Abstract
Background
Maternal diet during pregnancy has been suggested to be an important early life exposure that influences immune tolerance and the development of allergic diseases in the offspring.
Methods
We examined the relation between maternal dietary patterns assessed using 24 hr recalls and food diaries at 26-28 weeks of pregnancy and the subsequent development of allergic outcomes in the offspring in the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) birth cohort. Exploratory factor analysis was used to characterise maternal dietary patterns during pregnancy. During repeated visits in the first 36 months of life, questionnaires were administered to ascertain allergic symptoms, namely, eczema, rhinitis and wheezing. At ages 18 and 36 months, we administered skin prick testing to inhalant and food allergens.
Results
Of the three maternal dietary patterns that emerged, the Seafood and Noodle (SfN) pattern was associated with a reduced risk of developing allergen sensitization at both 18 months [odds ratio ( 95% confidence interval): 0.7 (0.5-0.9)] and 36 months [ odds ratio ( 95% confidence interval) 0.7 (0.6 -0.9)] after adjustment for family history of allergy, ethnicity, sex and maternal education levels. No associations between Vegetable, Fruit and white Rice and Pasta, Cheese and Processed meat patterns were observed with any of the allergic outcomes in the first 18 and 36 months of life.
Conclusion
Maternal diet during pregnancy can influence the subsequent development of allergic outcomes in offspring.
Keywords: maternal dietary patterns, allergic diseases, pregnancy, birth cohorts
Introduction
In recent decades, increasing attention has been placed on the non-inheritable factors relating to the programming of immune function in the child as suggested by the Developmental Origins of Health and Disease (DOHaD) concept [1]. Besides genetic predisposition increasing the risk of development of diseases, environmental exposures also play a role in modifying the risk [2]. This is evident from a study on monozygotic twin pairs where DNA methylation was influenced by maternal environment [3,4]. The early onset of paediatric allergic diseases raises the possibility that maternal diet during pregnancy may contribute to later disease development [5]. Maternal diet during pregnancy not only provides nutrients to the developing foetus but has also been suggested to be an important early life exposure that influences immune tolerance and the development of allergic diseases in the offspring [6]. Research on early life exposures such as maternal diet during pregnancy on the subsequent development of allergic disease can form preventive strategies and intervention plans to reduce childhood allergic diseases.
Maternal dietary patterns such as the Mediterranean diet pattern, typically consisting of fruits and vegetables, low to moderate amounts of eggs and dairy products, and minimal amount of red meat has been reported to have a protective effect on the development of allergic diseases such as asthma in children [7–10]. Most studies are either cross-sectional or case-control studies that recruit subjects in pre-school or school-going ages.[11–13]. In addition, some studies addressed the effect of nutrition in an atopic population where it is difficult to infer findings to the general population [14,15]. Moreover, other studies have only looked at outcomes in the first year of life [16,17]. Besides, studies that have looked at the contribution of maternal nutrition to the development of paediatric allergic outcomes in a prospective birth cohort setting are very limited in Asia as most studies on this topic are conducted in European countries [18,19] where dietary patterns and genetic constitution differ [20].
Here we aim to analyse the associations between maternal dietary patterns during pregnancy and the development of allergic diseases in the first 3 years of life in the Growing Up in Singapore Towards Healthy Outcomes cohort (GUSTO).
Materials and Methods
Questionnaires and allergic outcomes
The methodology of the GUSTO study has been described previously [21,22]. Briefly, we recruited 1237 healthy pregnant mothers who agreed to enroll their offspring for future follow-up. Interviewers gathered information on demographics, family history of allergy, social data and lifestyle factors. Definitions of allergic outcomes were standardized in the questionnaires administered at 3, 6, 9, 12, 15 18, 24 and 36 months to ensure consistency during interviews and home visits.
Physician-diagnosed atopic eczema was based on a positive answer to the written question: “Has your child ever been diagnosed with eczema?”. “Wheezing” was based on a positive answer to the written question “Has your child ever wheezed?”, while “rhinitis” was based on a positive response to the question “Has your child ever had sneezing, running nose, blocked or congested nose, snoring or noisy breathing during sleep or when awake that has lasted for 2 or more weeks duration?” Study team members called the subjects who reported rhinitis to collect information on the number of episodes of rhinitis and the duration of each episode. A case prior to 18 months required a single episode that lasted for at least 4 weeks or two or more episodes each lasting at least 2 weeks. New cases of rhinitis after 18 months were defined by one or more episodes lasting at least 2 weeks.
Allergen sensitization was determined by skin prick testing. Skin prick testing (SPT) to inhalant allergens (house dust mites Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Blomia tropicalis) and food allergens (egg, peanut and cow’s milk) was carried out at both 18 and 36 months. All of the allergens for skin prick testing were obtained from Greer Laboratories (Lenoir, NC, USA), except for B. tropicalis, which was obtained from our laboratory. All tests were interpreted as positive if the wheal was at least 3 mm, and a child was considered as SPT-positive if any one or more of the individual tests was positive with a positive reaction to the positive control (histamine) and a negative reaction to the negative control (saline).
Allergic clinical outcomes until 18 months were to the above-noted written questions in the first 18 months, combined with a positive SPT at 18 months. Allergic clinical outcomes until 36 months were defined as positive responses to the above-noted written questions in the first 36 months, combined with a positive SPT at 36 months. The allergic outcome was classified as absent when the answers for all visits were “no.” Family history of allergy was defined as positive if the mother, father or an older sibling ever had eczema, asthma or allergic rhinitis.
Ethics approval was obtained from the Domain Specific Review Board of Singapore National Healthcare Group and the Centralised Institutional Review Board of SingHealth. Conduct of this study was based on the guidelines in the Declaration of Helsinki.
