Abstract
Peri-prosthetic osteolysis remains as the main long-term complication of total joint replacement surgery. Research over four decades has established implant wear as the main culprit for chronic inflammation in the peri-implant tissues and macrophages as the key cells mediating the host reaction to implant-derived wear particles. Wear debris activated macrophages secrete inflammatory mediators that stimulate bone resorbing osteoclasts; thus bone loss in the peri-implant tissues is increased. However, the balance of bone turnover is not only dictated by osteoclast-mediated bone resorption but also by the formation of new bone by osteoblasts; under physiological conditions these two processes are tightly coupled. Increasing interest has been placed on the effects of wear debris on the cells of the bone-forming lineage. These cells are derived primarily from multipotent mesenchymal stem cells (MSCs) residing in bone marrow and the walls of the microvasculature. Accumulating evidence indicates that wear debris significantly impairs MSC-to-osteoblast differentiation and subsequent bone formation. In this review, we summarize the current understanding of the effects of biomaterial implant wear debris on MSCs. Emerging treatment options to improve initial implant integration and treat developing osteolytic lesions by utilizing or targeting MSCs are also discussed.
Keywords: total joint replacement, peri-prosthetic osteolysis, aseptic loosening, mesenchymal stem cells, macrophages
INTRODUCTION
Total joint replacement (TJR) is an excellent treatment for end-stage arthritis. This surgical procedure alleviates pain and restores functional ability and is the most cost effective and successful of surgical interventions.1,2 As a result of this success, TJR has become increasingly popular and the number of procedures has continued to increase. For example in The Organisation for Economic Co-operation and Development (OECD) countries, a 25% increase in the incidence of total hip replacement operations occurred between 2000 and 2009.3 The Center for Disease Control and Prevention reports that there were >300,000 total hip and 700,000 total knee replacements performed in 2010 in United States with 10–15% of these cases being revision procedures.4 Kurtz et al projected that between the years 2005 and 2030, the numbers of primary total hip and total knee replacements will increase by 174% and 673%, respectively.5,6 With the increasing number of primary total joint replacement operations, there will likely be a concomitant increase in the number of revision operations: the number of hip revision surgeries is expected to double by 2026 while the number of knee revisions doubled between 2005 and 2015 and is still expected to increase. The main cause for this impressive number of revision operations is peri-prosthetic osteolysis and aseptic loosening of implant components.7,8
Implant wear and subsequent release of biomaterial wear particles to the surrounding tissues are the main causes of peri-prosthetic osteolysis.2,9 It is well established that macrophages are the key cells mediating the chronic inflammatory reaction to biomaterial wear debris; wear particle-activated macrophages secrete chemokines and pro-inflammatory cytokines that lead to further macrophage recruitment, increased osteoclastogenesis, and ultimately peri-implant bone loss.10,11
While activated macrophages and osteoclasts play a key role in peri-prosthetic osteolysis, the balance of bone turnover in the peri-implant tissues is dictated not only by bone resorption but also by bone formation. Indeed, under physiological conditions, osteoclast-mediated bone resorption is tightly coupled to the formation of new bone by osteoblasts.12 There is growing evidence that the function of osteoblasts and their progenitor cells is significantly impaired in peri-prosthetic osteolysis contributing to the overall peri-implant bone loss. Similarly treatment strategies for aseptic osteolysis utilizing or targeting bone forming cells are emerging.
Osteoblasts are a part of the lineage of bone forming cells that include mesenchymal stem cells (MSCs), pre-osteoblasts, osteoblasts, and osteocytes.13–15 MSCs reside in bone marrow stroma, periosteum, and the walls of the local microvasculature, and they differentiate to bone-forming osteoblasts via specific stages. The sequence of MSC-to-osteoblast differentiation is incompletely understood but is characterized by transient expression of the transcription factors SOX9, RUNX2 and Osterix.13,15 The microenvironmental signals that initiate MSC-to-osteoblast differentiation are also poorly characterized but include soluble mediators in the Wnt, bone morphogenetic protein (BMP), and fibroblast growth factor (FGF) families.15 Osteoblasts produce the organic bone extracellular matrix including type 1 collagen that mineralizes with hydroxyapatite crystals. Once completely surrounded by the extracellular matrix, osteoblasts are termed osteocytes. Osteocytes form the majority of the cell mass in bone and are active participants in bone maintenance, forming a metabolically active intervening network of cells that expands throughout the bone tissue.
Osteoblast function is profoundly altered by both implant derived biomaterial wear debris and the inflammatory mediators secreted by debris-activated macrophages. A large number of in vitro studies have described reduced cell viability and impaired ability to produce mineralized bone matrix as a response to these adverse stimuli.16,17 Thus wear debris not only increases bone resorption by activating macrophages and osteoclasts but also directly impairs bone formation by inhibiting the function of osteoblasts. These effects of wear debris on osteoblasts and osteoblast-like cell-lines were recently reviewed elsewhere16,17 while the direct impact of biomaterial wear debris on MSCs and MSC-to-osteoblast differentiation is discussed in the sections below. Emerging treatment strategies for aseptic osteolysis utilizing or targeting MSCs are also summarized.
OVERVIEW OF THE MECHANISMS OF PERI-PROSTHETIC OSTEOLYSIS
Both mechanical and biological theories have been proposed to participate in the development of osteolysis around total joint replacements, while the exact mechanism of aseptic loosening is complex and likely multifactorial.18–20 Mechanical theories highlight cyclic implant loading affecting the bone-implant interface ultimately leading to the loss of implant fixation. First, micromotion between the implant and the surrounding bone has been suggested to result in remodeling of bone trabeculae and migration of fibroblasts into the empty spaces between implant surface and bone.18,21 Repeated stress can induce fibroblast proliferation and allow for the formation of a fibrous interface membrane.20,22 Bone remodeling around the implant could also occur due to non-physiological loading conditions in a phenomenon referred to as stress shielding and lead to bone loss in areas not subjected to loading.18 Other mechanical theories emphasize the role of high-pressure, oscillating synovial fluid as another source of bone erosion around the joint implant. Synovial fluid is synthesized inside the artificial joint by synovial-like macrophages and fibroblasts; as the joint is moved, pressure waves are generated to the synovial fluid.18,20,22,23 Micromotion between the implant and bone could facilitate access to the bone/implant interface allowing high-pressure joint fluid to seep between the bone and the implant; this could widen the interface, potentially harming osteocytes and local bone remodeling.
According to the established biological model of aseptic loosening, bone resorption is mainly driven by implant-derived wear particles.9,24 The exact properties of these wear particles are dependent on the mechanism of their formation. Most implant-related wear is generated by mechanical abrasion at the prosthetic joint articulation. Due to favorable tribological properties, the most common artificial joint articulations are composed on CoCr articulating against ultra-high molecular weight polyethylene (UHMWPE or simply PE). Most wear released from these metal-on-PE implants consists of PE particles of <1 μm in diameter. Additional wear debris can be released from bone/implant interface and is either polymethylmethacrylate (PMMA, bone cement), or titanium particles released from the osteoconductive, porous implant surface. Metal-on-metal articulations originally developed to avoid the problem of PE wear can release large amounts of nanometer sized cobalt and chromium particles, as well as significant amounts of Co2+ and Cr3+ ions due to combined effect of abrasion and corrosion. Similarly, significant metal release can occur from various modular junctions in some implants. Ceramic-on-ceramic couplings release minimal amount of wear byproducts.
These wear particles are dispersed along the bone-implant interface via the joint fluid.25,26 In the peri-prosthetic tissues, wear debris is phagocytized by macrophages which are subsequently activated to an inflammatory phenotype and secrete cytokines and chemokines such as TNF-α, IL-1β, IL-6, IL-8, CCL2/MCP-1, and CCL3/MIP-1α; these factors recruit more monocyte/macrophage lineage cells to the site of ongoing inflammation.2,10,27 Other cells residing in the peri-implant tissue including MSCs, fibroblasts, osteoblasts, lymphocytes, and endothelial cells are also impacted by the wear particles and the pro-inflammatory mediators produced from macrophages.9,20,26,28
The particle induced inflammatory cascade drives the differentiation and activation of osteoclasts either directly or indirectly, increasing the expression of Receptor Activator of Nuclear Factor kappa-B Ligand (RANKL) as well as suppressing the production of osteoprotegerin (OPG) in local stromal cells.19,29,30 RANKL is critical for the development and activation of osteoclasts, while OPG suppresses the effects of RANKL by acting as its decoy receptor.31 Thus, the increased RANKL/OPG ratio impacts the differentiation of osteoclast precursors and enhances osteoclast activity at the bone-implant interfaces.32 As wear particles are both resistant to enzymatic degradation and are continuously generated, a sustained chronic foreign body reaction and pro-inflammatory environment lead to progressive peri-implant osteolysis.
