Abstract
Bis(2-ethylhexyl)-tetrabromophthalate (BEH-TEBP; CAS No. 26040-51-7; PubChem CID: 117291; MW 706.15 g/mol, elsewhere: TeBrDEPH, TBPH, or BEHTBP) is used as an additive brominated flame retardant in consumer products.
Female Sprague Dawley rats eliminated 92–98% of [14C]-BEH-TEBP unchanged in feces after oral administration (0.1 or 10 μmol/kg). A minor amount of each dose (0.8–1%) was found in urine over 72 h. Disposition of orally administered BEH-TEBP in male B6C3F1/Tac mice was similar to female rats.
Bioaccumulation of [14C]-radioactivity was observed in liver and adrenals following 10 daily oral administrations (0.1 μmol/kg/day). These tissues contained 5- and 10-fold higher concentrations of [14C]-radioactivity, respectively, versus a single dose.
IV-administered [14C]-BEH-TEBP (0.1 μmol/kg) was slowly eliminated in feces, with >15% retained in tissues after 72 h. Bile and fecal extracts from these rats contained the metabolite mono-ethylhexyl tetrabromophthalate (TBMEHP).
BEH-TEBP was poorly absorbed, minimally metabolized, and eliminated mostly by the fecal route after oral administration. Repeated exposure to BEH-TEBP led to accumulation in some tissues. The toxicological significance of this effect remains to be determined. This work was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health (Project ZIA BC 011476).
Keywords: ADME, bioaccumulation, brominated flame retardant, persistent organic pollutant, phthalate ester
Introduction
To mitigate fire hazard and comply with regulations, flame retardant (FR) chemicals have been added to consumer products and building materials (Stapleton et al., 2012). Some FRs are environmental pollutants, having migrated out of the intended consumer product, and finally into humans and the environment (Carignan et al., 2013, Birnbaum and Staskal, 2004, Orta-Garcia et al., 2014, Klosterhaus et al., 2012). Many FR chemicals of concern contain bromines, chlorines, or a mix of the two species. Brominated FRs are structurally diverse (polybrominated diphenyl ethers (PBDEs), hexabromocyclododecanes, tetrabromobisphenol A (TBBPA), TBBPA derivatives) and have been used extensively in a variety of consumer products (IPCS/WHO, 1997, de Wit, 2002, USEPA, 2012). Penta- and octa-BDE formulations were voluntarily withdrawn from the US marketplace by their manufacturers at the end of 2004, and Deca-BDE at the end of 2013 (USEPA, 2010). As such, PBDEs are now considered ‘legacy’ FRs. Penta-BDE mixtures, previously and primarily used in polyurethane foams, bioaccumulate and have undesirable toxicity profiles with evidence for thyroid and liver, and neurotoxic, reproductive toxicities and carcinogenicity were also observed (Klosterhaus et al., 2012, La Guardia et al., 2012, Vorkamp and Riget, 2014, Chen et al., 2009, de Wit, 2002, Johnson et al., 2013, Scanlan et al., 2015, NTP, 2015). Polyurethane foam in soft furnishings purchased after 2005 contain mostly a mixture of brominated and chlorinated FRs, including BEH-TEBP 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (EH-TBB), and tris(1,3-dichloro-2-propyl) phosphate (Stapleton et al., 2012).
Halogenated FR products introduced in the early 2000s and used as replacements for pentaBDE mixtures are Firemaster 550 (FM550) and BZ-54 (Stapleton et al., 2012, Dodson et al., 2012). FM550 contains a 4:1 mixture of brominated components (EH-TBB and BEH-TEBP, respectively), as well as triaryl phosphate isomers, and triphenyl phosphate (Stapleton et al., 2008). BZ-54 is composed of a 5:2 mixture of EH-TBB & BEH-TEBP (Bearr et al., 2012). DP-45 is composed entirely of BEH-TEBP and is marketed as a FR and as a plasticizer for a variety of applications (GreatLakes, 2010). FM550 has been shown to be present in quantities up to 4.2% by weight in couch foam; PBDEs have been detected at similar levels (Stapleton et al., 2012). Like PBDEs, EH-TBB and BEH-TEBP are incorporated as additive FRs and are found in the environment with demonstrated human exposures (Stapleton et al., 2014, Ma et al., 2012, Hoffman et al., 2014).
BEH-TEBP is used as a FR or plasticizer in polyurethane foams, flexible polyvinyl chloride, adhesives, carpet backing, fabric coating, film and sheeting, wire and cable insulation, and wall coverings. BEH-TEBP is encountered in a variety of consumer products from furniture foam to baby products like mattresses and high-chair foam (Stapleton et al., 2011). As a consequence of this application, BEH-TEBP is a contaminant in household and office dust (Ali et al., 2012, Ali et al., 2011a, Ali et al., 2011b, Fromme et al., 2014, Hoffman et al., 2014, Peng et al., 2015, Sahlstrom et al., 2012, Schreder and La Guardia, 2014). BEH-TEBP has been detected in human serum and breast milk (Zhou et al., 2014, He et al., 2013). BEH-TEBP is also an environmental pollutant, outdoor dust and sediment, and wildlife (Klosterhaus et al., 2012).
BEH-TEBP is slated to undergo a full risk assessment under the Toxic Substances Control Act (TSCA) Work Plan and Action Plan (USEPA, 2011). Consequently, the US EPA has stated it will begin data collection activities to better understand the environmental fate of BEH-TEBP and other brominated phthalates (USEPA, 2012). 2012 Chemical Data Reporting database (http://java.epa.gov/oppt_chemical_search/) figures indicate that the US national production volume for BEH-TEBP was 1,000,000 – 10,000,000 lb/yr (USEPA, 2015a).
Commercial formulations of FRs containing BEH-TEBP may be complex mixtures and the biological effects ascribed to exposures in the models utilized thus far have been diverse (Patisaul et al., 2013, Bearr et al., 2010). BEH-TEBP was bioaccumulative when administered as a mixture (FM550 or BZ-54) in fathead minnows (Pimephales promelas), with BEH-TEBP remaining in a variety of tissues even 22 days after exposure to was FM550 stopped (Bearr et al., 2012). Repeated exposures to FM550 resulted in adverse effects in rodents including increased serum thyroxine and decreased hepatic esterase activity in dams treated with 1 mg/kg/day of FM550 (Patisaul et al., 2013). Furthermore, exposed offspring displayed high anxiety, especially in the female offspring. Female offspring had impaired glucose tolerance. The role of BEH-TEBP in these toxicities is uncertain; however, BEH-TEBP is a brominated analog of the known carcinogenic, teratogenic, and reproductive toxicant, bis(2-ethylhexyl) phthalate, commonly referred to as DEHP (Caldwell, 2012). Currently, the EPA predicts that BEH-TEBP will have moderate carcinogenic, reproductive, developmental, and neurological toxicities (USEPA, 2015a, USEPA, 2015b).
BEH-TEBP is highly lipophilic, with an estimated logP between 9.48 (Bergman et al., 2012) and 11.95 (USEPA, 2016), but little is known about its disposition, especially at environmentally relevant concentrations. Male SD rats, dosed with a high oral dose of Uniplex FRP-45 (~673 μmol/kg BEH-TEBP), metabolized a small fraction of BEH-TEBP to tetrabromophthalic acid which was detected in serum and urine; no further information on tissue deposition or routes of elimination for BEH-TEBP were reported (Silva et al., 2016). More information is needed regarding the disposition of BEH-TEBP to fully evaluate its potential toxicity and hazard effects.