Identifying maternal dietary patterns
The methods for maternal dietary assessment have been described previously [23]. Dietary intake was assessed at 26-28 weeks of pregnancy which was conducted by trained clinical staff with the use of the 5-stage multiple pass interviewing technique [24]. Visual aids such as food pictures of food portion sizes were presented to assist women in their quantification of food and beverage intake. Exploratory factor analysis was used to characterise maternal dietary patterns during pregnancy. Dietary patterns were derived by principal component extraction with varimax rotation on the 68 food groups. Three factors (i.e. dietary patterns) were retained and it was determined by the break point of the Scree plot and factor interpretability. The dietary pattern score for each participant was calculated by summing the standardised intake of food groups (g/d) weighted by their factor loadings using dietary records from 24 h recall. Factor loadings are correlation coefficients between each food group and the dietary pattern, hence higher dietary pattern scores indicate greater adherence to the derived pattern. Three maternal dietary patterns, namely the Vegetable, Fruit and white Rice (VFR) , Seafood and Noodles (SfN) and Pasta, Cheese and Processed meat (PCP) patterns have emerged [25] and details of the food items and factor loading scores of the dietary patterns are presented in Supplementary Table 1.
Statistical analysis
Statistical analysis was carried out using SPSS version 20.0 (IBM SPSS Statistics, Armonk, NY). Associations between maternal dietary pattern scores and subsequent allergic outcomes in the offspring were estimated using univariable as well as multivariable logistic regression adjusting for sex, ethnicity, maternal education and family history of allergy. These confounders were selected by a review of the literature [26,27].
Results
Description of the study cohort
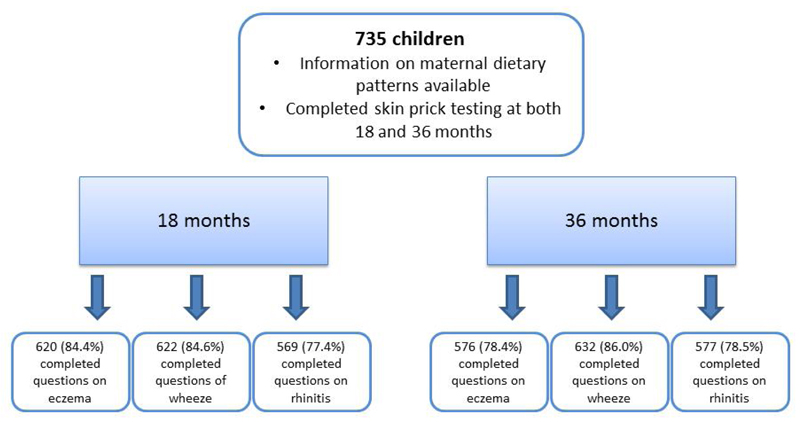
In this study, there were 735 children with information on maternal dietary patterns and completed skin prick testing at both 18 months and 36 months. At 18 months, 620 (84.4%) completed questions on eczema; 622 (84.6%)completed questions on wheeze and 569 (77.4%)completed questions on rhinitis. One hundred and three subjects (14.0%) had a positive SPT at 18 months; 6 (0.8%) had a positive SPT to cow’s milk; 8 (1.1%) to peanut; 24 (3.3%) to egg; 65 (8.8%) to Dermatophagoides pteronyssinus ; 53 (7.2%) to Dermatophagoides farinae and 8 (1.1%) to Blomia Tropicalis (Table 1). One hundred and thirty-two (21.3%) had eczema, 36 (5.8%) had eczema with a positive SPT, 107 (18.8%) had rhinitis, 22 (3.9%) had rhinitis with a positive SPT, 179 (28.8%) had wheezed, 26 (4.2%) wheezed and had a positive SPT.
Table 1. Sensitization rates to allergens in the first 3 years of life.
| Cow's milk | Peanut | Egg | Dermatophagoides pteronyssinus | Dermatophagoides farinae | Blomia Tropicalis | |
|---|---|---|---|---|---|---|
| N(%) | ||||||
| Month18 | 6 (0.8) | 8 (1.1) | 24 (3.3) | 65 (8.8) | 53 (7.2) | 8 (1.1) |
| Month 36 | 1 (0.1) | 8 (1.1) | 6 (1.1) | 144 (19.6) | 117 (15.9) | 20 (2.7) |
At 36 months, 576 (78.4%) completed questions on eczema; 577 (78.5%) completed questions on rhinitis and 632 (86.0%) completed questions on wheeze. One hundred and seventy-two subjects (23.4%) had a positive SPT at 36 months; 1 (0.1%) had a positive SPT to cow’s milk; 8 (1.1%) to peanut; 6 (1.1 %) to egg; 144 (19.6%) to Dermatophagoides pteronyssinus ; 117 (15.9%) to Dermatophagoides farinae and 20 (2.7%) to Blomia Tropicalis (Table 1). One hundred and fifty-seven (27.3%) had eczema, 59 (10.2%) had eczema with a positive SPT, 226 (39.2%) had rhinitis, 66 (11.4%) had rhinitis with a positive SPT, 258 (40.8%) had wheezed, 75 (10.2%) wheezed with a positive SPT.
A flow chart of the study population with responses to allergic outcomes in the first 36 months of life was illustrated in Figure 1. There are very regular follow up visits in this study and the main reason for non-completion of the questionnaires was the mothers’ not having been contactable at some point in the study and hence not having a home visit. The loss of follow up rates in our study are also similar to that in other studies [28,29]
Figure 1. Schematic diagram on the study population with responses to allergic outcomes in the first 3 years of life.
