Abstract
Previously, we reported widespread bilateral increases in stimulus-evoked functional magnetic resonance imaging signals in mouse brain to unilateral sensory paw stimulation. We attributed the pattern to arousal-related cardiovascular changes overruling cerebral autoregulation thereby masking specific signal changes elicited by local neuronal activity. To rule out the possibility that interhemispheric neuronal communication might contribute to bilateral functional magnetic resonance imaging responses, we compared stimulus-evoked functional magnetic resonance imaging responses to unilateral hindpaw stimulation in acallosal I/LnJ, C57BL/6, and BALB/c mice. We found bilateral blood-oxygenation-level dependent signal changes in all three strains, ruling out a dominant contribution of transcallosal communication as reason for bilaterality. Analysis of functional connectivity derived from resting-state functional magnetic resonance imaging, revealed that bilateral cortical functional connectivity is largely abolished in I/LnJ animals. Cortical functional connectivity in all strains correlated with structural connectivity in corpus callosum as revealed by diffusion tensor imaging. Given the profound influence of systemic hemodynamics on stimulus-evoked functional magnetic resonance imaging outcomes, we evaluated whether functional connectivity data might be affected by cerebrovascular parameters, i.e. baseline cerebral blood volume, vascular reactivity, and reserve. We found that effects of cerebral hemodynamics on functional connectivity are largely outweighed by dominating contributions of structural connectivity. In contrast, contributions of transcallosal interhemispheric communication to the occurrence of ipsilateral functional magnetic resonance imaging response of equal amplitude to unilateral stimuli seem negligible.
Keywords: functional magnetic resonance imaging, animal models, cerebral hemodynamics, anesthesia, blood-oxygenation-level dependent contrast, brain imaging, cerebral blood flow, diffusion tensor imaging, imaging, magnetic resonance, magnetic resonance imaging, physiology
Introduction
Functional magnetic resonance imaging (fMRI) responses to peripheral sensory input are expected to follow the functional topology of the somatosensory cerebral cortex. For example, unilateral stimuli in rats have been shown to predominantly elicit changes in blood oxygenation levels (BOLD) in contralateral somatosensory brain regions.1–8 Analogous studies in mice yielded conflicting results: Several groups reported contralateral dominance of the BOLD response to unilateral stimulation.9–11 Conclusions were based on the unilateral appearance of activated clusters in statistical maps and BOLD profiles of the affected (somatosensory cortical) region, whereas BOLD signal profiles in apparently unaffected regions were either not analyzed in those studies or not displayed. In contrast to the majority of published reports, we consistently observed rather widespread bilateral fMRI responses independent of the nature of stimulus,12–14 or type and depth of anesthesia including anesthesia protocols used by other laboratories.14 Only when using a very mild electrical stimulation paradigm with single pulses, we observed a weak BOLD response with contralateral dominance, though there were still considerable widespread signal changes involving both hemispheres.15 The occurrence of widespread signal led to the conclusion that mouse fMRI data comprise contributions from an arousal response potentially leading to widespread neural activity and hence fMRI responses and/or to stimulus-elicited changes in cardiac output.14 As neural activity-mediated signals are inherently weak, hemodynamic changes associated with fluctuations in physiological state may overrule cerebral autoregulation and affect fMRI readouts. Consequently, the specificity of the “fMRI signal” depends on the relative weight of contributions reflecting neural activity (neurogenic) and contributions independent thereof (non-neurogenic).
While arousal responses may largely account for the widespread fMRI patterns in mice following unilateral sensory stimuli, contributions from interhemispheric communication at a neuronal level cannot be excluded. In fact, ipsilateral activity might arise from parallel engagement of circuits within both hemispheres.16–18 Mechanisms involve communication via the corpus callosum (CC) and other interhemispheric white matter structures, as well as direct uncrossed afferent projections. Transcallosal contributions to the bilateral stimulus-evoked fMRI (se-fMRI) response could be evaluated by comparing mouse strains with different degrees of CC integrity, which might be quantitatively assessed using resting-state fMRI (rs-fMRI).19 In rodents displaying an intact CC, rs-fMRI connectivity analysis yielded network components displaying functional connectivity (FC) between corresponding brain areas in both hemispheres.20–23 Similar to se-fMRI responses, FC patterns derived from rs-fMRI analysis depend on both the underlying neural circuitry24,25 and efficiency of neurovascular coupling. Strain-specific cerebrovascular parameters might affect both amplitude and frequency spectrum of signal fluctuations and hence FC readouts.
