SUMMARY
SETTING
Although approximately 0.5 million cases of multidrug-resistant tuberculosis (MDR-TB) occur globally each year, surveillance data are limited. Botswana is one of the few high TB burden countries to have carried out multiple anti-tuberculosis drug resistance surveys (in 1995–1996, 1999 and 2002).
OBJECTIVE
In 2007–2008, we conducted the fourth national survey of anti-tuberculosis drug resistance in Botswana to assess anti-tuberculosis drug resistance, including trends over time. In the previous survey, 0.8% (95%CI 0.4–1.5) of new patients and 10.4% (95%CI 5.6–17.3) of previously treated patients had MDR-TB.
DESIGN
During the survey period, eligible specimens from all new sputum-smear positive TB patients and from all TB patients with history of previous anti-tuberculosis treatment underwent mycobacterial culture and anti-tuberculosis drug susceptibility testing (DST).
RESULTS
Of 924 new TB patients and 137 with previous anti-tuberculosis treatment with DST results, respectively 23 (2.5%, 95%CI 1.6–3.7) and 9 (6.6%, 95%CI 3.3–11.7) had MDR-TB. The proportion of new TB patients with MDR-TB has tripled in Botswana since the previous survey.
CONCLUSION
Combatting drug-resistant TB will require the scale-up of MDR-TB diagnosis and treatment to prevent the transmission of MDR-TB and strengthening of general TB control to prevent the emergence of resistance.
Keywords: tuberculosis, drug resistance, surveillance
Over the past two decades, global anti-tuberculosis drug resistance has steadily risen due to unstable supplies of drugs, inappropriate and inadequate treatment, and incomplete adherence.1,2 Multidrug-resistant tuberculosis (MDR-TB), defined as tuberculosis (TB) caused by Mycobacterium tuberculosis that is resistant to at least isoniazid (INH) and rifampin (RMP), is now one of the most important challenges to global TB control,2,3 with an estimated 450 000 new cases of MDR-TB (range 300 000–600 000) worldwide in 2012.3 While data on anti-tuberculosis drug resistance are now available for 70% of countries worldwide, data from Africa are limited, as drug susceptibility testing (DST) is not widely available and few African countries had conducted nationally representative drug resistance surveys (DRSs) before 2010–2103.3 Even fewer have trend data, and few have data on second-line drug resistance, including limited data on extensively drug-resistant TB (XDR-TB), defined as MDR-TB plus resistance to a fluoroquinolone and at least one of three injectable second-line drugs (amikacin, kanamycin or capreomycin).3
Botswana, a high TB burden country with an estimated TB prevalence rate that exceeded 500 per 100 000 population from 2000 to 2007,3 is unique for several reasons: 1) it has conducted three previous DRSs (1995–1996, 1999 and 2002), allowing for the assessment of trends in drug resistance;4–6 2) it shares borders and strong economic ties with South Africa, where 573 cases of XDR-TB (10.5% of MDR-TB cases) were reported in 2008;7 3) 84% of TB patients in Botswana also have human immunodeficiency virus (HIV) infection,8 making any rise in anti-tuberculosis drug resistance there a threat to public health gains in addressing the HIV epidemic; and 4) Botswana rolled out a national isoniazid preventive therapy (IPT) program for people living with HIV between 2001 and 2004, making the measurement of trends in INH resistance programmatically important. To address these related concerns, the Botswana National TB Programme (BNTP) and the Botswana National TB Reference Laboratory (NTRL), in collaboration with the US Centers for Disease Control and Prevention (CDC), conducted the fourth national anti-tuberculosis DRS in 2007–2008.
METHODS
Enrollment
Botswana has implemented the World Health Organization (WHO) recommended DOTS strategy since 2003, with TB diagnostic services available in all 24 health districts through a national network of 47 laboratories performing smear microscopy during the survey period. All TB diagnostic centers were sampled, such that all sputum specimens from persons presumed to have pulmonary TB (diagnostic specimens collected before treatment initiation) were assessed for eligibility to be included in the DRS according to criteria outlined below. Data for the DRS were abstracted from the routine mycobacteriology request forms that accompany specimens to the district or local laboratory; no additional data collection forms were used. Routine systems for specimen collection and transport were used. The period of enrollment for the DRS was between 10 September 2007 and 9 May 2008.