A schematic diagram on the study population that was included in this analysis was shown, showing number of subjects with responses to different allergic outcomes at 18 months and 36 months.
Tables 2 and 3 compare the characteristics of the children with complete information and those with missing data. There were some differences in ethnicity and maternal education levels within the analysis groups.
Table 2. Comparison of study subjects who completed questionnaires and SPT at 18 months vs other GUSTO subjects.
| N(%) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Completed SPT | Excluded | p-value | Completed question on eczema | Excluded | p-value | Completed questions on eczema and SPT | Excluded | p-value | Completed questions on wheeze | Excluded | p-value | Completed questions on wheeze and SPT | Excluded | p-value | Completed questions on rhinitis | Excluded | p-value | Completed questions on rhintis and SPT | Excluded | p-value | |
| Sex | |||||||||||||||||||||
| Male | 386 (52.5) | 56 (57.1) | 0.5 | 323 (52.1) | 63 (54.8) | 0.6 | 323 (52.1) | 63 (54.8) | 0.6 | 324 (52.1) | 62 (54.9) | 0.6 | 324 (52.1) | 62 (54.9) | 0.6 | 295 (51.8) | 91 (54.8) | 0.5 | 295 (51.8) | 91 (54.8) | 0.5 |
| Female | 349 (47.5) | 42 (42.9) | 297 (47.9) | 52 (45.2) | 297 (47.9) | 52 (45.2) | 298 (47.9) | 51 (45.1) | 298 (47.9) | 51 (45.1) | 274 (48.2) | 75 (45.2) | 274 (48.2) | 75 (45.2) | |||||||
| Ethnicity | |||||||||||||||||||||
| Chinese | 415 (56.5) | 57 (58.2) | 0.2 | 367 (59.2) | 48 (41.7) | <0.01 | 367 (59.2) | 48 (41.7) | <0.01 | 364 (58.5) | 51 (45.1) | <0.01 | 364 (58.5) | 51 (45.1) | <0.01 | 338 (59.4) | 77 (46.4) | 0.01 | 338 (59.4) | 77 (46.4) | 0.01 |
| Malay | 198 (26.9) | 19 (19.4) | 162 (26.1) | 36 (31.3) | 162 (26.1) | 36 (31.3) | 169 (27.2) | 29 (25.7) | 169 (27.2) | 29 (25.7) | 145 (25.5) | 53 (31.9) | 145 (25.5) | 53 (31.9) | |||||||
| Indian | 122 (16.6) | 22 (22.4) | 91 (14.7) | 31 (27.0) | 91 (14.7) | 31 (27.0) | 89 (14.3) | 33 (29.2) | 89 (14.3) | 33 (29.2) | 86 (15.1) | 36 (21.7) | 86 (15.1) | 36 (21.7) | |||||||
| Maternal Education Levels | |||||||||||||||||||||
| Less than 12 years | 295 (40.5) | 36 (37.1) | 0.6 | 236 (38.4) | 59 (52.2) | <0.01 | 236 (38.4) | 59 (52.2) | <0.01 | 243 (39.3) | 52 (47.3) | 0.1 | 243 (39.3) | 52 (47.3) | 0.1 | 212 (37.5) | 83 (50.9) | <0.01 | 212 (37.5) | 83 (50.9) | <0.01 |
| At least 12 years | 433 (59.5) | 61 (62.9) | 379 (61.6) | 54 (47.8) | 379 (61.6) | 54 (47.8) | 375 (60.7) | 58 (52.7) | 375 (60.7) | 58 (52.7) | 353 (62.5) | 80 (49.1) | 353 (62.5) | 80 (49.1) | |||||||
| Family history of allergy | |||||||||||||||||||||
| No family history of allergy | 252 (48.5) | 40 (61.5) | 0.049 | 211 (48.3) | 41 (49.4) | 0.9 | 211 (48.3) | 41 (49.4) | 0.9 | 209 (48.2) | 43 (50.0) | 0.8 | 209 (48.2) | 43 (50.0) | 0.8 | 191 (47.0) | 61 (53.5) | 0.2 | 191 (47.0) | 61 (53.5) | 0.2 |
| Family history of allergy | 268 (51.5) | 25 (38.5) | 226 (51.7) | 42 (50.6) | 226 (51.7) | 42 (50.6) | 225 (51.8) | 43 (50.0) | 225 (51.8) | 43 (50.0) | 215 (53.0) | 53 (46.5) | 215 (53.0) | 53 (46.5) | |||||||
Table 3. Comparison of study subjects who completed questionnaires and SPT at 36 months vs other GUSTO subjects.