These studies were designed to determine the disposition of one of the major brominated component of these novel flame retardant mixtures, BEH-TEBP at environmentally-relevant levels. Female SD rats were selected as the primary animal model with male B6C3F1/Tac mice as the secondary animal model. Female SD rats were administered a single dose of 0.1 or 10 μmol/kg by gavage or intravenously and the routes of elimination, distribution to tissues, and metabolite profiles in excreta were determined over 72 hours. Female SD rats were administered 1 or 10 daily doses of [14C]-BEH-TEBP (0.1 μmol/kg/day) to investigate the bioaccumulation potential of BEH-TEBP in rats. Distribution of [14C]-radioactivity to tissues was determined 24 h after the final dose in these animals. An exploratory assessment of species- or sex-related differences in the disposition of BEH-TEBP was performed using male B6C3F1/Tac mice using the model proposed by Buchanan et al. (1997).
Materials and Methods
Chemicals
[14C]-radiolabeled BEH-TEBP (carboxy-labeled; Figure 1) was purchased from Moravek Biochemicals, Inc. (La Brea, CA). [14C]-BEH-TEBP had a radiochemical purity of >99.4% with a specific activity of 60 mCi/mmol; chemical purity was determined to be >99% as compared to a BEH-TEBP reference standard (Accustandard, Inc., New Haven, CT). [14C]-BEH-TEBP was diluted in toluene to a stock concentration of 0.5 mCi/mL. Scintillation cocktails were obtained from MP Biomedicals (Ecolume; Santa Ana, CA) or Perkin-Elmer (Ultima-Flo M and PermaFluor E+; Torrance, CA). Corn oil (Mazola, ACH Food Companies, Inc., Memphis, TN) was purchased locally. Toluene, acetonitrile, ethyl acetate, and ethyl alcohol were purchased from Sigma-Aldrich (St. Louis, MO). Mono (2-ethylhexyl) tetrabromophthalate (TBMEHP) standard was generously provided by Dr. Heather Stapleton (Duke University, Durham NC). Tetrabromophthalic acid was procured from BOC Biosciences (Shirley, NY) and was >95% pure. All other reagents used in these studies were high performance liquid chromatography (HPLC) or analytical grade.
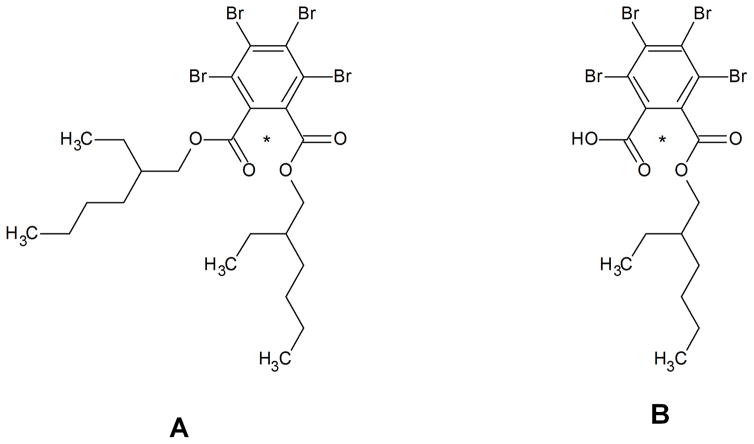
Figure 1.
Chemical structure of (A) BEH-TEBP and (B) TBMEHP; *[14C]-radiolabel on a single carbonyl carbon.
Animal model and dosing
Female Sprague-Dawley rats (SD; 10 weeks, ~200 g; Harlan Laboratories, Raleigh, NC) or male B6C3F1/Tac mice (10 weeks; ~20 g; Taconic Farms, Germantown, NY) were used in these studies. Animals were maintained in an AAALAC-approved animal care facility (humidity: ~49%, room temperature: ~72ºF, 12 h light/dark cycle). Animals were acclimated for 1 week prior to oral administration or 24 h prior to IV dosing. Food (NIH #31, Zeigler Brothers, Gardners, PA) and water were provided for ad libitum consumption. The NIH-31 Open Formula Autoclavable diet, a standard reference diet for biological and biomedical research, is formulated for maintenance, growth, reproduction, and lactation of both rats and mice and is not soy-free (Ziegler Brothers, 2010). All procedures were approved by the NIEHS Institutional Care and Use committee. Immediately following dosing, animals were housed individually in metabolism cages for collection of urine and feces. Airflow was maintained at approx. 600 mL/min when expired gasses were sampled (initial study). No expired [14C]-radioactivity was detected as [14C]-CO2 or [14C]-volatile organic compounds and all subsequent studies were conducted using openly ventilated metabolism cages.
Dose effect was investigated in female SD rats by administering a single dose of [14C]-BEH-TEBP by gavage (PO) at 0.1 (approx. 0.07 mg/kg, 6 μCi/kg) or 10 μmol/kg (approx. 7 mg/kg, 25 μCi/kg). Doses were selected based on estimates of high levels of human exposure to BEH-TEBP in dust (Patisaul et al., 2013). 4 animals were used per dose group and tissues were collected at 72 h post-dose, unless otherwise indicated. BEH-TEBP bioaccumulation potential in female SD rats was assessed by examining [14C]-radioactivity recoveries in excreta and tissues collected 24 h after 10 daily oral administrations of BEH-TEBP (0.1 μmol/kg, N=4). Male mice were administered a single dose of BEH-TEBP (0.1 μmol/kg, N=4) by gavage to determine 72 h elimination and tissue disposition. Doses containing 0.1 μmol/kg of BEH-TEBP provided approximately 6 μCi/kg of [14C]-BEH-TEBP while the 10 μmol/kg dose provided 25 μCi/kg (0.4 μmol/kg [14C]-labeled BEH-TEBP) and contained a balance of non-radiolabeled BEH-TEBP (9.6 μmol/kg). PO dosing solutions were prepared by adding appropriate volumes of [14C]-BEH-TEBP and a balance of non-radiolabeled BEH-TEBP dissolved in toluene and added to corn oil at a dose volume of 4 mL/kg. Toluene was removed by evaporation under a steady stream of nitrogen. Oral doses were administered using a #16 and #20 ball-tipped stainless steel feeding needle to rats and mice, respectively.
To determine the fate of systemically available BEH-TEBP, a single intravenous (IV) bolus was injected into the lateral tail vein. IV dosing solution (0.1 μmol/kg, 1 mL/kg) was prepared by adding [14C]-BEH-TEBP to Cremophore EL (Sigma-Aldrich, St. Louis MO) followed by evaporation of toluene under nitrogen and the addition of 1 part ethanol and 3 parts water. Final concentrations of [14C]-radioactivity were determined by assaying 5, 10, and 15 μL of dosing solution in triplicate by liquid scintillation counting using a Beckman Coulter LS6500 Multi-Purpose Scintillation Counter. Purity was confirmed each time by HPLC-radiometric analysis of dosing solution diluted in ethyl acetate.
