Abstract
Objective
Determine whether serum urate predicts ALS progression.
Methods
The study population comprised adult participants of EMPOWER (n=942), a phase III clinical trial to evaluate the efficacy of dexpramipexole to treat ALS. Urate was measured in blood samples collected during enrollment as part of the routine block chemistry.
Outcomes
Combined assessment of function and survival rank (CAFs), and time to death, by 12 months.
Results
In women there was not a significant relation between urate and outcomes. In men, outcomes improved with increasing urate (comparing highest to lowest urate quartile: CAFS was 53 points better with p for trend=0.04; and hazard ratio for death was 0.60 with p for trend=0.07), but with adjustment for body mass index (BMI) at baseline, a predictor of both urate levels and prognosis, associations were attenuated and no longer statistically significant. Overall, participants with urate levels equal to or above the median (5.1 mg/dL) appeared to have a survival advantage compared to those below (hazard ratio adjusted for BMI: 0.67; 95% confidence interval: 0.47 to 0.95).
Conclusion
These findings suggest that while the association between urate at baseline and ALS progression is partially explained by BMI, there may be an independent beneficial effect of urate.
Keywords: survival, progression, urate, body mass index, biomarkers
Introduction
Urate is a potent circulating antioxidant that has been associated with lower risk and slower progression of a number of neurological diseases. Initially, it was identified as a predictor of Parkinson's disease risk, where levels were lower in those who would become cases than in controls.(1, 2) Subsequently it was reported that within patient populations those with lower levels had a faster disease progression.(3, 4) Progression is also reportedly faster when urate is lower in patients with Huntington's disease(5) and multiple system atrophy.(6) Amyotrophic lateral sclerosis (ALS) is a fatal neurological disease that may involve oxidative processes. If it can be established that higher urate levels are associated with slower progression in ALS, then elevation of levels may prove to be a relatively safe, efficacious and inexpensive treatment.
The studies that examined ALS prognosis in relation to urate levels at diagnosis have inconsistent findings.(7-12) In two case-control studies with follow-up visits among cases, and in a population-based discovery series of cases, no association with plasma urate levels at enrollment was found with the revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) or with death.(7, 8, 10) In two recent analyses of data from randomized clinical trials, one with over 8,600 participants found a statistically significant but very small survival advantage with higher plasma urate levels,(11) while the other, with 251 participants, found a significant survival advantage in men, but not women.(9) The inconsistent findings in these earlier studies may result from variability in patient sample, study design, sample size, or the difference in strategies in dealing with confounding variables such as body mass index (BMI).(13) Therefore, in this study we sought to examine the relation between serum urate levels at baseline and ALS progression with the adjustment of potential confounding variables, using data collected from a randomized clinical trial.
Materials and Methods
Subjects
Participant description and criteria for enrollment have been described extensively previously.(14) Briefly, EMPOWER was a randomized, double-blind, placebo-controlled phase III clinical trial designed to evaluate the efficacy of dexpramipexole in the treatment of ALS. Participants were aged 18–80 years when recruited from 81 academic medical centers located in Australia, Belgium, Canada, France, Germany, Ireland, the Netherlands, Spain, Sweden, the UK, and the USA. To be eligible for enrollment, the first ALS symptom must have occurred within the 24 months before baseline, and a participant's upright slow vital capacity at a screening visit had to be at least 65% of the predicted value for his or her age, height, and sex. Assignment to dexpramipexole or placebo was in a one-to-one ratio stratified on location, onset site (bulbar vs other areas), and use of riluzole. Exclusion criteria included a number of co-morbidities: significant cognitive impairment, clinical dementia, psychiatric illness or other neurodegenerative diseases; clinically significant history of unstable or severe cardiac, oncological, hepatic, or renal disease or other medically significant illness; and pulmonary disorder not attributed to ALS. Abnormal neutrophil count (defined as <1.96 × 103 cells per μL) at screening or a documented history of neutropenia; aspartate aminotransferase or alanine aminotransferase concentrations more than 3.0 times the upper limit of normal; and/or creatinine clearance 50 mL/min or less were also criteria for exclusion. Participants were required to have no exposure to any other experimental drug (off-label use or investigational) during the 30 days before baseline, as well as no previous exposure to dexpramipexole, or present use of pramipexole or other dopamine agonists. The current analyses include all 942 participants who were randomized at baseline, 474 to dexpramipexole and 468 to placebo. The EMPOWER study is registered with ClinicalTrials.gov, number NCT01281189.
