Abstract
Spontaneous Ca2+ waves, also termed store-overload-induced Ca2+ release (SOICR), in cardiac cells can trigger ventricular arrhythmias especially in failing hearts. SOICR occurs when RyRs are activated by an increase in sarcoplasmic reticulum (SR) luminal Ca2+. Carvedilol is one of the most effective drugs for preventing arrhythmias in patients with heart failure. Furthermore, carvedilol analogues with minimal β-blocking activity also block SOICR showing that SOICR-inhibiting activity is distinct from that for β-block. We show here that carvedilol is a potent inhibitor of cADPR-induced Ca2+ release in sea urchin egg homogenate. In addition, the carvedilol analog VK-II-86 with minimal β-blocking activity also suppresses cADPR-induced Ca2+ release. Carvedilol appeared to be a non-competitive antagonist of cADPR and could also suppress Ca2+ release by caffeine. These results are consistent with cADPR releasing Ca2+ in sea urchin eggs by sensitizing RyRs to Ca2+ involving a luminal Ca2+ activation mechanism. In addition to action on the RyR, we also observed inhibition of inositol 1,4,5-trisphosphate (IP3)-induced Ca2+ release by carvedilol suggesting a common mechanism between these evolutionarily related and conserved Ca2+ release channels.
Introduction
Calcium (Ca2+) is a highly versatile intracellular signal that regulates a wide range of cellular functions, including exocytosis, contraction, metabolism, transcription, fertilization and cell proliferation1. Sources for Ca2+ signaling include both influx through channels at the plasma membrane and Ca2+ mobilization from Ca2+ storage organelles. A key question is how the ubiquitous Ca2+ signaling ion can control cellular processes with a high degree of specificity. One potential solution is the realization that there are multiple Ca2+ mobilizing messengers targeting specific Ca2+ release channels in distinct organelles. Three molecules have satisfied all the criteria for assignment as Ca2+ mobilizing second messengers. These are IP3, and the pyridine nucleotide metabolites, cADPR and NAADP 2. The first two molecules target Ca2+ release channels in the cell’s largest Ca2+ storage organelle, the endoplasmic reticulum (ER), whilst NAADP releases Ca2+ from organelles of the endolysosomal system likely through two-pore channels (TPCs) 3, 4.
IP3R and RyR channels are the evolutionarily related principal sarco-endoplasmic Ca2+ release channels mediating Ca2+ mobilization from this organelle in response to various stimuli 5. These channels are the main mediators of Ca2+-induced Ca2+release (CICR) 6, an autocatalytic mechanism by which cytoplasmic Ca2+ activates the release of Ca2+ from internal stores. This mechanism contributes to the globalization of intracellular Ca2+ signals in cells including propagating Ca2+ waves, since in the absence of such mechanisms buffering mechanisms greatly restrict the spatial diffusion of Ca2+ signals making them inherently local. IP3 and cADPR are thought to evoke openings of channels by sensitizing them to Ca2+ as a co-agonist 6. Two principal modes for triggering CICR have been proposed. The first is a cytosolic mode whereby an increase in cytosolic Ca2+ may activate Ca2+ release channels. The second is a luminal mode whereby an increase in intraluminal Ca2+ concentrations trigger the opening of Ca2+ release channels. The latter is associated with the phenomenon of spontaneous Ca2+ release from ER/SR7–9, which has been proposed as an important mechanism underlying various cardiac arrhythmias 10.
cADPR was first identified as a Ca2+ mobilizing molecule in sea urchin eggs and homogenates 11. Previous work had indicated that NAD was enzymatically converted to a Ca2+ mobilizing agent in sea urchin eggs and homogenates 12. Pharmacological studies showed that cADPR targeted RyRs but not IP3Rs based on cross-desensitization with RyR activators and inhibition by RyR blockers 13. Furthermore, both divalent cations and the RyR pharmacological activator caffeine, potentiate Ca2+ release by cADPR 14. Subsequent studies in many mammalian cell types support the link between cADPR and RyRs and, in cardiac cells, cADPR promotes the production of Ca2+ sparks and regulates contractility 15 16. An excess of cADPR may be pro-arrhythmic 16, and an inhibitor of the enzyme that synthesizes cADPR is proposed as a novel anti-arrhythmic drug 17. In summary, cADPR is considered a second messenger that acts by sensitizing RyRs to CICR 18.
It has long been recognized that spontaneous Ca2+ release occurs during SR Ca2+ overload, a process also known as store-overload induced Ca2+ release (SOICR). This is a luminal mechanism whereby as the ER (or SR) fills with Ca2+ when the intra-luminal Ca2+ concentration or amount reaches a certain level or threshold, the opening of RyRs is triggered 10. Such mechanisms have been suspected from studies on spontaneous Ca2+ release from the SR 7–9, but Chen and his colleagues demonstrated a drug that could block the process 19 and also pinpointed the molecular mechanism by which luminal Ca2+ triggers SOICR 10. Such spontaneous Ca2+ release may lead to propagating Ca2+ waves, essential for activation of eggs at fertilization, but potentially fatal in cardiac myocytes since they underlie arrhythmias 20. Chen and his colleagues proposed that, uniquely amongst β-blockers, carvedilol suppresses SOICR itself, in addition to its β-blocking action 19. Luminal amino-acid residues of the RyR have been identified as critical for SOICR, and these include the E4872 residue, which is highly conserved not only in RyR between species but also in IP3Rs 10. The proposal is that the RyR itself acts as a luminal Ca2+ sensor, rather than an accessory or interacting protein such as calsequestrin, as SOICR can persist in calsequestrin-null mice 21. The E4872Q mutation in a mouse model suppressed Ca2+ waves and ventricular tachyarrhythmias (VTs) 10.