Sample collections
Following administration of the compound, excreta and cage rinses were collected at 4, 8, 12, 24, 48, and 72 h in single-dose studies and at 24 h intervals in repeat-dose studies. Initial studies included the collection of [14C]-radioactivity in expired air, either as volatile organic compounds (VOC) or 14CO2, using the method described by Sanders et al. (2000) at 1, 4, 8, and 24 h. Euthanasia was realized by CO2 asphyxiation. Tissues (adipose, adrenals, brain, heart, kidneys, large intestine and contents, liver, lung, muscle, pancreas, ovaries [testes in male animals], skin, small intestine and contents, spleen, stomach and contents, thymus, thyroid, urinary bladder, and uterus) were dissected and placed in labeled pre-weighed vials. Blood samples were collected into heparinized syringes via cardiac puncture immediately following euthanasia. Plasma was isolated from heparinized blood by centrifugation (5 min at 2,300 x g). Bile samples were collected from rats administered BEH-TEBP by gavage or IV (tail vein) via an indwelling common bile-duct cannula at 1 h intervals up to 6 h post-dose (N=2/dose group). Samples were placed in labeled pre-weighed vials after all collections and maintained at −80ºC until analyses. Bile samples (~100 μL) were diluted with 1 mL water prior to analyses. Total mass of incompletely collected tissues were calculated as a fraction of body weight (adipose: 11%, muscle: 50%, skin: 16%, blood: 8%) using previously published values (Birnbaum et al., 1980). All other tissues were completely dissected and their weight determined gravimetrically. Samples were maintained at −80ºC until further analyses.
Analytical methods
Samples were analyzed in parallel for quantitative and qualitative differences. Quantitative analyses of total [14C]-radioactivity content was determined using a Beckman Coulter LS6500 Multi-Purpose Scintillation Counter. Total [14C]-radioactivity content of plasma (50 μL), urine (10–100 μL) and cage rinses (1 mL) was assayed in triplicate by liquid scintillation counting. Triplicate aliquots of whole blood were prepared and [14C]-radioactivity was quantified by combustion in a Packard 307 Biological Sample Oxidizer followed by LSC counting. Fecal samples were dried in a fume hood, weighed and ground to a powder using a mortar and pestle. Triplicate aliquots of feces and tissues (~25 mg) were weighed and [14C]-radioactivity was quantified by combustion. [14C]-radioactivity values for urine and cage rinses were combined for reporting purposes.
BEH-TEBP was quantified by UV/Vis absorbance and radiochemical detection following HPLC separation. The HPLC system was composed of an Agilent 1100 HPLC, a Restek Raptor biphenyl column (2.7 μm, 150 x 4.6 mm) with a Restek Raptor Biphenyl precolumn (5 μm) and an in-line INUS BetaRAM3 radiochemical detector. Mobile phases consisted of 0.1% formic acid in water (mobile phase A) and acetonitrile (mobile phase B). Sample separations were performed using a gradient; initial conditions (100% A) were reduced to 0% over 10 min then held at 0% A for 20 minutes. The column was returned to initial conditions and allowed to equilibrate for 2 minutes before re-use. HPLC mobile phase flow rates were 1 mL/min and scintillation cocktail (Flowlogic U, Lablogic Corp., Brandon FL) flow rate was 2 mL/min. Laura4 (Lablogic Corp.) software was used for instrument control and analysis.
Excreta and tissues containing [14C]-radioactivity were sampled and subjected to extractions for characterization by HPLC-radiochemical analyses. Feces selected for extractions were collected between 0–24 and 24–48 h post dose while liver tissues were collected at 24 or 72 h post dose. Prior to HPLC analysis, aliquots (~250 mg) of air-dried fecal samples were placed in labeled pre-weighed glass screw-top tubes and re-suspended in 5 mL toluene and mixed by vortexing for 1 minute. Samples were then centrifuged at 830 x g for 10 min using a Sorvall RC6+ centrifuge (Thermo Scientific, Inc., Waltham, MA) with a SH-3000 swing bucket rotor to sediment solids. This process was repeated twice, with the supernatants transferred and pooled. When recoveries were less than 95% of expected values, the pellet was further extracted with ethyl acetate (3 x 5 mL) and 1% HCl in 95% ethanol (3 × 5 mL). At each step, the samples were centrifuged and the supernatants transferred and pooled. Extracts were concentrated to approx. 100 μL using a Savant SPD1010 SpeedVac (Thermo Scientific, Inc.) without heating, and aliquots were analyzed by UV/radiometric HPLC. Remaining sample pellets were air dried, weighed, and unextracted [14C]-radioactivity remaining in the sample was quantified by combustion in a Packard 307 Biological Sample Oxidizer followed by LSC counting. Fecal extraction efficiencies from feces approached 100%; however, extraction efficiencies for liver samples from IV-dosed animals were 70%, similar to previously published tissue extraction efficiencies for other lipophilic brominated flame retardants (Sanders et al., 2013, Szabo et al., 2011, Szabo et al., 2010, Hakk et al., 2006, Hakk et al., 2009, Hakk et al., 2012).
Biliary & fecal analytes from IV-administered animals were further characterized by UPLC/MS analyses. The UPLC/MS consisted of a Waters H-Class UPLC and QDa single quadrupole detector equipped with an ESI source. Source conditions were: sheath gas = N2 at 20 L/min, capillary temperature = 400ºC, spray voltage = 0.5 kV, capillary voltage = −25 V. UPLC mobile phases were the same as described above. Bile samples were diluted and filtered with a 0.2 μm centrifugal filter (EMD Millipore, Billerica MA), prior to MS analysis. Following confirmation of the mass of TBMEHP (593.9 Da), full mass spectrometry (MS) scans were performed using a manual selection for the most abundant ions for isolation.
Statistical methods
The data were subjected to statistical analysis using paired t-tests or one-way ANOVA followed by the Tukey-Kramer test for pairwise comparisons (Graphpad Prism, Graphpad Software, Inc., La Jolla CA). Values were considered to be significantly different at p < 0.05.
Results
Single dose disposition studies
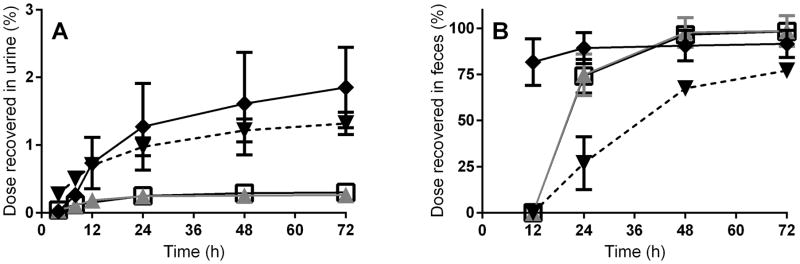
Feces contained ~75% of the dose after 24 h when BEH-TEBP was administered at the 0.1 μmol/kg dose level by gavage to female SD rats. Negligible differences in fecal recoveries were seen following a 100-fold increase in dose (Figure 2). Less than 0.3% of either BEH-TEBP dose was recovered in urine after oral administration to rats. [14C]-radioactivity recovered in feces exceeded 98% by 72 h after oral dosing, but recovery in feces reached only 78% after IV administration by 72 h post-dose. Total recoveries for both routes of administration approached 100%. The remainder of the IV-administered [14C]-radioactivity was recovered in tissues (described below). Male B6C3F1/Tac mice were observed to eliminate BEH-TEBP-derived radioactivity more rapidly via the fecal route than female rats. Higher but more variable amounts of [14C]-radioactivity were recovered in urine and cage rinses. A pilot study (N=2/dose group) using rats with an indwelling bile-duct cannula was conducted. In this study, ~0.1% of the dose was recovered in bile from animals administered BEH-TEBP by gavage, while 20–25% of IV-administered [14C] radioactivity was eliminated in the bile in 24 h, consistent with fecal recovery data from studies using conventional animals.