| N(%) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Completed SPT | Excluded | p-value | Completed question on eczema | Excluded | p-value | Completed questions on eczema and SPT | Excluded | p-value | Completed questions on wheeze | Excluded | p-value | Completed questions on wheeze and SPT | Excluded | p-value | Completed questions on rhinitis | Excluded | p-value | Completed questions on rhintis and SPT | Excluded | p-value | |
| Sex | |||||||||||||||||||||
| Male | 386 (52.5) | 43 (45.7) | 0.2 | 299 (51.9) | 87 (54.7) | 0.6 | 299 (51.9) | 87 (54.7) | 0.6 | 332 (52.5) | 54 (52.4) | 0.9 | 332 (52.5) | 54 (52.4) | 0.9 | 300 (52.0) | 86 (54.4) | 0.6 | 300 (52.0) | 86 (54.4) | 0.6 |
| Female | 349 (47.5) | 51 (54.3) | 277 (48.1) | 72 (45.3) | 277 (48.1) | 72 (45.3) | 300 (47.5) | 49 (47.6) | 300 (47.5) | 49 (47.6) | 277 (48.0) | 72 (45.6) | 277 (48.0) | 72 (45.6) | |||||||
| Ethnicity | |||||||||||||||||||||
| Chinese | 415 (56.5) | 51 (54.3) | 0.04 | 330 (57.3) | 85 (53.5) | 0.2 | 330 (57.3) | 85 (53.5) | 0.2 | 364 (57.6) | 51 (49.5) | <0.01 | 364 (57.6) | 51 (49.5) | <0.01 | 341 (59.1) | 74 (46.8) | 0.02 | 341 (59.1) | 74 (46.8) | 0.02 |
| Malay | 198 (26.9) | 18 (19.1) | 158 (27.4) | 40 (25.2) | 158 (27.4) | 40 (25.2) | 176 (27.8) | 22 (21.4) | 176 (27.8) | 22 (21.4) | 145 (25.1) | 53 (33.5) | 145 (25.1) | 53 (33.5) | |||||||
| Indian | 122 (16.6) | 25 (26.6) | 88 (15.3) | 34 (21.4) | 88 (15.3) | 34 (21.4) | 92 (14.6) | 30 (29.1) | 92 (14.6) | 30 (29.1) | 91 (15.8) | 31 (19.6) | 91 (15.8) | 31 (19.6) | |||||||
| Maternal Education Levels | |||||||||||||||||||||
| Less than 12 years | 295 (40.5) | 31 (34.1) | 0.3 | 226 (39.6) | 69 (43.9) | 0.4 | 226 (39.6) | 69 (43.9) | 0.4 | 249 (39.7) | 46 (45.5) | 0.3 | 249 (39.7) | 46 (45.5) | 0.3 | 215 (37.5) | 80 (51.6) | <0.01 | 215 (37.5) | 80 (51.6) | <0.01 |
| At least 12 years | 433 (59.5) | 60 (65.9) | 345 (60.4) | 88 (56.1) | 345 (60.4) | 88 (56.1) | 378 (60.3) | 55 (54.5) | 378 (60.3) | 55 (54.5) | 358 (62.5) | 75 (48.4) | 358 (62.5) | 75 (48.4) | |||||||
| Family history of allergy | |||||||||||||||||||||
| No family history of allergy | 252 (48.5) | 39 (54.2) | 0.4 | 195 (48.3) | 57 (49.1) | 0.9 | 195 (48.3) | 57 (49.1) | 0.9 | 207 (46.7) | 45 (58.4) | 0.1 | 207 (46.7) | 45 (58.4) | 0.1 | 190 (46.3) | 62 (56.4) | 0.1 | 190 (46.3) | 62 (56.4) | 0.1 |
| Family history of allergy | 268 (51.5) | 33 (45.8) | 209 (51.7) | 59 (50.9) | 209 (51.7) | 59 (50.9) | 236 (53.3) | 32 (41.6) | 236 (53.3) | 32 (41.6) | 220 (53.7) | 48 (43.6) | 220 (53.7) | 48 (43.6) | |||||||
Associations between maternal dietary patterns and allergic outcomes in the offspring
The maternal dietary pattern consisting of seafood and noodles was associated with a reduced risk of developing allergen sensitization at both 18 months [adjusted odds ratio 0.7 (0.5-0.9), Table 4] and 36 months [adjusted odds ratio 0.7 (0.6 -0.9), Table 5] after adjustment for family history of allergy, ethnicity, sex and maternal education levels. There were no significant associations between the maternal dietary patterns consisting of vegetable, fruit and white rice (VFR) and pasta, cheese and processed meat (PPM) on the development of allergen sensitization, eczema, rhinitis and wheeze at both 18 and 36 months.
Table 4. Univariable and multivariable associations between maternal dietary patterns and allergic outcomes in the offspring in the first 18 months of life.
| Univariable analysis | Allergen sensitization | Eczema | Eczema with a positive SPT | Wheeze | Wheeze with a positive SPT | Rhinitis | Rhinitis with a positive SPT |
|---|---|---|---|---|---|---|---|
| Maternal dietary patterns | OR (95% CI) | ||||||
| VFR | 0.9 (0.8-1.2) | 1.0 (0.8-1.2) | 1.0 (0.7-1.4) | 0.8 (0.7-0.97) | 0.8 (0.5-1.2) | 0.8 (0.7-1.0) | 1.0 (0.6-1.5) |
| SFN | 0.8 (0.7-1.0) | 1.1 (0.9-1.3) | 0.8 (0.5-1.1) | 0.9 (0.7-1.0) | 0.8 (0.5-1.3) | 0.9 (0.7-1.1) | 0.8 (0.5-1.2) |
| PCP | 0.9 (0.7-1.2) | 1.1 (0.9-1.3) | 0.7 (0.4-1.2) | 0.9 (0.7-1.1) | 0.7 (0.4-1.3) | 1.1 (0.9-1.3) | 1.0 (0.6-1.6) |
| Multivariable analysis | Allergen sensitization | Eczema | Eczema with a positive SPT | Wheeze | Wheeze with a positive SPT | Rhinitis | Rhinitis with a positive SPT |
| Maternal dietary patterns | |||||||
| VFR | 1.1 (0.8-1.4) | 0.8 (0.7-1.1) | 1.1 (0.7-1.7) | 0.9 (0.7-1.2) | 0.8 (0.5-1.4) | 0.8 (0.6-1.1) | 1.1 (0.6-2.0) |
| SFN | 0.7 (0.5-0.9) | 0.9 (0.6-1.2) | 0.6 (0.3-1.1) | 0.8 (0.6-1.1) | 0.6 (0.3-1.2) | 0.9 (0.6-1.2) | 0.8 (0.4-1.5) |
| PCP | 0.9 (0.7-1.2) | 1.1 (0.9-1.4) | 0.8 (0.4-1.5) | 0.8 (0.6-1.1) | 0.7 (0.3-1.4) | 1.1 (0.9-1.4) | 1.1 (0.7-1.7) |
Adjusted for sex, ethnicity, maternal education levels and family history of allergy
Table 5. Univariable and multivariable associations between maternal dietary patterns and allergic outcomes in the offspring in the first 3 years of life.
