included, 727 (53.1%) were graded T; 779 (56.9%) graded O and 799 (58.4%) graded B. In combination, 651 (47.6%), 113 (8.3%), 126 (9.2%) and 479 (35.0%) had three, two, one and no characteristics, respectively.
Confidence, expertise, image resolution and colonic site
In univariable analyses, test sensitivity was significantly greater with BCSP expertise (yes: 83.0% vs no: 64.1%, p<0.001). Confidence in polyp diagnosis (yes: 77.1% vs no: 72.0%, p=0.19) was not significant. However, neither expertise nor confidence were independently important influence in the final adjusted model. Colonoscopists reported high confidence assessing 78.1% of polyps (table 6). Further exploration of confidence in patients with two or more NICE signs showed no difference in polyp diagnoses with high and low confidence. Image resolution did not affect test sensitivity: univariable analyses test sensitivity for HD: 77.3% vs SD: 75.8% (p=0.65). Test sensitivity was not affected by the site of polyp in the colon.
External review of histopathology
Repeat histology was conducted on 193 polyps of which 189 were assessable (12% total polyps). The disagreement rate was 3.4% or 11.1% depending on narrow or inclusive definition of matching. The narrow definition compared only adenoma and hyperplasia matching; the inclusive definition included other categories used either by original or review histology. External review showed that histopathology did not provide a perfect reference standard.
Adverse events
During phase II, 55 adverse events were reported. Four were serious but only one (mild bleeding post polypectomy) was colonoscopy related. No perforations occurred among patients recruited to this trial.
Discussion
This largest multicentre prospective community study to date, evaluating the use of NBI-assisted optical diagnosis in routine clinical practice demonstrates that optical diagnosis in the hands of non-experts is not currently accurate enough to replace histology in determining surveillance for patients with colonic polyps. Regardless of the threshold employed, test sensitivity was significantly below required levels and below those reported in academic centres, which report concordance between optical and histology-based surveillance intervals of >90%.19 21
The Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) statement issued by the American Society of GI Endoscopy has issued advice on acceptable performance thresholds for real-time endoscopic assessment of diminutive polyps required before optical diagnosis should be recommended for routine clinical practice.22 The PIVI statement advises that optical diagnosis can be used for diminutive (1–5 mm) and histological diagnosis for small (6–9 mm) polyps and those summated results used to determine surveillance. In expert hands, optical diagnosis of small polyps using white light and NBI has been shown to be comparable to histology.19 21 A large meta-analysis showed per polyp sensitivity of 91% and specificity of 83%,23 but results from general settings have not replicated those values, with sensitivities ranging from 75% to 94% and specificity 65% to 76%.24–26 The present study used optical diagnosis for both small and diminutive polyps to determine surveillance interval but as 91% of patients had only diminutive polyps, the inclusion of small polyps did not significantly affect determination of surveillance interval. Accuracy of adenoma characterisation at the polyp level in the present study was 83%. The NHS BCSP provides a high standard of practice with colonoscopists accredited and regularly quality assured. In this study, performance was better for screening colonoscopists in univariable analysis but not in adjusted models. A meta-analysis reported that pooled negative predictive value was higher when optical diagnosis was made with high confidence as opposed to when no information on confidence was given (93% vs 88%) as well as higher agreement in surveillance intervals for high confidence (91% vs 79%).15 Non-experts in community practice made 49% of diagnoses with high confidence before training and 72% after training in optical diagnosis.27 Some studies assessing experienced endoscopists have reported high confidence optical diagnosis in over 85% of cases.28 The current study found that high confidence predictions were made in 78.1% of polyps but confidence did not influence test accuracy.
A Discard policy relies on accurate estimation of polyp size. It is recommended that polyp size is estimated against an instrument of known size such as an open biopsy forceps. Even using such an approach, estimation of size maybe inaccurate.29 Tools such as the endoscopic lesion measurement system have been developed. This consists of a graduated measurement device that can be passed down the biopsy channel and placed alongside the lesion to aid measurement and has been shown to be superior to clinician estimation.30 Such systems warrant further evaluation as size is a fundamental part of a potential Discard policy.
