Abstract
Importance
Contralateral prophylactic mastectomy (CPM) use is increasing among women with unilateral breast cancer, but little is known about treatment decision making or physician interactions in diverse patient populations.
Objective
To evaluate patient motivations, knowledge, and decisions, as well as the impact of surgeon recommendations, in a large, diverse sample of patients who underwent recent treatment for breast cancer.
Design/Setting/Participants
A survey was sent to 3631 women with newly diagnosed, unilateral stage 0, I, or II breast cancer between July 2013 and September 2014. Women were identified through the population-based Surveillance Epidemiology and End Results registries of Los Angeles County and Georgia. Data on surgical decisions, motivations for those decisions, and knowledge were included in the analysis. Logistic and multinomial logistic regression of the data were conducted to identify factors associated with (1) CPM vs all other treatments combined, 2) CPM vs unilateral mastectomy (UM), and (3) CPM vs breast-conserving surgery (BCS). Associations between CPM receipt and surgeon recommendations were also evaluated. All statistical models and summary estimates were weighted to be representative of the target population.
Main Outcome and Measures
Receipt of CPM was the primary dependent variable for analysis and was measured by a woman’s self-report of her treatment.
Results
Of the 3631 women selected to receive the survey, 2578 (71.0%) responded and 2402 of these respondents who did not have bilateral disease and for whom surgery type was known constituted the final analytic sample. The mean (SD) age was 61.8 (12) years at the time of the survey. Overall, 1301 (43.9%) patients considered CPM (601[24.8%] considered it very strongly or strongly; only 395 (38.1%) of them knew that CPM does not improve survival for all women with breast cancer. Ultimately, 1466 women (61.6%) received BCS, 508 (21.2%) underwent UM, and 428 (17.3%) received CPM. On multivariable analysis, factors associated with CPM included younger age (per 5-year increase: odds ratio [OR], 0.71; 95% CI, 0.65–0.77), white race (black vs white: OR, 0.50; 95% CI, 0.34–0.74), higher educational level (OR, 1.69; 95% CI, 1.20–2.40), family history (OR, 1.63; 95% CI, 1.22–2.17), and private insurance (Medicaid vs private insurance: OR, 0.47; 95% CI, 0.28–0.79). Among 1569 patients (65.5%) without high genetic risk or an identified mutation, 598 (39.3%) reported a surgeon recommendation against CPM, of whom only 12 (1.9%) underwent CPM, but among the 746 (46.8%) of these women who received no recommendation for or against CPM from a surgeon, 148 (19.0%) underwent CPM.
Conclusions and Relevance
Many patients consider CPM, but knowledge about the procedure is low and discussions with surgeons appear to be incomplete. Contralateral prophylactic mastectomy use is substantial among patients without clinical indications but is low when patients report that their surgeon recommended against it. More effective physician-patient communication about CPM is needed to reduce potential overtreatment.
INTRODUCTION
Contralateral prophylactic mastectomy (CPM) is a controversial procedure for patients diagnosed with unilateral breast cancer because no compelling evidence suggests a survival advantage1,2 and the risk of contralateral breast cancer development is low for most patients.1 Yet celebrity exposure and publicity have recently drawn attention to this approach for the management of early-stage unilateral breast cancer.3 Rates of this aggressive, costly, morbid, and burdensome procedure are increasing over time both at centers of excellence and in the broader community,2,4–9 even among patients without high genetic risk of a second primary breast cancer who would otherwise be candidates for breast-conserving therapy.
Few studies have evaluated patients’ decision-making experiences to illuminate why CPM rates have markedly increased; most studies have been limited in generalizability because they considered patients treated at a single institution or those of young age.10–14 Such studies have identified worrisome knowledge deficits among patients who chose CPM and noted that decisions for CPM appear to be patient-driven rather than shared or physician-driven.12,15,16 A prior study from our group described the surgical decision-making experiences of a population-based sample whose breast cancer was diagnosed around 2006 in an era when over two-thirds of patients received breast-conserving therapy and fewer than 10% received CPM.17 That study found that CPM receipt was significantly associated with genetic testing, strong family cancer history, receipt of magnetic resonance imaging, higher education, and greater worry about cancer recurrence. However, less is known about the knowledge regarding, motivations for, and recommendations perceived about CPM by diverse women treated more recently in the United States. Given that multiple studies have documented strong racial/ethnic and age-related differences in receipt of CPM,18 investigation of these questions in a large, diverse sample is particularly important to inform the design of targeted interventions that aim to reduce the use of aggressive treatments in patients with a favorable prognosis. Moreover, little is known about patient-physician communication in this context, including the prevalence of physician recommendations against CPM or their influence. Evaluating the impact of surgeon recommendations is particularly important given that prominent professional societies have long advocated for detailed discussions between surgeons and patients in this setting because of concerns that patients may overestimate risk. However, to our knowledge, the extent to which surgeon communication influences patients has not yet been evaluated in population-based samples.19
Therefore, we conducted a large-scale survey of patients with a diagnosis of early-stage breast cancer and identified through 2 population-based Surveillance Epidemiology and End Results (SEER) registries. We specifically sought to describe consideration and receipt of CPM, accuracy of knowledge, motivations, and correlates of CPM receipt, with particular attention to the impact of surgeon recommendations in this setting.