It has been shown that, compared to 14 other known β-blockers, carvedilol was the only one effective in inhibiting SOICR 19. This unique inhibitory action of carvedilol on SOICR needs further investigation, as this activity would be contributing to its β-blocking action. Single-channel recordings in lipid bilayers revealed that carvedilol has an impact on the gating properties of RyR2, reducing the duration and increased the frequency of channel openings. In this study, we have utilized the sea urchin egg homogenate preparation 12 which robustly express both IP3R and RyR Ca2+ release mechanisms on microsomal vesicles 22. Furthermore, ER-derived microsomes in sea urchin egg homogenates display spontaneous Ca2+ release due to Ca2+ overload, which can be effected by both IP3Rs and RyRs 9. This allowed us to examine the effect of carvedilol and analogs on SOICR mediated by the two principal ER-based Ca2+ release mechanisms. Although the interaction of IP3 with IP3Rs has now been elucidated at the molecular level 23, the mechanism by which cADPR activates RyRs is not well understood, with accessory cADPR binding proteins proposed 24–26. However, since cADPR is known to cause Ca2+ release by modulating RyRs 13, 14, it allows us to probe the role of SOICR in the mechanism by which cADPR activates RyRs. We find that carvedilol suppresses cADPR-evoked Ca2+ release, and also attenuates IP3-mediated Ca2+ release. A common mechanism by which carvedilol inhibits a conserved luminal Ca2+ gate for both Ca2+ release mechanisms is suggested.
Materials and Methods
Sea urchin egg homogenate preparation
Gamete shedding was stimulated by intracoelomic injections of 0.5 M KCl solution and sea urchin eggs from Lytechinus pictus were collected. Collection was carried out in artificial sea water (ASW). ASW: 435 mM NaCl, 10 mM KCl, 40 mM MgCl2, 15mM MgSO4, 11 mM CaCl2, 2.5 mM NaHCO3, 7 mM Tris base, 13 mM Tris-HCl, pH 8.0. Eggs were de-jellied by passage through 100 μM nylon mesh (Millipore), and then washed 4 times in Ca2+ -free ASW, with the first two washes containing 1 mM EGTA. Eggs were subsequently washed in intracellular-like medium, glucamine intracellular medium (GluIM). GluIM: 250 mM potassium gluconate, 250 mM N-methyl-D-glucamine, 20 mM Hepes (acid), and 1 mM MgCl2, pH 7.2 (pH adjusted with glacial acetic acid). Eggs were homogenized with a glass Dounce tissue homogenizer in ice-cold GluIM supplemented with 2 mM MgATP, 20 U/mL creatine phosphokinase (CPK), 20 mM phosphocreatine (PCr), Complete ™EDTA-free Protease Inhibitor tablets were from Roche. Homogenate (50%, v/v) was then centrifuged at 13,000 x g at 4 °C for 10 s. The supernatant were aliquoted into 0.5 ml portions and stored at -80 °C.
Fluorimetry to measure Ca2+ release
To measure Ca2+ changes, egg homogenate was diluted gradually 20-fold over 3 h in GluIM at 17°C supplemented with the ATP regenerating system (to facilitate optimal Ca2+ loading of the stores), fluorescent dyes were added (Fluo-3, 3 μM final) and fluorescence changes monitored in a 600 μl aliquot (2.5% v/v) in a stirred micro-cuvette in a Perkin Elmer LS-50B fluorimeter.
Fluo-3 was excited at 506 ± 3 nm and 526 ± 2 nm emission recorded.
The Fluo-3 fluorescence signal was converted to absolute Ca2+ concentrations by sequential addition of 0.5 mM EGTA and 10 mM CaCl2 to determine Fmin and Fmax, using the following equation:
where [Ca2+] is the extravesicular Ca2+ concentration, F is the fluorescence at any time, Fmin and Fmax are the fluorescence in the “absence” of Ca2+ and presence of saturating Ca2+, respectively. At the end of each experiment, Fmin and Fmax were empirically determined by the sequential addition of 500 μM EGTA and 10 mM Ca2+, respectively (6 μL of 50 mM EGTA; 6 μL of 1 M CaCl2).
For TPEN studies of effects on the kinetics of Ca2+ release, fluorescence (in units (U)/s) was normalized to the resting fluorescence (F0) to account for variability and therefore expressed as U.F0/s.
Source of reagents
cADPR and IP3 were purchased from Sigma-Aldrich and LC Laboratories, respectively. Fluo-3 (K+ salt) was purchased from Invitrogen and TPEN from Sigma-Aldrich. Carvedilol was purchased from Abcam Biochemicals. VK-II-86, the carvedilol analog, was synthesized as described previously19.
Statistical analysis
Two data sets were compared using Student’s t test and multiple groups using ANOVA and Dunnett’s multiple comparisons post test. Data were paired when needed and statistical significance assumed at P<0.05. Graphs were constructed and the following statistical conventions observed: P<0.05 (*), P<0.01 (**), P<0.001 (***), P<0.0001 (****) using Graphpad Prism. Representative fluorimetric traces were plotted as raw fluorescence (RFU) against time.
