Abstract
Introduction
Impaired coordination between breathing and swallowing (breathing–swallowing discoordination) may be a significant risk factor for the exacerbation of chronic obstructive pulmonary disease (COPD). We examined breathing–swallowing discoordination in patients with COPD using a non-invasive and quantitative technique and determined its association with COPD exacerbation.
Methods
We recruited 65 stable outpatients with COPD who were enrolled in our prospective observational cohort study and did not manifest an apparent swallowing disorder. COPD exacerbation was monitored for 1 year before and 1 year after recruitment. Swallowing during inspiration (the I-SW pattern) and swallowing immediately followed by inspiration (the SW-I pattern) were identified.
Results
The mean frequency of the I-SW and/or SW-I patterns (I-SW/SW-I rate) was 21.5%±25.5%. During the 2-year observation period, 48 exacerbation incidents (25 patients) were identified. The I-SW/SW-I rate was significantly associated with the frequency of exacerbation. During the year following recruitment, patients with a higher I-SW/SW-I frequency using thicker test foods exhibited a significantly higher probability of future exacerbations (p=0.002, log-rank test).
Conclusions
Breathing–swallowing discoordination is strongly associated with frequent exacerbations of COPD. Strategies that identify and improve breathing–swallowing coordination may be a new therapeutic treatment for patients with COPD.
Keywords: COPD Exacerbations, Respiratory Measurement, Lung Physiology
Introduction
Chronic obstructive pulmonary disease (COPD) is a common life-threatening lung disease worldwide. In the management of patients with COPD, prevention of COPD exacerbation is one of most important goals because exacerbation has serious impacts on the morbidity, mortality and healthcare costs associated with COPD.1 2 Therefore, identifying and reducing the risk factors associated with COPD exacerbation are major therapeutic targets in clinical settings.
In our previous study, gastro-oesophageal reflux (GER) symptoms were found to be associated with frequent COPD exacerbation,3 and various replication studies have been conducted.4–6 However, treating GER symptoms simply with a proton pump inhibitor does not reduce COPD exacerbation.7 This result suggests that something else is associated with GER that may be important to prevent COPD exacerbation rather than the GER symptom itself. Because most patients with COPD are old, they may exhibit various functional abnormalities, including swallowing abnormalities.3 8–10 In another previous report by our group,11 we elucidated the association between GER and impaired swallowing abnormalities in patients with COPD; the results suggested that swallowing abnormalities and subsequent aspiration may exacerbate COPD.
To assess these swallowing abnormalities, we recently developed a non-invasive and quantitative technique for measuring breathing–swallowing coordination.12 Using this technique, we can monitor breathing and swallowing as a time series and identify the respiratory phase sequence, overlap and delay of inspiration/expiration and swallowing. In particular, according to a recent study by Gross et al,13 swallowing during inspiration (I-SW pattern) and swallowing immediately followed by inspiration (SW-I pattern) may be notable signs of breathing–swallowing discoordination. However, there is not sufficient information about such discoordination in patients with COPD, and it remains unclear whether breathing–swallowing discoordination is a meaningful feature and useful index in patients with COPD.14 15
We hypothesised that breathing–swallowing coordination may be impaired in patients with COPD and that breathing–swallowing ‘discoordination’ may be a significant risk factor for frequent and/or further exacerbation.
Methods
Subjects
The present study was part of our prospective observational cohort study at Kyoto University. From patients who were enrolled in this cohort study, 65 stable outpatients with COPD who met the criteria and gave written informed consent were recruited into the present study (all male, 71.9±8.4 years old). Recruitment for the present study and swallowing monitoring measurements were conducted between May 2015 and October 2016. The present study was approved by the local ethical committee of Kyoto University (C841). The detailed inclusion and exclusion criteria for the present study are listed in online supplement e-table 1. Briefly, patients were aged 40 years or older, diagnosed with COPD by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria16 and in stable condition at the recruitment visit (day 0). In addition, patients with an apparent swallowing disorder, clinically evident cerebrovascular diseases, dementia or a cardiac pacemaker were excluded (online supplement e-table 1).
bmjresp-2017-000202supp001.pdf (144.4KB, pdf)
At the recruitment visit, patients conducted a swallowing function analysis (monitoring of breathing–swallowing coordination), pulmonary function test and questionnaires, which included respiratory symptoms, GER symptoms and their dietary situation.
To evaluate respiratory symptoms, the COPD Assessment Test (CAT)17 and modified Medical Research Council Dyspnoea Scale were used. GER symptoms were evaluated with a self-reported frequency scale for symptoms of GERD (FSSG) questionnaire.18 The cut-off score for GERD was set at 8 points. Subject’s dietary situations were assessed with the Food Intake Level Scale (FILS)19 and the Functional Oral Intake Scale (FOIS).20
Stable COPD was defined as a period of at least 4 weeks without any worsening respiratory symptoms assessed by a symptom diary, as in our previous studies.3 11
Exacerbation criteria
We evaluated the number of COPD exacerbation incidents from 1 year before and 1 year after the recruitment visit (day 0). The number of exacerbation-free days after day 0 was also investigated. All patients were asked to use a symptom diary that was described in our previous reports in detail.3 21 The diary includes records of ‘major’ symptoms and ‘minor’ symptoms, as well as the usage of additional treatments for COPD. COPD exacerbation was defined as the occurrence of two or more major symptoms or any one major symptom with any one minor symptom for at least 2 consecutive days, concomitant with a change in daily pharmacotherapy (see the online supplement).
Swallowing monitoring system and analysis methods
We used a non-invasive swallowing monitoring system using respiratory flow, swallowing sound and laryngeal movement.12 After setting up the swallowing monitoring system, patients assumed an upright sitting position with an intermediate head position22 and swallowed four types of test foods twice each (a total of eight times). Patients were instructed not to chew the test foods and to swallow voluntarily.