Figure 2.
Cumulative elimination of [14C]-radioactivity following a single administration of BEH-TEBP. N=4 per dose group, mean ± S.D. A: Recovery in urine, B: Recovery in feces (◆: 0.1 μmol/kg, PO, mouse; ▼: 0.1 μmol/kg, IV, rat; ▲:0.1 μmol/kg, oral, rat; ■: 10 μmol/kg, oral, rat).
Selected tissues were surveyed to determine any compound-specific sites of retention (Table 1). [14C]-radioactivity retained in tissues collected at 72 h following oral administration of BEH-TEBP was low (~1% of total dose in assayed tissues). However, approx. 20% of IV-administered [14C]-radioactivity was retained in tissues even 72 h after dosing, with the highest amounts found in liver (7%), muscle, skin (3%), and fat (2%). The gastrointestinal tract retained an additional 3% of the dose. Adrenal glands and liver retained the highest concentrations (pmol-eq/g tissue) of [14C]-radioactivity among the tissues analyzed after both IV and oral administration of BEH-TEBP.
Table 1.
[14C]-radioactivity in selected tissues and excreta following a single BEH-TEBP administration (72 h post dose).
| Dose recovered | 0.1 μmol/kg (Mouse, oral) | 0.1 μmol/kg (Rat, oral) | 10 μmol/kg (Rat, oral) | 0.1 μmol/kg (Rat, IV) | |
|---|---|---|---|---|---|
| Feces | % | 92 ± 7 | 98 ± 8 | 98 ± 1 | 77 ± 4 |
| Urine | % | 3 ± 2 | 0.3 ± 0.03 | 0.3 ± 0.04 | 1.3 ± 0.2 |
| Adipose | % | 0.1 ± 0.03 | 0.01 ± 0.01 | 0.03 ± 0.01 | 2 ± 0.4 |
| (pmol-eq/g) | (2 ± 1) | (0.04 ± 0.1) | (28 ± 10) | (15 ± 3) | |
| Adrenal | % | 0.01 ± 0.004 | 0.002 ± 0.002 | 0.004 ± 0.002 | 1 ± 0.1 |
| (pmol-eq/g) | (18 ± 6) | (7 ± 5) | (1314 ± 451) | (2207 ± 1246) | |
| Blood | % | 0.02 ± 0.01 | 0.4 ± 0.4 | 0.1 ± 0.1 | 1 ± 0.3 |
| (pmol-eq/g) | (0.02 ± 0.01) | (0.2 ± 0.2) | (3 ± 5) | (0.4 ± 0.1) | |
| Brain | % | 0.01 ± 0.01 | 0.0002 ± 0.0004 | 0.001 ± 0.001 | 0.04 ± 0.02 |
| (pmol-eq/g) | (1 ± 1) | (0.02 ± 0.05) | (13 ± 14) | (5 ± 2) | |
| Kidney | % | 0.01 ± 0.01 | 0.001 ± 0.001 | 0.002 ± 0.002 | 0.2 ± 0.03 |
| (pmol-eq/g) | (1 ± 1) | (0.1 ± 0.1) | (21 ± 32) | (22 ± 4) | |
| Liver | % | 0.3 ± 0.1 | 0.2 ± 0.2 | 0.4 ± 0.1 | 7 ± 1 |
| (pmol-eq/g) | (11 ± 0.4) | (4 ± 5) | (873 ± 328) | (144 ± 21) | |
| Lung | % | 0.01 ± 0.01 | 0.001 ± 0.001 | 0.002 ± 0.0004 | 0.1 ± 0.02 |
| (pmol-eq/g) | (2 ± 2) | (0.1 ± 0.2) | (29 ± 4) | (20 ± 5) | |
| Muscle | % | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.1 ± 0.1 | 5 ± 1 |
| (pmol-eq/g) | (0.4 ± 0.3) | (0.2 ± 0.4) | (10 ± 14) | (10 ± 2) | |
| Skin | % | 0.001 ± 0.002 | 0.01 ± 0.01 | 0.04 ± 0.02 | 3 ± 0.4 |
| (pmol-eq/g) | (0.01 ± 0.02) | (0.04 ± 0.1) | (24 ± 13) | (17 ± 3) |
Repeat dose studies
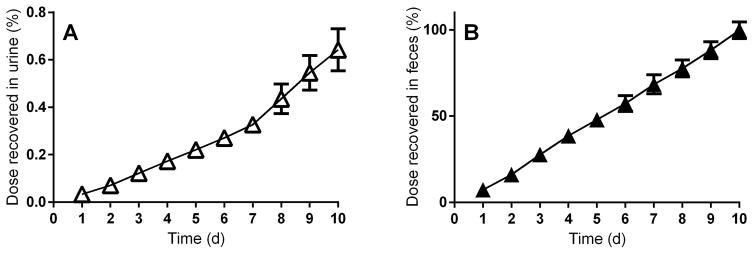
Ten daily oral administrations of [14C]-BEH-TEBP were used to estimate bioaccumulation potential of the chemical. Similar to single-dose studies, repeated administration of BEH-TEBP resulted in a small amount of the total dose excreted in urine, with the overwhelming majority recovered in feces (Figure 3). Total elimination was determined at 24 h intervals and compared to elimination from a group of animals administered a single dose and euthanized 24 h later (Table 2). Significantly more [14C]-radioactivity was present in liver after 10 doses (113±16 pmol-eq/g) than after one (23±4 pmol-eq/g). Concentrations in adrenal tissue increased more than 10-fold after 10 doses.
Figure 3.
Cumulative elimination of [14C]-radioactivity following repeated administration of BEH-TEBP to female SD rats. N=4 per dose group, mean ± S.D. A: Recovery in urine, B: Recovery in feces.
Table 2.