The rate of SSPs reported in this study is low when compared with the reported prevalence of 0.3–0.5%.31 One study has suggested that the use of NBI might improve the detection of SSPs although the increase detected did not reach statistical significance.32 In the present study, colonoscopists were asked to classify polyps as adenomas, hyperplastic, cancer or other and were not expected to specifically diagnose sessile serrated adenomas or polyps. Recent work has highlighted typical endoscopic features that may be used to distinguish SSPs from hyperplastic polyps.33 These features, together with the NICE classification, have been combined to develop the Workgroup serrAted polypS and Polyposis (WASP) classification. Using WASP, it has been shown that, following training, an accuracy of optical diagnosis for SSA/Ps 0.87 (95% CI 0.80 to 0.95) could be achieved when diagnoses were made with high confidence. Six months after training, accuracy was 0.84 (95% CI 0.81 to 0.88) when made with high confidence. The use of the WASP classification could be incorporated into future training modules in optical diagnosis.
Accurate adenoma identification by NBI was heavily dependent on identification of the three NICE polyp characteristics. If two or more features were present (55.9% of polyps), the sensitivity for correctly identifying adenomas exceeded 99%. The discrepancy between this finding and overall test sensitivity, combined with a high percentage of high confidence diagnoses, raises the possibility that endoscopists were relying on factors other than NICE criteria to make the optical diagnosis. Where NICE features were identified, accuracy was high but the present study cannot determine whether NICE features were not consistently present or features were incorrectly interpreted. Previous studies suggest a short learning curve for optical diagnosis16 34; however, training using still images and videos may not translate into accuracy in vivo. The current study is consistent with a previous study where 12/13 community-based gastroenterologists identified adenomas with >90% accuracy following training but only 3/12 managed this in vivo.25 This was a pragmatic study designed to examine whether NBI worked in clinical practice. While double reporting of histology was undertaken to assess reliability and training and feedback on NBI were given, it did not incorporate more formal testing of reliability and did not aim to test explanatory factors related to how NBI did or did not work.
As this was a pragmatic study generalisable to routine clinical practice, the protocol did not mandate use of HD or SD colonoscopes. Consequently, only 22% of patients (20% of polyps) were assessed with HD, although this did not significantly alter test sensitivity. Most studies that have achieved results comparable to reference standard have used HD systems15; however, a meta-analysis of all NBI studies reported that HD significantly decreased the performance of NBI, a possible explanation was that some of these studies also used magnification, making data more heterogeneous.14
Optical diagnosis remains an attractive idea because of the potential for reducing costs and streamlining care. This study demonstrates that correctly characterising diminutive polyps using optical diagnosis represents a major challenge. One method for improving accuracy could be the use of computer-aided diagnosis, which has been shown to be feasible in a pilot study.35 Should the accuracy of optical diagnosis be improved, validated accreditation programmes and on-going quality assurance would be required in order for it to be incorporated into routine practice.
Conclusions
Previous research, predominantly from single sites and academic groups, suggests that NBI-assisted optical diagnosis has acceptable accuracy to determine surveillance without histology. These findings were not replicated in this large, multicentre study of NBI use in routine practice, either at the polyp or patient level. The marked variation of accuracy according to the polyp characteristics detected is notable: either a proportion of polyps present without NBI detectable signs or colonoscopists vary in their ability to evaluate them. The first explanation would require imaging advances; the second further research into training and accreditation. NBI-assisted optical diagnosis of small polyps during colonoscopy cannot currently be recommended for routine use outside of specialist centres.
Footnotes
Collaborators: The study team wish to acknowledge the collaborators in this study: Laura Neilson, Adil Ahmed, Roisin Bevan, Mike Bradburn, Faheem Butt, Andrew Douglass, Vikki Edge, John Greenaway, Wendy Gregory, John Hancock, Lindsay Hurst, Babur Javaid, Diamond Joy, Deepak Kejariwal, Susan McConnell, Jestina Miles, Sarah Mills, David Oliver, Simon Panter, Francisco Porras-Perez, John Silcock, Joanne Topping, Christopher Wells; the hospital research teams that supported them; County Durham and Darlington, Cumbria, Northumbria, North Tees, South Tees and South Tyneside NHS Trusts for infrastructural support. In addition, we wish to thank staff at Durham Clinical Trial Unit, Jennifer Wilkinson and Catherine Frost; independent members of the Study Steering Committee, Greg Rubin (Chair), Janice Mulley, Yan Yiannakou; and the Data Monitoring Committee, Stephen Attwood (Chair), Mike Bramble and Adetayo Kasim.