METHODS
Sample
Between July 2013 and September 2014, we selected 3,880 women aged 20 to 79 who received a diagnosis of and were surgically treated for in situ or early-stage invasive breast cancer and were reported to the SEER registries of Georgia and Los Angeles County. African Americans, Asians and Latinas were oversampled in Los Angeles. As detailed in the eFigure in the Supplement, 249 women were subsequently found to be ineligible. Of the 3,631 eligible women, 2,578 (71%) completed a survey. The 2,402 of these respondents who did not have bilateral disease and for whom surgery type was known constituted the final analytic sample. This study was approved by the University of Michigan Institutional Review Board and received a waiver of documentation of informed consent. Financial compensation was provided to the patients.
Questionnaire design and content
Questionnaire content was developed based on a conceptual framework, research questions, and hypotheses. We selected established measures and developed new measures drawing from the literature and prior research from our group.17 We used standard techniques to assess content validity, including systematic review by design experts, cognitive pretesting with patients, and pilot studies in selected clinic populations.
Data Collection
Eligible patients were identified via initial surgical pathology reports from a list of definitive surgical procedures. The median (SD) time from diagnosis to survey completion was 6.4 (3.0) months. To encourage response, we provided a $20 cash incentive and used a modified Dillman method,20 including reminders to non-respondents. All materials were printed in English. We also included Spanish-translated materials for all women with surnames suggesting Latina ethnicity.21 Survey responses were merged with clinical data from the SEER registries.
Measures
CPM Consideration and Receipt
Receipt of CPM was our primary dependent variable for analysis and was measured by self-report. Specifically, patients reported the ultimate type of surgery they had received (lumpectomy, unilateral mastectomy [UM], or bilateral mastectomy with CPM). Consideration of CPM at the time of definitive surgical decision-making was measured through the patient survey using a 5-point response scale (very strongly, strongly, moderately, weakly, not at all).
Knowledge and Motivations
Patient knowledge was measured by inquiring (with response options of yes/no/don’t know) about whether removing the unaffected breast improves survival for all women with breast cancer and whether doing so reduces the risk of “the breast cancer coming back.”
Women who received CPM were asked to describe the level of importance of the following factors on their decision to choose CPM: their age, having a positive BRCA 1 or 2 genetic testing result, having a family history of breast cancer, wanting reconstruction to best match her breast, wanting reconstruction to change breast size, and peace of mind. Response options were “not at all,” “a little,” “somewhat,” “quite,” and “very.”
Surgeon recommendations
Surgeon recommendations were assessed with an item that asked patients how strongly the surgeons who the patient consulted recommended having a “mastectomy on both breasts.” Responses were grouped as having received a recommendation against CPM (either strong or weak), having received no recommendation for or against CPM, or having received a recommendation for CPM (either strong or weak).
Covariates
SEER registries provided cancer stage at diagnosis (0, I, or II); patients with stage III and IV disease were not sampled since the present analysis was part of a larger study on treatment experiences of patients with breast cancer with a favorable prognosis. Patients provided age at the time of the survey, information regarding race/ethnicity (non-Hispanic white, non-Hispanic black, non-Hispanic Asian, Latina), education (some high school, completed high school, attended or completed college), insurance coverage (Medicaid, Medicare, private/other insurance, not insured), income (grouped for analysis as <40,000, 40,000–89,999, ≥90,000), marital status (not married vs married or partnered), family history of breast cancer (grouped as present in ≥ first-degree relative vs no history), breast size (smaller [A, B, or C cup size] vs larger [D or larger]), and magnetic resonance imaging receipt (yes or no).
We divided patients into 2 groups, based on genetic risk of developing a contralateral primary breast cancer. We deliberately created a conservative measure of “average risk” by excluding from that group not only those who reported having been diagnosed with a deleterious mutation on germline genetic testing but also all those patients who would be considered at high risk for a mutation based on criteria derived from guidelines of the National Comprehensive Cancer Network.22 Specifically, we considered patients to be at high risk for a genetic mutation if they had one or more of the following factors: age at breast cancer diagnosis 45 years or younger; triple-negative breast cancer with age at diagnosis younger than 60 years; any relative with: ovarian cancer, sarcoma, or male breast cancer; 2 or more first-degree relatives with breast cancer; for patients diagnosed at age ≤50, ≥1 first-degree relative with breast cancer; Ashkenazi Jewish ancestry; or family history of a deleterious genetic mutation (BRCA1, BRCA2, or another mutation associated with high breast cancer risk, e.g., TP53). SEER registries provided the information on expression of estrogen receptor, progesterone receptor, and ERBB2 (formerly HER2) for this definition; patients self-reported the other information. Multiple variable analyses also controlled for geographic site (Georgia versus Los Angeles County).
Weights
Survey design and non-response weights were created to compensate for the differential probability of selecting patients by race/ethnicity, cancer stage, and SEER site and to adjust for survey non-response. The weights were normalized to equal to the observed sample size. All statistical models presented are weighted so that statistical inference is representative of our target population. All percentages reported herein are weighted, unless otherwise noted.
Statistical Methods
Weighted binomial logistic regression models and multinomial logistic regression models were constructed to compare surgical outcomes. Receipt of CPM was the primary outcome. Models included all of the theoretically prespecified covariates described above. To correct for the potential for bias due to item nonresponse when using complete-cases methods, values for missing items were imputed using sequential multiple imputations (SMI).23,24 Model results were compared between sequential multiple imputation analyses and complete-case analyses of the observed data for any meaningful differences. Odds ratios (ORs) and 95% CIs are reported; P ≤.05 was considered the level of significance; P values were 2-sided. Analysis was performed using Stata, release 14 (StataCorp).