Specimen processing and laboratory testing
Eligible specimens, defined as smear-positive sputum specimens from patients not previously treated for TB (new patients) and all specimens from patients previously treated for TB (retreatment patients), were forwarded to the NTRL for mycobacterial culture and DST.* One specimen per patient was processed using standard methods for mycobacterial culture on solid media (Löwenstein-Jensen [LJ]) and first-line anti-tuberculosis DST on LJ media using the 1% proportion method.9 INH was tested at two concentrations: 0.1 (low) and 0.4 (high) μg/ml. Identification was performed on LJ media with p-nitrobenzoic acid (0.5 mg/ml) incorporated, along with DST. Isolates that did not grow on p-nitrobenzoic acid media were reported as M. tuberculosis.9
The NTRL only performed first-line DST, including testing for resistance to INH, RMP, ethambutol (EMB) and streptomycin (SM).† Any DRS specimen found to have resistance to a first-line anti-tuberculosis drug at the NTRL was sent to the National Health Laboratory Services (NHLS) in South Africa for second-line DST.‡ The NHLS also provided external quality assurance (EQA) testing for first-line DST; this included first-line DST on all isolates found to have any resistance based on first-line DST results at the NTRL as well as on a 10% sample of isolates found to be susceptible on first-line DSTat the NTRL. Any specimen found to have discordant results between the NTRL and the NHLS was sent to the CDC laboratory in Atlanta, GA, USA, for re-testing.
The following algorithm was applied to determine the final first-line DST result for each individual drug: 1) if the NTRL result was available but no NHLS or CDC results were available, the NTRL result was used; 2) if the NTRL result was available and was concordant with the NHLS result, that result was used; 3) if the NTRL result and NHLS results were discordant, CDC results were used (agar proportion result if available, BACTEC™ MGIT 960™ TB System [BD, Sparks, MD, USA] result if agar proportion was not available, molecular testing result if neither available).
HIV testing
National policy in Botswana is to provide HIV testing for all persons presenting to a health care facility as part of routine care, unless the patient opts out; this includes people being evaluated for TB. HIV status was collected as part of the routine data collection on the mycobacteriology request form. Furthermore, HIV testing of anonymized sputum specimens using OraQuick (OraSure Technologies, Bethlehem, PA, USA), a visually read immunochromatographic test for the detection of HIV antibodies in oral secretions (previously validated in Botswana for use on sputum from persons with TB10), was performed to ensure that the estimate of the prevalence of HIV infection was unbiased, as routinely collected HIV results at the time of initial TB evaluation are often incomplete. Only specimen-level data were available for Ora-Quick results, as testing was performed on a de-identified aliquot of each specimen submitted to the NTRL for potential inclusion in this survey; subsequent data review for eligibility and cross-checking could therefore not be applied to the de-identified OraQuick test results, and these results could not be linked to the DST results.
Management of drug-resistant TB
At the time of the survey (2008), persons with confirmed or suspected drug-resistant TB were referred for treatment by specialists at one of two drug-resistant TB treatment initiating sites in Botswana.
Data checking and validation
BNTP staff worked with District TB Coordinators to collect data, check for transcriptional errors, and cross-check a 10% sample of records with district and health facility records. Survey data for patient category (new or retreatment) were also cross-checked against the National Electronic TB Register for approximately 60% of all records, including all those with drug resistance and a random sample of other records.
Sample size
Information from previous surveys and TB case data from the BNTP were used for sample size calculation, which was powered based on any RMP resistance (to detect with 80% power and an absolute difference of 1% for any RMP resistance), adjusted based on the culture-positive rate for new smear-positive patients from the previous survey (60%), and increased by 15% to account for contamination, loss, and absence of growth. This yielded a total sample size (new and retreatment patients) of 1441.
Analysis
We calculated the proportion of patients with any drug resistance, the proportion with resistance to each drug tested, and the proportion with MDR-TB and other combinations of drug resistance as well as 95% confidence intervals (CIs) for each proportion. We compared these proportions to those reported from the previous DRSs and calculated a χ2 for trend to assess for statistically significant trends. To determine whether resistance to INH had increased out of proportion to increases in resistance in other drugs, we analyzed data for patients with any resistance to INH, RMP or EMB, and determined the proportion of those with resistance to INH. We compared the results from this survey to those from the previous survey conducted in 2002, which was performed at a time when the IPT program was being piloted and IPT had not been implemented widely.
Ethics review
This project was reviewed by the CDC, and determined to be routine disease surveillance and not human subjects research requiring institutional ethics board review; it was also reviewed and approved by the Human Research Development Committee of the Botswana Ministry of Health, Gaborone, Botswana.