Contributors: CJR: Chief Investigator, designed the study, contributed to running of the study, recruited patients to the study, analysed results and is the main author of the manuscript. PTR, AW, BPS, JEE, MM, HC, RM, UM, HH and JMM: designed the study, contributed to running of the study, analysed results, reviewed and contributed to the writing of the manuscript. MDR: designed the study, contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript. AR, AD, AJ and CM: contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript.
Funding: This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-0407-13309). The Discard 2 study design was supported by the BSG Endoscopy Committee and supported by the NHS Bowel Cancer Screening Research Committee.
Competing interests: The Discard 2 study was entirely funded by NIHR Research for patient Benefit funding with no industry funding for this study. CJR, PTR, AW, MDR, BPS, JEE and AD have received research, travel and speaking funding from Olympus Medical. They have additionally received research, travel and speaking funding from other endoscopy companies.
Ethics approval: UK National Research Ethics Committee North East-Newcastle and North Tyneside.
Provenance and peer review: Not commissioned; internally peer reviewed.
Contributor Information
Collaborators: Laura Neilson, Adil Ahmed, Roisin Bevan, Mike Bradburn, Faheem Butt, Andrew Douglass, Vikki Edge, John Greenaway, Wendy Gregory, John Hancock, Lindsay Hurst, Babur Javaid, Diamond Joy, Deepak Kejariwal, Susan McConnell, Jestina Miles, Sarah Mills, David Oliver, Simon Panter, Francisco Porras-Perez, John Silcock, Joanne Topping, Christopher Wells, Jennifer Wilkinson, Catherine Frost, Greg Rubin, Janice Mulley, Yan Yiannakou, Stephen Attwood, Mike Bramble, and Adetayo Kasim
References
- 1.Edwards BK, Ward E, Kohler BA, et al. . Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–73. 10.1002/cncr.24760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelstein B, Fearon ER, Hamilton SR, et al. . Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525–32. 10.1056/NEJM198809013190901 [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN, et al. . The National Polyp Study. Eur J Cancer Prev 1993;2(Suppl 2):83–7. 10.1097/00008469-199306000-00014 [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467 10.1136/bmj.g2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeff LC, Richards TB, Shapiro JA, et al. . How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology 2004;127:1670–7. 10.1053/j.gastro.2004.09.051 [DOI] [PubMed] [Google Scholar]
- 6.Lieberman D, Moravec M, Holub J, et al. . Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology 2008;135:1100–5. 10.1053/j.gastro.2008.06.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rex DK, Overhiser AJ, Chen SC, et al. . Estimation of Impact of American College of Radiology Recommendations on CT Colonography Reporting for Resection of High-Risk Adenoma Findings. Am J Gastroenterol 2009;104:149–53. 10.1038/ajg.2008.35 [DOI] [PubMed] [Google Scholar]
- 8.Kamiński MF, Hassan C, Bisschops R, et al. . Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2014;46:435–49. 10.1055/s-0034-1365348 [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Bansal A, Rao D, et al. . Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc 2012;75:1022–30. 10.1016/j.gie.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 10.Gschwantler M, Kriwanek S, Langner E, et al. . High-grade dysplasia and invasive carcinoma in colorectal adenomas: a multivariate analysis of the impact of adenoma and patient characteristics. Eur J Gastroenterol Hepatol 2002;14:183–8. 10.1097/00042737-200202000-00013 [DOI] [PubMed] [Google Scholar]
- 11.Hirata M, Tanaka S, Oka S, et al. . Evaluation of microvessels in colorectal tumors by narrow band imaging magnification. Gastrointest Endosc 2007;66:945–52. 10.1016/j.gie.2007.05.053 [DOI] [PubMed] [Google Scholar]
- 12.Hewett DG, Kaltenbach T, Sano Y, et al. . Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012;143:599–607.e1. 10.1053/j.gastro.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 13.Rogart JN, Jain D, Siddiqui UD, et al. . Narrow-band imaging without high magnification to differentiate polyps during real-time colonoscopy: improvement with experience. Gastrointest Endosc 2008;68:1136–45. 10.1016/j.gie.2008.04.035 [DOI] [PubMed] [Google Scholar]