RESULTS
Table 1 reports the characteristics of the analytic sample by surgery received. Mean (SD) age was 61.8 (12) years. Overall, 428 (24.9%) patients had stage 0 disease (ductal carcinoma in situ), 1258 (46.9%) stage I disease, and 611 (24.7%) stage II disease. A total of 1292 (57.1%) women were white; 430 (18.0%) were black, 413 (13.7%) Latina, and 205 (8.6%) Asian. A total of 1260 (53.5%) had private insurance, but 682 (28.6%) had Medicare and 328 (12.8%) had Medicaid. The sample included a wide range of family income, and 1501 women (62.8%) were married. A total of 555 women (23.8%) reported having a first-degree family member with breast cancer. Preoperative MRI was performed in 1412 women (59.0%), and 1569 (65.5%) had neither a known deleterious mutation nor a high risk for a genetic mutation. Overall, 1466 (61.6%) received BCS, 508 (21.2%) underwent UM, and 428 (17.3%) bilateral mastectomy with CPM.
Table 1.
Clinical and Demographic Characteristics of the Sample, by Type of Surgery Received
| Variable | Total | BCS | UM | CPM |
|---|---|---|---|---|
| Age at survey: Mean {wMean} || wMean |
||||
| 61.7 {61.8} | 63.4 | 62.6 | 55.0 | |
| Stage: N (%) {w%} || row w% | ||||
| 0 | 428 (17.8) {24.9} | 62.9 | 21.6 | 15.4 |
| 1 | 1258 (52.4) {46.9} | 67.0 | 16.4 | 16.6 |
| 2 | 611 (25.4) {24.7} | 50.5 | 29.8 | 19.7 |
| Not reported | 105 (4.4) {3.4} | 58.5 | 19.3 | 22.2 |
| Race/Ethnicity: N (%) {w%} || row w% | ||||
| White | 1292 (53.8) {57.1} | 62.2 | 17.7 | 20.1 |
| Black | 430 (17.9) {18.0} | 63.8 | 23.3 | 12.9 |
| Latina | 413 (17.2) {13.7} | 64.9 | 20.7 | 14.4 |
| Asian | 205 (8.5) {8.6} | 49.1 | 35.0 | 15.9 |
| Other or Not reported | 62 (2.6) {2.5} | 57.6 | 37.4 | 5.1 |
| Education: N (%) {w%} || row w% | ||||
| High school graduate or less | 696 (29.0) {27.3} | 65.7 | 24.8 | 9.5 |
| Some college or greater | 1681 (70.0) {71.7} | 59.9 | 19.7 | 20.4 |
| Not reported | 25 (1.0) {1.0} | 72.7 | 24.7 | 2.6 |
| Insurance: N (%) {w%} || row w% | ||||
| None | 11 (0.5) {0.5} | 68.8 | 18.5 | 12.7 |
| Medicaid | 328 (13.7) {12.7} | 64.9 | 26.3 | 8.8 |
| Medicare | 682 (28.4) {28.6} | 68.6 | 21.7 | 9.7 |
| Other public | 30 (1.2) {1.2} | 46.0 | 28.7 | 25.3 |
| Private | 1260 (52.5) {53.5} | 57.1 | 19.1 | 23.8 |
| Not reported | 91 (3.8) {3.4} | 64.9 | 25.8 | 9.3 |
| Income: N (%) {w%} || row w% | ||||
| <40K | 733 (30.5) {29.3} | 65.0 | 22.2 | 12.8 |
| 40K to <90K | 659 (27.4) {28.3} | 58.2 | 22.2 | 19.7 |
| 90K+ | 587 (24.4) {25.7} | 59.7 | 16.4 | 23.9 |
| Not reported | 423 (17.6) {16.7} | 64.3 | 24.7 | 10.9 |
| Marital status: N (%) {w%} || row w% | ||||
| Not married | 872 (36.3) {35.9} | 64.1 | 22.6 | 13.3 |
| Married | 1501 (62.5) {62.8} | 59.9 | 20.3 | 19.8 |
| Not reported | 29 (1.2) {1.2} | 77.0 | 18.1 | 4.9 |
| Family history of breast cancer: N (%) {w%} || row w% | ||||
| No | 1670 (69.5) {68.8} | 61.0 | 23.1 | 15.9 |
| Yes | 555 (23.1) {23.8} | 62.6 | 15.2 | 22.1 |
| Not reported | 177 (7.4) {7.4} | 63.7 | 21.8 | 14.4 |
| Breast size (cup size): N (%) {w%} || row w% | ||||
| A or B | 760 (31.6) {31.8} | 58.9 | 24.2 | 16.9 |
| C | 743 (30.9) {31.0} | 62.7 | 21.4 | 16.0 |
| D | 479 (19.9) {19.7} | 64.9 | 18.0 | 17.1 |
| DD+ | 349 (14.5) {14.6} | 61.3 | 16.6 | 22.1 |
| Not reported | 71 (3.0) {2.9} | 59.1 | 28.2 | 12.7 |
| MRI: N (%) {w%} || row w% | ||||
| No | 781 (32.5) {33.0} | 63.6 | 21.9 | 14.5 |
| Yes | 1412 (58.8) {59.0} | 60.2 | 19.8 | 19.9 |
| Not reported | 209 (8.7) {8.0} | 63.6 | 27.1 | 9.4 |
| Genetic Risk: N (%) {w%} || row w% | ||||
| High risk or known genetic mutation | 676 (28.1) {28.4} | 55.7 | 18.0 | 26.4 |
| Neither (“Average Risk”) | 1569 (65.3) {65.5} | 63.8 | 22.0 | 14.2 |
| Not reported | 157 (6.5) {6.1} | 65.7 | 26.5 | 7.8 |
| Site: N (%) {w%} || row w% | ||||
| GA | 1265 (52.7) {53.8} | 58.1 | 19.5 | 22.4 |
| LA | 1137 (47.3) {46.2} | 65.7 | 23.0 | 11.3 |
w% = Weighted percentage. wMean = Weighted mean.