| Univariable analysis | Allergen sensitization | Eczema | Eczema with a positive SPT | Wheeze | Wheeze with a positive SPT | Rhinitis | Rhinitis with a positive SPT |
|---|---|---|---|---|---|---|---|
| Maternal dietary patterns | |||||||
| VFR | 1.0 (0.9-1.2) | 1.0 (0.8-1.2) | 1.0 (0.8-1.4) | 0.8 (0.7-0.96) | 0.9 (0.7-1.1) | 1.0 (0.8-1.1) | 1.1 (0.8-1.4) |
| SFN | 0.8 (0.7-1.0) | 1.2 (1.0-1.4) | 1.0 (0.8-1.3) | 0.9 (0.7-1.0) | 0.9 (0.7-1.1) | 1.0 (0.8-1.1) | 0.9 (0.7-1.2) |
| PCP | 0.9 (0.7-1.1) | 1.1 (0.9-1.3) | 0.9 (0.6-1.2) | 0.9 (0.8-1.1) | 0.9 (0.7-1.2) | 1.1 (0.9-1.3) | 1.0 (0.7-1.3) |
| Multivariable analysis | Allergen sensitization | Eczema | Eczema with a positive SPT | Wheeze | Wheeze with a positive SPT | Rhinitis | Rhinitis with a positive SPT |
| Maternal dietary patterns | |||||||
| VFR | 1.1 (0.9-1.4) | 0.8 (0.6-1.1) | 1.1 (0.8-1.6) | 0.9 (0.7-1.1) | 1.1 (0.8-1.5) | 0.9 (0.7-1.1) | 1.0 (0.7-1.5) |
| SFN | 0.8 (0.6-0.98) | 1.0 (0.7-1.3) | 0.9 (0.6-1.4) | 0.9 (0.7-1.2) | 0.8 (0.5-1.2) | 1.0 (0.7-1.2) | 0.9 (0.6-1.3) |
| PCP | 0.9 (0.8-1.2) | 1.1 (0.9-1.4) | 0.8 (0.6-1.3) | 0.8 (0.7-1.0) | 1.0 (0.7-1.3) | 1.0 (0.8-1.3) | 1.0 (0.7-1.3) |
Adjusted for sex, ethnicity, maternal education levels and family history of allergy
Discussion
In this observational study, we evaluated the effect of early life nutritional influences on allergic outcomes in infancy in a prospective birth cohort in Singapore. We found that maternal dietary pattern during pregnancy affects the risk of subsequent development of allergic diseases in the offspring.
Interestingly, we found that maternal “seafood-noodles” (SfN) dietary pattern intake reduce the risk of allergen sensitization in the children. There was also a trend of inverse association of this dietary pattern with other allergic outcomes although not statistically significant. A possible reason for our observations could be due to fatty acids that come largely from the fish and seafood products under this pattern. Fatty acids may play a role in the protective effect of early immunological development towards atopy sensitisation and allergy development [30]. In addition, the SfN dietary pattern also contains legumes and pulses which had been associated with reduced risk of allergic disease development in the offspring [31]. Supporting evidence for the protective effect of maternal consumption of legumes such as peanuts during pregnancy was showed by a longitudinal birth cohort study from the United States that studied 1277 mother-child pairs and found that higher maternal peanut intake was associated with decreased peanut allergic reaction in children when serum-specific IgE levels were tested in mid-childhood (mean age, 7.9 years) [31] . In addition, the Danish Birth cohort study also reported that maternal intake of peanuts and tree nuts were associated with reduced odds of development of asthma in children [19]. Maternal dietary antigens have been reported to be able to cross the placenta and affect T helper cell differentiation [32,33]. This protective effect may be particular to the food or dietary pattern due to the different composition of nutrients such as linolenic acid that has also been linked with T cell signalling and expression of MHC II molecules [30]. Due to the different nutrient profiles and synergistic effect between different foods in the SFN dietary pattern, the biological mechanism is complex and may involve many candidate nutrients that confer this protective effect. Other candidate nutrients include selenium [34], zinc [35] and vitamin E [36] that have also been associated with a protective effect for allergic diseases, possibly by influencing the T helper1/T helper 2 ratio through effects on cytokine production [37,38].
Similar to our findings, another prospective cohort study conducted in France that analysed 1500 children with follow up until 2 years reported no associations between maternal consumption of shellfish and childhood wheezing and eczema [39]. However, our findings were in contrast to a prospective study conducted in the Netherlands that recruited 2976 mothers with follow up of their offspring until 4 years that reported that maternal consumption of shellfish in the first trimester increased the risk of development of wheezing and eczema [40]. Possible reasons for the difference in our observations could be that we analyse dietary patterns, taking into account potential synergistic effect between food groups while the above mentioned studies looked at single food consumption in relation to the outcomes. In addition, there may also be a difference in genetic constitution and lifestyle between the populations.