[14C]-radioactivity in selected tissues following single or repeated oral BEH-TEBP administration (0.1 μmol/kg/day, 24 h after final dose).
| Tissue | Dose recovered | 1 dose | 10 doses |
|---|---|---|---|
| Feces | % | 91 ± 11 | 100 ± 5 |
| Urine | % | 0.3 ± 0.1 | 0.6 ± 0.1 |
| Gastrointestinal tract contents | % | 8 ± 11 | 0.5 ± 0.4 |
| Adipose | % | 0.4 ± 1 | 0.04 ± 0.1 |
| (pmol-eq/g) | (4 ± 5) | (8 ± 7) | |
| Adrenal | % | 0.01 ± 0.003 | 0.01 ± 0.004 |
| (pmol-eq/g) | (20 ± 5) | (207 ± 142) | |
| Blood | % | 0.2 ± 0.1 | 0.01 ± 0.003 |
| (pmol-eq/g) | (0.1 ± 0.1) | (0.1 ± 0.02) | |
| Brain | % | 0.01 ± 0.01 | 0.001 ± 0.002 |
| (pmol-eq/g) | (1 ± 1) | (1 ± 2) | |
| Kidney | % | 0.01 ± 0.01 | 0.002 ± 0.001 |
| (pmol-eq/g) | (1 ± 1) | (3 ± 1) | |
| Liver | % | 1 ± 0.2 | 0.4 ± 0.09 |
| (pmol-eq/g) | (23 ± 4) | (113 ± 16) | |
| Lung | % | 0.01 ± 0.01 | 0.01 ± 0.02 |
| (pmol-eq/g) | (1 ± 1) | (1 ± 1) | |
| Muscle | % | 0.3 ± 0.3 | 0.1 ± 0.1 |
| (pmol-eq/g) | (1 ± 1) | (1 ± 1) | |
| Skin | % | 0.1 ± 0.1 | 0.04 ± 0.02 |
| (pmol-eq/g) | (1 ± 1) | (3 ± 1) |
Metabolite analyses
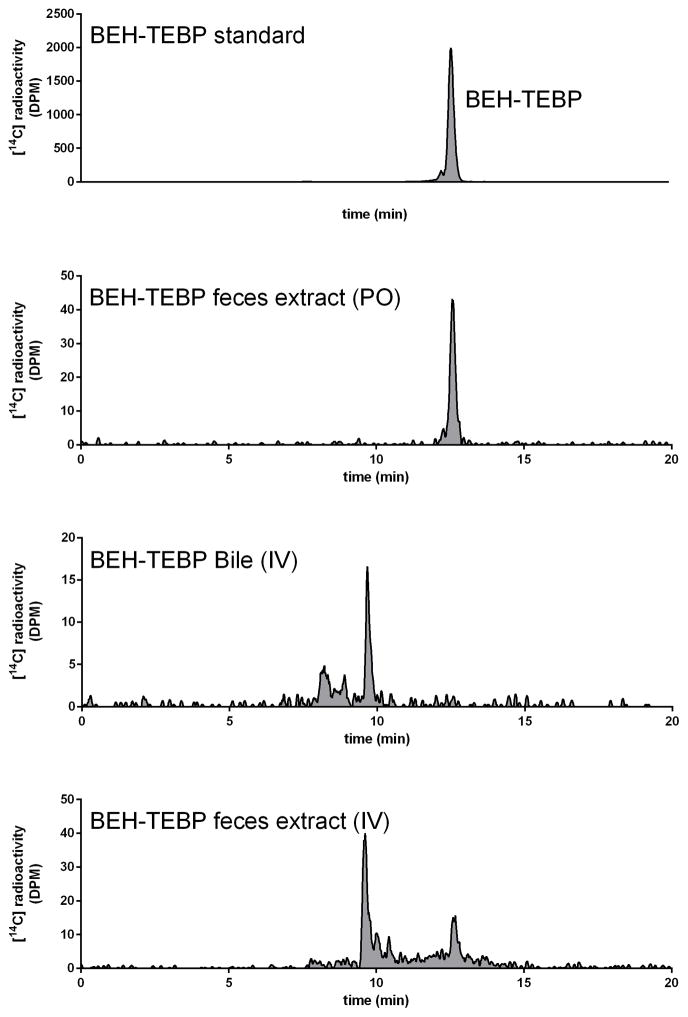
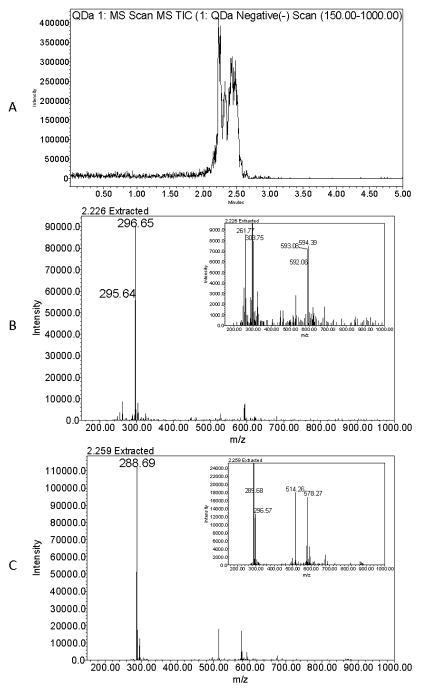
All extracts were subjected to HPLC-radiometric analyses. [14C]-Radioactivity in fecal and liver extracts, collected after oral dosing, was observed to co-elute with the [14C]-BEH-TEBP standard in a single [14C]-radiolabeled peak (Figure 4). Bile collected after IV dosing contained at least two [14C]-labeled metabolites, one had a mass consistent with a metabolite formed in vitro (TBMEHP), as reported by Roberts et al (2012) and another had a mass consistent with a de-methylated TBMEHP (Figure 5). Extracts from feces collected after IV-administration appeared to contain both [14C]-TBMEHP (~70%) and [14C]-BEH-TEBP (~30%).
Figure 4.
Representative HPLC-radiochromatograms of [14C]-radioactivity in excreta & bile following BEH-TEBP administration (0.1 μmol/kg) to female SD rats. A: [14C]-BEH-TEBP standard. B: [14C]-radioactivity in fecal extracts after oral administration. C: [14C]-radioactivity in bile after BEH-TEBP IV administration. D: [14C]-radioactivity in fecal extracts after BEH-TEBP IV administration.
Figure 5.
Representative mass spectra of bile following BEH-TEBP (0.1 μmol/kg) administration by IV to female SD rats. A: Total ion chromatogram for bile after UPLC separation. B: Full-scale extracted ion chromatograms for peak eluting at 2.226 min, m/z of 296.6 Da is consistent with that of a doubly charged TBMEHP [M+H]− ion (inset, lower abundance singly charged TBMEHP [M+H]− ion). C: Full-scale extracted ion chromatograms for peak eluting at 2.259 min m/z of 288.7 Da is consistent with that of a doubly charged demethylated TBMEHP [M+H]− ion (inset, lower abundance singly charged demethylated TBMEHP [M+H]− ion).
Discussion
BEH-TEBP is now an established ‘novel’ FR contaminant present in home and environmental exposures, with US households having the highest concentrations in dust worldwide (Stapleton et al., 2014). This is the first time the disposition of BEH-TEBP has been described separate from its proprietary FR mixtures. BEH-TEBP was poorly absorbed, minimally metabolized, and almost exclusively eliminated by the fecal route after oral administration at dose levels up to 10 μmol/kg. Liver and adrenal tissues retained the highest concentrations of [14C]-radioactivity at 72 h after a single oral dose of BEH-TEBP. Intravenously administered BEH-TEBP was very slowly excreted, indicating a limited hepatic capacity to metabolize or eliminate the dose. Muscle, skin, and fat appeared to be depots for systemically available BEH-TEBP. Repeated BEH-TEBP administration showed similar results, although significant increases in [14C]-radioactivity concentrations in liver and adrenal tissues were noted after 10 daily doses.