Results
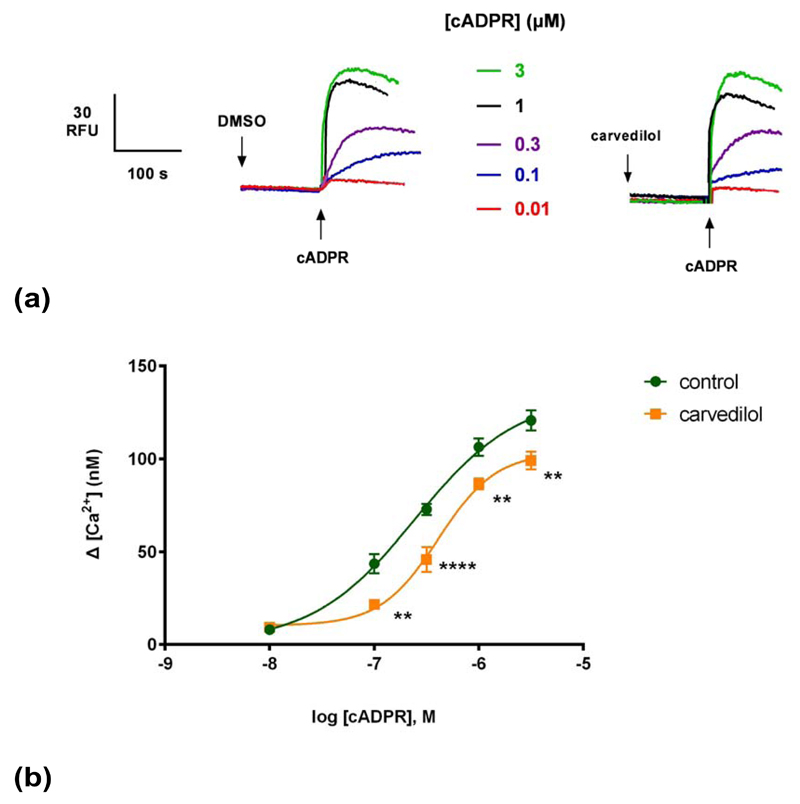
To gain further insight into the mechanism of cADPR-induced Ca2+ release, we examined the effect of carvedilol on Ca2+ release mechanisms in in sea urchin egg homogenates. cADPR-induced Ca2+ release was monitored in the presence of vehicle DMSO or carvedilol. A sub-maximal concentration of cADPR (0.3 μM) for Ca2+ release was determined by establishing a concentration-response curve of cADPR (EC50 ~ 0.25 μM; Fig. 2b). Preincubation with carvedilol for 2 minutes resulted in a concentration-dependent inhibition of cADPR-induced Ca2+ release with an approximate IC50 of 30 μM (Fig. 1b).
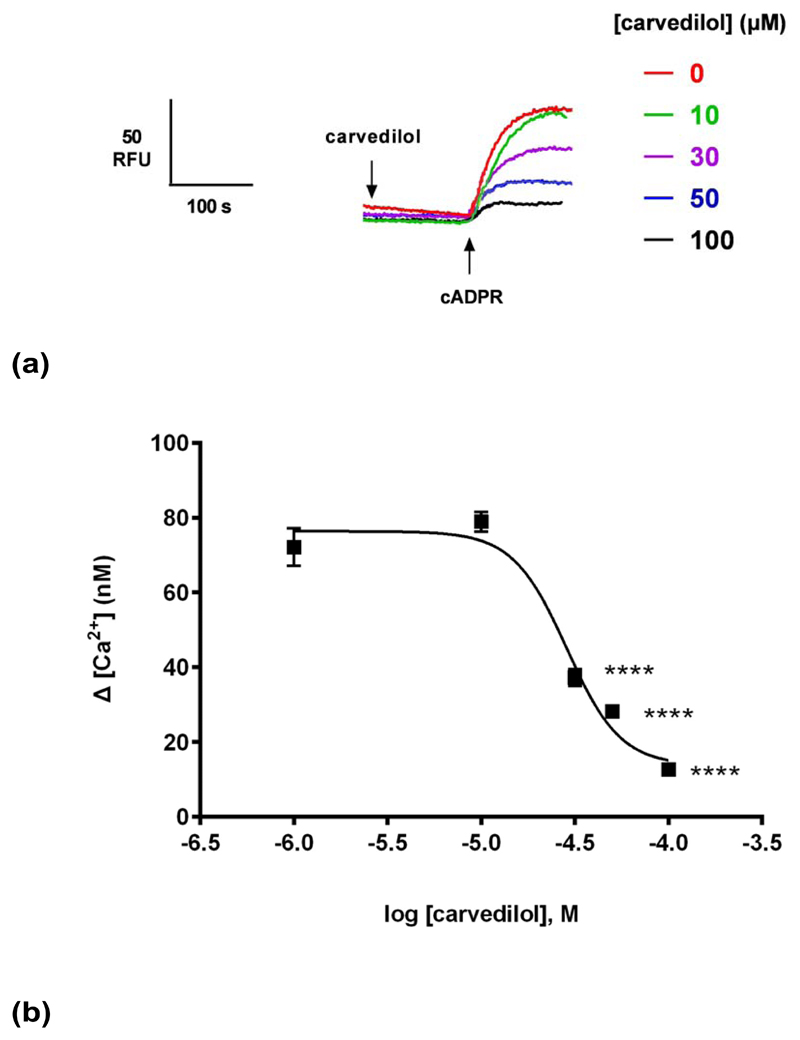
Fig. 2. Carvedilol inhibits cADPR-induced Ca2+ release.
(a) Summarized representative fluorimetric traces. (b) DMSO or 30 μM of carvedilol were preincubated for 2 minutes before the addition of different cADPR concentrations. Statistical significance was determined using a Student’s t test comparing control (n = 9) and carvedilol (n = 9) (b). Data were fit as a sigmoidal concentration-response (b). Data are represented as mean ± SEM.
Fig. 1. Carvedilol suppresses cADPR-induced Ca2+ release.
(a) Different concentrations of carvedilol or DMSO were preincubated for 2 minutes with a subsequent addition of a sub-maximal concentration of cADPR (0.3μM) and the raw control response of cADPR was 72 ± 5 nM (n = 9). Summarized representative fluorimetric traces shown in (a) and data fit as a sigmoidal concentration-response shown in (b). Statistical significance was determined with a Dunnett’s test in comparison to control response without carvedilol (b). Data are represented as mean ± SEM.
Generating a cADPR concentration-response curve in the presence or absence of a sub-maximal concentration of carvedilol revealed that carvedilol reduced the maximal response to cADPR without substantially changing the EC50 for cADPR (Fig. 2b), i.e. was likely a non-competitive blocker. The EC50 of cADPR with DMSO (and 95% confidence interval) was 250 nM (153–391 nM) and in the presence of carvedilol it was 410 nM (297–564 nM) (P>0.05).