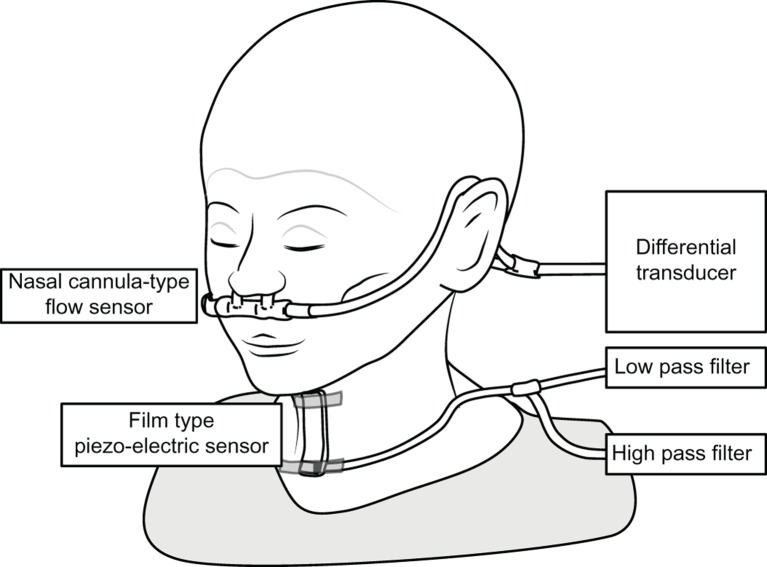
System configurations are presented in figure 1. The monitoring system consists of a nasal cannula-type flow sensor, a film-type piezoelectric sensor and signal processing units. Figure 2 shows an example of a breathing–swallowing coordination pattern analysed by this system. Using a series of the respiratory flow, the laryngeal sound and motion data, we evaluated (1) the duration of deglutition apnoea and swallowing latency and (2) breathing–swallowing coordination. Detailed information about the analysis is available in the online supplement. Figure 2A shows a usual pattern, such as the expiration–swallow–expiration pattern. Figure 2B,C shows two unusual patterns (I-SW and SW-I, respectively). These two unusual patterns may even occur in healthy subjects, although less frequently. The occurrence of these two unusual patterns (I-SW and/or SW-I) may indicate breathing–swallowing discoordination.13
Figure 1.
A schematic diagram of the swallowing monitor.
Figure 2.
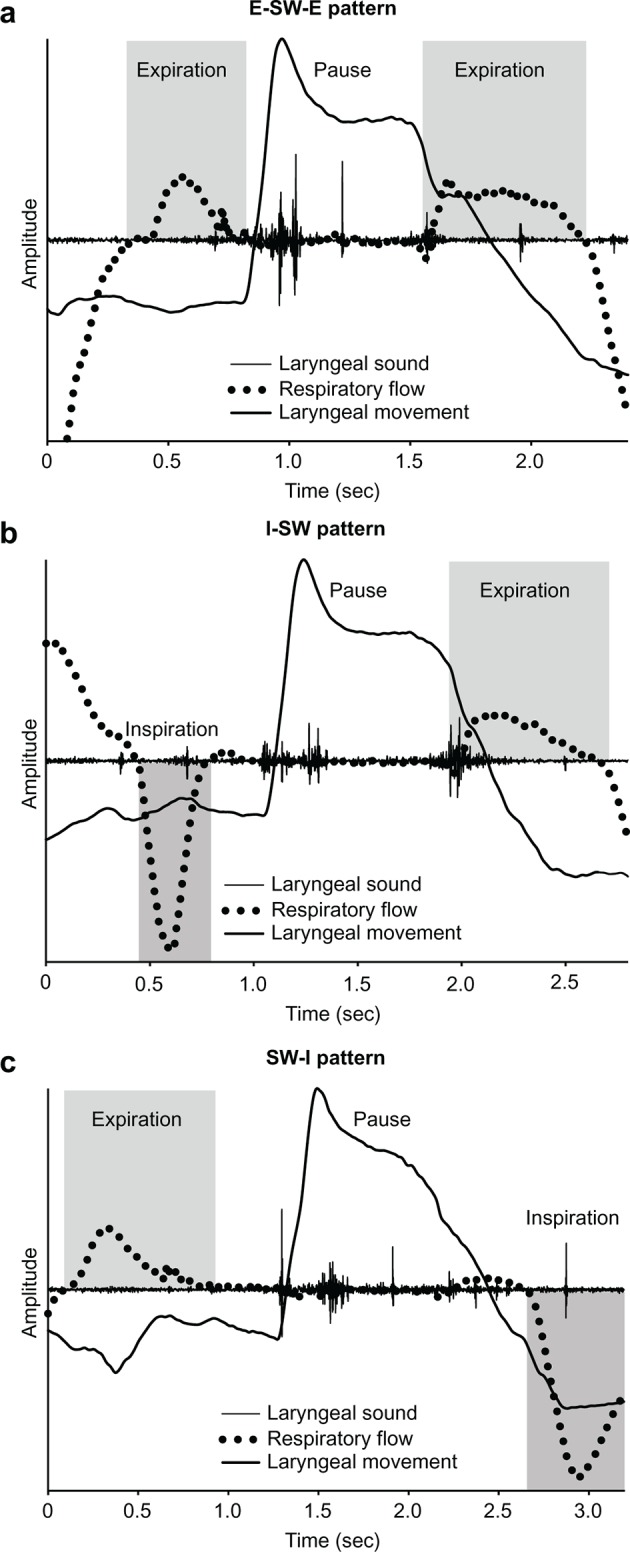
Coordination between breathing and swallowing. Swallowing occurs during expiration, and respiration resumes with expiration (A: E–SW–E pattern). Swallowing occurs during inspiration (B: I-SW pattern), and respiration resumes with inspiration (C: SW-I pattern). The I-SW and SW-I patterns are unusual patterns, but may even occur in healthy subjects. E–SW–E, expiration–swallow–expiration; I-SW, swallowing during inspiration; SW-I, swallowing immediately followed by inspiration.