Macrophages are key players initiating and regulating the inflammatory response to wear particles.10,33 In addition to wear particle characteristics (e.g., size, shape, material) various biomolecules attached to the particle surface appear to affect macrophage activation and cytokine production.34 The response of macrophages to wear particles is, in part, mediated by toll-like receptors (TLRs) expressed on the cell surface and endosomal compartment of macrophages.35–37 TLRs belong to the family of pattern recognition receptors (PRRs) essential for recognizing a wide spectrum of both exogenous microbial products, usually referred to as pathogen-associated molecular patterns (PAMPs), and endogenous danger signals also known as DAMPS or alarmins.38 Indeed, increased expression of TLRs has been documented in the peri-prosthetic tissues reflecting the possible presence of PAMPs or host derived alarmins adherent to particles.39,40 Both TLR2 and TLR4 have been raised as possible mediators of the inflammatory cascade in aseptic loosening; TLR2 recognizes gram positive bacterial structures such as lipoteichoic acid (LTA), and TLR4 acts as the primary receptor for gram negative bacteria-derived lipopolysaccharide (LPS).36,41 However, both of these receptors are also capable of sensing endogenous danger signals such as heat shock proteins (HSPs) and high mobility group box 1 (HMGB1).42 Wear particle mediated TLR activation promotes the production and release of pro-inflammatory mediators from macrophages especially when PAMPs are adhering to the particles.41,43 Further downstream, signaling through the MyD88 dependent pathway results in activation of Nuclear Factor-kappa B (NF-κB), a transcription factor pivotal to the production of pro-inflammatory cytokines and osteoclast differentiation.35 In addition to activating TLRs signaling, wear particles have been shown to cause endosomal damage, with subsequent activation of intracellular danger sensing machinery ultimately leading to NF-kB activation and the assembly of the NALP3 inflammasome.44,45 In addition, Co and Cr nanoparticles and high ion concentrations exert direct cytotoxic effects on macrophages and various other cell types.46 These ions can also form haptens with host proteins activating the type IV adaptive immune response in genetically susceptible individuals.47,48
Taken together, chronic inflammation stimulated by different wear particles results in changes in local tissue homeostasis that ultimately favors bone destruction. Mechanical forces likely contribute to the development of osteolysis by further accelerating the release of particle debris and affecting bone remodeling adversely. Although the essential role of macrophages in triggering the inflammatory response against particles is well established, many detailed cellular mechanisms leading to aseptic loosening are still under investigation. In particular, the impaired ability of MSCs to differentiate and form bone in aseptic loosening has become an active area of study with applications in the development of novel treatments for the condition.
MESENCHYMAL STEM CELLS
Mesenchymal stem cells were first identified from human bone marrow tissues. MSCs were initially defined as cells with the ability to develop into fibroblastic colony-forming cells (CFU-F) in vitro, and to regenerate heterotopic bone tissue in vivo.49–51 The exact definition of MSC remains controversial today despite the growing number of translational applications of MSC-based therapies.52 The International Society for Cellular Therapy has suggested the minimal criteria to define MSC as cells that (1) adhere to plastic in vitro cell culture; (2) express CD105, CD73, CD90, and lack expression of CD45, CD34, CD14, CD11b, CD79, CD19, and HLA-DR surface markers; and 3) have the ability to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro.53
Crisan et al. identified that the peri-vascular cells derived from multiple human organs (including skeletal muscle, pancreas, adipose tissue, and placenta) displayed the characteristics of MSCs, thus demonstrating that MSCs exist in bone marrow and multiple organs, and are closely associated with blood vessel walls.54 Since then, several distinct populations of peri-vascular MSCs with unique characteristics have been identified in transgenic mouse models.55,56 The nestin+ peri-vascular stromal cells located in the murine bone marrow niche are spatially associated with hematopoietic stem cells and are essential for their biological functions.55 These nestin+ cells were characterized as MSCs for the multilineage differentiation and self-renewal abilities. Zhou et al. reported a distinct MSC population with high leptin receptor and low nestin expression (LepR+/nestindim) located around sinusoids and arterioles in murine bone marrow.56 Unlike nestin+ MSCs, LepR+/nestindim MSCs were only expressed in adult bone marrow and represent the major source of bone and bone marrow fat in adult mice.
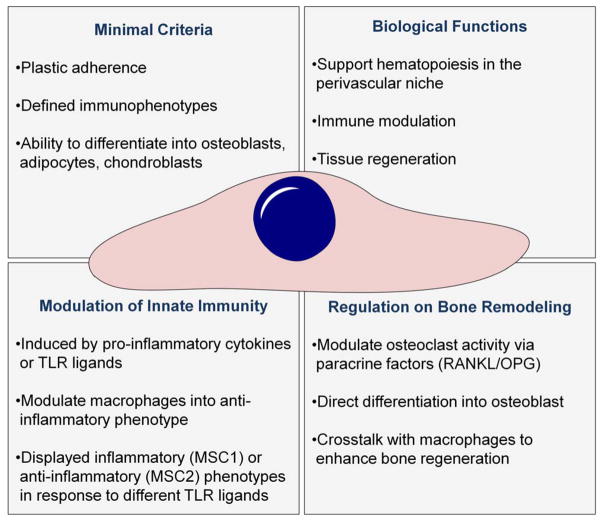
MSCs (1) support the hematopoietic stem cell in the peri-vascular niche; (2) modulate the innate and adaptive immune responses; (3) repair and regenerate damaged tissues including bone, cartilage, and blood vessels, and (4) can be easily harvested from adult tissues and expanded in vitro. As such, MSCs have generated great interest for tissue regenerative and translational applications. Indeed, MSC-based therapies have been applied to clinical trials for the treatment of myocardial infarction, graft-versus-host disease, diabetes, liver cirrhosis, and osteoarthritis, among others.57 In the following sections, we summarize the properties of MSCs most relevant for the context of peri-prosthetic osteolysis: the immunomodulatory functions of MSCs that they exert on macrophages, and the bone regenerative ability of MSCs in response to inflammatory stimuli (Fig. 1). The MSC effects on adaptive58 and other innate immune cells58,59 have been well documented elsewhere.
FIGURE 1.
Summary of the key characteristics of MSCs. MSCs ability to modulate the innate immune response, regulate bone turnover, and directly regenerate bone make them ideal candidates for the development of cell-based therapies for aseptic loosening and other conditions were bone loss is due to chronic inflammation.
Immunomodulation of innate immunity
The benefits of MSC-based therapy on autoimmune and inflammatory diseases are mainly mediated through modulation of the adaptive immune response (reviewed by Bernardo et al.).58 However, increasing evidence suggests that regulation of the innate immune response is also crucial during the MSC-mediated immunomodulation process.59 Interferon-γ and pro-inflammatory cytokines such as TNF-α or IL-1β synergistically enhance inducible nitric oxide synthase (iNOS)60 expression in murine MSC or indoleamine-2-oxygenese (IDO) in human MSCs,61 leading to apoptosis and reduced proliferation of T lymphocytes60,61 and a bystander effect to polarize macrophages into an anti-inflammatory phenotype.61 These findings suggest that innate immunity can activate the immunomodulatory functions in MSCs. In addition, activated MSCs can also initiate negative feedback regulation to mitigate excessive inflammatory responses. Transplantation of murine bone marrow MSC attenuated sepsis and increased IL-10 secreting macrophages in a mouse sepsis model.62 The protective effect of transplanted MSCs was diminished by pretreatment with IL-10 or IL-10 receptor blocking antibodies, or using the MSCs deficient in Toll-Like Receptor 4 (TLR4), MyD88, TNF-α receptor, or cycloxygenese-2 (Cox2). This mechanistic study indicated that MSCs secrete prostaglandin E2 (PGE2) to educate macrophages into a regulatory phenotype with high IL-10 secretion.62 Maggini et al. reported that the conditioned supernatants collected from MSCs decrease TNF-α, IL-6, IL-12p70, and IFN-γ, but increase IL-10 and IL-12p40 secretion in peritoneal macrophages stimulated with lipopolysaccharide (LPS).63 Secretion of PGE2 and the paracrine regulation of MSCs were suppressed by Cox2 inhibitor treatment. In the murine model of inflammation induced by subcutaneous implantation of a glass cylinder, MSCs recruited IL-10 secreting macrophages to alleviate tissue damage.63 Chen et al. reported that mouse and human MSCs secreted high levels of chemokines including MIP-1α and MIP-1β, induced macrophage migration in vitro and in vivo, and enhanced wound healing in mice.64 Kim et al. found that direct co-culture of MSCs enhanced anti-inflammatory phenotypes in macrophages compared to the transwell co-culture model, suggesting that paracrine factors and direct contact can both contribute to the immunomodulation effects of MSCs.65 The phagocytosis ability of macrophages was also increased in the same co-culture model with MSCs.65
MSCs display pro- or anti-inflammatory phenotypes in response to different stimuli.66 Short-term exposure (<1 h) of MSCs to LPS (TLR4 ligand) showed decreased ability to suppress T cell activation in the mixed lymphocyte reaction with peripheral blood mononuclear cells (PBMC). In contrast MSCs exposed to poly I:C (TLR3 ligand), remain capable of suppressing T cell activation and also secrete higher amounts of PGE2.62,63,66 These pro- and anti-inflammatory MSC phenotypes were termed MSC1 and MSC2, respectively, to reflect the similar phenomenon of T helper cell and macrophage polarization into distinct functional phenotypes.66
Taken together, MSCs can enhance the recruitment of macrophages and other immune cells to clear pathogens or damaged tissue.64,65 They also display significant immunomodulatory capabilities and educate surrounding immune cells, such as macrophages, into anti-inflammatory types.62,63,67 Therefore, temporal modulation of MSCs on the inflammatory response holds great promise to facilitate tissue repair and regeneration.58,59
MSCs in bone regeneration and remodeling
MSCs can modulate bone remodeling process via indirect paracrine regulation of osteoclast activation, or direct differentiation into osteoblasts. RANKL and its receptor RANK expressed on osteoclast precursors is essential for osteoclast differentiation and activation.68 RANKL is expressed in differentiated osteoblast-lineage cells and has also been reported to be expressed in MSCs isolated from aged mice.69 MSCs regulate osteoclast formation also by secreting osteoprotegerin (OPG), the RANKL decoy receptor. The balance of RANKL/OPG ratio determines osteolytic/osteoblastic activity and is regulated by MSC-lineage cells and the pro-inflammatory cytokines secreted by infiltrating immune cells.