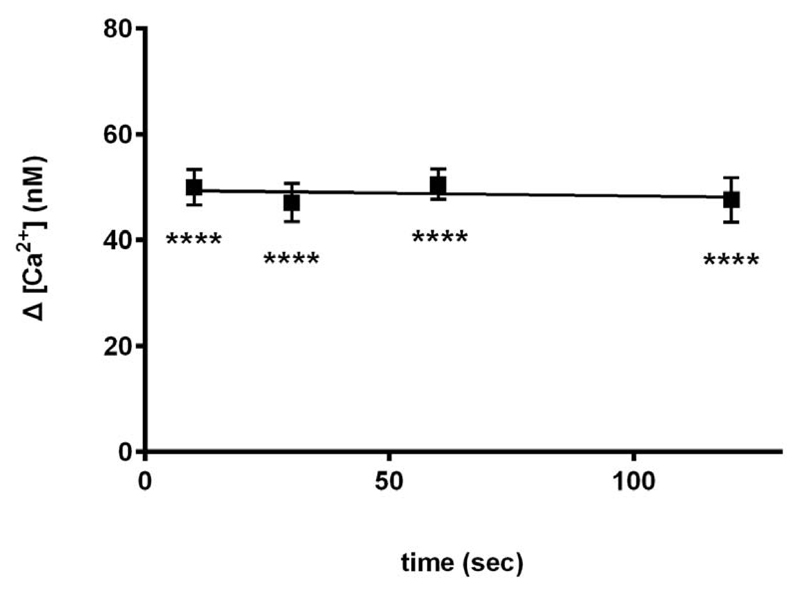
Next we examined the time course of inhibition by carvedilol. Carvedilol (30 μM) was preincubated with homogenate for 10, 30, 60 and 120 seconds. Our results show that carvedilol suppressed cADPR-induced Ca2+ release maximally within 10 seconds, and there was no further inhibition at longer incubations (Fig. 3).
Fig. 3. Carvedilol suppresses cADPR-induced Ca2+ release rapidly.
Effect of 30 μM carvedilol after preincubation with carvedilol for 10 s, 30 s, 60 s and 100 s is shown. Uncalibrated control ∆[ Ca2+ ] was 76 ±4 nM (n = 9). Statistical significance was determined using Dunnett’s test versus 0 μM of carvedilol. Data are represented as mean ±SEM.
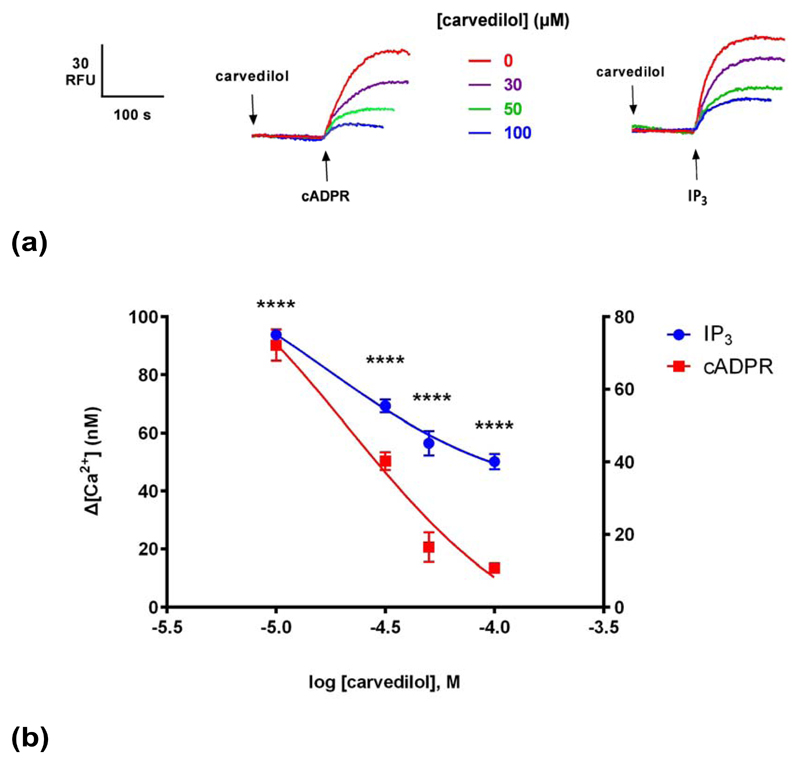
In order to investigate whether the effect of carvedilol was unique to cADPR- induced Ca2+ release and the RyR, we examined the effects of carvedilol on IP3-induced Ca2+ release. The proposed SOICR trigger of RyRs has been proposed to be conserved in IP3Rs 10. Carvedilol also suppressed IP3-induced Ca2+ release but the extent of inhibition of IP3 was less than that for cADPR (Fig. 4).
Fig. 4. Carvedilol inhibits IP3-induced Ca2+ release.
(a) Different concentrations of carvedilol were preincubated for 2 minutes before the addition of sub-maximal concentrations of either IP3 or cADPR (0.3 μM), which were determined by establishing dose-response curves. Raw control ∆[ Ca2+ ] with cADPR was 72 ±4 nM (n = 4–7) and with IP3 94 ±1 nM (n = 4–7).(b) Graph summarizing the results. Right Y axis applies for IP3, whereas left Y axis for cADPR. Statistical significance was determined using Student’s t test and data were fit as a sigmoidal concentration-response. Data are represented as mean ±SEM.