Assessment of Exposure
Urate level was part of the full chemistry panel measured at randomization. Samples were shipped by courier on the day of collection from site to the central laboratory at ambient temperature and processed on the same day. Levels in blood samples drawn were determined using an enzymatic assay performed at a central commercial clinical laboratory (Covance, Indianapolis, IN).
Statistical Analysis
The endpoints used were a combined assessment of function and survival (CAFS) rank, and time to death, both by 12 months. CAFS, the primary endpoint in EMPOWER, is a joint-rank test that considers change in ALSFRS-R scores between baseline and 12 months in survivors or time to death.(14) (15) (16) Participants who die are ranked according to time of death (earlier time of death ranked worse), while those who survive are ranked above those who die, with smaller declines in ALSFRS-R scores ranked better. The variable of interest was serum urate level. In the analyses, urate was analyzed as a continuous variable and entered the model in linear form. To explore potential nonlinear patterns, the effect of urate was also examined using aggregated values based on sex-specific quartiles. Tests for trend were conducted by including the sex-specific median of the quartile in a model as a continuous variable. The association between urate and CAFS was assessed with multivariate linear regression and that between urate and time to death at 12 months was assessed with the Cox proportional hazards model. Analyses were adjusted for baseline age (continuous), baseline ALSFRS-R (continuous), symptom duration at baseline (continuous), site of onset (bulbar: yes, no), riluzole use (yes/no), and body mass index (BMI: <18.5, 18.5<23, 23-<25, 25-<27.5, 27.5-<30, 30+ kg/m2). The significance level of 0.05 was used throughout the analysis. SAS version 9 was used for all analyses.
Results
Urate level was available in all 605 men (median: 5.6 mg/dL, range: 2.6 to 10.6) and in 336 of the 337 women (median: 4.3 mg/dL, range: 1.5 to 8.7) randomized at baseline. Overall, the average age was 57 years and average BMI was 26.1 kg/m2; 23% of participants had bulbar onset and 75% were using riluzole. In both men and women, BMI has a positive correlation with urate, while in men, age was negatively correlated with urate level. (Table 1)
Table 1. Patient Characteristics by Sex-specific Quartiles of Urate Measured at Randomization.
| Men | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| N | 152 | 157 | 148 | 148 |
| Urate, median (min, max) | 4.3 (2.6, 4.8) | 5.3 (4.8, 5.6) | 6.0 (5.6, 6.4) | 7.1 (6.4, 10.6) |
| Age, mean (sd) | 59.5 (10.6) | 55.8 (12.2) | 55.0 (11.6) | 53.8 (10.7) |
| BMI, mean (sd) | 25.0 (3.7) | 26.2 (3.9) | 26.3 (3.4) | 28.0 (4.2) |
| Bulbar onset (%) | 29.6 | 17.2 | 13.5 | 15.5 |
| Riluzole (%) | 77.0 | 76.4 | 80.4 | 70.3 |
| Baseline ALSFR-R, mean (sd) | 38.8 (5.1) | 38.3 (5.4) | 39.0 (5.3) | 38.9 (5.6) |
| Symptom duration mean (sd) | 14.9 (5.5) | 15.1 (5.5) | 15.2 (5.4) | 14.6 (5.1) |
|
| ||||
| Women | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|
| ||||
| N | 85 | 90 | 77 | 84 |
| Urate, median (min, max) | 3.1 (1.5, 3.6) | 4.0 (3.6, 4.3) | 4.6 (4.3, 5.0) | 5.6 (5.0, 8.7) |
| Age, mean (sd) | 58.7 (12.1) | 58.1 (10.7) | 58.4 (10.3) | 60.2 (10.7) |
| BMI, mean (sd) | 23.6 (3.7) | 24.5 (4.2) | 27.5 (5.9) | 27.1 (4.4) |
| Bulbar onset (%) | 28.2 | 41.1 | 33.8 | 20.2 |
| Riluzole (%) | 70.6 | 74.4 | 79.2 | 70.2 |
| Baseline ALSFR-R, mean (sd) | 37.5 (5.3) | 36.7 (6.6) | 37.6 (4.8) | 36.9 (5.2) |
| Symptom duration mean (sd) | 15.9 (4.9) | 15.4 (5.7) | 15.8 (5.1) | 15.9 (5.4) |
CAFS rank at 12 months
In men, there was a suggestion that baseline urate, modelled as continuous, was associated with better CAFS rank. In analyses adjusted for baseline values of age, ALSFRS-R, symptom duration, riluzole use, and for site of onset, for a 1mg/dL increase in urate CAFS rank was 15.1 units better (s.e.=8.3; p=0.07). However, after further adjustment for BMI, a predictor of urate level, the apparent advantage was attenuated and not significant (estimated difference=8.4 units; p=0.3). In women, urate modelled as continuous was not associated with CAFS rank, with or without adjustment for BMI.