Osteogenesis by MSCs is critical for successful fracture healing and successful osseointegration of implants. MSCs and macrophages interact during bone regeneration in these instances with the processes being regulated by the inflammatory microenvironment. Infiltrated macrophages or local tissue macrophages (osteomacs70) are crucial for bone regeneration during the acute inflammatory stage following bone injury.71 Depletion of macrophages impaired fracture healing in a murine model.71 Inflammatory macrophages (M1) directly enhanced MSC mediated osteogenesis in vitro via secretion of paracrine regulators including oncostatin M (OSM),72 BMP2, and TGF-β1.73 The polarization of macrophages into anti-inflammatory type (M2) by IL-4 at later time points (72–96 h) further enhanced osteogenesis in a co-culture model with MC3T3-E1 osteoprogenitor cells74 or primary mouse MSCs, suggesting that temporal modulation of the inflammatory microenvironment modulates bone formation.75 Interestingly, osteogenesis by MSCs can also be enhanced by preconditioning the MSCs with inflammatory stimuli which is comparable to the initial inflammatory status at the fracture site priming MSCs for optimal bone formation. Transient exposure of MSC to TNF-α, LPS, or specific TLR ligands increases osteogenesis in vitro, and is dependent on the dose, timing, and exposure period of the stimuli.76–85 However, unresolved inflammation may progress into harmful chronic inflammation if adverse stimuli persist. Continuous exposure to the chronic inflammatory environment causes osteoclast activation and impaired osteogenesis in MSCs86 and moves the balance of bone remodeling toward an osteolytic process.87
Taken together, MSCs play a crucial role in bone regeneration both by regulating osteoclast formation and by secreting the extracellular bone matrix. During fracture healing the functions of MSCs are regulated by complex crosstalk between macrophages and MSCs. Further understanding of this crosstalk can potentially be utilized to enhance bone regeneration and also treat osteolytic conditions such as peri-prosthetic osteolysis.
MESENCHYMAL STEM CELLS IN ASEPTIC LOOSENING
Impact of wear debris on MSCs
The pioneering work on characterizing the impact of biomaterial debris on MSC-to-osteoblast differentiation was done by Rocky Tuan’s group. They isolated human MSCs from resected femoral heads from patients’ undergoing hip replacement, and exposed the MSCs to submicron sized Ti and zirconium oxide (ZrO2) particles in vitro.88 Exposure to Ti particles reduced cell proliferation and viability in a dose-dependent manner. Diminished production of type 1 collagen, bone sialoprotein, and mineralization of the extracellular matrix was also observed indicating disruption of the MSC-to-osteoblast differentiation. The effect of ZrO2 particles of similar size and concentration was less pronounced, leading to moderate reduction in matrix mineralization. Next they showed that both Ti and ZrO2 particles-induced apoptosis rather than necrosis, thus compromising cell viability.89 The effect was time and dose dependent with Ti particles showing more pronounced adverse effects than ZrO2 particles at equivalent dosages. Particle-induced apoptosis was mediated in part by upregulation of tumor suppressor proteins p53 and p73. It was also demonstrated that conditioned medium from Ti treated MSCs, but not ZrO2- treated MSCs, induced cell apoptosis in a manner similar to direct particle treatment. Next the compromised MSC viability and impaired osteogenic differentiation were shown to be dependent on the phagocytosis of Ti particles; inhibiting particle phagocytosis with cytochalazin D mitigated these adverse effects.90 Finally, the group showed that Ti particles disrupted cytoskeletal organization and induced the secretion of IL-8 and of GM-CSF but not TNF-α, IL-1β, IL-6, or TGF-β from MSCs.91 Interestingly, recombinant GM-CSF was shown to reduce both cell viability and osteogenic differentiation in manner similar to Ti particles while IL-8, IL-6, IL-1β did not have these effects. Production of IL-8 from particle stimulated MSCs is important, as increased levels of this traditionally neutrophil related chemokine have been described in the peri-implant tissues.92,93 It has recently been reported that there is a positive correlation between high peri-prosthetic levels of IL-8 and early implant revision.94
Our group studied the kinetics of particle exposure on the MSC differentiation by challenging mouse bone marrow MSCs at different stages of osteogenic differentiation with increasing concentrations of PMMA particles.95,96 A dose-dependent reduction in osteogenic differentiation and cell proliferation were observed with higher particle concentrations, completely abolishing osteogenesis. In addition, particle exposure 5 days before or during the first 5 days of osteogenic differentiation significantly impaired cell proliferation, ALP expression, and matrix mineralization. If the cells were exposed to particles for <5 days during early differentiation, these effects were mitigated. Furthermore MSCs exposed to particles after 5 days of osteogenic differentiation were less sensitive to the particle stimulus showing reduced cell proliferation but had normal matrix mineralization. These effects were dependent on direct particle-cell interaction, as conditioned medium from particle stimulated MSCs did not recapitulate these effects. It was concluded that MSCs were most sensitive to PMMA particle challenge when exposed to particles before, or during the first 5 days of differentiation, with the length of the exposure playing a critical role. Particle exposure at the later stages of osteogenic differentiation was less harmful.
Subsequent studies have expanded these results initially obtained with Ti and PMMA particles to other clinically relevant orthopaedic wear particles. Chiu et al. studied the effects of joint replacement simulator derived submicron-sized UHMWPE particles on murine bone marrow derived MSCs.97 A dose-dependent reduction in MSC proliferation, ALP activity, osteocalcin production, and matrix mineralization was observed with higher particle concentrations completely inhibiting osteogenesis. Schofer et al. exposed human MSCs to varying concentrations of CoCrMo particles.98 MSCs were shown to phagocytose the particles with subsequent reduction in cell density and proliferation. An increased but morphologically abnormal deposition of osteocalcin and osteopontin was observed with immunohistochemistry; the authors concluded that the metal particles lead to dysregulation in bone matrix deposition. Jiang et al. compared the effects of TiAlV, PMMA, UHMWPE and CoCr particles on the viability and osteogenic differentiation of murine bone marrow MSCs.99 All of the particle types reduced MSC osteogenic differentiation with CoCr particles reducing osteogenesis the most and PMMA particles the least. The production of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 was induced by UHMWPE and CoCr particles while TiAlV and PMMA showed a more attenuated response. Interestingly RANKL was markedly upregulated and OPG down regulated by all particle types, with PMMA again showing the weakest adverse effect. The authors concluded that while various types of wear debris had distinct effects on MSCs, all of the particles examined suppressed MSC osteogenic differentiation, induced varying degrees of pro-inflammatory responses, and upregulated RANKL production. Hou et al. studied the effects of Ti nanoparticles on rat bone marrow MSCs.100 These nanoparticles impaired MSC viability, adhesion, cytoskeletal architecture, migration, and osteogenic differentiation with larger nanoparticles (196 and 108 nm) showing a stronger adverse effect than smaller particles (14 nm).
In contrast to these micron- and submicron-sized particulate debris, few studies have examined the effects of metal ions on MSC differentiation to osteoblasts. Schröck et al. observed that soluble Co2+ ions inhibited osteogenic differentiation of human MSCs in a dose-dependent manner.101 Rakow et al. determined the concentrations of dissociated Co2+ and Cr3+ ions in peri-implant tissues surrounding failed metal-on-metal (MoM) implants and exposed cultured human MSCs to a range of clinically relevant metal ion concentrations.102 A dose-dependent reduction in MSC viability, proliferation, ALP expression, and matrix mineralization was documented. Interestingly cobalt has been shown to induce a hypoxia-like response in MSCs and macrophages by stabilizing HIF-1α.103,104 It has been suggested that the pseudo-tumor formation occasionally seen around MoM implants might be in part due to growth factors such as VEGF secreted by cobalt-exposed cells.104 The effects of hypoxia in general on the MSC-to-osteoblast differentiation are currently controversial.105,106
These in vitro studies suggest that biomaterial wear particles of different types compromise viability, proliferation, cytoskeletal architecture, and osteogenic differentiation of MSCs. This effect is dependent on particle phagocytosis but is likely also mediated by auto- and paracrine effects of secreted factors released from particle stimulated MSCs. Exposure to wear debris early in the process of MSC-to-osteoblast differentiation seems to be more detrimental to osteogenesis than in later stages. Furthermore, particle stimulated MSCs secrete inflammatory mediators and modulate RANKL/OPG balance contributing to inflammation and osteoclast formation in the peri-prosthetic tissues (Fig. 2).
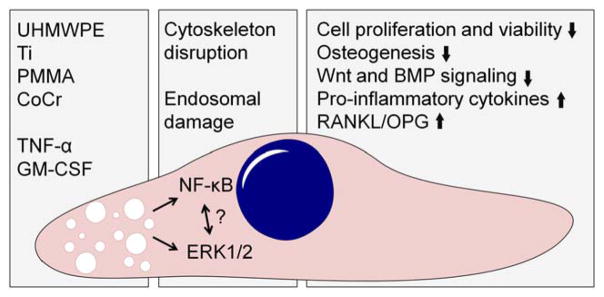
FIGURE 2.