RESULTS
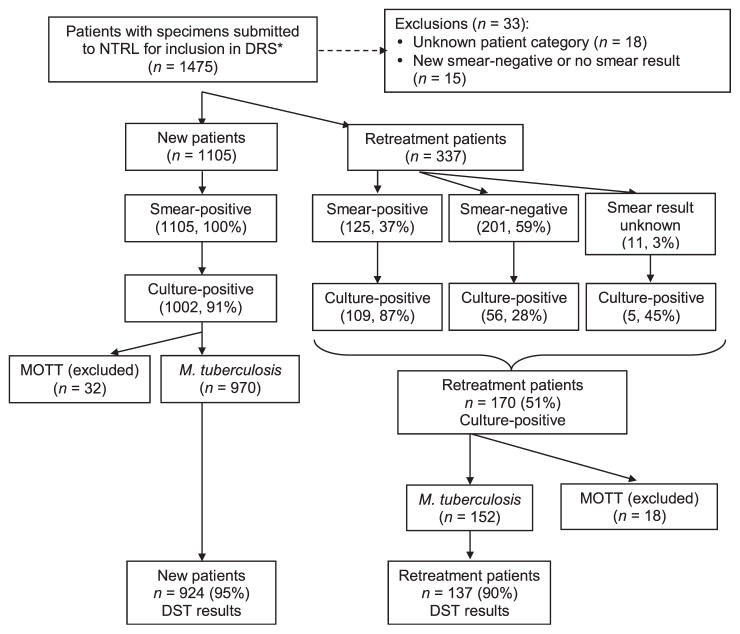
Between 10 September 2007 and 9 May 2008, we identified 1442 patients who met the inclusion criteria for the DRS. Of these, 1105 (77%) were new patients and 337 (23%) were retreatment patients (Figure 1). Among the new patients, the median age was 33 years (range 4–95) and 52% were male. Among the retreatment patients, the median age was 39 years (range 4–87), and 55% were male. Based on available HIV test results as reported on the mycobacteriology request form, 797 (55%) patients had a known HIV test result, of whom 573 (72%) were HIV-positive. During the survey period, 2030 de-identified specimens were submitted to the NTRL; these were HIV tested using OraQuick. Of these, 1988 (98%) had a valid HIV test result, and 1160 (58%) were HIV-positive (Table 1).
Figure 1.
Enrollment in Botswana’s fourth national anti-tuberculosis drug resistance survey, 2007–2008. * Initial eligibility for the DRS was decided by district/local laboratories, which selected all smear-positive diagnostic (month 0) specimens from new patients and all diagnostic (month 0) specimens (smear-positive or smear-negative) from retreatment patients and sent these to the NTRL for inclusion in the DRS. Final eligibility was determined at the NTRL. NTRL = National TB Reference Laboratory; DRS = drug resistance survey; MOTT = mycobacteria other than tuberculosis; DST = drug susceptibility testing.
Table 1.
Characteristics of new and retreated tuberculosis patients in Botswana’s national anti-tuberculosis drug resistance survey, 2007–2008
| All (n = 1442) n (%) |
New patients (n = 1105) n (%) |
Retreatment patients (n = 337) n (%) |
|
|---|---|---|---|
| Median age, years (range) | 35 (4–95) | 33 (4–95) | 39 (4–87) |
| Age categories, years | (n = 1348) | (n = 1042) | (n = 306) |
| 0–14 | 31 (2) | 23 (2) | 8 (3) |
| 15–24 | 211 (16) | 195 (19) | 16 (5) |
| 25–34 | 429 (32) | 358 (34) | 71 (23) |
| 35–44 | 335 (25) | 238 (23) | 97 (32) |
| ≥45 | 342 (25) | 228 (22) | 114 (37) |
| Male | 764 (53) | 578 (52) | 186 (55) |
| HIV results (as recorded on MH2011 form)* | |||
| HIV-positive | 573 (40) | 89 (35) | 184 (55) |
| HIV-negative | 224 (16) | 173 (16) | 51 (15) |
| HIV unknown† | 461 (32) | 394 (36) | 67 (20) |
| Missing‡ | 184 (13) | 149 (13) | 35 (10) |
| HIV-positive among persons with known HIV status | 797 (72) | 562 (69) | 235 (78) |
Results from anonymous testing of sputum specimens using OraQuick: 58% HIV-positive (1160/1988).
Recorded as unknown in the HIV section of the mycobacteriology request forms (MH2011 form).
Section on HIV of mycobacteriology request forms (MH2011 form) was not completed.
HIV = human immunodeficiency virus.