Without adjustment for BMI, men in the third and fourth quartiles of urate at baseline had better CAFS ranks than men in the first quartile: the mean CAFS rank was 29.8 (s.e.=28.5; p=0.3) points better in the second quartile, 71.3 (s.e.=29.1; p=0.014) better in the third, and 53.3 (s.e.=29.2; p=0.069) better in the fourth quartile (p for trend=0.038). With adjustment for BMI, these estimates were attenuated and the trend was no longer statistically significant (CAFS rank estimates were 20.4, 60.2, and 29.0 points better for the second, third and fourth quartile respectively, compared to the first quartile, with p for trend=0.22). In women, urate in quartiles was not associated with CAFS rank, with or without adjustment for BMI. (Table 2)
Table 2a. Hazard Ratios (95% Confidence Intervals) For Death at 12 Months According to Urate Measured at Randomization.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-trend | Per 1mg/dL increase in serum urate | |
|---|---|---|---|---|---|---|
| Men | Ref¥ | 0.74 (0.45, 1.22) | 0.73 (0.18, 3.01) | 0.60 (0.34, 1.05) | P=0.07 | 0.88 (0.75, 1.03) |
| Ref* | 0.80 (0.17, 3.79) | 0.81 (0.17, 4.02) | 0.68 (0.38, 1.24) | P=0.22 | 0.92 (0.78, 1.09) | |
| Women | Ref¥ | 1.12 (0.49, 2.57) | 1.37 (0.59, 3.15) | 0.51 (0.22, 1.18) | P=0.22 | 0.82 (0.61, 1.09) |
| Ref* | 1.39 (0.09, 20.9) | 1.93 (0.04, 83.9) | 0.63 (0.18, 2.20) | P=0.55 | 0.88 (0.75, 1.04) |
| Table 2b: Improvement (Standard Error) in CAFs1 Rank at 12 Months According to Urate Measured at Randomization | ||||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-trend | Per 1mg/dL increase in serum urate | |
| Men | Ref¥ | 29.8 (28.5) | 71.3 (29.1)‡ | 53.3 (29.2) ‡‡ | P=0.038 | 15.1 (8.3) ‡‡ |
| Ref* | 20.4 (28.8) | 60.2 (28.5)‡ | 29.0 (30.5) | P=0.22 | 8.4 (8.7) | |
| Women | Ref¥ | 56.3 (36.3) | -11.5 (37.5) | 38.9 (36.8) | P=0.56 | 10.5 (12.7) |
| Ref* | 34.5 (36.1) | -44.9 (39.1) | 18.6 (38.7) | P=0.92 | 5.97 (13.7) | |
Adjusted for baseline values of age, ALSFRS-R, symptom duration, riluzole use, and for site of onset.
Further adjusted for BMI at enrollment.
p-value <0.05,
p-value <0.10 .
CAFS= combined assessment of function and survival rank. P-for-trend uses the median of the quartile.
In further analyses, urate levels were dichotomized at the median among all participants (men and women combined: 5.1 mg/dL). After adjustment for baseline age, ALSFRS-R, symptom duration, riluzole use, site of onset, and BMI, men with urate equal to or above the median had a mean CAFS rank that was 46.3 units (s.e.=22.5; p=0.045) better than that of men below the median. In women findings were null (p=0.5).
