Abstract
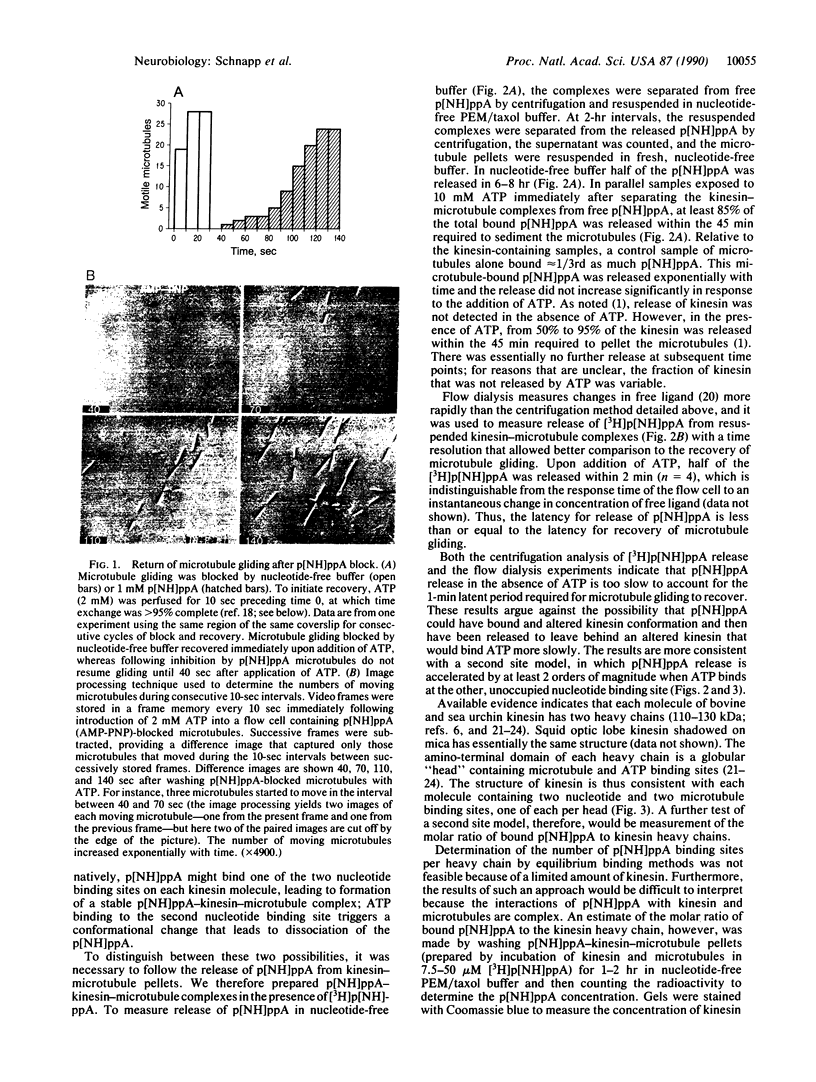
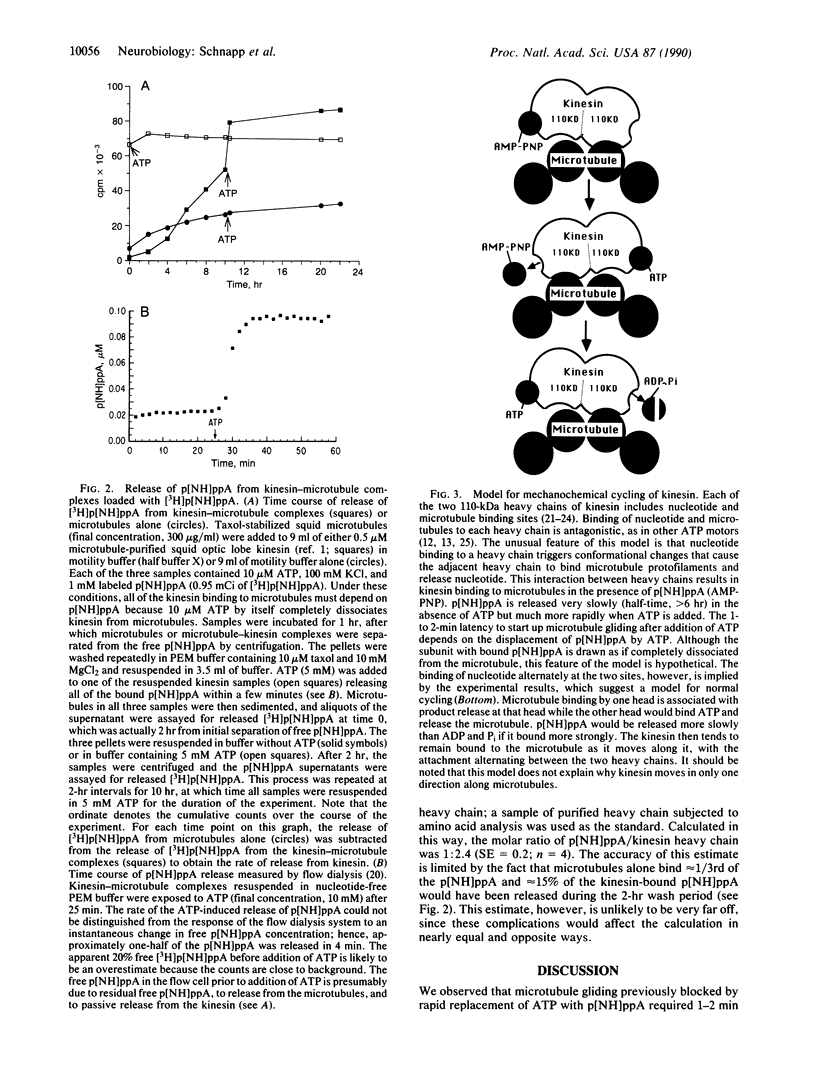
Kinesin is a microtubule-activated ATPase that moves objects toward the plus end of microtubules and makes microtubules glide along a glass surface. Here we investigate a remarkable effect of the nonhydrolyzable analogue of ATP, adenosine 5'-[beta,gamma-imido]triphosphate (p[NH]ppA), on kinesin-driven microtubule gliding. Microtubule gliding that has been blocked by rapid replacement of ATP with p[NH]ppA requires 1-2 min of exposure to ATP before microtubule gliding resumes. This latency is not shortened by prolonged washing of p[NH]ppA-blocked microtubules in nucleotide-free buffer for up to 15 min, suggesting that ATP binding to a second nucleotide binding site on kinesin triggers the release of bound p[NH]ppA. To test this hypothesis, the release of [3H]p[NH]ppA from kinesin-microtubule complexes was followed in parallel biochemical assays. In nucleotide-free buffer, the bound p[NH]ppA was released over several hours from the complexes. However, addition of ATP caused the release of p[NH]ppA from the kinesin-microtubule complexes within 2 min, which was similar to the latent period for start-up of microtubule gliding after p[NH]ppA inhibition. The stoichiometry of p[NH]ppA bound per kinesin heavy chain at saturation was estimated to be approximately 1:2. These results suggest a model in which each molecule of kinesin has at least two nucleotide binding sites that alternately bind nucleotide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C., Block S. M. A miniature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984 Nov;130(11):2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- Bloom G. S., Wagner M. C., Pfister K. K., Brady S. T. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988 May 3;27(9):3409–3416. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- Brady S. T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985 Sep 5;317(6032):73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Cohn S. A., Ingold A. L., Scholey J. M. Correlation between the ATPase and microtubule translocating activities of sea urchin egg kinesin. Nature. 1987 Jul 9;328(6126):160–163. doi: 10.1038/328160a0. [DOI] [PubMed] [Google Scholar]
- Cohn S. A., Ingold A. L., Scholey J. M. Quantitative analysis of sea urchin egg kinesin-driven microtubule motility. J Biol Chem. 1989 Mar 15;264(8):4290–4297. [PubMed] [Google Scholar]
- Colowick S. P., Womack F. C. Binding of diffusible molecules by macromolecules: rapid measurement by rate of dialysis. J Biol Chem. 1969 Feb 25;244(4):774–777. [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of the actin.subfragment 1 complex by adenyl-5'-yl imidodiphosphate, ADP, and PPi. J Biol Chem. 1980 Jan 25;255(2):543–548. [PubMed] [Google Scholar]