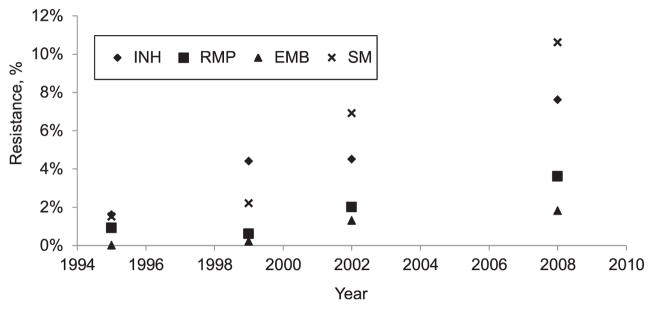
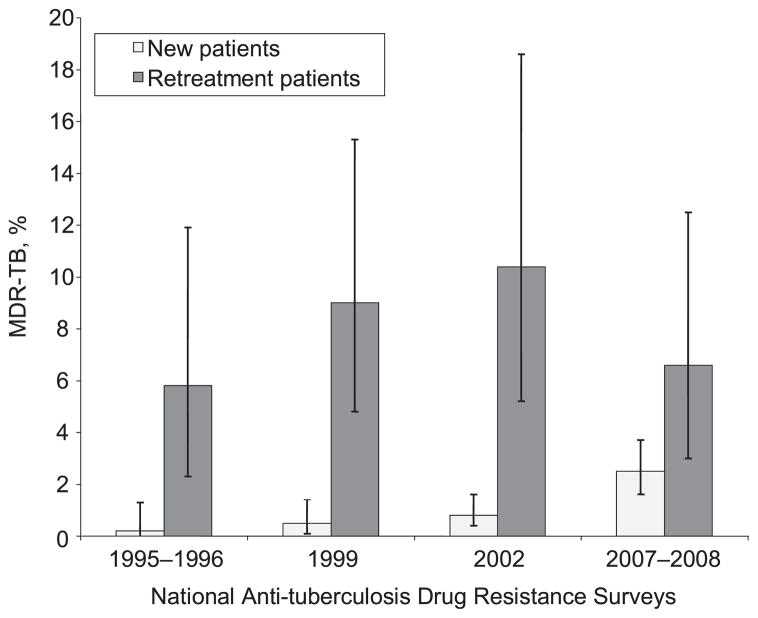
First-line DST results were available for 924 (95% of M. tuberculosis isolates) of the new patients and 137 (90% of those culture-positive for M. tuberculosis) of the retreatment patients (Figure 1). Among the 924 new patients with DST results, 82 (8.9%, 95%CI 7.2–10.8) were resistant to INH, RMP or EMB. Seventy (7.6%, 95%CI 6.0–9.4) had any resistance to INH; of these, 25 (36%) had low-level resistance to INH, while 45 (64%) had high-level resistance. Any resistance to RMP was identified in 33 (3.6%, 95%CI 2.5–4.9) and any resistance to EMB was identified in 17 (1.8%, 95%CI 1.1–2.9) (Table 2). Increasing trends in resistance to these first-line anti-tuberculosis drugs among new patients were observed compared to previous years (Figure 2). Compared to the previous survey in 2002, there was a 1.7-fold increase in the proportion of new patients with any INH resistance, a 1.8-fold increase in the proportion with any RMP resistance, a 1.4-fold increase in the proportion with any EMB resistance and a 1.5-fold increase in the proportion with SM resistance (Table 2). Among the 82 patients with any resistance to INH, RMP or EMB, 70 (85%) had resistance to INH compared to 75% (53/71) in the 2002 survey (P = 0.10). Of the 924 new patients, 23 (2.5%, 95%CI 3.3–11.7) had resistance to both INH and RMP (MDR-TB), with an increasing trend in MDR-TB observed among new patients compared to previous years (P < 0.0001) (Figure 3). The median age of the new MDR-TB patients (32 years, range 20–59) was similar to that of new patients without drug resistance; however, a smaller proportion were male (39%).
Table 2.