Time to death by 12 months
In men, there was a suggestion of a protective effect of urate comparing the fourth to the first quartile (hazard ratio [HR]=0.60; 95% confidence interval [CI]: 0.34, 1.05; p=0.073; p for trend=0.072), adjusted for baseline values of age, ALSFRS-R, symptom duration, riluzole use, and for site of onset. After further adjustment for BMI, the association was attenuated and null (p for trend=0.22). (Table 2) Among women, neither continuous nor quartiles of urate predicted time to death.
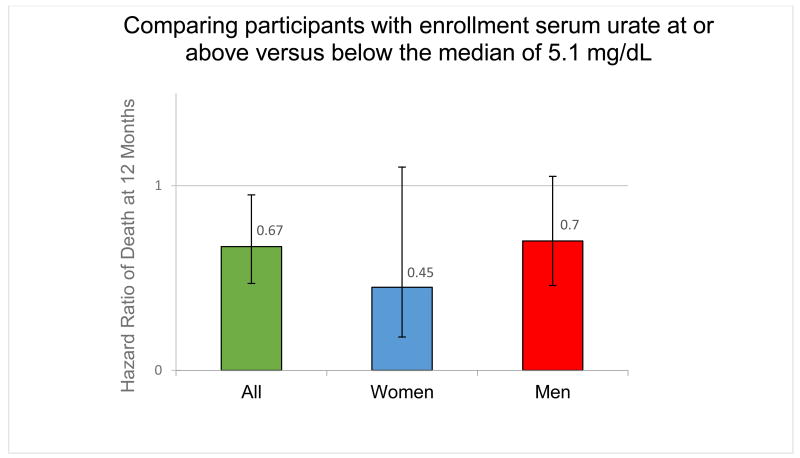
When urate levels were dichotomized, individuals with urate equal to or above the overall median (5.1 mg/dL) appeared to have a survival advantage compared to those below the median: HR=0.70 (95% CI: 0.46, 1.05; p=0.087) in men; HR=0.45 (95% CI: 0.18, 1.10; p=0.083) in women; and HR=0.67; (95% CI: 0.47, 0.95); p=0.029 in men and women, adjusted for BMI. (Figure)
Figure. Comparing participants with baseline serum urate at or above versus below the median of 5.1 mg/dL.
Associations were similar when adjusted for cholesterol level at baseline and in analyses restricted to the placebo arm.
BMI
BMI at randomization strongly predicted both CAFs rank and time to death by 12 months in men and women. CAFs rank at 12 months, adjusted for baseline values of age, ALSFRS-R, symptom duration, riluzole use, and for site of onset and sex was 7.3 units (s.e.=1.86; p<0.001) better for every 1 kg/m2 unit increase in BMI for men and women combined. There was also a positive association between BMI and time to death at 12 months: in similarly multivariable adjusted analyses the HR was 0.94 (95% CI: 0.90, 0.99; p=0.0117). Further adjustment for urate did not change the associations between BMI and either outcome.
Discussion
The studies that examined ALS prognosis in relation to urate levels at diagnosis have inconsistent findings.(7-12) Two case-control studies with follow-up visits for patients found no association overall between urate levels at enrollment and ALSFRS-R or death.(7, 8) Although one of the two reported that among 45 patients with repeated urate measurements there was a correlation between the change in patients' urate relative to that of controls and the change in ALSFRS-R.(7) An Italian population-based discovery cohort, comprising 712 patients recruited between January 1st, 2007 and December 31st, 2011, also reported no association between urate levels and disease progression.(10) However, a case-control study from Japan reported that among the 92 patients enrolled there was rapid worsening of annual ALS-FRS and FVC with lower serum urate levels after adjustment for age, sex and BMI.(12) Two recent studies that take advantage of existing data from randomized clinical trials also reported that urate levels at enrollment predicted ALS outcomes. In the PRO-ACT (Pooled Resource Open-Access ALS Clinical Trials) database, consisting of sixteen phase II and III trials with 8,600 participants, a statistically significant but very small (5.8% [converted from 0.1% per micromol/L to mg/dL, note: units are incorrect in original publication]) survival advantage was seen per each 1 mg/dL higher urate at trial entry.(11) In addition, higher urate was predictive of a slower drop in functional decline as measured by ALSFRS-R (0.06 points per each mg/dL higher urate, p=0.01). Again, the magnitude of the effect of urate level was small given that the overall rate of decline of ALSFRS-R was 1.02 (+/-2.3) points per month. PRO-ACT findings were not adjusted for BMI, a strong predictor of urate levels and of ALS progression. In contrast to the PRO-ACT data, but similar to the current study, a survival advantage of 39% (95% CI: [4% to 61%]; p=0.03) for a 1 mg/dL increase in urate was reported among men enrolled in two multicenter trials (n=251), adjusted for BMI.(9) However urate did not predict survival in women.