Figure 1 depicts the strength of consideration of CPM in this sample, among those with known deleterious mutations or high genetic risk, as well as the strength of consideration among all others, whom we consider to be at average risk of contralateral primary cancer development. Overall, 1056 patients (43.9%) considered CPM and 601 (24.8%) considered it strongly or very strongly. Consideration of CPM was more common among the higher risk patients (351 [52.0%]), but was also reported by 650 (40.9%) of those at average risk for a second primary breast cancer (P<0.001). Of the average-risk patients, 253 (15.7%) considered CPM very strongly, 82 (5.2%) strongly, 153 (9.7%) moderately, and 162 (10.4%) weakly.
Figure 1. Strength of consideration of CPM by risk for contralateral primary cancer.
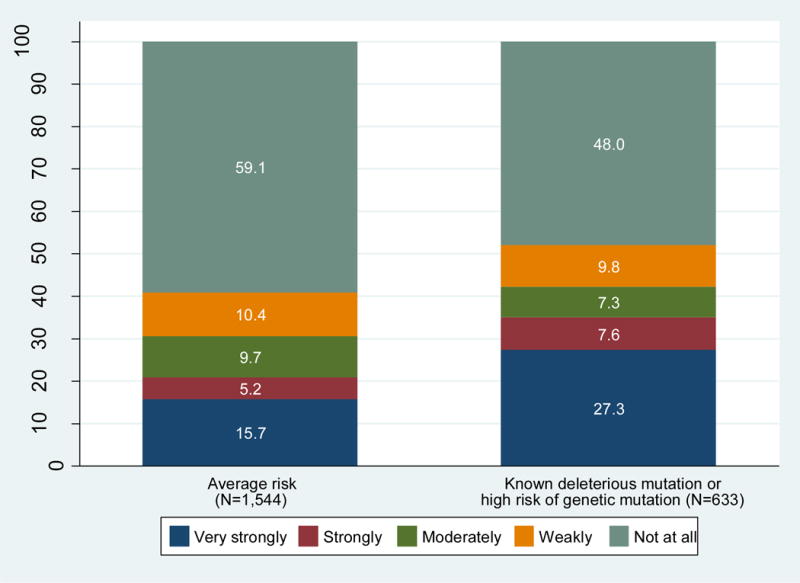
This figure depicts the proportion of patients sampled who reported consideration of CPM, along with the strength of that consideration, by risk groups defined using age, family history, and biologic subtype, derived from the contemporaneous NCCN guidelines for assessment of genetic risk.
Among patients who considered CPM, 395 (38.1%) knew that it does not improve survival for all women with breast cancer (23.8% believed it did and 38.1% did not know); 462 (43.5%) knew that removing the breast without cancer does not prevent cancer from recurring for all women with breast cancer (17.0% thought it did, and 39.5% did not know). Among women who actually received CPM, 158 (37.3%) believed it improves survival for all women with breast cancer.
Overall, 180 (26.4%) of 676 higher risk patients and 236 of 1569 individuals (14.2%) at average risk received CPM. As shown in Figure 2, almost all (96.3%) of patients endorsed peace of mind as very or quite important in motivating them to receive CPM. Substantial minorities cited their age, family history, or a desire to have reconstruction for symmetry as motivating factors; smaller minorities cited their BRCA mutation status or a desire to change the size of their breasts.
Figure 2. Motivations for CPM receipt.
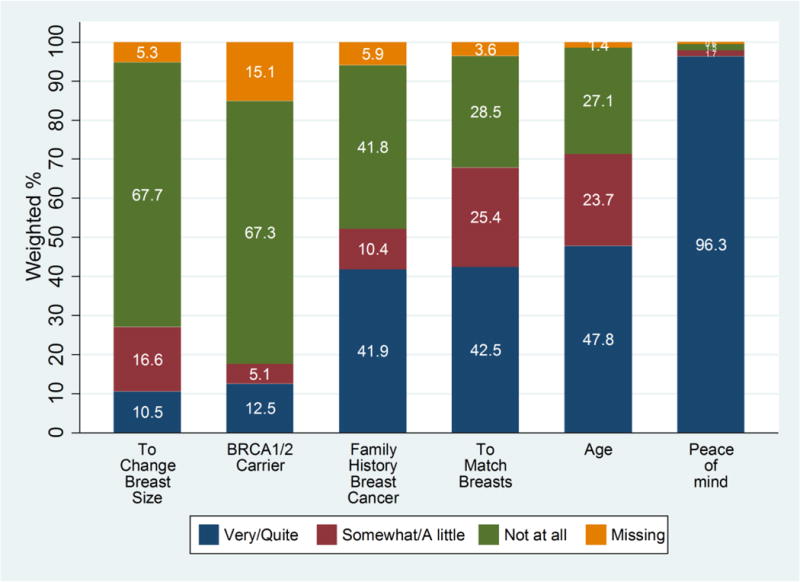
The 428 women in our sample who chose CPM were asked the importance of various factors in their decision to have the procedure. This figure depicts the distribution of their responses for wanting reconstruction to change the size of the breasts, having a positive BRCA 1 or 2 test result, having a family history of breast cancer, wanting reconstruction to best match the breasts, age, and wanting peace of mind.