Test foods
We adopted the Japanese ‘Level’ system to describe the specifications of the test foods, which was standardised by the Japanese Society of Dysphagia Rehabilitation.23 The test foods we used included level 0 (soft jelly), level 2 (hard jelly), level 3 (purée) and water. The properties (hardness, cohesion and adhesion) of these test foods were strictly controlled.12 One piece of test food weighed approximately 3 g, and 3 mL of water was injected using a syringe.
Statistical analyses
The results associated with patient characteristics are presented as frequencies for categorical variables and means±SDs and ranges for continuous variables unless otherwise specified. Comparisons of the variables between patients with and without exacerbation during the 2-year observation trial were performed using the χ2 test or Fisher’s exact test for categorical variables and t-tests for continuous variables. Univariate analyses between the frequency of exacerbation and the breathing–swallowing coordination index, pulmonary function or other parameters were performed, and multivariate regression analyses using three different models were subsequently performed.
To evaluate the predictive ability of the breathing–swallowing coordination index, we also used the Kaplan-Meier method, the log-rank test and the Cox proportional hazards model, using time-to-event data of exacerbation incidents during half of the observation period (1 year after the recruitment).
All statistical analyses were performed using JMP11 (SAS Institute Inc., Cary, NC, USA). In all instances, a two-sided p value <0.05 was considered to indicate statistical significance.
Results
Patients’ characteristics
The anthropometric, symptomatic and pathophysiological patient characteristics are shown in table 1. One-third of patients (n=21) were classified as having severe or very severe airflow limitation (GOLD III or IV), and all subjects were at FILS level 10 and FOIS level 7, confirming that the subjects did not have an apparent swallowing disorder at recruitment. All patients completed the entire 2 -year observation period for COPD exacerbation. In total, 25 out of 65 patients experienced COPD exacerbation (48 incidents). The frequency of exacerbation was 0.37 incidents per patient per year in the present study. Patients who experienced exacerbation showed significantly more severe pulmonary dysfunction (forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC), diffusing capacity of carbon monoxide (DLco)), severe respiratory symptoms (CAT) and a higher prevalence of GER symptoms (FSSG ≥8) than patients who did not experience exacerbation (table 2).
Table 1.
Patients’ characteristics
| Variable | Mean±SD (range) |
| Total number of patients | 65 |
| Age, years | 71.9±8.4 (46–89) |
| Sex, male/female | 65/0 |
| Height, cm | 164.6±6.3 (145.3–178) |
| Weight, kg | 60.5±9.4 (41.5–88) |
| BMI, kg/m2 | 22.3±3.0 (16–31.6) |
| Smoking status, former/current | 60/5 |
| Smoking history, pack-years | 63.2±32.4 (18–184) |
| FSSG score | 6.1±6.04 (0–36) |
| GER symptoms (FSSG ≥8) | 20/45 |
| CAT | 11.28±6.97 (1–28) |
| mMRC Dyspnoea Scale, 0/1/2/3/4 | 21/28/13/2 |
| GOLD stage, I/II/III/IV | 10/34/14/7 |
| FEV1, L | 1.63±0.6 (0.62–2.91) |
| FEV1, % predicted | 58.87±19.74 (17.7–96.9) |
| FVC, L | 3.2±0.8 (1.76–4.99) |
| FEV1/FVC, % | 49.94±13.5 (0.23–100) |
| VC, L | 3.5±0.7 (2.01–4.95) |
| VC, % predicted | 99.61±16 (63–131.7) |
| IC/TLC, % | 37.84±6.63 (24.24–52.91) |
| RV/TLC, % | 40.9±7.5 (27–67) |
| DLco, mL/min/mm Hg | 13.32±5.41 (3.6–28.27) |
The continuous variables are presented as the mean±SD (range).
BMI, body mass index; CAT, Chronic Obstructive Pulmonary Disease Assessment Test; DLco, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 s; FSSG, frequency scale for symptoms of gastro-oesophageal reflux disease; FVC, forced vital capacity; GER, gastro-oesophageal reflux; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IC, inspiratory capacity; mMRC, modified Medical Research Council; RV, residual volume; TLC, total lung capacity; VC, vital capacity.
Table 2.