The absence of a strong, consistent inverse relationship between urate levels and ALS progression in women is similar to findings in studies of progression of PD,(3, 4) and may result from the small proportion of women with urate levels at a potentially therapeutic concentration. In addition, urate levels in women may have more intra-individual variation given the interplay of urate and sex hormones.(17) Our finding that urate level was lower in men and women with bulbar onset has been reported previously, and it has been hypothesized that lower levels could be due to impaired nutrition.(18) Hyperuricemia, defined as urate levels of >7.0 mg/dl in men and >5.7 mg/dl in women, was low in the studied population.(19) In Table 1, we report the median of the top quartile for men and women is 7.1 mg/dl and 5.6 mg/dl. Therefore, the prevalence of hyperuricemia is considerably lower than the prevalence reported in NHANES III: 21% in adult men and 22% in adult women, rising to 28% in ages 60-69, and 32% in ages 70-79 years.(19) Only ten male (1.7%) and no female participants were taking plasma urate lowing drugs (Zyloprim, Allopurinol, Zyloric) at enrollment.
To summarize, variability in urate-ALS progression studies may result in part from small numbers in earlier studies, differences in patient populations recruited (for example: prevalence of use of riluzole and average baseline ALSFRS-R, prevalence of bulbar onset), and inconsistent adjustment for determinants of urate level and for survival, for example BMI, dietary or genetic factors.
For two reasons, it should be noted that weak or null associations between baseline urate and ALS outcomes do not exclude the possibility that individuals with higher levels of urate may have a lower ALS risk and, more importantly, that urate elevation could indeed be beneficial in individuals with ALS. The first reason is that participation in EMPOWER, and thus inclusion in our analyses, was by design conditional on clinical status (ALS with at least 65% vital capacity). This conditioning would be expected to induce a spurious correlation between the factors that are causally related to ALS risk or prognosis.(20) For example, if higher urate levels reduced ALS risk, factors unrelated to urate that increase ALS risk or worsen prognosis would be enriched among those individuals with high urate in our study. The second reason is that the possibility of a beneficial effect of urate on ALS progression was obfuscated by unmeasured confounders. The investigation of the relation between serum urate in otherwise healthy individuals and their future ALS risk will help address these questions.
A more definitive answer on whether urate elevation could be beneficial in ALS, however, would require a randomized trial. Oxidative stress has been implicated in motor neuron degeneration and astrocyte dysfunction in ALS pathogenesis.(21, 22) Urate is a powerful antioxidant circulating in humans at almost the limit of its solubility.(23) Several in vitro and in vivo studies suggest a neuroprotective effect of urate and its precursor, inosine.(24) For example, urate has been shown to protect spinal cord (MAP-2+) neurons in astrocytic co-culture.(25) Further, when urate is added to neuron cultures before and during treatment with MPP(+), the loss of dopaminergic neurons in neuron-enriched cultures is attenuated, and fully prevented in neuron-astrocyte cultures. Neurons prepared from mice over-expressing urate oxidase were more susceptible to MPP(+) toxicity.(26) Moreover, mice with a constitutive mutation of the urate oxidase gene have increased concentrations of brain urate and exhibit attenuated toxic effects of intrastriatal 6-hydroxydopamine. Conversely, genetically manipulated mice with overexpression of urate oxidase showed exacerbates morphological, neurochemical, and functional lesions of the dopaminergic nigrostriatal pathway.(27) A randomized clinical trial of urate elevation in early Parkinson's disease patients found inosine was generally safe, tolerable, and effective in raising serum and cerebrospinal fluid urate levels.(28) While prospective studies have reported that more cardiovascular events occur in individuals with higher levels of peripheral urate, this association is most likely due to confounding by other risk factors, because genetically determined variations in blood urate are not associated with diabetes, cardiovascular heart disease, or heart failure.(29)
Conclusion
In this large longitudinal investigation among patients with ALS participating in a randomized clinical trial of dexpramipexole, we found only a modest inverse relation between serum urate at enrollment and clinical outcomes during 12 months of follow-up. Overall, after adjustment for BMI, there was not a significant trend of improved survival or slower disease progression with increasing urate levels, whether urate was considered as a continuous variable or categorized in a priori defined gender-specific quartiles. On the other hand, in both men and women, those patients with urate at or above the population median of 5.1 mg/dL had significantly better outcomes than those below the median. Although these results provide only modest support for the hypothesis that urate elevation could be beneficial in ALS, considering the lack of effective treatments and the safety of elevating urate levels under close medical monitoring(28) a trial of urate elevation in patients with ALS and urate levels below the median should be considered.