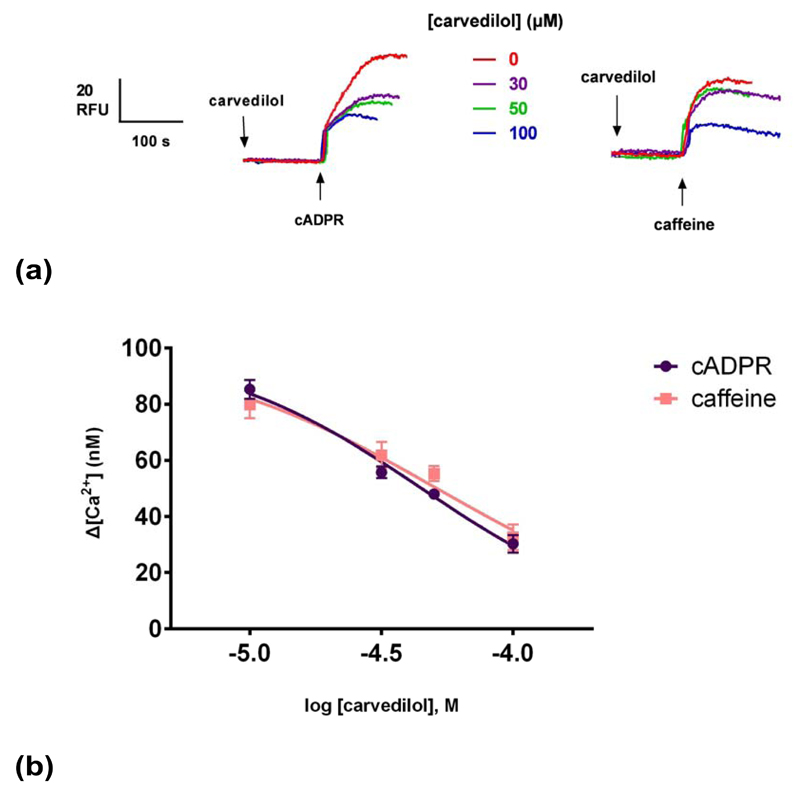
Caffeine is also a commonly used pharmacological RyR agonist 27. Caffeine has also shown to release Ca2+ in sea urchin egg homogenates and eggs by activating RyRs 13, 28. We compared the effects of carvedilol on cADPR- and caffeine-induced Ca2+ release. Different concentrations of carvedilol were preincubated for 2 minutes prior to the addition of 10 mM caffeine13 or 0.3 μM cADPR (Fig. 5a). Carvedilol suppressed caffeine-induced Ca2+ release with an IC50 indistinguishable from to cADPR with IC50s of 45 μM (for cADPR) and 51 μM for caffeine. The differences were not statistically significant.
Fig. 5. Carvedilol suppresses caffeine-induced Ca2+ release.
Different concentrations of carvedilol were preincubated for 2 minutes before the addition of sub-maximal concentrations of either caffeine (10 mM) or cADPR (0.3 μM). Raw control ∆[Ca2+ ] with caffeine was 80 ±5 nM (n = 4–7) and with cADPR 85 ±3 (n = 4–7). Statistical significance (P>0.05) was determined using Student’s t test and data were fit as a sigmoidal concentration-response. Summarized representative fluorimetric traces are shown in (a). Data are represented as mean ±SEM.
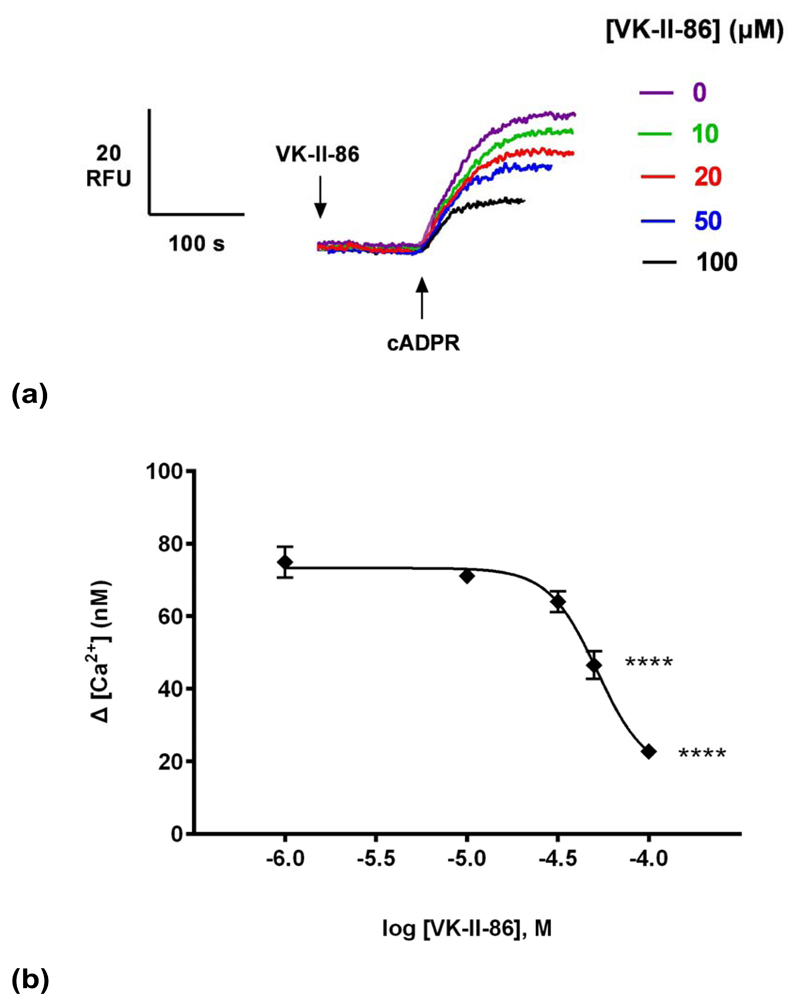
VK-II-86 is a carvedilol analog but with minimal β-blocking activity, demonstrating that the pharmacophore for SOICR inhibition can be separated from that for β-blockade 19, 29. Like Carvedilol, VK-II-86 inhibited cADPR-induced Ca2+ release in a concentration-dependent manner (IC50 of 52 μM; Fig. 6b), demonstrating that the β-blocking activity of carvedilol was dispensable for blocking Ca2+ release in homogenates.