Patient characteristics stratified by patients who did and did not experience exacerbation during the 2-year observation period
| Exacerbation during the 2-year observation period | |||
| Variable | Patients with exacerbation | Patients without exacerbation | p Value |
| Total number of patients | 25 | 40 | |
| Age, years | 73.2±9.1 (46–88) | 71.1±7.92 (46–89) | 0.321 |
| Height, cm | 163.1±7.3 (145.3–175.9) | 165.6±5.5 (149.8–178) | 0.118 |
| Weight, kg | 59.0±11.0 (41.5–88) | 61.4±8.2 (41.9–85) | 0.332 |
| BMI, kg/m2 | 22.1±3.6 (16–31.6) | 22.4±2.5 (16.3–27.1) | 0.782 |
| Smoking status, former/current | 25/0 | 35/5 | 0.147 |
| Smoking history, pack-years | 57.2±29.8 (24.8–153) | 67.0±33.7 (18–184) | 0.239 |
| FSSG score | 7.3±5.8 (0–19) | 5.3±6.1 (0–36) | 0.186 |
| GER symptoms (FSSG ≥8) | 12/25 | 8/40 | 0.017 |
| CAT | 13.4±7.2 (1–28) | 9.9±6.6 (1–27) | 0.047 |
| mMRC Dyspnoea Scale, 0/1/2/3/4 | 4/15/5/1/0 | 17/13/8/1/0 | 0.106 |
| GOLD stage, I/II/III/IV | 2/14/5/4 | 7/21/10/2 | 0.358 |
| FEV1, L | 1.41±0.61 (0.62–2.82) | 1.75±0.61 (0.66–2.91) | 0.028 |
| FEV1, %predicted | 53.7±19.9 (17.7–96.9) | 62.1±19.2 (22.7–92.9) | 0.095 |
| FVC, L | 3.03±0.7 (1.8–4.3)±0.7(1.8–4.3) | 3.3±0.8(1.8–5.0) | 0.147 |
| FEV1/FVC, % | 45.5±13.0 (23.3–65.9) | 52.6±13.2 (0.3–100) | 0.037 |
| VC, L | 3.2±0.7 (2.0–4.5) | 3.7±0.7 (2.5–5.0) | 0.011 |
| VC, % predicted | 95.2±17.5 (63–124.1) | 102.4±14.53 (73.3–131.7) | 0.083 |
| IC/TLC, % | 35.8±7.1 (24.2–47.1) | 39.1±6.1 (26.1–52.9) | 0.051 |
| RV/TLC, % | 42.0±7.7 (27.1–54.8) | 40.2±7.43 (28.9–67.2) | 0.373 |
| DLco, mL/min/mm Hg | 11.5±4.4 (6.7–21.8) | 14.4±5.7 (3.6–28.3) | 0.032 |
| I-SW/SW-I rate | 32.5±31.1 (0–87.5) | 14.6±18.7 (0–75) | 0.005 |
| Swallowing latency, s | 519.6±584.2 (−99.9–2031.5) | 276.8±362.8 (−141.9–1641.5) | 0.042 |
| Apnoea duration, ms | 1.6±0.8 (0.5–3.1) | 1.3±0.6 (0.4–2.6) | 0.074 |
The continuous variables are presented as the mean±SD (range).
BMI, body mass index; CAT, Chronic Obstructive Pulmonary Disease Assessment Test; DLco, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 s; FSSG, frequency scale for symptoms of gastro-oesophageal reflux disease; GER, gastro-oesophageal reflux; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IC, inspiratory capacity; I-SW, swallowing during inspiration; mMRC, modified Medical Research Council; RV, residual volume; SW-I, swallowing immediately followed by inspiration; TLC, total lung capacity; VC, vital capacity.
From the breathing–swallowing monitoring, the frequencies of the I-SW and/or SW-I patterns in patients with COPD were 21.5%±25.5%. Patients with COPD in the present study seemed to have relatively higher frequencies of I-SW/SW-I than healthy subjects in our previous report (11.6%±18.0%).12 This breathing–swallowing discoordination did not show significant associations with pulmonary dysfunction or respiratory symptoms (online supplement e-table 2); however, the frequency of I-SW/SW-I was significantly higher in patients who experienced exacerbation (p<0.001, table 2).
bmjresp-2017-000202supp002.pdf (263.2KB, pdf)
Univariate and multivariate analyses of COPD exacerbation
Table 3 shows the results of univariate analyses in which the dependent variable is the frequency of exacerbation. The frequencies of I-SW/SW-I, %FEV1, FEV1/FVC, %vital capacity (%VC) and DLco were significantly associated with the frequency of exacerbation. In addition, patients with GER symptoms (FSSG ≥8) showed a high frequency of exacerbation (0.6 vs 0.25/year, p<0.05).
Table 3.
Univariate analysis in terms of the frequency of exacerbation
| Factor | r | 95% CI | p Value |
| Age | 0.21 | −0.037 to 0.43 | 0.095 |
| BMI | −0.010 | −0.33 to 0.15 | 0.435 |
| Smoking history pack-year | −0.06 | −0.30 to 0.19 | 0.648 |
| I-SW/SW-I rate | 0.37 | 0.14 to 0.56 | 0.002 |
| Deglutition apnoea duration | 0.27 | 0.029 to 0.48 | 0.028 |
| Swallowing latency | 0.29 | 0.046 to 0.50 | 0.020 |
| FSSG score | 0.12 | −0.13 to 0.35 | 0.341 |
| CAT | 0.28 | 0.04 to 0.49 | 0.025 |
| mMRC Dyspnoea Scale score | 0.19 | −0.06 to 0.42 | 0.131 |
| FEV1 | −0.30 | −0.51 to −0.06 | 0.013 |
| %FEV1 | −0.24 | −0.45 to 0.008 | 0.057 |
| FVC | −0.21 | −0.43 to 0.035 | 0.093 |
| FEV1/FVC | −0.27 | −0.48 to −0.03 | 0.028 |
| VC | −0.32 | −0.53 to −0.09 | 0.007 |
| %VC | −0.25 | −0.47 to −0.001 | 0.049 |
| IC/TLC | −0.35 | −0.54 to −0.11 | 0.004 |
| RV/TLC | 0.23 | −0.01 to 0.45 | 0.064 |
| DLco | −0.36 | −0.55 to −0.12 | 0.003 |
BMI, body mass index; CAT, Chronic Obstructive Pulmonary Disease Assessment Test; DLco, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 s; FSSG, frequency scale for symptoms of gastro-oesophageal reflux disease; FVC, forced vital capacity; IC, inspiratory capacity; I-SW, swallowing during inspiration; mMRC, modified Medical Research Council; r, Pearson’s correlation coefficient; RV, residual volume; SW-I, swallowing immediately followed by inspiration; TLC, total lung capacity; VC, vital capacity.
We subsequently performed multivariate analyses using three different sets of independent variables (table 4). We selected the I-SW/SW-I rate, GER symptoms, %FEV1 and inspiratory capacity (IC)/total lung capacity (TLC) as candidates for independent variables, taking their significance in the univariate analysis as well as previous reports into account. Multivariate analyses with the set of I-SW/SW-I rate, %FEV1 and IC/TLC (model 1), with the set of variables replacing I-SW/SW-I rate with GER symptoms in model 1 (model 2) and with all candidates (model 3) indicated that both the I-SW/SW-I rate and IC/TLC were good independent predictors of COPD exacerbation (p<0.05).