BEH-TEBP exhibited differential routes of elimination following IV and oral administration. With less than 0.1% of a 0.1 μmol/kg oral dose eliminated in bile over the first 24 h, it was clear that BEH-TEBP was very poorly absorbed. Dose escalation over a hundred-fold difference did not noticeably alter BEH-TEBP uptake or metabolism after oral administration, as only parent compound was extracted from feces. In contrast, systemically available [14C]-radioactivity (e.g., when BEH-TEBP was administered by IV) was largely eliminated in the feces as a mixture of parent and metabolite(s), with a significant proportion of the dose retained in tissues. Few sex- or species-dependent differences were observed when data from female SD rats were compared to disposition data from male B6C3F1/Tac mice in an exploratory study. Mice appeared to excrete a similar proportion of the dose, albeit more rapidly, in the feces.
Accumulation of xenobiotics in a variety of tissues has been well documented, but physiologic explanations of tissue-specific affinities are scant. At least two PBDEs, 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) and decabromodiphenyl ether (decaBDE), accumulate in liver, adrenal, and other tissues (Morck et al., 2003, Hakk et al., 2002). Apparent liver bioaccumulation likely results from “slow” hepatic metabolism of parent molecule coupled with biliary elimination. Along with BDE-99 and decaBDE, one other minimally absorbed and poorly metabolized BFR, tetrabromobisphenol A bis (2,3 dibromopropyl ether) is sequestered in the liver prior to metabolism and eventual biliary elimination (Knudsen et al., 2007). Systemically available BEH-TEBP appeared to be primarily eliminated by biliary secretion, which may help explain hepatic bioaccumulation. Localization to the adrenal gland is less intuitive, although the impact of endocrine disrupting chemicals (like BEH-TEBP and other BFRs) on steroid synthesis may eventually yield an explanation of the phenomenon. Imaging studies have shown decaBDE localizes to the adrenal cortex and ovarian stroma in exposed rats although it was unclear whether brominated components detected in the cortex were present as parent chemical or metabolites (Seyer et al., 2010). Other studies of [14C]-labeled xenobiotics found adrenal extracts co-eluted with cholesteryl ester with evidence for their degradation and incorporation into endogenous substances (Darnerud and Brandt, 1985).
In addition to biotic metabolism to TBMEHP, BEH-TEBP may also undergo photolytic debromination to form tri- and di-brominated products, although complete degradation to DEHP in the environment has not been established (Davis and Stapleton, 2009). While BEH-TEBP appeared to be poorly absorbed, debromination is likely to increase absorption, as evidenced by the high level of DEHP absorption following gastrointestinal exposure (Daniel and Bratt, 1974). DEHP is carcinogenic in rats and mice (Caldwell, 2012). The primary metabolite of BEH-TEBP, mono-(2-ethylhexyl) tetrabromophthalate, possesses endocrine disrupting potential (Springer et al., 2012).
Small children may ingest dust through hand-mouth contact, and as such are at a greater risk for repeated exposure to BEH-TEBP, with unknown biological outcomes (Stapleton et al., 2014). BEH-TEBP adsorbed to dust may also be absorbed through the skin or inhaled and absorbed in the lung. Future studies may explore these additional exposure routes to more fully assess exposure risks. So, while poorly absorbed and metabolized, continuous exposure may lead to tissue accumulation.
Conclusions
BEH-TEBP was administered to female Sprague Dawley rats by intragastric or intravenous bolus and disposition was assessed at 0.1 or 10 μmol/kg dose levels. Male B6C3F1/Tac mice were dosed with BEH-TEBP by gavage (0.1 μmol/kg). The results of the current study indicate poor absorption and rapid elimination of BEH-TEBP as parent compound. Systemically available BEH-TEBP (by IV injection) was largely metabolized to mono-ethylhexyl tetrabromophthalate and eliminated slowly via biliary excretion; approx. 20% of the dose remained in tissues 72 h after dosing. Daily repeated oral administration led to accumulation in tissues, most notably liver and adrenal tissues. However, small amounts of [14C]-radioactivity were retained in tissues 72 h after a single oral dose, leading to investigation of bioaccumulation after repeated exposures. Subsequent studies of repeated oral exposures showed that while only a small amount of BEH-TEBP is absorbed, it has the potential to accumulate in adrenal and liver tissue. This accumulated chemical was determined to be largely parent, but upwards of 30% of the BEH-TEBP-derived material retained in the liver was non-extractable. The biological and toxicological significance of this effect remains to be determined.
Acknowledgments
The authors would like to thank Ms. Sherry Coulter, Ms. Samantha Hall, Mr. Ethan Hull, Ms. Katelyn McIntosh, and Mr. Abdella Sadik for technical assistance. This work was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health (Project ZIA BC 011476).
References
- ALI N, DIRTU AC, VAN DEN EEDE N, GOOSEY E, HARRAD S, NEELS H, MANNETJE TA, COAKLEY J, DOUWES J, COVACI A. Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere. 2012;88:1276–82. doi: 10.1016/j.chemosphere.2012.03.100. [DOI] [PubMed] [Google Scholar]
- ALI N, HARRAD S, GOOSEY E, NEELS H, COVACI A. “Novel” brominated flame retardants in Belgian and UK indoor dust: implications for human exposure. Chemosphere. 2011a;83:1360–5. doi: 10.1016/j.chemosphere.2011.02.078. [DOI] [PubMed] [Google Scholar]
- ALI N, HARRAD S, MUENHOR D, NEELS H, COVACI A. Analytical characteristics and determination of major novel brominated flame retardants (NBFRs) in indoor dust. Anal Bioanal Chem. 2011b;400:3073–83. doi: 10.1007/s00216-011-4966-7. [DOI] [PubMed] [Google Scholar]
- BEARR JS, MITCHELMORE CL, ROBERTS SC, STAPLETON HM. Species specific differences in the in vitro metabolism of the flame retardant mixture, Firemaster(R) BZ-54. Aquat Toxicol. 2012;124–125:41–7. doi: 10.1016/j.aquatox.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARR JS, STAPLETON HM, MITCHELMORE CL. Accumulation and DNA damage in fathead minnows (Pimephales promelas) exposed to 2 brominated flame-retardant mixtures, Firemaster 550 and Firemaster BZ-54. Environ Toxicol Chem. 2010;29:722–9. doi: 10.1002/etc.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMAN A, RYDEN A, LAW RJ, DE BOER J, COVACI A, ALAEE M, BIRNBAUM L, PETREAS M, ROSE M, SAKAI S, VAN DEN EEDE N, VAN DER VEEN I. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ Int. 2012;49:57–82. doi: 10.1016/j.envint.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRNBAUM LS, DECAD GM, MATTHEWS HB. Disposition and excretion of 2,3,7,8-tetrachlorodibenzofuran in the rat. Toxicol Appl Pharmacol. 1980;55:342–52. doi: 10.1016/0041-008x(80)90096-4. [DOI] [PubMed] [Google Scholar]