Table 2 presents a simple logistic regression model evaluating factors associated with receipt of CPM in comparison with all other treatments, as well as a multinomial logistic regression model that allows more nuanced comparison of CPM with UM, CPM with BCS, and UM with BCS. Because the models were generally consistent, we summarize here the results of the simpler model. Older patients were significantly less likely to have CPM (per 5-year increase, OR, 0.71; 95% CI, 0.65–0.77; P < .001). Black patients were significantly less likely to have CPM than white patients (OR, 0.50; 95% CI, 0.34–0.74; P < .001). Patients who had attended at least some college were more likely to receive CPM (OR, 1.69; 95% CI, 1.20–2.40; P = .003) compared with less educated patients. Patients with Medicaid vs private insurance were significantly less likely to receive CPM (OR, 0.47; 95% CI, 0.28–0.79; P = .005). Patients reporting at least 1 first-degree relative with breast cancer were significantly more likely to receive CPM (OR, 1.63; 95% CI, 1.22–2.17; P < .001). Patients with larger breast size were significantly more likely to receive CPM (OR, 1.31; 95% CI, 1.01–1.71; P = .046). Having controlled for age and family history, we found that patients known to be deleterious mutation carriers or at high risk of genetic mutations were only marginally more likely to receive CPM, and this finding did not achieve statistical significance (OR, 1.33; 95% CI, 0.99–1.79; P = .056). Finally, patients in Los Angeles County were significantly less likely to receive CPM (OR, 0.45; 95% CI, 0.32–0.64; P < .001) than patients in Georgia.
Table 2.
Multiple Variable Model Results Regarding Factors Associated with CPM Receipt (N=2,375)†.
| OR [95% CI] (p-value) | ||||
|---|---|---|---|---|
| LOGISTIC REGRESSION MODEL | MULTINOMIAL LOGISTIC REGRESSION MODEL | |||
| Variable | CPM vs all others | CPM vs UM | CPM vs BCS | UM vs BCS |
| Age: +5 year increase | 0.71 [0.65 – 0.77] (<0.001) | 0.71 [0.65 – 0.79] (<0.001) | 0.7 [0.64 – 0.77] (<0.001) | 0.98 [0.92 – 1.05] (0.625) |
| Cancer stage | ||||
| 0 vs 1 | 0.83 [0.59 – 1.17] (0.299) | 0.66 [0.43 – 0.99] (0.047) | 0.89 [0.63 – 1.27] (0.523) | 1.36 [1.00 – 1.84] (0.049) |
| 2 vs 1 | 1.21 [0.92 – 1.61] (0.172) | 0.64 [0.46 – 0.89] (0.008) | 1.55 [1.16 – 2.08] (0.003) | 2.43 [1.89 – 3.14] (<0.001) |
| Race/ethnicity | ||||
| Black vs White | 0.50 [0.34 – 0.74] (<0.001) | 0.43 [0.27 – 0.68] ((<0.001) | 0.53 [0.36 – 0.78] (0.002) | 1.23 [0.88 – 1.72] (0.224) |
| Latina vs White | 0.98 [0.60 – 1.59] (0.932) | 0.81 [0.46 – 1.42] (0.458) | 1.04 [0.63 – 1.72] (0.875) | 1.29 [0.87 – 1.91] (0.208) |
| Asian vs White | 1.08 [0.62 – 1.90] (0.778) | 0.55 [0.30 – 1.04] (0.066) | 1.48 [0.83 – 2.63] (0.183) | 2.67 [1.74 – 4.09] (<0.001) |
| Other vs White | 0.21 [0.03 – 1.24] (0.085) | 0.12 [0.02 – 0.78] (0.027) | 0.26 [0.04 – 1.55] (0.139) | 2.27 [1.06 – 4.89] (0.036) |
| Education: Some College or More vs High School or Less | 1.69 [1.20 – 2.40] (0.003) | 1.89 [1.27 – 2.81] (0.002) | 1.62 [1.13 – 2.32] (0.009) | 0.86 [0.65 – 1.13] (0.281) |
| Insurance | ||||
| Medicaid vs Private | 0.47 [0.28 – 0.79] (0.005) | 0.44 [0.25 – 0.79] (0.006) | 0.49 [0.29 – 0.84] (0.009) | 1.11 [0.76 – 1.63] (0.576) |
| Medicare vs Private | 1.07 [0.73 – 1.57] (0.713) | 1.04 [0.66 – 1.63] (0.865) | 1.1 [0.74 – 1.62] (0.647) | 1.05 [0.76 – 1.46] (0.755) |
| Income, $ | ||||
| 40,000–89,999 vs <40,000 | 1.09 [0.78 – 1.52] (0.629) | 0.98 [0.66 – 1.46] (0.923) | 1.13 [0.80 – 1.61] (0.487) | 1.16 [0.84 – 1.59] (0.367) |
| ≥90,000 vs <40,000 | 1.04 [0.71 – 1.52] (0.839) | 1.12 [0.68 – 1.87] (0.646) | 1.02 [0.68 – 1.53] (0.913) | 0.91 [0.55 – 1.50] (0.691) |
| Marital status: Not Married vs Married or Partnered | 1.16 [0.86 – 1.55] (0.334) | 1.19 [0.84 – 1.68] (0.333) | 1.14 [0.84 – 1.55] (0.385) | 0.96 [0.75 – 1.24] (0.764) |
| Any family history of breast cancer in a first degree relative: Yes vs No | 1.63 [1.22 – 2.17] (0.001) | 2.19 [1.52 – 3.16] (<0.001) | 1.48 [1.10 – 2.00] (0.009) | 0.68 [0.50 – 0.91] (0.011) |
| Breast size: Larger vs Smaller1 | 1.31 [1.01 – 1.71] (0.046) | 1.60 [1.16 – 2.22] (0.005) | 1.23 [0.94 – 1.61] (0.14) | 0.77 [0.60 – 0.98] (0.036) |
| MRI receipt: Yes vs No | 1.22 [0.93 – 1.6] (0.152) | 1.24 [0.90 – 1.71] (0.189) | 1.21 [0.92 – 1.60] (0.179) | 0.98 [0.77 – 1.24] (0.838) |
| Risk Status: High risk or genetic carrier vs All others | 1.33 [0.99 – 1.79] (0.056) | 1.31 [0.91 – 1.88] (0.141) | 1.34 [0.99 – 1.82] (0.058) | 1.02 [0.78 – 1.35] (0.867) |
| Site: LA vs GA | 0.45 [0.32 – 0.64] (<0.001) | 0.54 [0.36 – 0.82] (0.004) | 0.43 [0.30 – 0.60] (<0.001) | 0.78 [0.59 – 1.04] (0.093) |
Multiply imputed data, weighted for survey design and for non-response, excluding cases with no insurance or other public insurance (N=2,375 on average, minimum 2,374, maximum 2,376).