Trends in first-line anti-tuberculosis drug resistance by patient treatment category in Botswana’s national anti-tuberculosis DRS, 1995–2008
| 1995–1996
|
1999
|
2002
|
2007–2008
|
||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| n (%) | 95%CI | n (%) | 95%CI | n (%) | 95%CI | n (%) | 95%CI | P value* | |
| New patients | (n = 430) | (n = 638) | (n = 1182) | (n = 924)† | |||||
| Any first-line drug resistance | 16 (3.7) | 2.2–5.8 | 40 (6.3) | 4.6–8.4 | 123 (10.4) | 4.6–8.4 | 156 (16.9) | 14.6–19.4 | <0.0001 |
| Any INH, RMP, EMB resistance | 10 (2.3) | 1.2–4.1 | 30 (4.7) | 3.3–6.6 | 71 (6.0) | 4.8–7.5 | 82 (8.9) | 7.2–10.8 | <0.0001 |
| Any INH | 7 (1.6) | 0.7–3.2 | 28 (4.4) | 3.0–6.2 | 53 (4.5) | 3.4–5.8 | 70 (7.6)‡ | 6.0–9.4 | <0.0001 |
| Any RMP | 4 (0.9) | 0.3–2.2 | 4 (0.6) | 0.2–1.5 | 24 (2.0) | 1.3–3.0 | 33 (3.6)‡ | 2.5–4.9 | <0.0001 |
| Any EMB | 0 | 0–0.9 | 1 (0.2) | 0–0.8 | 15 (1.3) | 0.7–2.1 | 17 (1.8)‡ | 1.1–2.9 | 0.0001 |
| Any SM§ | 6 (1.4) | 0.6–2.9 | 14 (2.2) | 1.3–3.6 | 82 (6.9) | 5.6–8.5 | 96 (10.6)‡ | 8.7–12.8 | <0.0001 |
| Any PZA | NA | NA | 1 (0.1) | 27¶ | NA | ||||
| Monoresistance | |||||||||
| Any (apart from PZA) | 15 (3.5) | 2.0–5.6 | 34 (5.3) | 3.6–7.3 | 86 (7.3) | 5.9–8.9 | 120 (13.0) | 10.9–15.3 | <0.0001 |
| INH | 6 (1.4) | 0.6–2.9 | 23 (3.6) | 2.4–5.3 | 22 (1.9) | 1.2–2.8 | 35 (3.8) | 2.7–5.2 | 0.09 |
| RMP | 3 (0.7) | 0.2–1.9 | 1 (0.2) | 0–0.8 | 10 (0.8) | 0.4–1.5 | 10 (1.1) | 0.6–1.9 | 0.13 |
| EMB | 0 | 0–0.9 | 0 | 0–0.6 | 2 (0.2) | 0–0.6 | 1 (0.1) | 0–0.5 | NA |
| SM | 6 (1.4) | 0.6–2.9 | 10 (1.6) | 0.8–2.8 | 52 (4.4) | 3.3–5.7 | 74 (8.2) | 6.5–10.1 | <0.0001 |
| PZA | NA | NA | 1 (0.1) | 15¶ | NA | ||||
| Any multidrug resistance** | 1 (0.2) | 0–1.3 | 3 (0.5) | 0.1–1.3 | 10 (0.8) | 0.4–1.5 | 23 (2.5)‡ | 1.6–3.7 | <0.0001 |
| Previously treated patients | (n = 121) | (n = 145) | (n = 106) | (n = 137)† | |||||
| Any first-line drug resistance | 18 (14.9) | 9.3–22.1 | 33 (22.8) | 16.5–30.1 | 24 (22.6) | 15.4–31.3 | 32 (23.4) | 16.8–31.0 | 0.13 |
| Any INH, RMP, EMB resistance | 15 (12.4) | 7.4–19.2 | 30 (20.7) | 14.7–27.9 | 19 (17.9) | 11.5–26.1 | 23 (16.8) | 11.2–23.8 | 0.55 |
| Any INH | 12 (9.9) | 5.5–16.3 | 24 (16.6) | 11.2–23.3 | 15 (14.2) | 8.5–21.8 | 14 (10.2)# | 5.9–16.2 | 0.88 |
| Any RMP | 10 (8.3) | 4.3–14.2 | 19 (13.1) | 8.3–19.4 | 13 (12.3) | 7.0–19.6 | 18 (13.1)# | 8.2–19.6 | 0.3 |
| Any EMB | 6 (5.0) | 2.0–10.0 | 4 (2.8) | 0.9–6.5 | 9 (8.5) | 4.2–15.0 | 9 (6.6)# | 3.3–11.7 | 0.24 |
| Any SM | 10 (8.3) | 4.3–14.3 | 7 (4.8) | 2.1–9.3 | 17 (16.0) | 10.0–24.0 | 16 (11.8)# | 7.1–18.0 | 0.07 |
| Any PZA | NA | NA | 0 | 3¶ | NA | ||||
| Monoresistance | |||||||||
| Any (apart from PZA) | 9 (7.4) | 3.7–13.2 | 18 (12.4) | 7.8–18.6 | 7 (6.6) | 3.0–12.6 | 20 (14.6) | 9.4–21.3 | 0.18 |
| INH | 4 (3.3) | 1.1–7.8 | 9 (6.2) | 3.1–11.1 | 0 | 0–3.4 | 3 (2.2) | 0.6–5.8 | 0.17 |
| RMP | 2 (1.7) | 0.3–5.4 | 6 (4.1) | 1.7–8.4 | 0 | 0–3.4 | 8 (5.8) | 2.7–10.8 | 0.19 |
| EMB | 0 | 0–3.0 | 0 | 0–2.5 | 2 (1.9) | 0.3–6.1 | 0 | 0–2.7 | NA |
| SM | 3 (2.5) | 0.6–6.6 | 3 (2.1) | 0.5–5.5 | 5 (4.7) | 1.7–10.1 | 9 (6.6)† | 3.3–11.8 | 0.05 |
| PZA | NA | NA | 0 | 0¶ | NA | ||||
| Any multidrug resistance** | 7 (5.8) | 2.6–11.1 | 13 (9.0) | 5.1–14.5 | 11 (10.4) | 5.6–17.3 | 9 (6.6)# | 3.3–11.7 | 0.81 |
χ2 test for linear trend.