Although high maternal intake of fruits and vegetables was found to be protective in developing asthma symptoms in two previous cohort studies [41,42], we did not find any associations between maternal “vegetables, fruits and rice-based” dietary pattern and allergic outcomes in our study. In agreement with our observations, another study which looked at two birth cohorts from Spain and Greece, recruiting 1771 and 745 mother-child pairs respectively, also showed no association between maternal Mediterranean diet during pregnancy and infantile wheeze or eczema at 1 years old [43]. Besides this, a longitudinal cohort study which recruited 1376 mother-child pairs from Project Viva in the United States also found no significant associations between “Prudent” and “Western” dietary patterns (comparable to the Mediterranean diet and processed diet respectively) during pregnancy with recurrent wheeze at 3 years of age [44]. Additionally, a birth cohort with data from 2832 children showed no associations between maternal vegetable consumption and childhood wheeze or asthma at 8 years old [45]. However, a Spanish birth cohort study showed that adherence to Mediterranean diet in pregnancy was associated with a lower risk of persistent wheeze and atopy in 460 children who had skin prick testing at age 6.5 years [8]. This suggests merit in following up the children in our study over a longer term to track atopic sensitizations and allergic outcomes in the future.
The strengths of this study are that we analyse dietary pattern of food consumption which allows holistic analyses of the synergistic effects of foods and nutrients. In addition, this is a prospective birth cohort study with regular collection of information at multiple time points. The collection of maternal dietary information during pregnancy also reduces recall and response bias as this information is collected prior to the assessment of infant allergic outcomes.
However the weaknesses of this study are the incomplete response rates to the questionnaires due to subjects being uncontactable at some point in the study. Another limitation is that some allergic outcomes are parental reported which may be subjected to recall bias and misclassifications. Hence we combined it with an objective assessment of allergen sensitization.
In conclusion, we found evidence that maternal diet during pregnancy play a role in the subsequent development of allergic outcomes in the offspring.
Supplementary Material
Acknowledgements
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. This work is also supported by the National Medical Research Council, NMRC/CSA/022/2010 and NRF370062-HUJ-NUS (Project 10). Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. KMG is funded by the NIHR through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreement numbers 289346 and 613977.
The co-authors acknowledge the contribution of the rest of the GUSTO study group which includes Pratibha Agarwal, Dennis Bier, Arijit Biswas, Shirong Cai, Jerry Kok Yen Chan, Cornelia Yin Ing Chee, Helen Y. H Chen, Audrey Chia, Amutha Chinnadurai, Chai Kiat Chng, Shang Chee Chong, Mei Chien Chua, Chun Ming Ding, Eric Andrew Finkelstein, Doris Fok, Marielle Fortier, Yam Thiam Daniel Goh, Joshua J. Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Joanna D Holbrook, Chin-Ying Hsu, Hazel Inskip, Jeevesh Kapur, Birit Leutscher-Broekman, Sok Bee Lim, Seong Feei Loh, Yen-Ling Low, Iliana Magiati, Lourdes Mary Daniel, Michael Meaney, Susan Morton, Cheryl Ngo, Krishnamoorthy Niduvaje, Anqi Qiu, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Jenny L. Richmond, Anne Rifkin-Graboi, Allan Sheppard, Borys Shuter, Leher Singh, Wing Chee So, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Soek Hui Tan, Rob M. van Dam, Sudhakar K. Venkatesh, Inez Bik Yun Wong, P. C. Wong, George Seow Heong Yeo.
Footnotes
Conflict of interest
Peter D. Gluckman, Keith M. Godfrey, Shek Pei-Chi, Lynette and Chong Yap-Seng have received reimbursement for speaking at conferences sponsored by companies selling nutritional products.
Peter D. Gluckman, Keith M. Godfrey, and Chong Yap-Seng are part of an academic consortium that has received research funding from Abbot Nutrition, Nestec and Danone. Shek Pei-Chi Lynette has received research funding from Danone.
References
- 1.Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, Landrigan PJ, Sly PD, Suk W, Cory Slechta D, Thompson C, et al. Developmental origins of health and disease: Integrating environmental influences. Endocrinology. 2015;156:3416–3421. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harb H, Amarasekera M, Ashley S, Tulic MK, Pfefferle PI, Potaczek DP, Martino D, Kesper DA, Prescott SL, Renz H. Epigenetic regulation in early childhood: A miniaturized and validated method to assess histone acetylation. International archives of allergy and immunology. 2015;168:173–181. doi: 10.1159/000442158. [DOI] [PubMed] [Google Scholar]
- 3.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, Maecker HT, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160:37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterland RA, Kellermayer R, Laritsky E, Rayco-Solon P, Harris RA, Travisano M, Zhang W, Torskaya MS, Zhang J, Shen L, Manary MJ, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS genetics. 2010;6:e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan TK, Palmer DJ, Prescott SL. In-utero exposures and the evolving epidemiology of paediatric allergy. Current opinion in allergy and clinical immunology. 2015;15:402–408. doi: 10.1097/ACI.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 6.West CE, D'Vaz N, Prescott SL. Dietary immunomodulatory factors in the development of immune tolerance. Current allergy and asthma reports. 2011;11:325–333. doi: 10.1007/s11882-011-0200-0. [DOI] [PubMed] [Google Scholar]