Large breast size defined as D cup or larger.
Figure 3 depicts the distribution of patient-reported surgeon recommendations regarding CPM by treatment receipt. Overall, 859 (37.0%) patients reported that their surgeons recommended against CPM, of whom 19 (2.1%) received it; 1131 (46.3%) reported receiving no surgeon recommendation regarding CPM, of whom 240 (20.9%) received it; and 282 (11.1%) reported a surgeon recommendation for CPM, of whom 162 (58.9%) received it. Among the subset of patients without high genetic risk or a known deleterious mutation, 598 (39.3%) reported a surgeon recommendation against CPM, of whom only 12 (1.9%) received it, but among the 746 of these patients (46.8%) who received no recommendation for CPM, 148 (19.0%) received it.
Figure 3. Receipt of CPM by surgeon recommendation.
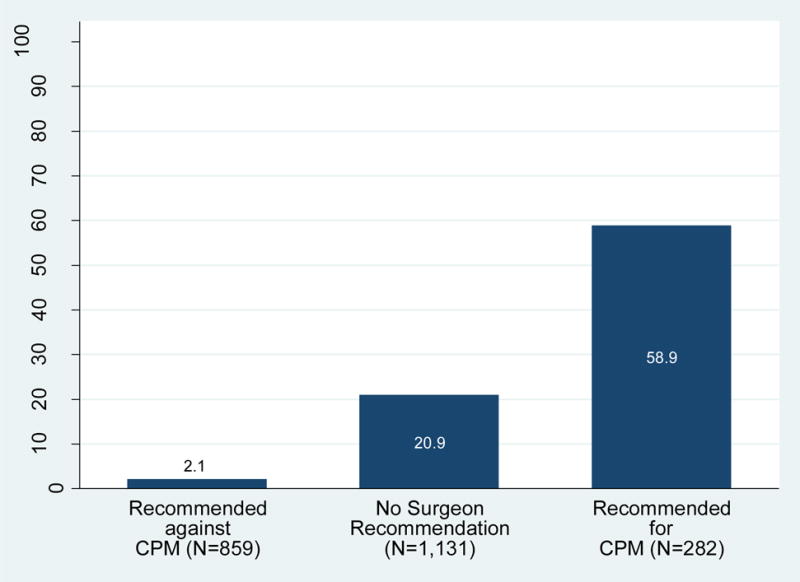
This figure demonstrates the rates of CPM receipt among patients reporting a surgeon recommendation against it, no surgeon recommendation for nor against it, and surgeon recommendation for it.
DISCUSSION
In this large survey of a recent sample of women with newly diagnosed breast cancer identified through population-based registries, nearly half of all patients considered CPM and 1 in 5 received it. Even among patients without known deleterious genetic mutations or elevated risk of a genetic mutation, 40.9% considered CPM and 14.2% received it. This strong patient interest in CPM and the substantial use of this aggressive surgical procedure by patients who are unlikely to develop a second breast cancer is sobering. Patient knowledge about CPM was low, even among those who considered or received it. Surgeon recommendations were strongly associated with treatment receipt, with only 1.9% of average-risk patients who perceived a surgeon recommendation against CPM receiving it, but 19.0% of those who reported receiving no surgeon recommendation doing so.
The rates of consideration and receipt of CPM are substantially higher in the current study than they were in a study conducted in the same geographic regions from 2005–2007. Our observation of higher rates of CPM consideration and receipt is concerning because second primary breast cancer rates in patients without elevated genetic risk have plummeted: this trend has been attributed to increasingly effective systemic therapy that not only reduces recurrence but also the development of subsequent new primaries.25,26 Breast conservation now results in very low in-breast event rates, especially among patients with hormonally-sensitive disease,27 but it is unclear whether patients accurately understand their risks in this context. Smaller studies of selected patients have suggested that knowledge deficits exist.12,15 Our findings in this large, diverse sample confirm that many patients misunderstand crucial information for surgical decision making.