Table 4.
Multivariate regression analysis in terms of the frequency of exacerbation
| Model 1 | Model 2 | Model 3 | |||||||
| β | 95% CI | p Value | β | 95% CI | p Value | β | 95% CI | p Value | |
| I-SW/SW-I rate | 0.37 | 0.69 to 2.62 | <0.001 | — | — | — | 0.33 | 0.48 to 2.49 | 0.004 |
| GER symptom (FSSG ≥8) | — | — | — | 0.23 | 0.0001 to 1.12 | 0.049 | 0.13 | −0.22 to 0.88 | 0.243 |
| %FEV1 | −0.07 | −1.86 to 0.98 | 0.535 | −0.06 | −1.88 to 1.12 | 0.613 | −0.06 | −1.79 to 1.04 | 0.597 |
| IC/TLC | −0.31 | −1.17 to −0.31 | 0.013 | −0.28 | −9.37 to −0.41 | 0.032 | −0.30 | −9.42 to −0.30 | 0.016 |
| Cumulative R2 | 0.27 | 0.18 | 0.29 | ||||||
FEV1, forced expiratory volume in 1 s; FSSG, frequency scale for symptoms of gastro-oesophageal reflux disease; GER, gastro-oesophageal reflux; IC, inspiratory capacity; I-SW, swallowing during inspiration; SW-I, swallowing immediately followed by inspiration; TLC, total lung capacity.
In addition, we evaluated whether the occurrence of the I-SW/SW-I patterns differed depending on the type of test food. As shown in table 5, the number of patients with I-SW/SW-I patterns was the lowest for level 0 jelly, that is, the safest test food, and largest for level 3 purée, the food most difficult to swallow among the test foods. Interestingly, the associations between the frequency of I-SW/SW-I and the frequency of exacerbation were strongest for level 0 jelly (r=0.44, p<0.001).
Table 5.
Association between the occurrence of the I-SW/SW-I patterns and the frequency of exacerbation in each test food
| Occurrence of I-SW/SW-I pattern* | Frequency of I-SW/SW-I pattern | r† |
95% CI | p Value | |
| Level 0 | 15/65 | 14.8±29.7 | 0.44 | 0.22 to 0.62 | <0.001 |
| Level 2 | 19/65 | 20.2±34.2 | 0.29 | 0.05 to 0.50 | 0.018 |
| Level 3 | 24/65 | 28.1±41.2 | 0.32 | 0.08 to 0.53 | 0.009 |
| Water | 23/65 | 23.2±34.4 | 0.08 | −0.17 to 0.32 | 0.521 |
Level 0, smooth jelly food without protein; level 2, rough jelly surface; level 3, purée.
*Number of patients showing I-SW and/or SW-I pattern.
†r, Pearson’s correlation coefficient.
Kaplan-Meier curve
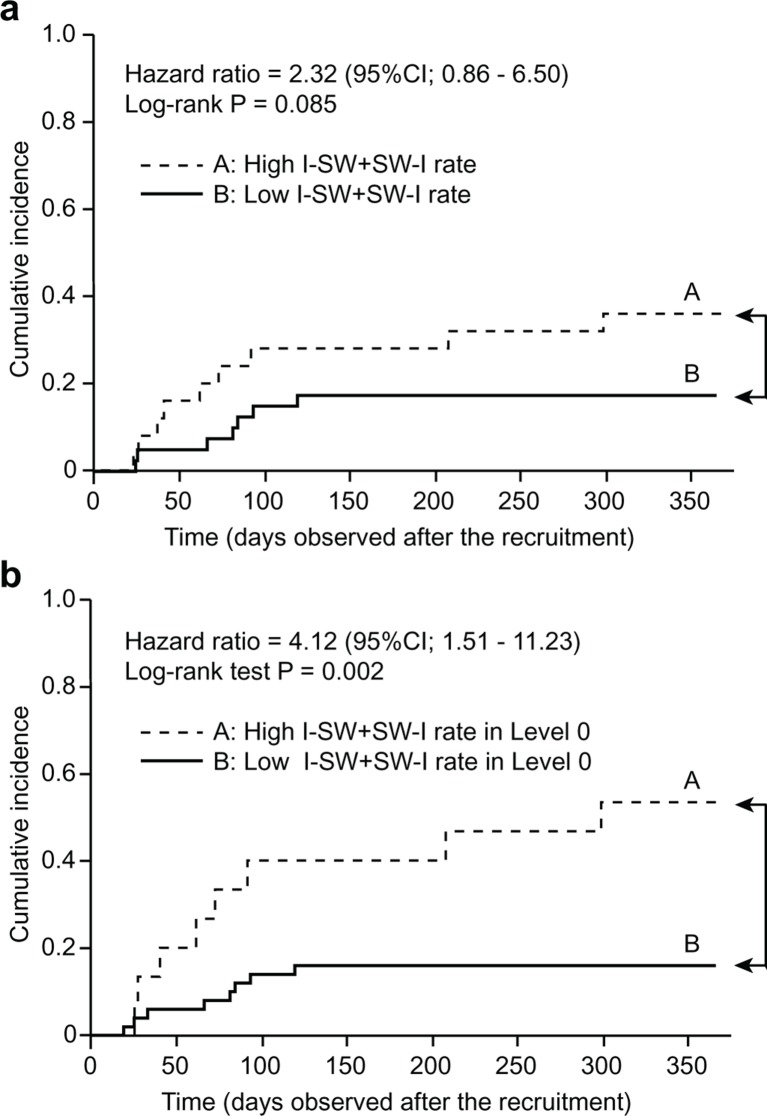
To evaluate the predictive ability of breathing–swallowing discoordination for future exacerbation, we performed an additional investigation using only the latter half of the observation period, 1 year after the measurement of breathing–swallowing coordination at recruitment. During this later observation period, COPD exacerbation occurred in 16 patients. We selected a 25% average frequency of I-SW/SW-I as the cut-off value according to the quartile value of a healthy subject,12 and patients with an I-SW/SW-I rate ≥25% were defined as having high breathing–swallowing discoordination. Figure 3A shows the Kaplan-Meier curves of the high discoordination group (group A, n=25) and the low discoordination group (group B, n=40). Patients with COPD with high discoordination had a higher risk for future exacerbation than patients with low discoordination (HR, 2.32; 95% CI 0.86 to 6.50; p=0.085 by log-rank test). Furthermore, interestingly, if we stratified patients using the I-SW/SW-I rate with only level 0 test foods, the high breathing–swallowing discoordination group (positive occurrence of I-SW/SW-I, group A; n=15) was at a significantly higher risk for future exacerbation than the low discoordination group (no occurrence of I-SW/SW-I, group B; n=50) (HR, 4.12; 95% CI 1.51 to 11.23; p=0.002 by log-rank test, figure 3B).