- 7.Chatzi L, Apostolaki G, Bibakis I, Skypala I, Bibaki-Liakou V, Tzanakis N, Kogevinas M, Cullinan P. Protective effect of fruits, vegetables and the mediterranean diet on asthma and allergies among children in crete. Thorax. 2007;62:677–683. doi: 10.1136/thx.2006.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatzi L, Torrent M, Romieu I, Garcia-Esteban R, Ferrer C, Vioque J, Kogevinas M, Sunyer J. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507–513. doi: 10.1136/thx.2007.081745. [DOI] [PubMed] [Google Scholar]
- 9.Chatzi L, Kogevinas M. Prenatal and childhood mediterranean diet and the development of asthma and allergies in children. Public health nutrition. 2009;12:1629–1634. doi: 10.1017/S1368980009990474. [DOI] [PubMed] [Google Scholar]
- 10.Grieger JA, Clifton VL, Tuck AR, Wooldridge AL, Robertson SA, Gatford KL. In utero programming of allergic susceptibility. International archives of allergy and immunology. 2016;169:80–92. doi: 10.1159/000443961. [DOI] [PubMed] [Google Scholar]
- 11.Castro-Rodriguez JA, Garcia-Marcos L, Alfonseda Rojas JD, Valverde-Molina J, Sanchez-Solis M. Mediterranean diet as a protective factor for wheezing in preschool children. The Journal of pediatrics. 2008;152:823–828. doi: 10.1016/j.jpeds.2008.01.003. 828 e821-822. [DOI] [PubMed] [Google Scholar]
- 12.de Batlle J, Garcia-Aymerich J, Barraza-Villarreal A, Anto JM, Romieu I. Mediterranean diet is associated with reduced asthma and rhinitis in mexican children. Allergy. 2008;63:1310–1316. doi: 10.1111/j.1398-9995.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 13.Rice JL, Romero KM, Galvez Davila RM, Meza CT, Bilderback A, Williams DL, Breysse PN, Bose S, Checkley W, Hansel NN, Investigators GS Association between adherence to the mediterranean diet and asthma in peruvian children. Lung. 2015;193:893–899. doi: 10.1007/s00408-015-9792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sicherer SH, Wood RA, Stablein D, Lindblad R, Burks AW, Liu AH, Jones SM, Fleischer DM, Leung DY, Sampson HA. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. The Journal of allergy and clinical immunology. 2010;126:1191–1197. doi: 10.1016/j.jaci.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann ME, Dannemann A, Gruters A, Radisch B, Dudenhausen JW, Bergmann R, Coumbos A, Weitzel HK, Wahn U. Prospective study of the atopy preventive effect of maternal avoidance of milk and eggs during pregnancy and lactation. European journal of pediatrics. 1996;155:770–774. doi: 10.1007/BF02002904. [DOI] [PubMed] [Google Scholar]
- 16.Erkkola M, Nwaru BI, Kaila M, Kronberg-Kippila C, Ilonen J, Simell O, Veijola R, Knip M, Virtanen SM. Risk of asthma and allergic outcomes in the offspring in relation to maternal food consumption during pregnancy: A finnish birth cohort study. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2012;23:186–194. doi: 10.1111/j.1399-3038.2012.01272.x. [DOI] [PubMed] [Google Scholar]
- 17.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, Arshad SH, Dean T. Factors associated with maternal dietary intake, feeding and weaning practices, and the development of food hypersensitivity in the infant. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2009;20:320–327. doi: 10.1111/j.1399-3038.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 18.Nwaru BI, Ahonen S, Kaila M, Erkkola M, Haapala AM, Kronberg-Kippila C, Veijola R, Ilonen J, Simell O, Knip M, Virtanen SM. Maternal diet during pregnancy and allergic sensitization in the offspring by 5 yrs of age: A prospective cohort study. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2010;21:29–37. doi: 10.1111/j.1399-3038.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 19.Maslova E, Granstrom C, Hansen S, Petersen SB, Strom M, Willett WC, Olsen SF. Peanut and tree nut consumption during pregnancy and allergic disease in children-should mothers decrease their intake? Longitudinal evidence from the danish national birth cohort. The Journal of allergy and clinical immunology. 2012;130:724–732. doi: 10.1016/j.jaci.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Hadley C. Food allergies on the rise? Determining the prevalence of food allergies, and how quickly it is increasing, is the first step in tackling the problem. EMBO reports. 2006;7:1080–1083. doi: 10.1038/sj.embor.7400846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soh SE, Lee SS, Hoon SW, Tan MY, Goh A, Lee BW, Shek LP, Teoh OH, Kwek K, Saw SM, Godfrey K, et al. The methodology of the gusto cohort study: A novel approach in studying pediatric allergy. Asia Pacific allergy. 2012;2:144–148. doi: 10.5415/apallergy.2012.2.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stunkel W, Holbrook JD, Kwek K, Chong YS, Saw SM. Cohort profile: Growing up in singapore towards healthy outcomes (gusto) birth cohort study. International journal of epidemiology. 2013 doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 23.Chong MF, Chia AR, Colega M, Tint MT, Aris IM, Chong YS, Gluckman P, Godfrey KM, Kwek K, Saw SM, Yap F, et al. Maternal protein intake during pregnancy is not associated with offspring birth weight in a multiethnic asian population. The Journal of nutrition. 2015;145:1303–1310. doi: 10.3945/jn.114.205948. [DOI] [PubMed] [Google Scholar]
- 24.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the us department of agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. The American journal of clinical nutrition. 2003;77:1171–1178. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 25.de Seymour J, Chia A, Colega M, Jones B, McKenzie E, Shirong C, Godfrey K, Kwek K, Saw SM, Conlon C, Chong YS, et al. Maternal dietary patterns and gestational diabetes mellitus in a multi-ethnic asian cohort: The gusto study. Nutrients. 2016;8 doi: 10.3390/nu8090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyake Y, Okubo H, Sasaki S, Tanaka K, Hirota Y. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in japanese infants aged 16-24 months: The osaka maternal and child health study. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2011;22:734–741. doi: 10.1111/j.1399-3038.2011.01176.x. [DOI] [PubMed] [Google Scholar]