Some patients may pursue CPM for cosmetic symmetry or other reasons. However, it is not clear that average-risk patients who choose CPM truly understand that it will not improve survival or alter recurrence risk. Far higher proportions of patients choosing this procedure prioritize “peace of mind” than other potential reasons for its use, suggesting that they do believe—whether rationally or emotionally—that there is a meaningful impact of more aggressive surgery on the ultimate risk of recurrence or survival. Physicians must recognize that peace of mind motivates many patients who choose CPM, suggesting that it may be particularly important to explain to patients considering CPM how other therapeutic interventions, such as endocrine therapy in appropriate patients (which may be less easy to understand than a simple surgical intervention, or may not be discussed until after surgical decisions are complete), can offer meaningful benefits and increase the peace of mind that these patients ultimately seek, without the risks of more aggressive surgery.
Our observations that CPM receipt continues to be more common among advantaged groups (those who are white, have higher educational levels, and have private rather than Medicaid insurance) are consistent with other studies to date.8,9,17,28 Prior research from our group and others has suggested that when patients participate more in their breast cancer surgical decisions, they more often receive aggressive treatment.16,29 Ironically, a physician’s desire to support patient autonomy may result in excessive surgery if patients are misinformed, as our results suggest is common. Shared decision making requires that physicians participate actively in ensuring that patients’ knowledge is accurate. Otherwise, deference to the patient’s wishes constitutes an abdication of a hallowed professional obligation.
Our results are particularly noteworthy because, to our knowledge, they are the first population-based data that suggest a strong influence of surgeons on CPM receipt: approximately one-third of patients at average risk of contralateral primary cancers reported that their surgeons recommended against CPM, and of these, very few received it. Yet many patients reported that they perceived no recommendation from their surgeons, and these women were much more likely to receive CPM. The results of this study are observational and measured through patient self-report; thus, it is possible that some patients were so clearly committed to CPM that physicians feared to alienate them by offering alternatives, or that they did not recall a surgeon’s recommendation against CPM. Nonetheless, it is compelling that so few patients who perceived a surgeon’s active recommendation against CPM received it. This finding suggests that physicians can influence patients against a surgical option that may be more extensive than is clinically indicated. In the context of studies suggesting that surgeon involvement in decision making is associated with less aggressive treatment,13,29,30 along with studies reporting surgeons’ knowledge deficits about CPM and contralateral breast cancer risk,31 our findings suggest that surgeons’ knowledge and communication practices are targets for quality improvement interventions.
Strengths and Limitations
Aspects of the study merit comment. Strengths include the large, diverse, recent population-based sample of women with newly diagnosed, early-stage breast cancer. We incorporated highly valid measures of treatment, an extensive array of patient attributes, and granular measures of patient experiences. Limitations of the study include potential biases due to survey nonresponse. However, our response rate was high, and we used weighting to ensure that our findings are representative of the targeted population, along with multiple imputation to minimize the impact of missing data due to item nonresponse. Measurement errors may exist in self-reported data; however, we conducted extensive pre-testing and relied on validated measures wherever possible.32 Patients’ recall of communication experiences may not be perfectly accurate, but their perceptions provide a critically important perspective. Finally, our results may not be generalizable to other geographic areas. We found substantial differences in rates of CPM in Los Angeles County vs. Georgia, but we investigated interaction effects and found that the correlates of use, knowledge, and impact of surgeon recommendation appeared generally consistent across the 2 sites. The mechanisms underlying the observed site differences may be particularly illuminating; therefore, further research is warranted to investigate the extent to which these differences are explained by differences in the treating surgeons’ practice settings, attitudes, and resources.
CONCLUSIONS
Rates of CPM are substantial even in a diverse, population-based sample, and patient knowledge in this context is poor. When they do not perceive a surgeon’s recommendation against it, even patients without high genetic risk for a second primary breast cancer choose CPM at an alarmingly high rate (nearly 1 in 5). However, CPM rates are very low among patients who report a surgeon’s recommendation against it. Our findings should motivate surgeons to broach these difficult conversations with their patients, to make their recommendations clear, and to promote patients’ peace of mind by emphasizing how other treatments complement surgery to reduce the risk of both tumor recurrence and subsequent cancer development. These findings should also motivate efforts to inform and support surgeons in this challenging communication context, understand surgeons’ perspectives more fully, and design physician-facing interventions to reduce excessive treatment.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute (NCI) of the National Institutes of Health under award number P01CA163233 to the University of Michigan. Reshma Jagsi and Kent Griffith had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Allison W. Kurian has received research funding for work performed outside of the current study from Myriad Genetics, Invitae, Ambry Genetics, GeneDx, and Genomic Health.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the NCI’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California (USC), and contract HHSN261201000034C awarded to the Public Health Institute. The collection of cancer incidence data in Georgia was supported by contract HHSN261201300015I, Task Order HHSN26100006 from the NCI and cooperative agreement 5NU58DP003875-04-00 from the CDC. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the NCI, and the CDC or their Contractors and Subcontractors is not intended nor should be inferred.
We acknowledge the outstanding work of our project staff (Mackenzie Crawford and Kiyana Perrino from the Georgia Cancer Registry; Jennifer Zelaya, Pamela Lee, Maria Gaeta, Virginia Parker, and Renee Bickerstaff-Magee from USC; Rebecca Morrison, Rachel Tocco, Alexandra Jeanpierre, Stefanie Goodell, and Rose Juhasz from the University of Michigan). We acknowledge with gratitude our survey respondents.
Footnotes
Conflict of Interest: Allison W. Kurian has received research funding for work performed outside of the current study from Myriad Genetics, Invitae, Ambry Genetics, GeneDx, and Genomic Health. The remaining authors have no conflict of interest to report.