Fig. 6. VK-II-86 suppresses cADPR-induced Ca2+ release.
(a) Different concentrations of VK-II-86 or DMSO were preincubated for 2 minutes with a subsequent addition of a sub-maximal concentration of cADPR (0.3 μM) and the raw control response of cADPR was 75 ±4 nM (n = 9). Summarized representative fluorimetric traces shown in (a) and data fit as a sigmoidal concentration-response shown in (b). Statistical significance was determined with a Dunnett’s test in comparison to control response without carvedilol (b). Data are represented as mean ±SEM.
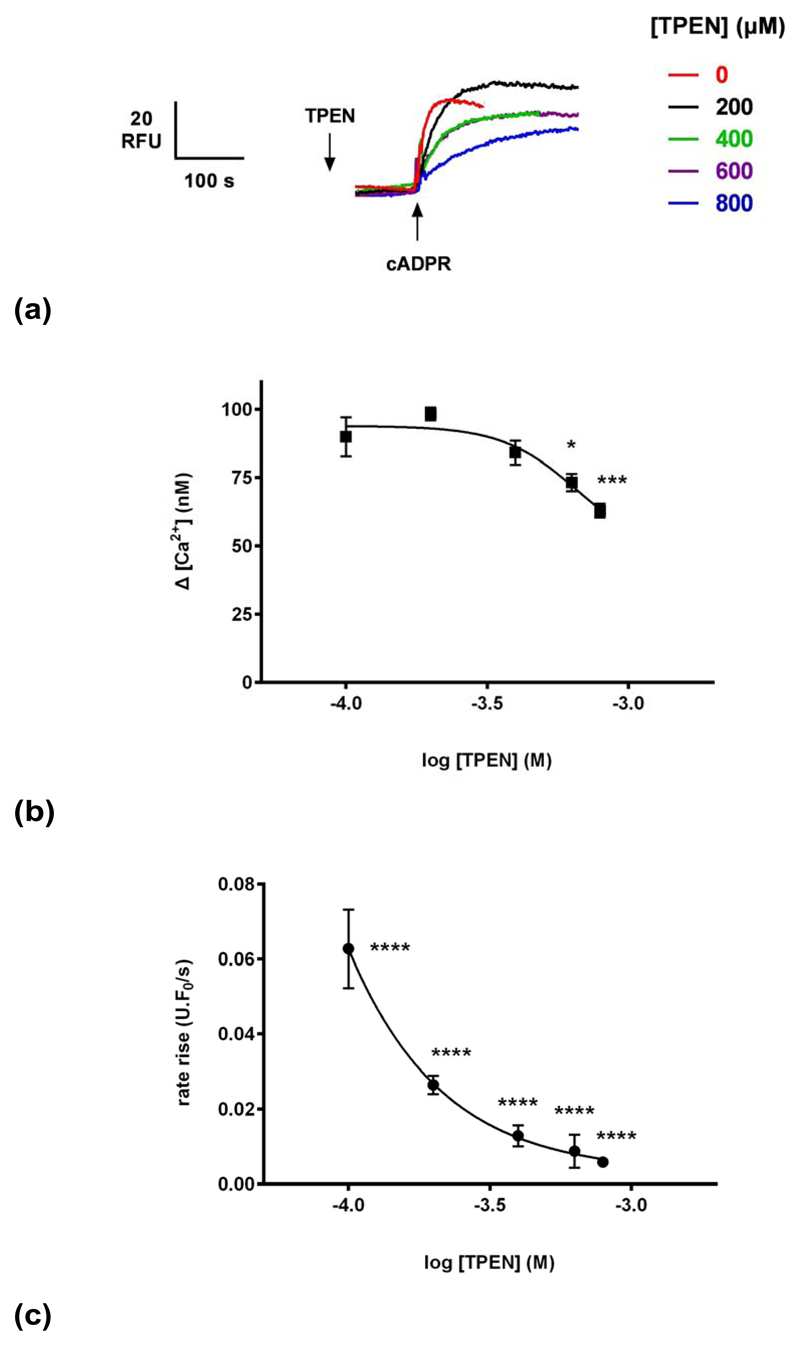
To investigate the proposed luminal mechanism of action of carvedilol, the membrane-permeant low affinity Ca2+ chelator TPEN (N,N,N’,N’- tetrakis(2-pyridylmethyl)ethylenediamine) was employed to allow the manipulation of luminal Ca2+ as previously shown 30. Different concentrations of TPEN (or ethanol vehicle) were preincubated with homogenates for 2 minutes prior to the addition of 0.3 μM cADPR (Fig. 7a). We found that TPEN inhibited cADPR-induced Ca2+ release, both in terms of the magnitude and kinetics of response but have a greater impact on kinetics (estimated carvedilol IC50 of 9 μM and 663 μM for kinetics (Fig. 7c) and magnitude respectively (Fig. 7b). The inhibition by TPEN is consistent with a perturbation of luminal Ca2+ as previously observed for IP3 30. However, the effect of TPEN and carvedilol are dissimilar, as the kinetic data may indicate an additional site of action for carvedilol, and which at high concentrations almost completely inhibits Ca2+ release in response to cADPR.
Fig. 7. TPEN inhibits cADPR-induced Ca2+ release.
(a) Different concentrations of TPEN (or vehicle ethanol) were preincubated with egg homogenate for 2 minutes prior to the addition of 0.3 μM cADPR.
(b) TPEN reduces the magnitude of cADPR-evoked Ca2+ release.
(c) TPEN affects the kinetics of cADPR-evoked Ca2+ release. Fluorescence (in units (U)/s) was normalized to the resting fluorescence (F0) to account for variability and therefore expressed as U.F0/s. Statistical significance was determined using Student’s t test and data were fit as a sigmoidal concentration-response. Data are represented as mean ±SEM.