The study objective was to evaluate the relative importance of neural and vascular factors in determining se-fMRI responses elicited by unilateral sensory stimulation as well as rs-fMRI patterns in mice. We therefore compared three mouse strains: I/LnJ mice lacking interhemispheric callosal communication, BALB/c mice displaying a propensity for compromized CC integrity,26 and C57BL/6 mice with fully developed CC. In addition to varying interhemispheric connectivity at a neuronal level, differences in cerebrovascular parameters and hence in neurovascular coupling efficiency among the strains allowed investigating vascular contributions to se- and rs-fMRI signals. Accordingly, FC data derived from rs-fMRI have been evaluated in the context of basic physiological parameters such as cerebral blood volume (CBV), vascular reactivity, and reserve capacity, which determine the ability of vessels to adapt on demand. Our results indicate that communication via the CC did not significantly contribute to the unspecific bilateral se-fMRI response to unilateral peripheral stimulation, while bilateral homotopic FC patterns derived from rs-fMRI reflect structural connectivity at a neural level via the CC and appear to be rather robust toward strain-specific variation of vascular parameters.
Material and methods
Animals
The in vivo experiments were carried out in compliance with the Swiss law of animal protection under a license (#ZH243/14) of the Cantonal Veterinary Office of the Canton of Zurich and in compliance with the ARRIVE guidelines. Female I/LnJ (The Jackson Laboratory, Bar Harbor, Maine, USA), C57BL/6 and BALB/c mice (Janvier, Le Genest-St Isle, France) were studied at 3–4 months of age, and 17.5–22 g of body weight. Data were acquired with randomly alternating the strains. Different effect size for the different parameters was expected. Group size was chosen such that clear effects (structural connectivity) could be captured with certainty while there was a reasonable chance to also capture weaker effects (cerebrovascular effects).
Animal preparation
Anesthesia was induced with 4% isoflurane (Abbott, Cham, Switzerland) in a 20% O2/80% air mixture, before endotracheal intubation under 2% isoflurane for mechanical ventilation. After transfer to the animal support (Bruker Biospin GmbH, Ettlingen, Germany), the mice were connected to a small animal ventilator (CWE, Ardmore, USA) and kept at 2% isoflurane until all animal preparation steps had been completed. The mouse head was positioned with the incisors secured over a bite bar and fixed with stereotactic ear bars. Ophthalmic ointment was applied and a rectal temperature probe inserted to monitor and maintain body temperature at 36.0℃. The tail vein was cannulated for intravenous administration of the neuromuscular blocking agent pancuronium bromide (Sigma-Aldrich, Steinheim, Germany), administration of contrast agent (CA) and acetazolamide for CBV measurement, and for infusion of medetomidine (Domitor, medetomidine hydrochloride; Pfizer Pharmaceuticals, Sandwich, UK) in selected mice. Pancuronium bromide was administered as a bolus at a dose of 0.75 mg/kg during animal preparation. The infusion line was prepared such that several tubing segments of volume-adjusted length were attached to each other using a 30G cannula needle, whereby the different solutions to be applied (second bolus of pancuronium bromide, CA, saline for flushing, acetazolamide, saline leading to the infusion pump) have been separated by a small air bubble to prevent mixing. The infusion pump (Harvard Apparatus, Hollistan, USA) was set to the respective volumes for each substance manually prior to its administration. For electrical stimulation performed during the magnetic resonance imaging (MRI) experiment and for the reflex test in bench-top measurements, a pair of needle electrodes was inserted subcutaneously into the right hindpaw with a distance of 2 mm between the needles. Throughout the course of the experiment, the animals were ventilated with a 20% O2/80% air mixture at a rate of 80 breaths/min, with a respiration cycle of 25% inhalation, 75% exhalation, and an inspiration volume of 1.8 ml/min. For the principal MRI experiments (rs-fMRI, se-fMRI, CBV measurement, DTI), isoflurane was adjusted to 1.1%. To control for effects mediated by anesthesia depth, in a set of separate se-fMRI only experiments, the level of isoflurane was adjusted to 1.2% and 1.5% for C57BL/6 mice and 1.5% for I/LnJ mice, respectively. In a set of separate rs-fMRI only experiments, the level of isoflurane was adjusted to 1.3% and 1.5% for all three mouse strains, respectively, or alternatively a combination of medetomidine—0.05 mg/kg for the bolus and 0.1 mg/kg/h for the infusion—and low dose isoflurane (0.5%) was administered as described previously.22 To maintain the level of muscle relaxation throughout the duration of the MRI experiment (2 h), a second injection of 0.5 mg/kg pancuronium bromide was (ca. 70 min after first bolus) infused together with the CA prior to starting the CBV measurements. Bench-top measurements of reflexes during electrical stimulation as indicator of anesthesia depth, were performed without applying muscle relaxant. After the experiments, time for recovery was provided for all the animals.