References
- 1.Portschy PR, Kuntz KM, Tuttle TM. Survival outcomes after contralateral prophylactic mastectomy: a decision analysis. Journal of the National Cancer Institute. 2014;106(8) doi: 10.1093/jnci/dju160. [DOI] [PubMed] [Google Scholar]
- 2.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(16):2158–2164. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 3.Borzekowski DL, Guan Y, Smith KC, Erby LH, Roter DL. The Angelina effect: immediate reach, grasp, and impact of going public. Genetics in medicine : official journal of the American College of Medical Genetics. 2014;16(7):516–521. doi: 10.1038/gim.2013.181. [DOI] [PubMed] [Google Scholar]
- 4.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Annals of surgical oncology. 2009;16(10):2697–2704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 5.Jones NB, Wilson J, Kotur L, Stephens J, Farrar WB, Agnese DM. Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Annals of surgical oncology. 2009;16(10):2691–2696. doi: 10.1245/s10434-009-0547-9. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 7.Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(9):919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurian AW, Lichtensztajn DY, Keegan TH, Nelson DO, Clarke CA, Gomez SL. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. Jama. 2014;312(9):902–914. doi: 10.1001/jama.2014.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi M, Hunt KK, Arun BK, et al. Factors impacting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer prevention research (Philadelphia, Pa) 2010;3(8):1026–1034. doi: 10.1158/1940-6207.CAPR-09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker PA, Peterson SK, Bedrosian I, et al. Prospective Study of Surgical Decision-making Processes for Contralateral Prophylactic Mastectomy in Women With Breast Cancer. Annals of surgery. 2016;263(1):178–183. doi: 10.1097/SLA.0000000000001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beesley H, Holcombe C, Brown SL, Salmon P. Risk, worry and cosmesis in decision-making for contralateral risk-reducing mastectomy: analysis of 60 consecutive cases in a specialist breast unit. Breast (Edinburgh, Scotland) 2013;22(2):179–184. doi: 10.1016/j.breast.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Fisher CS, Martin-Dunlap T, Ruppel MB, Gao F, Atkins J, Margenthaler JA. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol. 2012;19(10):3246–3250. doi: 10.1245/s10434-012-2525-x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SM, Sepucha K, Ruddy KJ, et al. Local Therapy Decision-Making and Contralateral Prophylactic Mastectomy in Young Women with Early-Stage Breast Cancer. Annals of surgical oncology. 2015;22(12):3809–3815. doi: 10.1245/s10434-015-4572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King L, O’Neill SC, Spellman E, et al. Intentions for bilateral mastectomy among newly diagnosed breast cancer patients. J Surg Oncol. 2012 doi: 10.1002/jso.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med. 2013;159(6):373–381. doi: 10.7326/0003-4819-159-6-201309170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nekhlyudov L, Bower M, Herrinton LJ, et al. Women’s decision-making roles regarding contralateral prophylactic mastectomy. Journal of the National Cancer Institute Monographs. 2005;35:55–60. doi: 10.1093/jncimonographs/lgi038. [DOI] [PubMed] [Google Scholar]
- 17.Hawley SJR, Morrow M, Janz NK, Hamilton A, Graff JJ, Katz SJ. Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surgery. 2014 doi: 10.1001/jamasurg.2013.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimmer L, Liederbach E, Velasco J, Pesce C, Wang CH, Yao K. Variation in Contralateral Prophylactic Mastectomy Rates According to Racial Groups in Young Women with Breast Cancer, 1998 to 2011: A Report from the National Cancer Data Base. Journal of the American College of Surgeons. 2015;221(1):187–196. doi: 10.1016/j.jamcollsurg.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano AE, Boolbol S, Degnim A, Kuerer H, Leitch AM, Morrow M. Society of Surgical Oncology: position statement on prophylactic mastectomy. Approved by the Society of Surgical Oncology Executive Council, March 2007. Annals of surgical oncology. 2007;14(9):2425–2427. doi: 10.1245/s10434-007-9447-z. [DOI] [PubMed] [Google Scholar]
- 20.Dillman D, Smyth J, Christian L. Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method. 3rd. Hoboken, NY: John Wiley & Sons; 2009. [Google Scholar]
- 21.Hamilton AS, Hofer TP, Hawley ST, et al. Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(7):2022–2029. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daly MB, Pilarski R, Axilbund JE, et al. Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2015. Journal of the National Comprehensive Cancer Network : JNCCN. 2016;14(2):153–162. doi: 10.6004/jnccn.2016.0018. [DOI] [PubMed] [Google Scholar]
- 23.Raghunathan T, Lepkowski J, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 24.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 1987. [Google Scholar]
- 25.Nichols HB, Berrington de Gonzalez A, Lacey JV, Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(12):1564–1569. doi: 10.1200/JCO.2010.32.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet (London, England) 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26(14):2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 28.Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Annals of surgical oncology. 2010;17(10):2554–2562. doi: 10.1245/s10434-010-1091-3. [DOI] [PubMed] [Google Scholar]
- 29.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. Journal of the National Cancer Institute. 2009;101(19):1337–1347. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(24):5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 31.Yao K, Belkora J, Sisco M, et al. Survey of the Deficits in Surgeons’ Knowledge of Contralateral Prophylactic Mastectomy. JAMA Surg. 2015:1–3. doi: 10.1001/jamasurg.2015.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CanSORT. Measures Appendix. University of Michigan; 2015. pp. 1–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