Discussion
In the present study we have demonstrated that carvedilol inhibits cADPR-induced Ca2+ release in sea urchin egg homogenates. cADPR has been characterized as a second messenger regulating the sensitivity of RyRs to activation by Ca2+ in sea urchin eggs 13, 14, and widely in plant and animal cells 2. However, the mechanism of action of cADPR, from sea urchin egg to cardiac cells, remains undefined 31. A possible mechanism is for cADPR to activate the RyR indirectly by binding to a protein that is part of the RyR macromolecular complex or to a protein that either translocates itself to the RyR or influences a signalling pathway that regulates RyR activity 26. Another possible mechanism is that cADPR stimulates an increase in the level of SR Ca2+, perhaps with the involvement of SERCA 31, resulting in a Ca2+ overload response. Due to the recent elucidation of the action of carvedilol actions on RyRs, we tested the effect of this drug on cADPR-induced Ca2+ release in sea urchin egg homogenates. We found that carvedilol was an effective inhibitor of cADPR-induced Ca2+ release. Carvedilol caused a substantial inhibition (82%) of cADPR-induced Ca2+ release (Fig. 1). This is unlikely to be due to blocking the cADPR binding site since carvedilol inhibition is likely non-competitive, and carvedilol also inhibits caffeine and also IP3-induced Ca2+ release. It is known that although cADPR, caffeine and ryanodine can release Ca2+ by activating RyRs, a selective inhibitor of cADPR, 8-NH2-cADPR does not block Ca2+ release by caffeine or ryanodine implying action at different but interacting sites 32. Therefore we propose also that carvedilol and cADPR act at distinct sites.
VK-II-86 also was found to suppress cADPR-induced Ca2+ release and this is consistent with the notion that the effect of carvedilol on cADPR-evoked Ca2+ release is independent of its β-blocking activity.
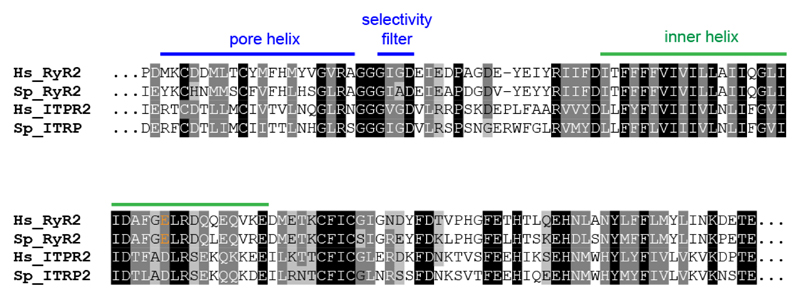
It has been proposed that carvedilol inhibits spontaneous Ca2+ release from the SR by a mechanism dependent on a luminal trigger site on RyRs. Amino acid residues important for such triggering have been identified and include a cluster of negatively charged residues at the C-terminal part of the predicted inner helix of the RyR 10. These include the residue E4872 conserved between RyRs, including that for sea urchins (Fig. 8). Importantly, acidic residues at the putative luminal sensor are also conserved in sea urchin IP3Rs. We have shown for the first time that carvedilol also inhibits IP3-induced Ca2+ release in urchin homogenates (Fig. 4), although to a lesser degree (47% versus >80%) than that for either cADPR or caffeine-induced Ca2+ release. Since IP3-mediated Ca2+ signaling has also been implicated in arrhthymogenesis 33, this may also be pertinent to the clinical effects of carvedilol. Our novel finding that carvedilol can suppress IP3-induced Ca2+ release also suggests that carvedilol may be a potential therapeutic agent for treating other IP3R-associated disorders in addition 34.
Fig. 8. Key amino acid residues for the proposed luminal Ca2+ sensor of RyRs and IP3Rs are conserved in sea urchins.
Alignment of amino acid sequences of the pore and inner helix region of RyR2 and IP3R2 from human (Hs) and sea urchin (Sp; Strongylocentrotus purpuratus). The acidic nature of residue E4872 (in orange) is conserved between RyR2 and IP3R2. Sequences (HsRyR2: Q92736; SpRyR2: XP_011670306; HsITPR2: Q14571; SpITPR2: XP_011682423) were aligned using Clustal Omega.
A luminal Ca2+ regulation site is consistent with the inhibition of a low affinity membrane-permeant Ca2+ chelator, TPEN. Not only do IP3Rs and RyRs share the conserved acidic residues, but they also share sensitivity to TPEN 30 (and this study). Moreover, since TPEN by modulating luminal Ca2+ similarly inhibits Ca2+ release by both IP3 and cADPR in a similar way to carvedilol, a common mechanism based on a luminal mechanism is suggested. We previously have proposed that TPEN does not simply reduce the electrochemical gradient for Ca2+ because ionomycin-evoked Ca2+ release is relatively insensitive to TPEN 30. Again, this implies a modulatory role of TPEN on Ca2+ release channels by lowering luminal Ca2+, whereas carvedilol has a similar effect by blocking the luminal Ca2+ sensor on Ca2+ release channels.