The impact of wear debris on MSCs. Biomaterial wear particles released from the implant are phagocytosed by MSCs. Particle phagocytosis induces disruption of the actin cytoskeleton and likely damage to endosomal membranes. This particle induced cell stress leads to activation of inflammatory signaling pathways, including NF-kB and ERK1/2. Activation of these pathways compromises both the viability and osteogenic differentiation ability of MSCs. Furthermore, production of selected pro-inflammatory cytokines as well as the ratio of RANKL to OPG is increased, both changes that can contribute to local osteoclast formation. In addition to direct effect of particles, also pro-inflammatory cytokines such as TNF-α and GM-CSF produced either by MSCs themselves or local macrophages likely contribute to the overall effect of wear debris. The role of Toll-like receptors and other pattern recognition receptors in the particle induced activation of inflammatory intracellular signaling pathways remain to be determined as does the detailed cross-talk between these pathways.
Signaling pathways in wear debris activated MSCs
Little is known about the cellular signaling pathways mediating wear particle-induced suppression of MSC differentiation. In the context of wear particle and metal ion-stimulated macrophages both cell membrane and intracellular danger sensing pattern recognition receptors as well as endosomal damage have been implicated.36,45 Signaling from these receptors activates the transcription factor NF-κB and the NALP3 inflammasome. Similar signaling pathways have been implicated also in wear particle stimulated MSCs but the evidence is limited. Using both mouse bone marrow and human MSCs challenged with UHMWPE particles, Lin et al. provided indirect evidence of the involvement of NF-κB signaling pathway in the abolition of osteogenesis in particle-treated MSCs.86 UHMWPE particles reduced MSC viability, osteogenic differentiation, and diminished OPG production. These adverse effects were largely prevented by decoy oligodeoxynucleotide (ODN) that specifically blocks NF-κB signaling by preventing the binding of the transcription factor to its target regions in the DNA. Furthermore, ODN treatment increased OPG production and was shown to be dependent on the upregulation of TGF-β1 in ODN-treated MSCs. These results suggest that the adverse effects of UHMWPE particles on MSCs are in part mediated by NF-kB activation. Indeed, chronic NF-κB activation was recently described as one mechanism by which osteogenesis is impaired in aged MSCs, providing further support for the adverse role of chronically active NF-κB signaling in MSC-to-osteoblast differentiation.69 Lee et al. provided evidence that Ti particle phagocytosis and subsequent disruption in the intracellular acting cytoskeleton in human MSCs and MC3T3-E1 pre-osteoblasts was associated with the activation of ERK1/2–CEBP-β pathway leading to production of IL-6 and COX-2 but not TNF-α or IL-1β.107 Activation of ERK signaling was also shown in the mouse calvarial model of particle induced osteolysis. Importantly, a specific inhibitor of ERK signaling prevented particle-induced inflammation both in vitro and in vivo, further implicating ERK signaling as a mediator of particle-induced osteolysis. Wang et al. showed that Ti-stimulated mouse MSCs down-regulate Wnt/β–catenin signaling pathway, and reduced the levels of cytoplasmic and nuclear β–catenin levels that are involved in MSC-to-osteoblast differentiation.108 It was shown that the compound Icariin that activates Wnt/β–catenin signaling attenuated these effects both in vitro and in vivo.
To summarize, several different intracellular signaling pathways have been implicated mediating the adverse effect of wear debris on MSCs but the number of studies is limited. The relative importance and complex interactions among these signaling pathways in the wear particle responses of MSCs demands further study.
MSC–macrophage interactions
Since wear particle-activated macrophages play a key role in peri-prosthetic osteolysis, understanding the interactions of wear particle activated-macrophages and MSCs is of great importance. In the context of fracture healing and bone regeneration, macrophages regulate the recruitment and function of MSCs.75 However, relatively little is currently known about the interactions of these two cell types in the context of wear particle disease. Pro-inflammatory cytokines have complex effects on MSCs and MSC-to-osteoblast differentiation and it can be speculated that secreted factors from particle stimulated macrophages have an impact on MSC function.79–84,109 In an elegant series of experiments, Lee et al. studied the effects of conditioned media from Ti particle-stimulated RAW 264.7 cells on pre-osteoblast cell line MC3T3-E1 and human primary MSCs.110 First, it was shown that conditioned media from particle simulated macrophages inhibited osteogenic differentiation of these cells evident by a reduction of RUNX2 expression, ALP activity, and matrix mineralization. These effects were mediated by macrophage secreted TNF-α with subsequent activation of NF-κB signaling in MSCs, upregulation of sclerostin, and inhibition of Wnt and BMP signaling pathways.
Huang et al. showed that conditioned media from PMMA but not UHMWPE particle stimulated RAW 264.7 cell-induced MSC chemotaxis and that this effect was due to chemokine MIP-1α (CCL3) as it could be inhibited by a CCL3 blocking antibody.111 Migration of THP-1 monocytes toward particle-stimulated macrophages was dependent on MCP-1 (CCL2). Gibon et al. further demonstrated, using the in vivo continuous intramedullary particle infusion model, that reporter MSCs injected to the systemic circulation migrate to the area of particle induced inflammation.112 Similar to the findings of Huang et al. this effect was dependent on the chemokine receptor CCR1, and presumably from its ligand CCL3. Interestingly blocking of CCR1 resulted in the exacerbation of the particle induced osteolysis suggesting that continuous recruitment of MSCs to the area of inflammation helps to limit the inflammation and potentially regenerate the local bone stock.
Taken together, it seems that cytokines and chemokines secreted from particle activated macrophages impact MSC recruitment and differentiation. The net effects of these various paracrine signals on MSCs remain to be determined. Also the potential role of MSC-derived signals reciprocally regulating macrophage function is a subject for further study.
Impact of mechanical strain on MSCs
MSCs and their lineage-specific differentiation are greatly impacted by the mechanical properties of the microenvironment. The effects of mechanical strain on MSCs have been thoroughly reviewed elsewhere, but in general, mechanical strain either in the form of fluid flow, hydrostatic pressure, or compression enhances the osteogenic or chondrogenic differentiation of MSCs.113,114 Indeed, exposing mice to microgravity compromises the ability of bone marrow MSCs to differentiate.115 However, little is known about the effect of mechanical strain on MSC-to-osteoblast differentiation in the context of peri-prosthetic osteolysis. Lee et al. showed that cyclical equi-biaxial tensile strain of 5000 and 30,000 με applied at 1 Hz with Ti particles synergistically increased the production of IL-6 and M-CSF by MC3T3-E1 cells and primary osteoblasts.116 These effects were associated with disorganized actin cytoskeleton and damage to cell membrane leading to the activation of the ERK signaling pathway. However, the strain itself was not sufficient to induce these inflammatory changes. The osteogenic differentiation potential of cells exposed to strain with or without Ti particles was not evaluated. Overall the impact of the peri-prosthetic mechanical microenvironment on local MSCs and MSC-to-osteoblast differentiation remains thus largely unknown. Based on the beneficial effects of mechanical strain on MSC differentiation, it can be speculated that the stress-induced bone loss is mainly mediated by increased osteoclast activity.
Retrieval studies
The majority of the current knowledge of the impact of wear debris and peri-implant microenvironment on MSC differentiation is derived from in vitro experiments. Two recent seminal retrieval studies have characterized MSCs from peri-prosthetic tissue surrounding loosening total joint replacement implants. Marguelis et al. isolated MSCs from bone marrow and fibrous tissue immediately adjacent to loose implants and compared their properties to cells obtained from bone marrow during primary joint replacement operations.117 MSCs from revision operations showed diminished capacity for self-renewal and reduced expression of several MSC-related markers including PDGFRα, CD51, ALCAM, endoglin, nestin, CMG1, and nucleostemin. In contrast, the expression of OPG and IL-6 were increased. Importantly, both the osteogenic and adipogenic differentiation ability of revision MSCs was diminished. Rakow et al. isolated MSCs from bone marrow surrounding revised MoM implants.102 High metal ion and particle concentration in the tissues surrounding the implant was demonstrated and subsequently the revision-derived MSCs displayed reduced osteogenic potential with diminished ALP activity; interestingly reduced ALP levels were detected also from the patients’ circulation. MSC viability, proliferation, and migration as well as chrondro- and adipogenic differentiation ability were intact.
Taken together, these two studies suggest that MSC function is severely impaired due to the various microenvironmental signals surrounding loosening implant such as wear debris and macrophage-derived inflammatory cytokines. Indeed, Marquelies and colleagues suggested that impaired quality of MSCs contribute to less robust bone stock and compromised healing ability observed after revision surgeries.