Missing results: new patients: RMP (n = 1), EMB (n = 3), SM (n = 21); retreatment patients: SM (n = 1).
NTRL results for new patients: any INH resistance (n = 70: low INH resistance [n = 25], high INH resistance [n = 45]); any RMP resistance (n = 35); any EMB resistance (n = 18); any SM resistance (n = 169); any MDR-TB (n = 23).
While SM is no longer considered a first-line drug, for the purposes of reporting the results of this DRS we retained its classification as a first-line drug to allow for comparison of results from the three previous DRSs.
As DSTagainst PZA was performed only on a subset of patient isolates sent to NHLS for first-line EQA and SLD DST (PZA results available for 130 new patients and for 22 retreatment patients), the proportion resistant was not calculated.
NTRL results for retreatment patients: any INH resistance (n =14: low INH resistance [n =4], high INH resistance [n =10]), any RMP resistance (n =18); any EMB resistance (n = 8); any SM resistance (n = 25); any MDR-TB (n = 10).
Defined as resistance to at least INH and RMP, with or without other drug resistance.
DRS =drug resistance survey; CI =confidence interval; INH =isoniazid; RMP =rifampicin; EMB =ethambutol; SM =streptomycin; PZA =pyrazinamide; NA =not available; NTRL = National TB Reference Laboratory; MDR-TB = multidrug-resistant tuberculosis; DST = drug susceptibility testing; NHLS = National Health Laboratory Services; EQA = external quality assurance; SLD = second-line drug.
Figure 2.
Trends in first-line anti-tuberculosis drug resistance among new patients in Botswana’s national anti-tuberculosis drug resistance surveys, 1995–2008. INH =isoniazid; RMP =rifampicin; EMB = ethambutol; SM = streptomycin.
Figure 3.
Trends in proportion (and 95% confidence intervals) of patients with MDR-TB by patient treatment category in Botswana’s national anti-tuberculosis drug resistance surveys, 1995–2008. MDR-TB = multidrug-resistant tuberculosis.
Among the 137 retreatment patients with DST results, 23 (16.8%, 95%CI 11.2–23.8) were resistant to INH, RMP or EMB; 14 (10.2%, 95%CI 5.9–16.2) had resistance to INH, including 4 (29%) with low-level resistance and 10 (71%) with high-level resistance. Any resistance to RMP was identified in 18 (13.1%, 95%CI 8.2–19.6) patients and any resistance to EMB was identified in 9 (6.6%, 95%CI 3.3–11.7). Of the 137 patients, 9 (6.6%, 95%CI 3.3–11.7) had MDR-TB. None of these were statistically significant increases compared to previous years (Table 2). The median age of the retreatment patients with MDR-TB (30 years, range 23–54) was lower than that of retreatment patients without drug resistance, and a larger proportion were male (67%).
Of the 23 new patients with MDR-TB, 17 had second-line DST results available; of these, 2 (11.8%) had any fluoroquinolone resistance and 1 (5.9%) had any KM resistance. Of the 9 retreatment patients with MDR-TB, 7 had second-line DST results available; of these, none of the isolates were found to be resistant to fluoroquinolones or KM (Table 3). No XDR-TB cases were identified in this survey; however, as second-line DST results were not available for all persons with MDR-TB, XDR-TB cases may have been missed. No data on resistance to second-line anti-tuberculosis drugs were available for comparison from the previous surveys, as this was the first time second-line DST had been included in the DRS.
Table 3.