References
- 1.Berridge M, Lipp P, Bootman M. The versatility and universality of calcium signalling. Nature Mol Cell Biol Rev. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- 3.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel S. Function and dysfunction of two-pore channels. Sci Signal. 2015;8:re7. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- 5.Seo MD, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, Taylor CW. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–12. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roderick HL, Berridge MJ, Bootman MD. Calcium-induced calcium release. Curr Biol. 2003;13:R425. doi: 10.1016/s0960-9822(03)00358-0. [DOI] [PubMed] [Google Scholar]
- 7.Palade P, Mitchell RD, Fleischer S. Spontaneous calcium release from sarcoplasmic reticulum. General description and effects of calcium. J Biol Chem. 1983;258:8098–107. [PubMed] [Google Scholar]
- 8.Missiaen L, Taylor CW, Berridge MJ. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991;352:241–4. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- 9.Galione A, McDougall A, Busa WB, Willmott N, Gillot I, Whitaker M. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science. 1993;261:348–352. doi: 10.1126/science.8392748. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, Zhou Q, Xie C, Zhang J, Guo A, Tian X, et al. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med. 2014;20:184–92. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DL. Structural determination of a cyclic metabolite of NAD with intracellular calcium-mobilizing activity. J Biol Chem. 1989;264:1608–1615. [PubMed] [Google Scholar]
- 12.Clapper DL, Walseth TF, Dargie PJ, Lee HC. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem. 1987;262:9561–9568. [PubMed] [Google Scholar]
- 13.Galione A, Lee HC, Busa WB. Ca2+-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- 14.Lee HC. Potentiation of calcium- and caffeine-induced calcium release by cyclic ADP-ribose. J Biol Chem. 1993;268:293–299. [PubMed] [Google Scholar]
- 15.Iino S, Cui Y, Galione A, Terrar DA. Actions of cADP-ribose and its antagonists on contraction in guinea pig isolated ventricular myocytes. Influence of temperature. Circ Res. 1997;81:879–84. doi: 10.1161/01.res.81.5.879. [DOI] [PubMed] [Google Scholar]
- 16.Rakovic S, Cui Y, Iino S, Galione A, Ashamu GA, Potter BV, Terrar DA. An antagonist of cADP-ribose inhibits arrhythmogenic oscillations of intracellular Ca2+ in heart cells. J Biol Chem. 1999;274:17820–7. doi: 10.1074/jbc.274.25.17820. [DOI] [PubMed] [Google Scholar]
- 17.Kannt A, Sicka K, Kroll K, Kadereit D, Gogelein H. Selective inhibitors of cardiac ADPR cyclase as novel anti-arrhythmic compounds. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:717–27. doi: 10.1007/s00210-012-0750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galione A, Cui Y, Empson R, Iino S, Wilson H, Terrar D. Cyclic ADP-ribose and the regulation of calcium-induced calcium release in eggs and cardiac myocytes. Cell Biochem Biophys. 1998;28:19–30. doi: 10.1007/BF02738307. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–9. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffe LF. Fast calcium waves. Cell Calcium. 2010;48:102–13. doi: 10.1016/j.ceca.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–20. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan AJ, Galione A. Preparation and use of sea urchin egg homogenates. Methods Mol Biol. 2014;1128:161–73. doi: 10.1007/978-1-62703-974-1_10. [DOI] [PubMed] [Google Scholar]
- 23.Fan G, Baker ML, Wang Z, Baker MR, Sinyagovskiy PA, Chiu W, Ludtke SJ, Serysheva II. Gating machinery of InsP3R channels revealed by electron cryomicroscopy. Nature. 2015;527:336–41. doi: 10.1038/nature15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HC. Specific binding of cyclic ADP-ribose to calcium-storing microsomes from sea-urchin eggs. J Biol Chem. 1991;266:2276–2281. [PubMed] [Google Scholar]
- 25.Walseth TF, Aarhus R, Kerr JA, Lee HC. Identification of cyclic ADP-ribose-binding proteins by photoaffinity labeling. J Biol Chem. 1993;268:26686–26691. [PubMed] [Google Scholar]
- 26.Zhang K, Sun W, Huang L, Zhu K, Pei F, Zhu L, Wang Q, Lu Y, Zhang H, Jin H, Zhang LH, et al. Identifying glyceraldehyde 3-phosphate dehydrogenase as a cyclic adenosine diphosphoribose binding protein by photoaffinity protein-ligand labeling approach. J Am Chem Soc. 2017;139:156–170. doi: 10.1021/jacs.6b08088. [DOI] [PubMed] [Google Scholar]
- 27.Meissner G, Lai FA, Anderson K, Xu L, Liu QY, Herrmann-Frank A, Rousseau E, Jones RV, Lee HB. Purification and reconstitution of the ryanodine- and caffeine-sensitive Ca2+ release channel complex from muscle sarcoplasmic reticulum. Adv Exp Med Biol. 1991;304:241–56. doi: 10.1007/978-1-4684-6003-2_20. [DOI] [PubMed] [Google Scholar]
- 28.McPherson SM, McPherson PS, Mathews L, Campbell KP, Longo FJ. Cortical localization of a calcium release channel in sea urchin eggs. J Cell Biol. 1992;116:1111–21. doi: 10.1083/jcb.116.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith CD, Wang A, Vembaiyan K, Zhang J, Xie C, Zhou Q, Wu G, Chen SR, Back TG. Novel carvedilol analogues that suppress store-overload-induced Ca2+ release. J Med Chem. 2013;56:8626–55. doi: 10.1021/jm401090a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan AJ, Parrington J, Galione A. The luminal Ca2+ chelator, TPEN, inhibits NAADP-induced Ca2+ release. Cell Calcium. 2012;52:481–7. doi: 10.1016/j.ceca.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venturi E, Pitt S, Galfre E, Sitsapesan R. From eggs to hearts: what is the link between cyclic ADP-ribose and ryanodine receptors? Cardiovasc Ther. 2012;30:109–16. doi: 10.1111/j.1755-5922.2010.00236.x. [DOI] [PubMed] [Google Scholar]
- 32.Walseth TF, Lee HC. Synthesis and characterization of antagonists of cyclic-ADP-ribose-induced Ca2+ release. Biochim Biophys Acta. 1993;1178:235–42. doi: 10.1016/0167-4889(93)90199-y. [DOI] [PubMed] [Google Scholar]
- 33.Roderick HL, Bootman MD. Pacemaking, arrhythmias, inotropy and hypertrophy: the many possible facets of IP3 signalling in cardiac myocytes. J Physiol. 2007;581:883–884. doi: 10.1113/jphysiol.2007.133819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hisatsune C, Mikoshiba K. IP3 receptor mutations and brain diseases in human and rodents. J Neurochem. 2017 doi: 10.1111/jnc.13991. in press. [DOI] [PubMed] [Google Scholar]