MSC-TARGETED TREATMENT OPTIONS OF ASEPTIC LOOSENING
As MSCs are easy to isolate from adult tissues, expand in vitro, and are relatively safe compared to other stem cells, they are being studied in the treatment of variety of pathologic states including, myocardial infarction, diabetes, graft versus host disease, and liver cirrhosis.57 MSCs can be injected at the site of injury, engrafted into damaged tissue via biocompatible scaffolds, or even administered systemically to subsequently home to sites of inflammation.118–121 By directly introducing MSCs to the site of injury, it is possible to exploit MSCs’ ability to exert context-specific anti-inflammatory and pro-regenerative effects via both paracrine signaling and direct contact65; furthermore MSCs could potentially regenerate tissues directly by differentiating into various mesenchymal cell linages as guided by the local microenviromental signals and other cues.122
Accumulating evidence indicates that MSCs are active participants in peri-prosthetic bone loss and implant loosening. As precursors for bone forming cells that possess immunomodulatory abilities, they are attractive targets for novel treatments for aseptic implant loosening. Indeed, MSC-based therapies have been widely explored for bone regeneration and bone tissue engineering but their applications to treat peri-prosthetic osteolysis are only now emerging.118 Based on current literature, the following MSC-targeted treatment strategies are outlined: (1) utilizing implanted MSCs to enhance initial implant integration; (2) pharmacologically targeting MSCs to limit inflammation and recover bone formation in wear particle challenged peri-implant tissues; (3) implanting MSCs to fill osteolytic lesions during the revision surgery to reconstitute the bone stock and optimize implant integration. The next section summarizes the current efforts of MSC-targeted interventions to optimize implant integration and treat peri-prosthetic osteolysis.
MSCs for improving initial implant integration
Poor initial implant fixation leads to micromotion, compromised osseointegration, and implant loosening. Thus proper initial fixation and subsequent osseointegration are crucial for the long-term success of the implant. Several preclinical studies have utilized implanted MSCs to enhance the initial implant integration. Gao et al. studied the integration of a titanium coated rod into the femur of osteopetrotic FGFR3−/− mice.123 These mice display similar defects in the quality of bone as seen in aging human bone. MSCs derived from healthy donor mice and transplanted into the femoral canal were shown to significantly enhance implant integration. Dozza et al. observed similar effects for transplanted MSCs in a sheep model.124 MSCs with platelet lysate were mixed either with fibrin or a collagen scaffold and transplanted into the femoral canal prior to press-fit implantation of a titanium femoral component. Transplanted MSCs significantly improved the implant integration evident by increased bone formation and enhanced bone/implant contact area. Ogawa et al. seeded rabbit MSCs on the surface of CoCr implant and differentiated them into bone forming osteoblasts in vitro prior to implantation in vivo.125 Implant integration to bone was significantly enhanced as evaluated by histomorphometry and mechanical testing of the bone implant interface. Kalia et al.126 and Coathup et al.127 sprayed autologous and allogenic MSCs or differentiated MSC-derived osteoblasts in fibrin glue onto hydroxyapatite coated ingrowth collars of massive bone tumor implants and studied the integration of these implants in a sheep model.126,127 Autologous MSCs and MSC-derived osteoblasts but not allogenic MSCs increased implant integration. Thus transplanted autologous MSCs can be utilized to enhance initial implant integration but clinical studies in humans remain to be conducted.
Pharmacological targeting of MSCs to limit peri-prosthetic osteolysis
In addition to directly implanting MSCs at the site of injury, another strategy is to enhance the function of wear particle challenged MSCs. Several successful pharmacological treatments to limit adverse reaction to wear debris have been reported. Since many of the adverse effects of particles on MSCs are mediated by particle phagocytosis it has been suggested that inhibiting MSC phagocytosis of such particles—instead leaving particulates for macrophage clearance—may mitigate the particulate-driven reduction in bone formation.90 Kann et al. showed in vitro that BMP-7 was effective in recovering osteogenesis in PMMA particle challenged pre-osteoblast MC3T3-E1 cells.128 Similarly, activation of Wnt/β-catenin signaling with the compound Icariin was shown to rescue the Ti particle-induced reduction in MSC-mediated osteogenesis both in vitro and in vivo.104 Lin et al. showed that blocking NF-kB signaling with decoy ODN limited wear particle responses both in vitro and in vivo.86,129 Finally, Lee et al. showed that AZD6244, a clinically relevant inhibitor of ERK pathway, attenuated Ti particle-induced reduction in MSC function in vitro and in vivo.103 Although successful in cell cultures and animal models, the translational potential of these concepts remains to be determined. Furthermore, many of the signaling pathways targeted by these treatments are not specific for MSCs making the delineation of effects difficult.
Implantation of MSCs to reconstitute peri-implant bone stock during implant revision
Poor quality of remaining bone bed as well as filling out the osteolytic lesions to obtain adequate implant stability can be major challenges during revision total joint replacement surgery. MSCs are highly attractive options to reconstitute the bone stock and promote the integration of the new implant in the context of revision surgery; as compromised MSC quality and bone forming ability have been characterized around loosened implants, reconstructing the MSC stock with healthy transplanted MSCs is intriguing. Indeed, preliminary attempts to this end have been made. Korda et al. demonstrated that autologous MSCs mixed with allograft bone enhanced the integration of a femoral component in a sheep hemiarthroplasty model.130 Hernigou et al. treated patients undergoing revision of acetabular component for large peri-acetabular osteolysis with allograft bone loaded with autologous bone marrow MSCs.131 Increased graft union rate, reduced graft resorption, and reduced rate of failures were observed in the MSC-treated group compared to the group treated with allograft alone. Similar results in the reconstitution of peri-acetabular bone defects following aseptic loosening were reported by Vulcano et al. in a series of only 5 patients with no controls.132 A case report by Jäger et al. described the treatment of peri-acetabular osteolysis with a BMP2/MSC composite; progressive healing with a satisfactory functional outcome was reported.133 Finally, Korda et al. demonstrated that MSCs are able to withstand standard forces during impaction allografting further demonstrating that the approach of mixing MSCs with allograft bone might be an effective means for MSC delivery to the osteolytic lesions around implants.134
Future directions
MSCs play a significant role in modulating inflammation and bone remodeling; these properties suggest great potential as a therapeutic target in inflammation-associated bone disorders such as peri-prosthetic osteolysis. Transplantation of exogenous MSCs enhances osseointegration and could potentially mitigate osteolysis around the implants through paracrine regulation. However, the long-term viability of transplanted MSCs remains questionable, and the direct effects to regenerate new bone formation are still in debate. Furthermore, there are several obstacles remaining to be resolved before moving forward into clinical treatment applications. First, mouse and human MSCs are different in many respects including growth pattern, paracrine secretion profile, and the response to inflammatory stimuli.58,61 Indeed, some of the discoveries in murine models remain to be confirmed in human cells. Second, the biological activities and cell viability of MSCs can be significantly impaired during the natural aging process.69,135–137 The protective effects of MSCs might be weakened in elderly patients due to reduced number and decreased differentiation ability. Finally, MSC may play different roles during the chronic inflammatory and tissue regenerative processes. Further investigation on how MSCs sense and response to the environmental stimuli is essential to optimize the protective functions of exogenous or endogenous MSCs. Nevertheless, innovative treatment approaches utilizing the immunomodulatory capabilities of MSCs to mitigate peri-prosthetic osteolysis will undoubtedly be investigated in the future. Similarly, use of genetically or otherwise modified MSCs as targeted drug delivery vehicles hold great promise but remain unexplored in the context of peri-prosthetic osteolysis; as MSCs have the capacity to migrate to the site of inflammation from circulation, utilizing MSCs as targeted drug delivery vehicles might prove to be of interest. Increasing the recruitment of therapeutic MSCs to the area of inflammation by selectively utilizing chemokines such as CCL3 is another strategy that remains to be explored by further studies.107,108
CONCLUSIONS
It is well established that several types of implant-derived wear debris elicit inflammatory response from MSCs, impair MSC viability, and severely compromise MSC-to-osteoblast differentiation. These effects are due in part to direct phagocytosis of particles and the secretion of pro-inflammatory mediators acting in an auto- and paracrine manner. Relevant intracellular signaling pathways include NF-kB, ERK1/2 and perhaps others. Although the majority of these studies have been conducted in cell culture models, there is evidence that the function of MSCs retrieved from peri-implant tissues are compromised, validating the in vitro concepts. Thus it seems that MSCs clearly are targets of biomaterial wear debris and contribute to peri-prosthetic bone loss. However, there still exist significant gaps in our understanding of the specific roles of MSCs in peri-prosthetic osteolysis. In particular, the interactions of the various intracellular signaling pathways as well as cross-talk of MSCs and macrophages in this condition remain poorly characterized. A summary of the suggested role of the MSCs in the sequence of events of aseptic implant loosening is shown in Figure 3.
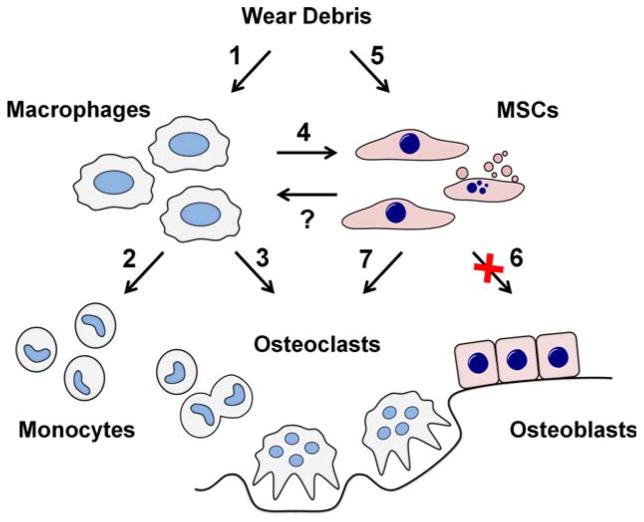
FIGURE 3.