Figure 3.
Kaplan-Meier curves of the probability of chronic obstructive pulmonary disease exacerbation during the year after recruitment: (A) stratified by the average I-SW/SW-I frequency with all four test foods; (B) stratified by the occurrence of I-SW/SW-I with level 0 test foods. I-SW, swallowing during inspiration; SW-I, swallowing immediately followed by inspiration.
Discussion
We conducted an observational study of patients with COPD to gain insight into the clinical significance of breathing–swallowing discoordination for frequent COPD exacerbation. Our results showed that, even for those who did not have apparent dysphagia, breathing–swallowing discoordination, as indicated by the I-SW/SW-I rate, contributed to frequent exacerbation. Our results further indicate that breathing–swallowing discoordination is associated with the risk of aspiration, and interventions focusing on the improvement of breathing–swallowing discoordination will be good candidates for the treatment of patients with COPD.
It has been previously shown that swallowing abnormalities are more frequently observed in patients with COPD,11 14 and swallowing abnormalities are associated with cough sensitivity and GER symptoms.11 Because GER symptoms are one of the most important extrapulmonary predictive factors of frequent COPD exacerbation, we can assume that the relationship between GER symptoms and swallowing abnormalities, as well as aspiration disorders, will have clinical impacts including a high risk of COPD exacerbation. It remains unclear whether it is possible to improve breathing–swallowing discoordination in patients with COPD, although Martin-Harris et al showed that it was possible to improve swallowing function with a biofeedback approach for breathing–swallowing discoordination in postoperative patients with head and neck cancer.24 Therefore, breathing–swallowing discoordination may be a therapeutic target for the reduction of exacerbation in patients with COPD.
Swallowing abnormalities in patients with COPD have been reported by our colleague11 and Cassiani et al 14 previously; however, these reports employed invasive or cumbersome techniques, such as injections through a nasal catheter and videofluoroscopy, which require additional imaging procedures or radiation exposure. We used a new non-invasive and quantitative technique to evaluate breathing–swallowing discoordination and found the frequencies of the I-SW and/or SW-I patterns to be a promising index for the risk of COPD exacerbation. As mentioned above, our method has certain advantages, such as quantitative capability, non-invasiveness and easiness to repeat, compared with other techniques. In addition, even in patients who did not show apparent swallowing problems, our technique successfully revealed latent breathing–swallowing discoordination in patients with COPD. Moreover, the index of discoordination (I-SW/SW-I rate) was revealed as a good predictive marker for frequent COPD exacerbation (figure 3).
Although we did not compare patients with COPD with normal healthy subjects directly in the present study, the frequencies of I-SW/SW-I seemed to be higher in patients with COPD than normal healthy subjects.12 A similar pattern was already reported in patients with COPD13 as well as patients with Parkinson’s disease,25 and patients with Parkinson's disease showed a significant association between breathing–swallowing discoordination and the risk of laryngeal penetration/aspiration.26 We still need to clarify whether this breathing–swallowing abnormality is associated with physiological abnormalities due to respiratory disease. We speculated that breathing–swallowing discoordination may be affected by pulmonary hyperinflation and dyspnoea, but the frequency of I-SW/SW-I was not significantly associated with the severity of airflow limitation. Therefore, we can assume that the I-SW and SW-I patterns are more related to the subject’s ‘extrapulmonary’ traits, which could be affected by age or various functional abnormalities or diseases (eg, neuromuscular disease or cerebrovascular diseases).
Breathing–swallowing discoordination with different food ingestion
In the present study, the occurrence of inspiration immediately before or after swallows was most frequently observed in level 3 purée swallows (table 5). This finding is in agreement with a previous study showing that patients with COPD had a significantly higher rate of inhaling after swallowing pudding and needed more transit time in the pharynx.13 14 These findings indicated the sensitivity of our evaluation, which could detect the influence of the test food properties on breathing–swallowing coordination. Interestingly, the level 0 jelly swallow, for which the number of patients with I-SW/SW-I patterns was lowest, was most associated with exacerbation. In the Kaplan-Meier analysis, a high prevalence of I-SW/SW-I with only level 0 test food attained significant differences between groups in the risk for future exacerbation 1 year after recruitment, whereas the average I-SW/SW-I rate with all four test foods was not significantly different (figure 3A, B). The results suggest that level 0 jelly, the safest food against aspiration, is most useful for detecting the risk of exacerbation. Moreover, in terms of predicting future exacerbation, we could employ only one type of test food in our evaluations.