Acknowledgments
Funding for this study includes NIH grant NS045893 awarded to AA. MAS is funded by Target ALS. SP is funded by an NIH Career Development Award (2K12HD001097-16). The EMPOWER trial was designed by Biogen Idec and Knopp Biosciences and sponsored by Biogen Idec. We thank the study participants and their families and caregivers, the study investigators and site staff. (14)
Footnotes
Author Contributions: DL had had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept or design: MEC, AA.
Drafting/revising the manuscript for content: EO'R, AA, SP, MEC, MAS.
Analysis or interpretation of data: EO'R, DL, ML, DJ, AA.
Disclosures: Éilis J. O'Reilly reports no disclosures.
Dawei Liu is an employee of Biogen Idec.
Donald R. Johns is an employee of Biogen Idec.
Merit E. Cudkowicz has served as a consultant for AstraZenica, Biogen Idec, Cytokinetics, GlaxoSmithKline, Voyager, Denali, and Genentech.
Sabrina Paganoni reports no disclosures.
Michael A. Schwarzschild reports no disclosures.
Melanie Leitner is an employee of Biogen Idec.
Alberto Ascherio reports no disclosures.
Disclosures of Interest: AA, EO'R, SP, MAS: none. ML, DJ, DL are employees of Biogen Idec. MEC has served as a consultant for AstraZenica, Biogen Idec, Cytokinetics, GlaxoSmithKline, Voyager, Denali, and Genentech.
References
- 1.Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson' disease. Am J Epidemiol. 2007;166:561–7. doi: 10.1093/aje/kwm127. Epub 2007/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. Epub 2005/10/22. [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66:1460–8. doi: 10.1001/archneurol.2009.247. Epub 2009/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarzschild MA, Schwid SR, Marek K, Watts A, Lang AE, Oakes D, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65:716–23. doi: 10.1001/archneur.2008.65.6.nct70003. Epub 2008/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auinger P, Kieburtz K, McDermott MP. The relationship between uric acid levels and Huntington' disease progression. Mov Disord. 2010;25:224–8. doi: 10.1002/mds.22907. Epub 2010/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JE, Song SK, Sohn YH, Lee PH. Uric acid as a potential disease modifier in patients with multiple system atrophy. Mov Disord. 2011;26:1533–6. doi: 10.1002/mds.23556. Epub 2011/05/05. [DOI] [PubMed] [Google Scholar]
- 7.Keizman D, Ish-Shalom M, Berliner S, Maimon N, Vered Y, Artamonov I, et al. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci. 2009;285:95–9. doi: 10.1016/j.jns.2009.06.002. Epub 2009/06/26. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z, Guo X, Wei Q, Song W, Cao B, Huang R, et al. Serum uric acid level is associated with the prevalence but not with survival of amyotrophic lateral sclerosis in a Chinese population. Metab Brain Dis. 2014;29:771–5. doi: 10.1007/s11011-014-9510-y. Epub 2014/03/01. [DOI] [PubMed] [Google Scholar]
- 9.Paganoni S, Zhang M, Quiroz Zarate A, Jaffa M, Yu H, Cudkowicz ME, et al. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J Neurol. 2012;259:1923–8. doi: 10.1007/s00415-012-6440-7. Epub 2012/02/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chio A, Calvo A, Bovio G, Canosa A, Bertuzzo D, Galmozzi F, et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: a population-based study. JAMA neurology. 2014;71:1134–42. doi: 10.1001/jamaneurol.2014.1129. Epub 2014/07/23. [DOI] [PubMed] [Google Scholar]
- 11.Atassi N, Berry J, Shui A, Zach N, Sherman A, Sinani E, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology. 2014;83:1719–25. doi: 10.1212/WNL.0000000000000951. Epub 2014/10/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda K, Hirayama T, Takazawa T, Kawabe K, Iwasaki Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med. 2012;51:1501–8. doi: 10.2169/internalmedicine.51.7465. Epub 2012/06/26. [DOI] [PubMed] [Google Scholar]