MRI
The principal MRI study was carried out under 1.1% isoflurane and comprised—in the order specified—BOLD fMRI (rs-fMRI, and se-fMRI using an electrical hindpaw stimulation paradigm), DTI, baseline CBV measurement, and the assessment of dynamic CBV response to the injection of acetazolamide (with 5 min baseline recording used for CBV rs-fMRI analysis). To evaluate the influence of anesthesia on FC patterns, additional se-fMRI and rs-fMRI measurements have been carried out using different anesthesia protocols, as outlined in the previous paragraph.
MRI experiments were conducted on a Bruker Biospec 94/30 small animal MR system (Bruker BioSpin MRI, Ettlingen, Germany) operating at 400 MHz (9.4 T), using a two-element receive-transmit cryogenic quadrature coil (Bruker BioSpin AG, Fällanden, Switzerland). Sixteen adjacent coronal slices of 0.5-mm thickness were positioned such that the first slice was placed 2.5 mm rostrally of bregma according to a stereotaxic mouse brain atlas.27 An anatomical reference scan was acquired using a spin echo (Turbo-RARE) sequence: field of view (FOV) = 20 × 20 mm2, matrix dimension (MD) = 200 × 200, repetition time (TR) = 3500 ms, echo time (TE) = 13 ms, effective echo time (TEeff) = 39 ms, RARE factor = 8, number of averages (NA) = 2. Prior to fMRI data acquisition the local field homogeneity has been optimized using acquired field maps. BOLD fMRI and CBV data were acquired using a gradient-echo echo-planar imaging (GE-EPI) sequence: FOV = 16 × 7 mm2, MD = 80 × 35, yielding an in-plane voxel dimension of 200 × 200 µm2, flip angle (FA) = 60°, TR = 1000 ms, TE = 2 ms (BOLD)/8.7 ms (CBV), NA = 1, and 16 slices, yielding a temporal resolution of 1 s per image data set. Rs-fMRI scans comprised 360 (BOLD) and 300 (CBV) repetitions. When applying the stimulation paradigm, the length of scan (se-fMRI) was set to 740 repetitions.
For CBV measurements, after acquisition of a pre-contrast scan superparamagnetic iron oxide nanoparticles (Endorem®, Laboratoire Guerbet SA, Roissy, France) were used as an intravascular CA and injected at a dose of 30 mg Fe/kg in a volume of 3 µl saline/g body weight. After a waiting period of 10 min to allow the CA to reach its plasma steady-state level28 data acquisition for the evaluation of baseline CBV (CBVbsl) and dynamic CBV response was started. Total scan duration was 20 min, i.e. 1200 repetitions. At 5 min after start of acquisition, the carbonic anhydrase inhibitor acetazolamide (Diamox® parenteral, Goldshield Pharmaceuticals Ltd, Croydon, UK) was infused at a dose of 15 mg/kg in a volume of 1.5 µl saline/g body weight and at a rate of 0.5 ml/min. Injections of Endorem and acetazolamide were followed by a 20 µl saline flush.
DTI data were acquired using a multi-shot DTI-EPI sequence: FOV = 20 × 17.5 mm2, MD = 128 × 128, yielding an in-plane voxel dimension of 263 × 233 µm2, FA = 90°, TR = 2000 ms, TE = 22 ms, four segments. Five image data sets were acquired with b = 0 s/mm2, followed by 36 gradient-encoded images with b = 1000 s/mm2.