The association between exacerbation and lung function
Exacerbation becomes more frequent in patients with COPD as the severity of disease increases,4 27 and the mortality rate is strongly correlated with FEV1,28 29 IC/TLC and DLco.30 These associations are consistent with the present results showing that lung functions such as %FEV1, FEV1/FVC, VC, IC/TLC and DLco were significantly related to exacerbation (table 3). In the multivariate regression analysis, the I-SW/SW-I rate and GER symptoms were also revealed as significant variables associated with the frequency of exacerbation (table 4). Because of the small sample size, definitive conclusions could not be made, although the I-SW/SW-I rate may serve as a better prognostic index for COPD exacerbation than other known indexes.
The association between exacerbation and GER symptoms
Previous large-scale cohort studies and our previous cohort study have indicated that the existence of GER symptoms is a major predictor for COPD exacerbation.4 11 31 32 Therefore, we employed an evaluation of GER symptoms because GER symptoms are confounding variables for frequent exacerbation. By performing multivariate regression analyses with different models, we identified the significant impact of GER symptoms on exacerbation (model 2), as well as breathing–swallowing discoordination (model 1). In model 3, using all possible variables, the I-SW/SW-I rate was a significant variable, whereas GER symptoms were not (table 4). In addition, unexpectedly, the I-SW/SW-I rate did not show a significant association with FSSG (online supplement e-table 2). These results could be interpreted to indicate that pre-existing breathing–swallowing discoordination causes microaspirations and subsequently frequent coughs and increased sputum, causing COPD exacerbation. Moreover, GER symptoms may have direct influences on gastro-oesophageal problems, not only on swallowing disorders.
Limitations
First, the number of subjects enrolled in our study was relatively small, and only men were included. Further, the observation period may not have been long enough, as the number of exacerbation events was small (48 exacerbation incidents in 65 patients). However, because the average frequency of exacerbation (0.37 per patient year) was almost the same as in previous reports in Japanese patients33 and because we found that GER symptoms had a significant impact on frequent exacerbation, we can assume that our observations were consistent and can be extrapolated to other clinical settings. Nevertheless, a large-scale cohort study is required to establish the significance of breathing–swallowing coordination for COPD exacerbation.
Second, breathing–swallowing discoordination was evaluated only once. We do not know if temporal changes exist for breathing–swallowing coordination, whether exacerbation affects breathing–swallowing coordination and whether pulmonary rehabilitation ameliorates breathing–swallowing coordination. Future studies should focus on interventions to improve breathing–swallowing coordination, and prospective evaluations should be conducted to determine whether the improvement of breathing–swallowing coordination decreases the likelihood of exacerbation.
Third, as mentioned above, we did not compare the patients with healthy subjects in the present study. In addition, we could not find a significant association between the I-SW/SW-I rate and a disease-specific index, such as airflow limitation. Breathing–swallowing discoordination occurs even in healthy subjects, and its frequency may increase in older populations or people with certain disease states (eg, Parkinson’s disease). Moreover, we do not know the physiological mechanism of breathing–swallowing discoordination. Thus, further studies are necessary to elucidate the mechanisms of breathing–swallowing discoordination in patients with COPD.
Conclusions
We conclude that breathing–swallowing discoordination is an independent, strong predictor of exacerbation in patients with COPD. Even in patients without an apparent swallowing disorder, the evaluation and early intervention for breathing–swallowing discoordination may prevent exacerbation and consequently improve the prognosis of patients with COPD.
bmjresp-2017-000202supp003.pdf (289.4KB, pdf)
Footnotes
Contributors: SN contributed to the protocol design, data collection and analysis and writing of the manuscript. YO contributed to the protocol design, data analysis and writing of the manuscript. NY contributed to the data analysis and the review of the manuscript. SS contributed to the protocol design, data collection and analysis and editing of the manuscript. RU contributed to the data analysis and review of the manuscript. SMO contributed to the data analysis and review of the manuscript. YY contributed to the data analysis and review of the manuscript. JK contributed to the data analysis and review of the manuscript. KT contributed to the data analysis and review of the manuscript. RT contributed to the data analysis and supervised the study. SMU contributed to the protocol design, data collection and analysis and writing of the manuscript.
Funding: YO, NY and SN received financial support from FoodCare and CareIdo. Swallowing monitors and sensors were provided by Murata Manufacturing. This work was supported by grants from JSPS KAKENHI: NumbersJP16K01546 and JP16K16264.
Patient consent: Obtained.
Ethics approval: The study was approved by the institutional ethics committee of the Kyoto University (approval number, C841).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Indigenous protocols may limit the sharing of data for this paper.