Role of MSCs in the aseptic loosening. Implant-derived wear debris is phagocytosed both by macrophages and MSCs. (1) Macrophages are induced to produce chemokines such as CCL2 and CCL3 as well as pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6. (2) Chemokines continuously recruit additional monocytes and osteoclast precursors to the peri-implant tissue. (3) Pro-inflammatory cytokines stimulate osteoclast formation and (4) contribute to the impairment of MSC function. (5) Phagocytosed wear particles directly compromise both the viability and (6) osteogenic differentiation of MSCs. (7) Upregulation of pro-inflammatory cytokines and RANKL/OPG ratio in particle stimulated MSCs contributes to the osteoclast formation and activity. With increased osteoclast activity and suppressed osteoblast formation and function the balance of bone remodeling in the peri-prosthetic tissue turns to bone resorption with progressive peri-implant bone loss. (?) The possible effects and the therapeutic potential of MSCs reciprocally regulating macrophage function remain to be determined.
MSC have several characteristics—including the ability to regenerate mesenchymal tissues, regulate bone metabolisms, and modulate inflammation—that make them highly attractive candidates for cell based-therapies. Indeed, MSC-targeted treatment strategies to improve initial implant integration and prevent peri-prosthetic osteolysis are emerging. In preclinical models, MSCs coated on the implant surface significantly improve implant integration. Similarly in pre-clinical models, pharmacological interventions are effective in recovering bone forming ability of particle challenged MSCs both in vitro and in vivo. Limited case series describe the use of MSCs to improve the function of allograft bone in reconstituting bone stock during revision surgeries. Nevertheless, the full potential of MSCs for bone regeneration and local immunomodulation remain to be delineated more fully in future studies.
Acknowledgments
Contract grant sponsor: NIH; contract grant number: 2R01AR055650, 1R01AR063717
Contract grant sponsor: The Ellenburg Chair in Surgery at Stanford University
Contract grant sponsor: Jane and Aatos Erkko foundation (J.P.)
References
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century: Total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Pajarinen J, Lin TH, Sato T, Yao Z, Goodman S. Interaction of materials and biology in total joint replacement - successes, challenges and future directions. J Mater Chem B Mater Biol Med. 2014;2:7094–7108. doi: 10.1039/C4TB01005A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OECD. Health at a glance 2011: OECD indicators. 4.7. [Accessed on November 12th, 2016];Hip and knee replacements. 2011 :92–93. http://www.oecd.org/els/health-systems/49105858.pdf.
- 4.CDC. [Accessed on November 12th, 2016];Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age. 2010 https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberpro-cedureage.pdf.
- 5.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 7.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich SD, Seyler TM, Bennett D, Delanois RE, Saleh KJ, Thongtrangan I, Kuskowski M, Cheng EY, Sharkey PF, Parvizi J, Stiehl JB, Mont MA. Total hip arthroplasties: What are the reasons for revision? Int Orthop. 2008;32:597–604. doi: 10.1007/s00264-007-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo J, Goodman SB, Konttinen YT, Raska M. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19:213–224. doi: 10.1177/1753425912451779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nich C, Takakubo Y, Pajarinen J, Ainola M, Salem A, Sillat T, Rao AJ, Raska M, Tamaki Y, Takagi M, Konttinen YT, Goodman SB, Gallo J. Macrophages-key cells in the response to wear debris from joint replacements. J Biomed Mater Res A. 2013;101:3033–3045. doi: 10.1002/jbm.a.34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 14.Väänänen HK. Mesenchymal stem cells. Ann Med. 2005;37:469–479. doi: 10.1080/07853890500371957. [DOI] [PubMed] [Google Scholar]
- 15.Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112:3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman SB, Ma T, Chiu R, Ramachandran R, Smith RL. Effects of orthopaedic wear particles on osteoprogenitor cells. Biomaterials. 2006;27:6096–6101. doi: 10.1016/j.biomaterials.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill SC, Queally JM, Devitt BM, Doran PP, O’Byrne JM. The role of osteoblasts in peri-prosthetic osteolysis. Bone Joint J. 2013;95-B:1022–1026. doi: 10.1302/0301-620X.95B8.31229. [DOI] [PubMed] [Google Scholar]
- 18.Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006;77:177–197. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Amer Y, Darwech I, Clohisy JC. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther. 2007;9:S6. doi: 10.1186/ar2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiri Gallo YTK, Stuart B, Goodman, Jacob P, Thyssen E, Gibon J, Pajarinen Y, Takakubo P, Schalock Z, Mackiewicz E, Jämsen M, Petrek R, Trebse A, Coer M. Takagi Aseptic loosening of total hip arthroplasty as a result of local failure of tissue homeostasis. In: Fokter SK, editor. Recent Advances in Arthroplasty. Rijeka, Croatia: InTech; 2012. pp. 319–362. [Google Scholar]
- 21.Goodman SB. The effects of micromotion and particulate materials on tissue differentiation. Bone chamber studies in rabbits. Acta Orthop Scand Suppl. 1994;258:1–43. doi: 10.3109/17453679409155227. [DOI] [PubMed] [Google Scholar]
- 22.Greenfield EM, Bechtold J Implant Wear Symposium 2007 Biologic Work Group. What other biologic and mechanical factors might contribute to osteolysis? J Am Acad Orthop Surg. 2008;16(Suppl 1):S56–S62. doi: 10.5435/00124635-200800001-00012. [DOI] [PubMed] [Google Scholar]
- 23.Aspenberg P, van der Vis H. Fluid pressure may cause periprosthetic osteolysis. Particles are not the only thing. Acta Orthop Scand. 1998;69:1–4. doi: 10.3109/17453679809002344. [DOI] [PubMed] [Google Scholar]
- 24.Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;460:240–252. doi: 10.1097/BLO.0b013e31804b4147. [DOI] [PubMed] [Google Scholar]
- 25.Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. 1992;74:849–863. [PubMed] [Google Scholar]
- 26.Revell PA. The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J R Soc Interface. 2008;5:1263–1278. doi: 10.1098/rsif.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman SB, Ma T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials. 2010;31:5045–5050. doi: 10.1016/j.biomaterials.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman SB, Gibon E, Yao Z. The basic science of periprosthetic osteolysis. Instr Course Lect. 2013;62:201–206. [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchlin CT, Schwarz EM, O’Keefe RJ, Looney RJ. RANK, RANKL and OPG in inflammatory arthritis and periprosthetic osteolysis. J Musculoskelet Neuronal Interact. 2004;4:276–284. [PubMed] [Google Scholar]
- 30.Looney RJ1, Schwarz EM, Boyd A, O’Keefe RJ. Periprosthetic osteolysis: An immunologist’s update. Curr Opin Rheumatol. 2006;18:80–87. doi: 10.1097/01.bor.0000198004.88568.96. [DOI] [PubMed] [Google Scholar]
- 31.Khosla S. Minireview: The OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 32.Clohisy JC, Frazier E, Hirayama T, Abu-Amer Y. RANKL is an essential cytokine mediator of polymethylmethacrylate particle-induced osteoclastogenesis. J Orthop Res. 2003;21:202–212. doi: 10.1016/S0736-0266(02)00133-X. [DOI] [PubMed] [Google Scholar]
- 33.Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 34.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res A. 2001;16:2082–2091. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 35.Pearl JI, Ma T, Irani AR, Huang Z, Robinson WH, Smith RL, Goodman SB. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32:5535–5542. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenfield EM. Do genetic susceptibility, Toll-like receptors, and pathogen-associated molecular patterns modulate the effects of wear? Clin Orthop Relat Res. 2014;472:3709–3717. doi: 10.1007/s11999-014-3786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bechtel CP, Gebhart JJ, Tatro JM, Kiss-Toth E, Wilkinson JM, Greenfield EM. Particle-induced osteolysis is mediated by TIRAP/Mal in vitro and in vivo: Dependence on adherent pathogen-associated molecular patterns. J Bone Joint Surg Am. 2016;98:285–294. doi: 10.2106/JBJS.O.00736. [DOI] [PubMed] [Google Scholar]
- 38.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 39.Tamaki Y, Takakubo Y, Goto K, Hirayama T, Sasaki K, Konttinen YT, Goodman SB, Takagi M. Increased expression of toll-like receptors in aseptic loose periprosthetic tissues and septic synovial membranes around total hip implants. J Rheumatol. 2009;36:598–608. doi: 10.3899/jrheum.080390. [DOI] [PubMed] [Google Scholar]
- 40.Lähdeoja T, Pajarinen J, Kouri VP, Sillat T, Salo J, Konttinen YT. Toll-like receptors and aseptic loosening of hip endoprosthesis-a potential to respond against danger signals? J Orthop Res. 2010;28:184–190. doi: 10.1002/jor.20979. [DOI] [PubMed] [Google Scholar]