- 13.O'Reilly EJ, Wang H, Weisskopf MG, Fitzgerald KC, Falcone G, McCullough ML, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2013;14:205–11. doi: 10.3109/21678421.2012.735240. Epub 2012/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cudkowicz ME, van den Berg LH, Shefner JM, Mitsumoto H, Mora JS, Ludolph A, et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol. 2013;12:1059–67. doi: 10.1016/S1474-4422(13)70221-7. Epub 2013/09/27. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med. 1999;18:1341–54. doi: 10.1002/(sici)1097-0258(19990615)18:11<1341::aid-sim129>3.0.co;2-7. Epub 1999/07/10. [DOI] [PubMed] [Google Scholar]
- 16.Berry JD, Miller R, Moore DH, Cudkowicz ME, Van Den Berg LH, Kerr DA, et al. The Combined Assessment of Function and Survival (CAFS): A new endpoint for ALS clinical trials. Amyotrophic lateral sclerosis and frontotemporal degeneration. 2013;14:162–8. doi: 10.3109/21678421.2012.762930. Epub 2013/01/18. [DOI] [PubMed] [Google Scholar]
- 17.Mumford SL, Dasharathy SS, Pollack AZ, Perkins NJ, Mattison DR, Cole SR, et al. Serum uric acid in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. Hum Reprod. 2013;28:1853–62. doi: 10.1093/humrep/det085. Epub 2013/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoccolella S, Simone IL, Capozzo R, Tortelli R, Leo A, D'Errico E, et al. An exploratory study of serum urate levels in patients with amyotrophic lateral sclerosis. J Neurol. 2011;258:238–43. doi: 10.1007/s00415-010-5735-9. Epub 2010/09/16. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136–41. doi: 10.1002/art.30520. Epub 2011/07/30. [DOI] [PubMed] [Google Scholar]
- 20.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–78. doi: 10.1093/aje/kwi187. Epub 2005/07/01. [DOI] [PubMed] [Google Scholar]
- 21.Turner MR, Bowser R, Bruijn L, Dupuis L, Ludolph A, McGrath M, et al. Mechanisms, models and biomarkers in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2013;14(Suppl 1):19–32. doi: 10.3109/21678421.2013.778554. Epub 2013/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65:509–27. doi: 10.1016/j.freeradbiomed.2013.06.029. Epub 2013/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. Epub 1981/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cipriani S, Bakshi R, Schwarzschild MA. Protection by inosine in a cellular model of Parkinson' disease. Neuroscience. 2014;274:242–9. doi: 10.1016/j.neuroscience.2014.05.038. Epub 2014/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y, Chen CP, Tseng CY, Eisenberg Y, Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55:463–72. doi: 10.1002/glia.20472. Epub 2007/01/05. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani S, Desjardins CA, Burdett TC, Xu Y, Xu K, Schwarzschild MA. Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson' disease. PloS one. 2012;7:e37331. doi: 10.1371/journal.pone.0037331. Epub 2012/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Burdett TC, Desjardins CA, Logan R, Cipriani S, Xu Y, et al. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:300–5. doi: 10.1073/pnas.1217296110. Epub 2012/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, Hare JM, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA neurology. 2014;71:141–50. doi: 10.1001/jamaneurol.2013.5528. Epub 2013/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keenan T, Zhao W, Rasheed A, Ho WK, Malik R, Felix JF, et al. Causal Assessment of Serum Urate Levels in Cardiometabolic Diseases Through a Mendelian Randomization Study. J Am Coll Cardiol. 2016;67:407–16. doi: 10.1016/j.jacc.2015.10.086. Epub 2016/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]