- 27.Shaheen SO, Northstone K, Newson RB, Emmett PM, Sherriff A, Henderson AJ. Dietary patterns in pregnancy and respiratory and atopic outcomes in childhood. Thorax. 2009;64:411–417. doi: 10.1136/thx.2008.104703. [DOI] [PubMed] [Google Scholar]
- 28.Smithers LG, Brazionis L, Golley RK, Mittinty MN, Northstone K, Emmett P, McNaughton SA, Campbell KJ, Lynch JW. Associations between dietary patterns at 6 and 15 months of age and sociodemographic factors. European journal of clinical nutrition. 2012;66:658–666. doi: 10.1038/ejcn.2011.219. [DOI] [PubMed] [Google Scholar]
- 29.Oddy WH, de Klerk NH, Sly PD, Holt PG. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. The European respiratory journal. 2002;19:899–905. doi: 10.1183/09031936.02.00103602. [DOI] [PubMed] [Google Scholar]
- 30.Yaqoob P, Calder PC. Fatty acids and immune function: New insights into mechanisms. The British journal of nutrition. 2007;98(Suppl 1):S41–45. doi: 10.1017/S0007114507832995. [DOI] [PubMed] [Google Scholar]
- 31.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Camargo CA, Jr, Gillman MW, Gold DR, Litonjua AA. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. The Journal of allergy and clinical immunology. 2014;133:1373–1382. doi: 10.1016/j.jaci.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devereux G. The increase in the prevalence of asthma and allergy: Food for thought. Nature reviews Immunology. 2006;6:869–874. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 33.Loibichler C, Pichler J, Gerstmayr M, Bohle B, Kisst H, Urbanek R, Szepfalusi Z. Materno-fetal passage of nutritive and inhalant allergens across placentas of term and pre-term deliveries perfused in vitro. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2002;32:1546–1551. doi: 10.1046/j.1365-2222.2002.01479.x. [DOI] [PubMed] [Google Scholar]
- 34.Devereux G, McNeill G, Newman G, Turner S, Craig L, Martindale S, Helms P, Seaton A. Early childhood wheezing symptoms in relation to plasma selenium in pregnant mothers and neonates. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2007;37:1000–1008. doi: 10.1111/j.1365-2222.2007.02757.x. [DOI] [PubMed] [Google Scholar]
- 35.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA, Jr, Weiss ST, Gillman MW, Gold DR. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. The American journal of clinical nutrition. 2006;84:903–911. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devereux G, Turner SW, Craig LC, McNeill G, Martindale S, Harbour PJ, Helms PJ, Seaton A. Low maternal vitamin e intake during pregnancy is associated with asthma in 5-year-old children. American journal of respiratory and critical care medicine. 2006;174:499–507. doi: 10.1164/rccm.200512-1946OC. [DOI] [PubMed] [Google Scholar]
- 37.Malmberg KJ, Lenkei R, Petersson M, Ohlum T, Ichihara F, Glimelius B, Frodin JE, Masucci G, Kiessling R. A short-term dietary supplementation of high doses of vitamin e increases t helper 1 cytokine production in patients with advanced colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:1772–1778. [PubMed] [Google Scholar]
- 38.Li-Weber M, Giaisi M, Treiber MK, Krammer PH. Vitamin e inhibits il-4 gene expression in peripheral blood t cells. European journal of immunology. 2002;32:2401–2408. doi: 10.1002/1521-4141(200209)32:9<2401::AID-IMMU2401>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Pele F, Bajeux E, Gendron H, Monfort C, Rouget F, Multigner L, Viel JF, Cordier S. Maternal fish and shellfish consumption and wheeze, eczema and food allergy at age two: A prospective cohort study in brittany, france. Environmental health : a global access science source. 2013;12:102. doi: 10.1186/1476-069X-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leermakers ET, Sonnenschein-van der Voort AM, Heppe DH, de Jongste JC, Moll HA, Franco OH, Hofman A, Jaddoe VW, Duijts L. Maternal fish consumption during pregnancy and risks of wheezing and eczema in childhood: The generation r study. European journal of clinical nutrition. 2013;67:353–359. doi: 10.1038/ejcn.2013.36. [DOI] [PubMed] [Google Scholar]
- 41.Fitzsimon N, Fallon U, O'Mahony D, Loftus BG, Bury G, Murphy AW, Kelleher CC, Lifeways Cross Generation Cohort Study Steering G Mothers' dietary patterns during pregnancy and risk of asthma symptoms in children at 3 years. Irish medical journal. 2007;100:suppl 27–32. [PubMed] [Google Scholar]
- 42.Willers SM, Devereux G, Craig LC, McNeill G, Wijga AH, Abou El-Magd W, Turner SW, Helms PJ, Seaton A. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007;62:773–779. doi: 10.1136/thx.2006.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatzi L, Garcia R, Roumeliotaki T, Basterrechea M, Begiristain H, Iniguez C, Vioque J, Kogevinas M, Sunyer J, group Is, group Rs Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: Inma (spain) and rhea (greece) mother-child cohort studies. The British journal of nutrition. 2013;110:2058–2068. doi: 10.1017/S0007114513001426. [DOI] [PubMed] [Google Scholar]
- 44.Lange NE, Rifas-Shiman SL, Camargo CA, Jr, Gold DR, Gillman MW, Litonjua AA. Maternal dietary pattern during pregnancy is not associated with recurrent wheeze in children. The Journal of allergy and clinical immunology. 2010;126:250–255. doi: 10.1016/j.jaci.2010.05.009. 255 e251-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willers SM, Wijga AH, Brunekreef B, Kerkhof M, Gerritsen J, Hoekstra MO, de Jongste JC, Smit HA. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. American journal of respiratory and critical care medicine. 2008;178:124–131. doi: 10.1164/rccm.200710-1544OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.