- 41.Greenfield EM, Beidelschies MA, Tatro JM, Goldberg VM, Hise AG. Bacterial pathogen-associated molecular patterns stimulate biological activity of orthopaedic wear particles by activating cognate Toll-like receptors. J Biol Chem. 2010;285:32378–32384. doi: 10.1074/jbc.M110.136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho DR, Shanbhag AS, Hong CY, Baran GR, Goldring SR. The role of adsorbed endotoxin in particle-induced stimulation of cytokine release. J Orthop Res. 2002;20:704–713. doi: 10.1016/S0736-0266(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 44.Caicedo MS, Desai R, McAllister K, Reddy A, Jacobs JJ, Hallab NJ. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: A novel mechanism for implant debris reactivity. J Orthop Res. 2009;27:847–854. doi: 10.1002/jor.20826. [DOI] [PubMed] [Google Scholar]
- 45.Burton L, Paget D, Binder NB, Bohnert K, Nestor BJ, Sculco TP, Santambrogio L, Ross FP, Goldring SR, Purdue PE. Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation. J Orthop Res. 2013;31:73–80. doi: 10.1002/jor.22190. [DOI] [PubMed] [Google Scholar]
- 46.Gill HS, Grammatopoulos G, Adshead S, Tsialogiannis E, Tsiridis E. Molecular and immune toxicity of CoCr nanoparticles in MoM hip arthroplasty. Trends Mol Med. 2012;18:145–155. doi: 10.1016/j.molmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Hallab NJ, Anderson S, Stafford T, Glant T, Jacobs JJ. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005;23:384–391. doi: 10.1016/j.orthres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 49.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 50.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 52.Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. Open Orthop J. 2011;5:242–248. doi: 10.2174/1874325001105010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 54.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 60.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 61.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 62.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PloS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesen-chymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PloS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eggenhofer E, Hoogduijn MJ. Mesenchymal stem cell-educated macrophages. Transpl Res. 2012;1:12. doi: 10.1186/2047-1440-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 69.Lin TH, Gibon E, Loi F, Pajarinen J, Cordova LA, Nabeshima A, Lu L, Yao Z, Goodman SB. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-κB activity. J Orthop Res. 2016 doi: 10.1002/jor.23270. (in press) [DOI] [PubMed] [Google Scholar]
- 70.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 71.Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, Maugham ML, Gregory LS, Steck R, Pettit AR. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 2014;184:3192–3204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J, Richards CD, Chevalier S, Rédini F, Heymann D, Gascan H, Blanchard F. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 73.Champagne CM, Takebe J, Offenbacher S, Cooper LF. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone. 2002;30:26–31. doi: 10.1016/s8756-3282(01)00638-x. [DOI] [PubMed] [Google Scholar]
- 74.Loi F, Córdova LA, Zhang R, Pajarinen J, Lin TH, Goodman SB, Yao Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016;7:15. doi: 10.1186/s13287-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45:367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- 77.Mountziaris PM, Tzouanas SN, Mikos AG. Dose effect of tumor necrosis factor-alpha on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials. 2010;31:1666–1675. doi: 10.1016/j.biomaterials.2009.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mountziaris PM, Dennis Lehman E, Mountziaris I, Sing DC, Kasper FK, Mikos AG. Effect of temporally patterned TNF-α delivery on in vitro osteogenic differentiation of mesenchymal stem cells cultured on biodegradable polymer scaffolds. J Biomater Sci Polym Ed. 2013;24:1794–1813. doi: 10.1080/09205063.2013.803455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, Kim CD, Jung JS. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010;223:168–177. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- 80.Croes M, Oner FC, Kruyt MC, Blokhuis TJ, Bastian O, Dhert WJ, Alblas J. Proinflammatory mediators enhance the osteogenesis of human mesenchymal stem cells after lineage commitment. PloS One. 2015;10:e0132781. doi: 10.1371/journal.pone.0132781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu Z, Wang G, Dunstan CR, Chen Y, Lu WY, Davies B, Zreiqat H. Activation and promotion of adipose stem cells by tumour necrosis factor-α preconditioning for bone regeneration. J Cell Physiol. 2013;228:1737–1744. doi: 10.1002/jcp.24330. [DOI] [PubMed] [Google Scholar]
- 82.Lu Z, Wang G, Dunstan CR, Zreiqat H. Short-term exposure to tumor necrosis factor-alpha enables human osteoblasts to direct adipose tissue-derived mesenchymal stem cells into osteogenic differentiation. Stem Cells Dev. 2012;21:2420–2429. doi: 10.1089/scd.2011.0589. [DOI] [PubMed] [Google Scholar]
- 83.Briolay A, Lencel P, Bessueille L, Caverzasio J, Buchet R, Magne D. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-α in human mesenchymal stem cells. Biochem Biophys Res Commun. 2013;430:1072–1077. doi: 10.1016/j.bbrc.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 84.Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol. 2014;5:48. doi: 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: Implications on therapeutic potential. Mediators Inflamm. 2010;2010:865601. doi: 10.1155/2010/865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin TH, Sato T, Barcay KR, Waters H, Loi F, Zhang R, Pajarinen J, Egashira K, Yao Z, Goodman SB. NF-κB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle. Tissue Eng Part A. 2015;21:875–883. doi: 10.1089/ten.tea.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin TH, Tamaki Y, Pajarinen J, Waters HA, Woo DK, Yao Z, Goodman SB. Chronic inflammation in biomaterial-induced peri-prosthetic osteolysis: NF-κB as a therapeutic target. Acta Biomater. 2014;10:1–10. doi: 10.1016/j.actbio.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang ML, Nesti LJ, Tuli R, Lazatin J, Danielson KG, Sharkey PF, Tuan RS. Titanium particles suppress expression of osteoblastic phenotype in human mesenchymal stem cells. J Orthop Res. 2002;20:1175–1184. doi: 10.1016/S0736-0266(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 89.Wang ML, Tuli R, Manner PA, Sharkey PF, Hall DJ, Tuan RS. Direct and indirect induction of apoptosis in human mesenchymal stem cells in response to titanium particles. J Orthop Res. 2003;21:697–707. doi: 10.1016/S0736-0266(02)00241-3. [DOI] [PubMed] [Google Scholar]
- 90.Okafor CC, Haleem-Smith H, Laqueriere P, Manner PA, Tuan RS. Particulate endocytosis mediates biological responses of human mesenchymal stem cells to titanium wear debris. J Orthop Res. 2006;24:461–473. doi: 10.1002/jor.20075. [DOI] [PubMed] [Google Scholar]
- 91.Haleem-Smith H, Argintar E, Bush C, Hampton D, Postma WF, Chen FH, Rimington T, Lamb J, Tuan RS. Biological responses of human mesenchymal stem cells to titanium wear debris particles. J Orthop Res. 2012;30:853–863. doi: 10.1002/jor.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jämsen E, Kouri VP, Olkkonen J, Cör A, Goodman SB, Konttinen YT, Pajarinen J. Characterization of macrophage polarizing cytokines in the aseptic loosening of total hip replacements. J Orthop Res. 2014;32:1241–1246. doi: 10.1002/jor.22658. [DOI] [PubMed] [Google Scholar]
- 93.Lassus J, Waris V, Xu JW, Li TF, Hao J, Nietosvaara Y, Santavirta S, Konttinen YT. Increased interleukin-8 (IL-8) expression is related to aseptic loosening of total hip replacement. Arch Orthop Trauma Surg. 2000;120:328–332. doi: 10.1007/s004020050475. [DOI] [PubMed] [Google Scholar]
- 94.Jämsen E, Kouri VP, Ainola M, Goodman SB, Nordström DC, Eklund KK, Pajarinen J. Correlations between macrophage polarizing cytokines, inflammatory mediators, osteoclast activity, and toll-like receptors in tissues around aseptically loosened hip implants. J Biomed Mater Res a. 2016 doi: 10.1002/jbm.a.35913. In press. [DOI] [PubMed] [Google Scholar]
- 95.Chiu R, Ma T, Smith RL, Goodman SB. Polymethylmethacrylate particles inhibit osteoblastic differentiation of bone marrow osteoprogenitor cells. J Biomed Mater Res a. 2006;77:850–856. doi: 10.1002/jbm.a.30697. [DOI] [PubMed] [Google Scholar]
- 96.Chiu R, Ma T, Smith RL, Goodman SB. Kinetics of polymethyl-methacrylate particle-induced inhibition of osteoprogenitor differentiation and proliferation. J Orthop Res. 2007;25:450–457. doi: 10.1002/jor.20328. [DOI] [PubMed] [Google Scholar]
- 97.Chiu R, Ma T, Smith RL, Goodman SB. Ultrahigh molecular weight polyethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. J Biomed Mater Res A. 2009;89:242–247. doi: 10.1002/jbm.a.32001. [DOI] [PubMed] [Google Scholar]
- 98.Schofer MD, Fuchs-Winkelmann S, Kessler-Thönes A, Rudisile MM, Wack C, Paletta JR, Boudriot U. The role of mesenchymal stem cells in the pathogenesis of Co-Cr-Mo particle induced aseptic loosening: An in vitro study. Biomed Mater Eng. 2008;18:395–403. doi: 10.3233/BME-2008-0556. [DOI] [PubMed] [Google Scholar]