References
- 1. Seemungal TA, Donaldson GC, Paul EA, et al. . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–22.doi:10.1164/ajrccm.157.5.9709032 [DOI] [PubMed] [Google Scholar]
- 2. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. . Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925–31.doi:10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Terada K, Muro S, Sato S, et al. . Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax 2008;63:951–5.doi:10.1136/thx.2007.092858 [DOI] [PubMed] [Google Scholar]
- 4. Hurst JR, Vestbo J, Anzueto A, et al. . Evaluation of copd longitudinally to identify predictive surrogate endpoints investigators. susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128–38. [DOI] [PubMed] [Google Scholar]
- 5. Mokhlesi B, Morris AL, Huang CF, et al. . Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest 2001;119:1043–8.doi:10.1378/chest.119.4.1043 [DOI] [PubMed] [Google Scholar]
- 6. Rascon-Aguilar IE, Pamer M, Wludyka P, et al. . Role of gastroesophageal reflux symptoms in exacerbations of COPD. Chest 2006;130:1096–101.doi:10.1378/chest.130.4.1096 [DOI] [PubMed] [Google Scholar]
- 7. Baumeler L, Papakonstaninou E, Stolz D. Gastroesophageal reflux and COPD exacerbations: is cholinergic-mediated oesophago-bronchial reflex a possible link?—Reply. Respirology 2016;21:1497–8.doi:10.1111/resp.12898 [DOI] [PubMed] [Google Scholar]
- 8. Cvejic L, Harding R, Churchward T, et al. . Laryngeal penetration and aspiration in individuals with Stable COPD. Respirology 2011;16:269–75.doi:10.1111/j.1440-1843.2010.01875.x [DOI] [PubMed] [Google Scholar]
- 9. Mokhlesi B, Logemann JA, Rademaker AW, et al. . Oropharyngeal deglutition in stable COPD. Chest 2002;121:361–9.doi:10.1378/chest.121.2.361 [DOI] [PubMed] [Google Scholar]
- 10. Teramoto S, Matsuse T, Fukuchi Y, et al. . Simple two-step swallowing provocation test for elderly patients with aspiration pneumonia. Lancet 1999;353:1243doi:10.1016/S0140-6736(98)05844-9 [DOI] [PubMed] [Google Scholar]
- 11. Terada K, Muro S, Ohara T, et al. . Abnormal swallowing reflex and COPD exacerbations. Chest 2010;137:326–32.doi:10.1378/chest.09-0482 [DOI] [PubMed] [Google Scholar]
- 12. Yagi N, Nagami S, Lin MK, et al. . A noninvasive swallowing measurement system using a combination of respiratory flow, swallowing sound, and laryngeal motion. Med Biol Eng Comput 2016doi:10.1007/s11517-016-1561-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gross RD, Atwood CW, Ross SB, et al. . The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;179:559–65.doi:10.1164/rccm.200807-1139OC [DOI] [PubMed] [Google Scholar]
- 14. Cassiani RA, Santos CM, Baddini-Martinez J, et al. . Oral and pharyngeal bolus transit in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015;10:489–96.doi:10.2147/COPD.S74945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghannouchi I, Speyer R, Doma K, et al. . Swallowing function and chronic respiratory diseases: systematic review. Respir Med 2016;117:54–64.doi:10.1016/j.rmed.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 16. Grobal initiative for chronic obstructive lung disease (GOLD) Online. 2015. http://goldcopd.org/.
- 17. Jones PW, Harding G, Berry P, et al. . Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648–54.doi:10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 18. Kusano M, Shimoyama Y, Sugimoto S, et al. . Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol 2004;39:888–91.doi:10.1007/s00535-004-1417-7 [DOI] [PubMed] [Google Scholar]
- 19. Kunieda K, Ohno T, Fujishima I, et al. . Reliability and validity of a tool to measure the severity of dysphagia: the Food Intake LEVEL Scale. J Pain Symptom Manage 2013;46:201–6.doi:10.1016/j.jpainsymman.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 20. Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005;86:1516–20.doi:10.1016/j.apmr.2004.11.049 [DOI] [PubMed] [Google Scholar]
- 21. Patel IS, Seemungal TA, Wilks M, et al. . Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002;57:759–64.doi:10.1136/thorax.57.9.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okada S, Saitoh E, Palmer JB, et al. . What is the chin-down posture? A questionnaire survey of speech language pathologists in Japan and the United States. Dysphagia 2007;22:204–9.doi:10.1007/s00455-006-9073-0 [DOI] [PubMed] [Google Scholar]
- 23. Cichero JA, Steele C, Duivestein J, et al. . The need for International terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: foundations of a global initiative. Curr Phys Med Rehabil Rep 2013;1:280–91.doi:10.1007/s40141-013-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin-Harris B, McFarland D, Hill EG, et al. . Respiratory-swallow training in patients with head and neck Cancer. Arch Phys Med Rehabil 2015;96:885–93.doi:10.1016/j.apmr.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gross RD, Atwood CW, Ross SB, et al. . The coordination of breathing and swallowing in Parkinson's disease. Dysphagia 2008;23:136–45.doi:10.1007/s00455-007-9113-4 [DOI] [PubMed] [Google Scholar]
- 26. Troche MS, Huebner I, Rosenbek JC, et al. . Respiratory-swallowing coordination and swallowing safety in patients with Parkinson's disease. Dysphagia 2011;26:218–24.doi:10.1007/s00455-010-9289-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Enright Md P. Office-based DLCO tests help pulmonologists to make important clinical decisions. Respir Investig 2016;54:305–11.doi:10.1016/j.resinv.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 28. Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986;133:14–20.doi:10.1164/arrd.1986.133.1.14 [DOI] [PubMed] [Google Scholar]
- 29. Hansen EF, Phanareth K, Laursen LC, et al. . Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:1267–71.doi:10.1164/ajrccm.159.4.9807121 [DOI] [PubMed] [Google Scholar]
- 30. Tanimura K, Sato S, Fuseya Y, et al. . Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. novel chest computed tomography-derived index for prognosis. Ann Am Thorac Soc 2016;13:334–41.doi:10.1513/AnnalsATS.201507-446OC [DOI] [PubMed] [Google Scholar]
- 31. Ingebrigtsen TS, Marott JL, Vestbo J, et al. . Gastro-esophageal reflux disease and exacerbations in chronic obstructive pulmonary disease. Respirology 2015;20:101–7.doi:10.1111/resp.12420 [DOI] [PubMed] [Google Scholar]
- 32. Martinez CH, Okajima Y, Murray S, et al. . Impact of self-reported gastroesophageal reflux disease in subjects from COPDGene cohort. Respir Res 2014;15:62.doi:10.1186/1465-9921-15-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukuchi Y, Fernandez L, Kuo HP, et al. . Efficacy of tiotropium in COPD patients from Asia: a subgroup analysis from the UPLIFT trial. Respirology 2011;16:825–35.doi:10.1111/j.1440-1843.2011.01982.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2017-000202supp001.pdf (144.4KB, pdf)
bmjresp-2017-000202supp002.pdf (263.2KB, pdf)
bmjresp-2017-000202supp003.pdf (289.4KB, pdf)