Second-line DST results* by patient category in Botswana’s fourth national anti-tuberculosis drug resistance survey, 2007–2008
| MDR-TB (n = 17) n (%) |
DR-TB, not MDR-TB (n = 55) n (%) |
Drug-susceptible TB (n = 85) n (%) |
|
|---|---|---|---|
| New patients | |||
| Any second-line drug resistance | 11 (64.7) | 4 (7.3) | 8 (9.4) |
| Any FQ resistance† | 2 (11.8) | 2 (3.6) | 0 |
| Any KM resistance | 1 (5.9) | 1 (1.8) | 0 |
| Any ETH resistance | 11 (64.7) | 5 (9.1) | 8 (9.4) |
| XDR-TB‡ | 0 | NA | NA |
| Retreatment patients | (n = 7) | (n = 9) | (n = 11) |
| Any second-line drug resistance | 2 (28.6) | 2 (33.3) | 1 (9.0) |
| Any FQ resistance† | 0 | 0 | 0 |
| Any KM resistance | 0 | 0 | 0 |
| Any ETH resistance | 2 (28.6) | 2 (22.2) | 1 (9.0) |
| XDR-TB‡ | 0 | NA | NA |
Second-line DST was performed only on a subset of specimens sent to the National Reference Laboratory, South Africa, for additional testing, which included all those with resistance to isoniazid, rifampicin, ethambutol or streptomycin, and a 10% sample of those without resistance (susceptible); totals (n) listed in Table show the number per category with second-line DST results.
Ofloxacin.
Defined as MDR-TB plus resistance to a FQ and resistance to any of the second-line injectable drugs (AMK, KM, capreomycin). Note: seven new patients underwent DST for AMK and no resistance to AMK was identified.
DST = drug susceptibility testing; MDR-TB = multidrug-resistant tuberculosis; DR-TB = drug-resistant TB; FQ = fluoroquinolones; KM = kanamycin; ETH = ethionamide; XDR-TB = extensively drug-resistant TB; NA = not available; AMK = amikacin.
EQA results were available from the NHLS for 230 patients. Among these, 21 (9.1%) had results discordant for INH,§ 4 (1.7%) for RMP and 8 (3.5%) for EMB.
DISCUSSION
In the 2007–2008 national anti-tuberculosis DRS in Botswana, we found that resistance to INH, RMP, EMB and SM continued to rise among new TB patients. The proportion of new patients with any INH or RMP resistance had increased 1.7-fold, and MDR-TB had increased 3.1-fold since 2002. There was no statistically significant change in the proportion of retreatment patients with MDR-TB.
Misclassification of some retreatment patients as new patients could account for these findings. However, we undertook efforts to verify data and avoid misclassification, and do not think that these results can be explained by potential misclassification. Furthermore, these findings are consistent with estimates of the proportion of MDR-TB among persons with TB in the WHO Africa Region of 2.3% (range 0.2–4.4) among new patients and 11% (range 4.4–17) among retreatment patients.3
The common dogma for MDR-TB control is to ‘turn off the tap’ by improving general TB control. However, it must be recognized that there are now at least two taps: 1) the generation of new drug resistance due to suboptimal treatment; and 2) transmission of MDR-TB to others.11 Turning off the first tap by improving general TB control, while an essential component of the strategy, is not sufficient to prevent cases of primary MDR-TB. To prevent these cases, efforts must be aimed at reducing the transmission of MDR-TB.
In 2001–2004, Botswana introduced a nationwide IPT program for people living with HIV. One of the potential barriers to the implementation of the IPT programs is concern by clinicians and some public health officials about drug resistance. Data from clinical trials have not shown a statistically significant increased risk for INH resistance in people who receive IPT;12,13 similarly, the Botswana IPT trial comparing 6 vs. 36 months of IPT found that the proportion of participants with incident TB disease having any INH resistance did not differ from the expected proportion.14 However, no data from large-scale programs in resource-limited settings were previously available. In this survey, we found a trend of increasing resistance to INH, RMP and EMB among new patients, but did not find that resistance to INH had increased out of proportion to resistance to the other first-line drugs when data from this survey (post-initiation of IPT program) were compared to those of the previous surveys (mostly preinitiation of IPT program).
The increasing trend in SM resistance among new patients to levels similar to those of retreatment patients suggests ongoing transmission of SM-resistant M. tuberculosis strains. One possible explanation for this would be the use of SM in empiric retreatment (Category II) regimens before the availability of rapid testing for drug resistance. However, we can only hypothesize about this, as our data cannot assess the cause of the increase.
This survey was subject to a number of limitations. Sputum collection from new TB patients was limited to persons with sputum smear-positive TB, as recommended by the WHO.15 However, given the high prevalence of HIV infection in Botswana and the fact that persons living with HIV are more likely to have smear-negative pulmonary TB than those without HIV infection,16–18 this may account for the lower HIV prevalence found in this survey as compared to previously reported data from baseline evaluation of HIV testing of TB patients.8 These differences were also evident in the HIV results obtained from the mycobacteriology request form and the OraQuick HIV test results. These discrepancies also likely reflect both the incompleteness of data routinely collected from persons with suspected TB and the limitations of HIV testing of sputum samples where sensitivity can be impacted if there is a delay in specimen processing.10
Botswana’s ongoing efforts to scale up MDR-TB treatment, to implement and expand facility- and community-based interventions to reduce TB transmission, and to strengthen TB control will be critical to addressing the increasing trend in anti-tuberculosis drug resistance. Expansion and strengthening of laboratory capacity, including the implementation of rapid diagnostic methods, and continued surveillance for drug resistance are needed to determine the impact of these efforts.