- 14.Wanders LK, East JE, Uitentuis SE, et al. . Diagnostic performance of narrowed spectrum endoscopy, autofluorescence imaging, and confocal laser endomicroscopy for optical diagnosis of colonic polyps: a meta-analysis. Lancet Oncol 2013;14:1337–47. 10.1016/S1470-2045(13)70509-6 [DOI] [PubMed] [Google Scholar]
- 15.Abu Dayyeh BK, Thosani N, Konda V, et al. . ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2015;81:502.e1–e16. 10.1016/j.gie.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 16.Ignjatovic A, Thomas-Gibson S, East JE, et al. . Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc 2011;73:128–33. 10.1016/j.gie.2010.09.021 [DOI] [PubMed] [Google Scholar]
- 17.[No authors listed] The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58(6 Suppl):S3–43. [DOI] [PubMed] [Google Scholar]
- 18.Atkin WS, Saunders BP. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut 2002;51(Suppl 5):V6–9. 10.1136/gut.51.suppl_5.v6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ignjatovic A, East JE, Suzuki N, et al. . Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol 2009;10:1171–8. 10.1016/S1470-2045(09)70329-8 [DOI] [PubMed] [Google Scholar]
- 20.Jones SR, Carley S, Harrison M. An introduction to power and sample size estimation. Emerg Med J 2003;20:453–8. 10.1136/emj.20.5.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology 2009;136:1174–81. 10.1053/j.gastro.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 22.Rex DK, Kahi C, O'Brien M, et al. . The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2011;73:419–22. 10.1016/j.gie.2011.01.023 [DOI] [PubMed] [Google Scholar]
- 23.McGill SK, Evangelou E, Ioannidis JP, et al. . Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics. Gut 2013;62:1704–13. 10.1136/gutjnl-2012-303965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuiper T, Marsman WA, Jansen JM, et al. . Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol 2012;10:1016–20; quiz e79 10.1016/j.cgh.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 25.Ladabaum U, Fioritto A, Mitani A, et al. . Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology 2013;144:81–91. 10.1053/j.gastro.2012.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paggi S, Rondonotti E, Amato A, et al. . Resect and discard strategy in clinical practice: a prospective cohort study. Endoscopy 2012;44:899–904. 10.1055/s-0032-1309891 [DOI] [PubMed] [Google Scholar]
- 27.Rastogi A, Rao DS, Gupta N, et al. . Impact of a computer-based teaching module on characterization of diminutive colon polyps by using narrow-band imaging by non-experts in academic and community practice: a video-based study. Gastrointest Endosc 2014;79:390–8. 10.1016/j.gie.2013.07.032 [DOI] [PubMed] [Google Scholar]
- 28.Repici A, Hassan C, Radaelli F, et al. . Accuracy of narrow-band imaging in predicting colonoscopy surveillance intervals and histology of distal diminutive polyps: results from a multicenter, prospective trial. Gastrointest Endosc 2013;78:106–14. 10.1016/j.gie.2013.01.035 [DOI] [PubMed] [Google Scholar]
- 29.Rex DK, Rabinovitz R. Variable interpretation of polyp size by using open forceps by experienced colonoscopists. Gastrointest Endosc 2014;79:402–7. 10.1016/j.gie.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 30.Leng Q, Jin HY. Measurement system that improves the accuracy of polyp size determined at colonoscopy. World J Gastroenterol 2015;21:2178–82. 10.3748/wjg.v21.i7.2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.East JE, Vieth M, Rex DK. Serrated lesions in colorectal cancer screening: detection, resection, pathology and surveillance. Gut 2015;64:991–1000. 10.1136/gutjnl-2014-309041 [DOI] [PubMed] [Google Scholar]
- 32.Rex DK, Clodfelter R, Rahmani F, et al. . Narrow-band imaging versus White light for the detection of proximal colon serrated lesions: a randomized, controlled trial. Gastrointest Endosc 2016;83:166–71. 10.1016/j.gie.2015.03.1915 [DOI] [PubMed] [Google Scholar]
- 33.Hazewinkel Y, López-Cerón M, East JE, et al. . Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution White-light endoscopy and narrow-band imaging. Gastrointest Endosc 2013;77:916–24. 10.1016/j.gie.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 34.Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc 2010;72:572–6. 10.1016/j.gie.2010.03.1124 [DOI] [PubMed] [Google Scholar]
- 35.Tischendorf JJ, Gross S, Winograd R, et al. . Computer-aided classification of colorectal polyps based on vascular patterns: a pilot study. Endoscopy 2010;42:203–7. 10.1055/s-0029-1243861 [DOI] [PubMed] [Google Scholar]