- Hackney D. D. Kinesin ATPase: rate-limiting ADP release. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6314–6318. doi: 10.1073/pnas.85.17.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Pfister K. K., Yorifuji H., Wagner M. C., Brady S. T., Bloom G. S. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989 Mar 10;56(5):867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J., Vale R. D. Movement of microtubules by single kinesin molecules. Nature. 1989 Nov 9;342(6246):154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- Ingold A. L., Cohn S. A., Scholey J. M. Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J Cell Biol. 1988 Dec;107(6 Pt 2):2657–2667. doi: 10.1083/jcb.107.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayalar C., Rosing J., Boyer P. D. An alternating site sequence for oxidative phosphorylation suggested by measurement of substrate binding patterns and exchange reaction inhibitions. J Biol Chem. 1977 Apr 25;252(8):2486–2491. [PubMed] [Google Scholar]
- Kuznetsov S. A., Gelfand V. I. Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Vaisberg E. A., Shanina N. A., Magretova N. N., Chernyak V. Y., Gelfand V. I. The quaternary structure of bovine brain kinesin. EMBO J. 1988 Feb;7(2):353–356. doi: 10.1002/j.1460-2075.1988.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Vaisberg Y. A., Rothwell S. W., Murphy D. B., Gelfand V. I. Isolation of a 45-kDa fragment from the kinesin heavy chain with enhanced ATPase and microtubule-binding activities. J Biol Chem. 1989 Jan 5;264(1):589–595. [PubMed] [Google Scholar]
- Lasek R. J., Brady S. T. Attachment of transported vesicles to microtubules in axoplasm is facilitated by AMP-PNP. Nature. 1985 Aug 15;316(6029):645–647. doi: 10.1038/316645a0. [DOI] [PubMed] [Google Scholar]
- Porter M. E., Scholey J. M., Stemple D. L., Vigers G. P., Vale R. D., Sheetz M. P., McIntosh J. R. Characterization of the microtubule movement produced by sea urchin egg kinesin. J Biol Chem. 1987 Feb 25;262(6):2794–2802. [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey J. M., Heuser J., Yang J. T., Goldstein L. S. Identification of globular mechanochemical heads of kinesin. Nature. 1989 Mar 23;338(6213):355–357. doi: 10.1038/338355a0. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Porter M. E., Grissom P. M., McIntosh J. R. Identification of kinesin in sea urchin eggs, and evidence for its localization in the mitotic spindle. Nature. 1985 Dec 5;318(6045):483–486. doi: 10.1038/318483a0. [DOI] [PubMed] [Google Scholar]
- Schroer T. A., Schnapp B. J., Reese T. S., Sheetz M. P. The role of kinesin and other soluble factors in organelle movement along microtubules. J Cell Biol. 1988 Nov;107(5):1785–1792. doi: 10.1083/jcb.107.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. T., Laymon R. A., Goldstein L. S. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989 Mar 10;56(5):879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]