- 99.Jiang Y, Jia T, Gong W, Wooley PH, Yang SY. Effects of Ti, PMMA, UHMWPE, and Co-Cr wear particles on differentiation and functions of bone marrow stromal cells. J Biomed Mater Res a. 2013;101:2817–2825. doi: 10.1002/jbm.a.34595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hou Y, Cai K, Li J, Chen X, Lai M, Hu Y, Luo Z, Ding X, Xu D. Effects of titanium nanoparticles on adhesion, migration, proliferation, and differentiation of mesenchymal stem cells. Int J Nanomedicine. 2013;8:3619–3630. doi: 10.2147/IJN.S38992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schrock K, Lutz J, Mandl S, Hacker MC, Kamprad M, Schulz-Siegmund M. Co(II)-mediated effects of plain and plasma immersion ion implanted cobalt-chromium alloys on the osteogenic differentiation of human mesenchymal stem cells. J Orthop Res. 2015;33:325–333. doi: 10.1002/jor.22765. [DOI] [PubMed] [Google Scholar]
- 102.Rakow A, Schoon J, Dienelt A, John T, Textor M, Duda G, Perka C, Schulze F, Ode A. Influence of particulate and dissociated metal-on-metal hip endoprosthesis wear on mesenchymal stromal cells in vivo and in vitro. Biomaterials. 2016;98:31–40. doi: 10.1016/j.biomaterials.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 103.Ciavarella C, Fittipaldi S, Pasquinelli G. CoCl2 administration to vascular MSC cultures as an in vitro hypoxic system to study stem cell survival and angiogenesis. Methods Mol Biol. 2016;1516:309–317. doi: 10.1007/7651_2016_350. [DOI] [PubMed] [Google Scholar]
- 104.Samelko L, Caicedo MS, Lim SJ, la-Valle C, Jacobs J, Hallab NJ. Cobalt-alloy implant debris induce HIF-1alpha hypoxia associated responses: A mechanism for metal-specific orthopedic implant failure. PLoS One. 2013;8:e67127. doi: 10.1371/journal.pone.0067127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsu SH, Chen CT, Wei YH. Inhibitory effects of hypoxia on metabolic switch and osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2013;31:2779–2788. doi: 10.1002/stem.1441. [DOI] [PubMed] [Google Scholar]
- 106.Zhou J, Zhao L. Hypoxia-mimicking Co doped TiO2 microporous coating on titanium with enhanced angiogenic and osteogenic activities. Acta Biomater. 2016;43:358–368. doi: 10.1016/j.actbio.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 107.Lee HG, Minematsu H, Kim KO, Celil Aydemir AB, Shin MJ, Nizami SA, Chung KJ, Hsu AC, Jacobs CR, Lee FY. Actin and ERK1/2-CEBPβ signaling mediates phagocytosis-induced innate immune response of osteoprogenitor cells. Biomaterials. 2011;32:9197–9206. doi: 10.1016/j.biomaterials.2011.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J, Tao Y, Ping Z, Zhang W, Hu X, Wang Y, Wang L, Shi J, Wu X, Yang H, Xu Y, Geng D. Icariin attenuates titanium-particle inhibition of bone formation by activating the Wnt/β-catenin signaling pathway in vivo and in vitro. Sci Rep. 2016;6:23827. doi: 10.1038/srep23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kotake S, Nanke Y. Effect of TNFα on osteoblastogenesis from mesenchymal stem cells. Biochim Biophys Acta. 2014;1840:1209–1213. doi: 10.1016/j.bbagen.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 110.Lee SS, Sharma AR, Choi BS, Jung JS, Chang JD, Park S, Salvati EA, Purdue EP, Song DK, Nam JS. The effect of TNFα secreted from macrophages activated by titanium particles on osteogenic activity regulated by WNT/BMP signaling in osteoprogenitor cells. Biomaterials. 2012;33:4251–4263. doi: 10.1016/j.biomaterials.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Huang Z, Ma T, Ren PG, Smith RL, Goodman SB. Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells. J Biomed Mater Res a. 2010;94:1264–1269. doi: 10.1002/jbm.a.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gibon E, Yao Z, Rao AJ, Zwingenberger S, Batke B, Valladares R, Smith RL, Biswal S, Gambhir SS, Goodman SB. Effect of a CCR1 receptor antagonist on systemic trafficking of MSCs and polyethylene particle-associated bone loss. Biomaterials. 2012;33:3632–3638. doi: 10.1016/j.biomaterials.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Steward AJ, Kelly DJ. Mechanical regulation of mesenchymal stem cell differentiation. J Anat. 2015;227:717–731. doi: 10.1111/joa.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Delaine-Smith RM, Reilly GC. Mesenchymal stem cell responses to mechanical stimuli. Muscles Ligaments Tendons J. 2012;2:169–180. [PMC free article] [PubMed] [Google Scholar]
- 115.Blaber EA, Dvorochkin N, Torres ML, Yousuf R, Burns BP, Globus RK, Almeida EA. Mechanical unloading of bone in micro-gravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 2014;13:181–201. doi: 10.1016/j.scr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 116.Lee HG, Hsu A, Goto H, Nizami S, Lee JH, Cadet ER, Tang P, Shaji R, Chandhanayinyong C, Kweon SH, Oh DS, Tawfeek H, Lee FY. Aggravation of inflammatory response by costimulation with titanium particles and mechanical perturbations in osteo-blast- and macrophage-like cells. Am J Physiol Cell Physiol. 2013;304:C431–C439. doi: 10.1152/ajpcell.00202.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Margulies BS, DeBoyace SD, Parsons AM, Policastro CG, Ee JS, Damron TS. Functionally deficient mesenchymal stem cells reside in the bone marrow niche with M2-macrophages and amyloid-beta protein adjacent to loose total joint implants. J Orthop Res. 2015;33:615–624. doi: 10.1002/jor.22790. [DOI] [PubMed] [Google Scholar]
- 118.Nöth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev. 2010;62:765–783. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 119.Nöth U, Steinert AF, Tuan RS. Technology insight: Adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371–380. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- 120.Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 121.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 122.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: An update. Cell Transpl. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 123.Gao C, Seuntjens J, Kaufman GN, Tran-Khanh N, Butler A, Li A, Wang H, Buschmann MD, Harvey EJ, Henderson JE. Mesenchymal stem cell transplantation to promote bone healing. J Orthop Res. 2012;30:1183–1189. doi: 10.1002/jor.22028. [DOI] [PubMed] [Google Scholar]
- 124.Dozza B, Di Bella C, Lucarelli E, Giavaresi G, Fini M, Tazzari PL, Giannini S, Donati D. Mesenchymal stem cells and platelet lysate in fibrin or collagen scaffold promote non-cemented hip prosthe-sis integration. J Orthop Res. 2011;29:961–968. doi: 10.1002/jor.21333. [DOI] [PubMed] [Google Scholar]
- 125.Ogawa M, Tohma Y, Ohgushi H, Takakura Y, Tanaka Y. Early fixation of cobalt-chromium based alloy surgical implants to bone using a tissue-engineering approach. Int J Mol Sci. 2012;13:5528–5541. doi: 10.3390/ijms13055528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kalia P, Blunn GW, Miller J, Bhalla A, Wiseman M, Coathup MJ. Do autologous mesenchymal stem cells augment bone growth and contact to massive bone tumor implants? Tissue Eng. 2006;12:1617–1626. doi: 10.1089/ten.2006.12.1617. [DOI] [PubMed] [Google Scholar]
- 127.Coathup MJ, Kalia P, Konan S, Mirza K, Blunn GW. A comparison of allogeneic and autologous mesenchymal stromal cells and osteoprogenitor cells in augmenting bone formation around massive bone tumor prostheses. J Biomed Mater Res A. 2013;101:2210–2218. doi: 10.1002/jbm.a.34536. [DOI] [PubMed] [Google Scholar]
- 128.Kann S, Chiu R, Ma T, Goodman SB. OP-1 (BMP-7) stimulates osteoprogenitor cell differentiation in the presence of polymethylmethacrylate particles. J Biomed Mater Res A. 2010;94:485–488. doi: 10.1002/jbm.a.32712. [DOI] [PubMed] [Google Scholar]
- 129.Lin TH, Pajarinen J, Sato T, Loi F, Fan C, Cordova LA, Nabeshima A, Gibon E, Zhang R, Yao Z, Goodman SB. NF-κB decoy oligodeoxynucleotide mitigates wear particle-associated bone loss in the murine continuous infusion model. Acta Biomater. 2016;41:273–281. doi: 10.1016/j.actbio.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Korda M, Blunn G, Goodship A, Hua J. Use of mesenchymal stem cells to enhance bone formation around revision hip replacements. J Orthop Res. 2008;26:880–885. doi: 10.1002/jor.20598. [DOI] [PubMed] [Google Scholar]
- 131.Hernigou P, Pariat J, Queinnec S, Homma Y, Flouzat Lachaniette CH, Chevallier N, Rouard H. Supercharging irradiated allografts with mesenchymal stem cells improves acetabular bone grafting in revision arthroplasty. Int Orthop. 2014;38:1913–1921. doi: 10.1007/s00264-014-2285-2. [DOI] [PubMed] [Google Scholar]
- 132.Vulcano E, Murena L, Falvo DA, Baj A, Toniolo A, Cherubino P. Bone marrow aspirate and bone allograft to treat acetabular bone defects in revision total hip arthroplasty: Preliminary report. Eur Rev Med Pharmacol Sci. 2013;17:2240–2249. [PubMed] [Google Scholar]
- 133.Jäger M, Emami R, Thorey F, Krauspe R. Saving implants BMP-2 application in revision total hip surgery. Int J Biomed Sci. 2006;2:187–195. [PMC free article] [PubMed] [Google Scholar]
- 134.Korda M, Blunn G, Phipps K, Rust P, Di Silvio L, Coathup M, Goodship A, Hua J. Can mesenchymal stem cells survive under normal impaction force in revision total hip replacements? Tissue Eng. 2006;12:625–630. doi: 10.1089/ten.2006.12.625. [DOI] [PubMed] [Google Scholar]
- 135.Asumda FZ, Chase PB. Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol. 2011;12:44. doi: 10.1186/1471-2121-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 137.Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol. 2011;23:518–524. doi: 10.1016/j.coi.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]