- BIRNBAUM LS, STASKAL DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHANAN JR, BURKA LT, MELNICK RL. Purpose and guidelines for toxicokinetic studies within the National Toxicology Program. Environ Health Perspect. 1997;105:468–71. doi: 10.1289/ehp.105-1469880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL JC. DEHP: genotoxicity and potential carcinogenic mechanisms-a review. Mutat Res. 2012;751:82–157. doi: 10.1016/j.mrrev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- CARIGNAN CC, HEIGER-BERNAYS W, MCCLEAN MD, ROBERTS SC, STAPLETON HM, SJODIN A, WEBSTER TF. Flame retardant exposure among collegiate United States gymnasts. Environ Sci Technol. 2013;47:13848–56. doi: 10.1021/es4037868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN SJ, MA YJ, WANG J, CHEN D, LUO XJ, MAI BX. Brominated flame retardants in children’s toys: concentration, composition, and children’s exposure and risk assessment. Environ Sci Technol. 2009;43:4200–6. doi: 10.1021/es9004834. [DOI] [PubMed] [Google Scholar]
- DANIEL JW, BRATT H. The absorption, metabolism and tissue distribution of di(2-ethylhexyl)phthalate in rats. Toxicology. 1974;2:51–65. doi: 10.1016/0300-483x(74)90042-0. [DOI] [PubMed] [Google Scholar]
- DARNERUD PO, BRANDT I. Pitfalls in the interpretation of whole-body autoradiograms: long-time retention in brain and adrenal cortex caused by metabolic incorporation of 14C from various labelled xenobiotics. Acta Pharmacol Toxicol (Copenh) 1985;56:55–62. doi: 10.1111/j.1600-0773.1985.tb01253.x. [DOI] [PubMed] [Google Scholar]
- DAVIS EF, STAPLETON HM. Photodegradation pathways of nonabrominated diphenyl ethers, 2-ethylhexyltetrabromobenzoate and di(2-ethylhexyl)tetrabromophthalate: identifying potential markers of photodegradation. Environ Sci Technol. 2009;43:5739–46. doi: 10.1021/es901019w. [DOI] [PubMed] [Google Scholar]
- DE WIT CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- DODSON RE, PEROVICH LJ, COVACI A, VAN DEN EEDE N, IONAS AC, DIRTU AC, BRODY JG, RUDEL RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46:13056–66. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROMME H, HILGER B, KOPP E, MISEROK M, VOLKEL W. Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and “novel” brominated flame retardants in house dust in Germany. Environ Int. 2014;64:61–8. doi: 10.1016/j.envint.2013.11.017. [DOI] [PubMed] [Google Scholar]
- GREATLAKES. DP-45 Technical Data Sheet. Chemtura Corporation; 2010. www.greatlakes.com. [Google Scholar]
- HAKK H, HUWE J, LOW M, RUTHERFORD D, LARSEN G. Tissue disposition, excretion and metabolism of 2,2′,4,4′,6-pentabromodiphenyl ether (BDE-100) in male Sprague-Dawley rats. Xenobiotica. 2006;36:79–94. doi: 10.1080/00498250500491675. [DOI] [PubMed] [Google Scholar]
- HAKK H, HUWE JK, LARSEN GL. Absorption, distribution, metabolism and excretion (ADME) study with 2,2′,4,4′,5,6′-hexabromodiphenyl ether (BDE-154) in male Sprague-Dawley rats. Xenobiotica. 2009;39:46–56. doi: 10.1080/00498250802546853. [DOI] [PubMed] [Google Scholar]
- HAKK H, LARSEN G, KLASSON-WEHLER E. Tissue disposition, excretion and metabolism of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) in the male Sprague-Dawley rat. Xenobiotica. 2002;32:369–82. doi: 10.1080/00498250110119117. [DOI] [PubMed] [Google Scholar]
- HAKK H, SZABO DT, HUWE J, DILIBERTO J, BIRNBAUM LS. Novel and distinct metabolites identified following a single oral dose of alpha- or gamma-hexabromocyclododecane in mice. Environ Sci Technol. 2012;46:13494–503. doi: 10.1021/es303209g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE S, LI M, JIN J, WANG Y, BU Y, XU M, YANG X, LIU A. Concentrations and trends of halogenated flame retardants in the pooled serum of residents of Laizhou Bay, China. Environ Toxicol Chem. 2013;32:1242–7. doi: 10.1002/etc.2172. [DOI] [PubMed] [Google Scholar]
- HOFFMAN K, FANG M, HORMAN B, PATISAUL HB, GARANTZIOTIS S, BIRNBAUM LS, STAPLETON HM. Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster(R) 550. Environ Health Perspect. 2014;122:963–9. doi: 10.1289/ehp.1308028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCS/WHO. Flame retardants: a general introduction. Geneva: World Health Organization; 1997. [Google Scholar]
- JOHNSON PI, STAPLETON HM, MUKHERJEE B, HAUSER R, MEEKER JD. Associations between brominated flame retardants in house dust and hormone levels in men. Sci Total Environ. 2013;445–446:177–84. doi: 10.1016/j.scitotenv.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOSTERHAUS SL, STAPLETON HM, LA GUARDIA MJ, GREIG DJ. Brominated and chlorinated flame retardants in San Francisco Bay sediments and wildlife. Environ Int. 2012;47:56–65. doi: 10.1016/j.envint.2012.06.005. [DOI] [PubMed] [Google Scholar]
- KNUDSEN GA, JACOBS LM, KUESTER RK, SIPES IG. Absorption, distribution, metabolism and excretion of intravenously and orally administered tetrabromobisphenol A [2,3-dibromopropyl ether] in male Fischer-344 rats. Toxicology. 2007;237:158–67. doi: 10.1016/j.tox.2007.05.006. [DOI] [PubMed] [Google Scholar]
- LA GUARDIA MJ, HALE RC, HARVEY E, MAINOR TM, CIPARIS S. In situ accumulation of HBCD, PBDEs, and several alternative flame-retardants in the bivalve (Corbicula fluminea) and gastropod (Elimia proxima) Environ Sci Technol. 2012;46:5798–805. doi: 10.1021/es3004238. [DOI] [PubMed] [Google Scholar]
- MA Y, VENIER M, HITES RA. 2-Ethylhexyl tetrabromobenzoate and bis(2-ethylhexyl) tetrabromophthalate flame retardants in the Great Lakes atmosphere. Environ Sci Technol. 2012;46:204–8. doi: 10.1021/es203251f. [DOI] [PubMed] [Google Scholar]
- MORCK A, HAKK H, ORN U, KLASSON WEHLER E. Decabromodiphenyl ether in the rat: absorption, distribution, metabolism, and excretion. Drug Metab Dispos. 2003;31:900–7. doi: 10.1124/dmd.31.7.900. [DOI] [PubMed] [Google Scholar]
- NTP. Draft NTP Technical Report on the toxicology of a pentabromodiphenyl ether mixture [DE-71 (technical grade)] (CAS No. 32534-81-9) in F344/N rats and B6C3F1/N mice and toxicology and carcinogenesis studies of a pentabromodiphenyl ether mixture [DE-71 (technical grade)] in Wistar Han [Crl:WI)Han)] rats and B6C3F1/N mice (gavage studies) [Online] [Accessed 11/5/2015 2015];2015 https://ntp.niehs.nih.gov/results/pubs/longterm/reports/longterm/index.html. Available: http://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2015/june/tr589_peerdraft.pdf.