Footnotes
The NTRL performed smear microscopy, mycobacterial culture and first-line DST at the time of the survey. Implementation of external quality assurance (EQA) for mycobacterial cultures and DST started in 2007. Quality assurance for smear microscopy was piloted in 2007, with full implementation in 2008 with the implementation of the National EQA Plan. In 2007–2008, laboratory turnaround time was ≤48 h for 97.5% of laboratories.
While SM is no longer considered a first-line drug, we retained its classification as a first-line drug for the purposes of reporting the results of this DRS to allow for comparisons with results of the three previous DRS.
Second-line drugs tested included kanamycin (KM), ofloxacin (OFX), ethionamide (ETH) and pyrazinamide (PZA).
Discordance for INH was due to both misclassification of level of resistance and misclassification of no resistance vs. any resistance.
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
References
- 1.Mitchison DA. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. Int J Tuberc Lung Dis. 1998;2:10–15. [PubMed] [Google Scholar]
- 2.Matteelli A, Migliori GB, Cirillo D, Centis R, Girardi E, Raviglione M. Multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis: epidemiology and control. Expert Rev Anti Infect Ther. 2007;5:857–871. doi: 10.1586/14787210.5.5.857. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global tuberculosis report, 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 4.Kenyon TA, Mwasekaga MJ, Huebner R, Rumisha D, Binkin N, Maganu E. Low levels of drug resistance amidst rapidly increasing tuberculosis and human immunodeficiency virus co-epidemics in Botswana. Int J Tuberc Lung Dis. 1999;3:4–11. [PubMed] [Google Scholar]
- 5.Talbot EA, Kenyon TA, Mwasekaga MJ, Moeti TL, Mallon V, Binkin NJ. Control of anti-tuberculosis drug resistance in Botswana. Int J Tuberc Lung Dis. 2003;7:72–77. [PubMed] [Google Scholar]
- 6.Nelson LJ, Talbot EA, Mwasekaga MJ, et al. Anti-tuberculosis drug resistance and anonymous HIV surveillance in tuberculosis patients in Botswana, 2002. Lancet. 2005;366:488–490. doi: 10.1016/S0140-6736(05)67062-6. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2010.3. [Google Scholar]
- 8.Gammino VM, Mboya JJ, Samandari T, et al. Baseline evaluation of routine HIV testing among tuberculosis patients in Botswana. Int J Tuberc Lung Dis. 2008;12(Suppl 1):S92–S94. [PubMed] [Google Scholar]
- 9.World Health Organization. Guidelines for surveillance of drug resistance in tuberculosis. 3. Geneva, Switzerland: WHO; 2003. WHO/CDS/2003.320. [Google Scholar]
- 10.Talbot EA, Hone NM, Moffat HJ, et al. The validity of HIV testing using sputum from suspected tuberculosis patients, Botswana, 2001. Int J Tuberc Lung Dis. 2003;7:710–713. [PubMed] [Google Scholar]
- 11.Caminero JA. Multidrug-resistant tuberculosis: epidemiology, risk factors, and case finding. Int J Tuberc Lung Dis. 2010;14:382–390. [PubMed] [Google Scholar]
- 12.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12:744–751. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halsema CL, Fielding KL, Chihota VN, et al. Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. AIDS. 2010;24:1051–1055. doi: 10.1097/QAD.0b013e32833849df. [DOI] [PubMed] [Google Scholar]
- 14.Samandari T, Agizew TB, Nyirenda S, et al. 6-month vs. 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomized, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Guidelines for surveillance of drug resistance in tuberculosis. 4. Geneva, Switzerland: WHO; 2009. WHO/HTM/TB/2009.422. [Google Scholar]
- 16.Harries AD, Banda HT, Boeree MJ, et al. Management of pulmonary tuberculosis suspects with negative sputum smears and normal or minimally abnormal chest radiographs in resource-poor settings. Int J Tuberc Lung Dis. 1998;2:999–1004. [PubMed] [Google Scholar]
- 17.Mtei L, Matee M, Herfort O, Ba, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 18.Smith RL, Yew K, Berkowitz KA, Aranda CP. Factors affecting the yield of acid-fast sputum smears in patients with HIV and tuberculosis. Chest. 1994;106:684–686. doi: 10.1378/chest.106.3.684. [DOI] [PubMed] [Google Scholar]