- ORTA-GARCIA S, PEREZ-VAZQUEZ F, GONZALEZ-VEGA C, VARELA-SILVA JA, HERNANDEZ-GONZALEZ L, PEREZ-MALDONADO I. Concentrations of persistent organic pollutants (POPs) in human blood samples from Mexico City, Mexico. Sci Total Environ. 2014;472:496–501. doi: 10.1016/j.scitotenv.2013.11.059. [DOI] [PubMed] [Google Scholar]
- PATISAUL HB, ROBERTS SC, MABREY N, MCCAFFREY KA, GEAR RB, BRAUN J, BELCHER SM, STAPLETON HM. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27:124–36. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENG H, SAUNDERS DM, SUN J, CODLING G, WISEMAN S, JONES PD, GIESY JP. Detection, identification, and quantification of hydroxylated bis(2-ethylhexyl)-tetrabromophthalate isomers in house dust. Environ Sci Technol. 2015;49:2999–3006. doi: 10.1021/es505743d. [DOI] [PubMed] [Google Scholar]
- ROBERTS SC, MACAULAY LJ, STAPLETON HM. In vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem Res Toxicol. 2012;25:1435–41. doi: 10.1021/tx300086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHLSTROM L, SELLSTROM U, DE WIT CA. Clean-up method for determination of established and emerging brominated flame retardants in dust. Anal Bioanal Chem. 2012;404:459–66. doi: 10.1007/s00216-012-6160-y. [DOI] [PubMed] [Google Scholar]
- SANDERS JM, KNUDSEN GA, BIRNBAUM LS. The fate of beta-hexabromocyclododecane in female C57BL/6 mice. Toxicol Sci. 2013;134:251–7. doi: 10.1093/toxsci/kft121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCANLAN LD, LOGUINOV AV, TENG Q, ANTCZAK P, DAILEY KP, NOWINSKI DT, KORNBLUH J, LIN XX, LACHENAUER E, ARAI A, DOUGLAS NK, FALCIANI F, STAPLETON HM, VULPE CD. Gene Transcription, Metabolite and Lipid Profiling in Eco-Indicator Daphnia magna Indicate Diverse Mechanisms of Toxicity by Legacy and Emerging Flame-Retardants. Environ Sci Technol. 2015 doi: 10.1021/acs.est.5b00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHREDER ED, LA GUARDIA MJ. Flame retardant transfers from U.S. households (dust and laundry wastewater) to the aquatic environment. Environ Sci Technol. 2014;48:11575–83. doi: 10.1021/es502227h. [DOI] [PubMed] [Google Scholar]
- SEYER A, RIU A, DEBRAUWER L, BOURGES-ABELLA N, BRUNELLE A, LAPREVOTE O, ZALKO D. Time-of-flight secondary ion mass spectrometry imaging demonstrates the specific localization of deca-bromo-diphenyl-ether residues in the ovaries and adrenal glands of exposed rats. J Am Soc Mass Spectrom. 2010;21:1836–45. doi: 10.1016/j.jasms.2010.06.019. [DOI] [PubMed] [Google Scholar]
- SILVA MJ, HILTON D, FURR J, GRAY LE, PREAU JL, CALAFAT AM, YE X. Quantification of tetrabromo benzoic acid and tetrabromo phthalic acid in rats exposed to the flame retardant Uniplex FPR-45. Arch Toxicol. 2016;90:551–7. doi: 10.1007/s00204-015-1489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRINGER C, DERE E, HALL SJ, MCDONNELL EV, ROBERTS SC, BUTT CM, STAPLETON HM, WATKINS DJ, MCCLEAN MD, WEBSTER TF, SCHLEZINGER JJ, BOEKELHEIDE K. Rodent thyroid, liver, and fetal testis toxicity of the monoester metabolite of bis-(2-ethylhexyl) tetrabromophthalate (tbph), a novel brominated flame retardant present in indoor dust. Environ Health Perspect. 2012;120:1711–9. doi: 10.1289/ehp.1204932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAPLETON HM, ALLEN JG, KELLY SM, KONSTANTINOV A, KLOSTERHAUS S, WATKINS D, MCCLEAN MD, WEBSTER TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 2008;42:6910–6. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- STAPLETON HM, KLOSTERHAUS S, KELLER A, FERGUSON PL, VAN BERGEN S, COOPER E, WEBSTER TF, BLUM A. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45:5323–31. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAPLETON HM, MISENHEIMER J, HOFFMAN K, WEBSTER TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014;116:54–60. doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAPLETON HM, SHARMA S, GETZINGER G, FERGUSON PL, GABRIEL M, WEBSTER TF, BLUM A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46:13432–9. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO DT, DILIBERTO JJ, HAKK H, HUWE JK, BIRNBAUM LS. Toxicokinetics of the flame retardant hexabromocyclododecane gamma: effect of dose, timing, route, repeated exposure, and metabolism. Toxicol Sci. 2010;117:282–93. doi: 10.1093/toxsci/kfq183. [DOI] [PubMed] [Google Scholar]
- SZABO DT, DILIBERTO JJ, HAKK H, HUWE JK, BIRNBAUM LS. Toxicokinetics of the flame retardant hexabromocyclododecane alpha: effect of dose, timing, route, repeated exposure, and metabolism. Toxicol Sci. 2011;121:234–44. doi: 10.1093/toxsci/kfr059. [DOI] [PubMed] [Google Scholar]
- USEPA; ASSESSMENT, N. C. F. E, editor. An exposure assessment of polybrominated diphenyl ethers. Washington, DC: 2010. [Google Scholar]
- USEPA; O. O. P. P. A. T, editor. DESIGN FOR THE ENVIRONMENT PROGRAM. Washington, DC: 2011. Design for the Environment Program Alternatives Assessment Criteria for Hazard Evaluation, Version 2.0. [Google Scholar]
- USEPA; TSCA Work Plan Chemicals: Methods Document. O. O. P. P. A, editor. TOXICS. 2012 http://www.epa.gov/oppt/existingchemicals/pubs/wpmethods.pdf.
- USEPA. [Accessed 10/5/2015 2015];Chemical Data Access Tool (CDAT), Search Query: CAS No. 26040-51-7 [Online] 2015a http://java.epa.gov/oppt_chemical_search/
- USEPA; O. O. P. P. A. T, editor DESIGN FOR THE ENVIRONMENT PROGRAM. 2015b. EPA Report No. 744-R-15-002: Flame retardants used in flexible polyurethane foam: an alternatives assessement update. [Google Scholar]
- USEPA. Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11. 2016 Available from, as of 25 March 2016: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface.
- VORKAMP K, RIGET FF. A review of new and current-use contaminants in the Arctic environment: evidence of long-range transport and indications of bioaccumulation. Chemosphere. 2014;111:379–95. doi: 10.1016/j.chemosphere.2014.04.019. [DOI] [PubMed] [Google Scholar]
- ZHOU SN, BUCHAR A, SIDDIQUE S, TAKSER L, ABDELOUAHAB N, ZHU J. Measurements of selected brominated flame retardants in nursing women: implications for human exposure. Environ Sci Technol. 2014;48:8873–80. doi: 10.1021/es5016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIEGLER BROTHERS, I. Rodent NIH-31 Open Formula Auto. 2010 http://www.zeiglerfeed.com/product_literature/lab%20research%20literature_Rodent/Rodent%20NIH-31%20Open.pdf.