Electrical stimulation
The pair of bipolar platinum needle electrodes (Genuine Grass Instruments, West Warwick, USA) placed subcutaneously in the hindpaw was connected to a current stimulus isolator (A365D, World Precision Instruments Inc., Sarasota, USA). Stimulus delivery was controlled with custom-written LabVIEW software (National Instruments, Austin, USA), and synchronized to the onset of the fMRI sequence. A fixed set of stimulation parameters was used: current amplitude = 0.5 mA (in all the three strains at 1.1% isoflurane) or 0.7 mA (in C57BL/6 mice at 1.2% and 1.5% isoflurane, in I/LnJ mice at 1.5% isoflurane, respectively), pulse duration = 0.5 ms, pulse frequency = 5 Hz. The stimulation paradigm consisted of a block design starting with 180 s baseline followed by four cycles of 20 s stimulus and 120 s post-stimulus period.
Systemic physiological parameters at rest and of reflex activity during electrical hindpaw stimulation
For the bench-top physiological parameter measurements, the animal’s left thigh was shaved and a fiber-optic pulse oximeter (MouseOx, STARR Life Science, Oakmont, USA) fixed to the flank in order to record heart rate (in beats per minute, bpm), pulse distention (in µm), and O2 saturation (in %). All other preparations followed the procedure previously described. After the animal preparation data were acquired for 30 min at resting conditions. To estimate anesthesia depth under all the different isoflurane doses administered a set of animals—without prior injection of muscle relaxant—was used for reflex tests during electrical hindpaw stimulation at 0.5 mA and/or 0.7 mA, respectively.
Data analysis
fMRI preprocessing was performed in AFNI (http://afni.nimh.nih.gov/): The first 20 volumes of each data set were discarded to account for the T1 relaxation. Data were slice-time corrected. Motion correction was performed by realigning all scan volumes to the first repetition and coregistration of all animals to a strain-specific template. Further, a weak Gaussian blur (FWHM = 0.3 mm) was applied. For rs-fMRI, data were corrected with global signal regression and band pass filtered for 0.01 Hz to 0.3 Hz. For se-fMRI, the data were scaled to percent signal change relative to baseline and detrended by adding polynomials (to the fourth order) as regressors to a general linear model (GLM).
For the se-fMRI scans, statistical parametric maps were generated using the GLM. As a model for the evoked BOLD response, the SPM basis functions were convolved with the stimulus time course. The beta values of the GLM analysis using the gamma variate as a regressor entered the group statistics (one-sample t-test) for each strain. Maps are shown as color-coded beta-values. For the analysis of time courses of se-fMRI, signal changes regions-of-interest (ROIs) were defined according to a stereotaxic mouse brain atlas27 for the contralateral (cl) as well as ipsilateral (il) primary somatosensory hindlimb cortices (S1HL), and for a control region (visual cortex, V1) using MATLAB based software Aedes (http://aedes.uef.fi). All further steps were performed using custom-written MATLAB code (The MathWorks, Natick, MA), and average time courses from all animals per strain were computed. The maximum amplitude of the response to the first electrical stimulation block in the contralateral S1HL cortex was taken as measure for maximum evoked BOLD signal change (ΔBOLDmax(cl)). Correspondingly, the maximum amplitude for the ipsilateral S1HL BOLD signal change (ΔBOLDmax(il)) was quantified in addition to compute the ratio between contra- and ipsilateral se-fMRI responses (ΔBOLDmax(il)/ΔBOLDmax(cl)).
For statistical parametric maps representing resting-state signal patterns, the seeds (2 × 2 voxels) were positioned in the primary somatosensory cortex region (S1), more precisely in the anterior parietal cortex (aPc) of the brain, and maps were computed in FSL 5.0 (FMRIB, Oxford, UK). Group-level statistical analysis was performed with one-sample t-test. Maps are shown as color-coded t-statistics. Network analysis was conducted using an independent component analysis (ICA)-based atlas derived from Grandjean et al.22 to extract time series. Pearson’s correlations between the time series were computed and corrected with Fisher’s z correction for bounded values, and are indicated as z-scores, referred to as FC for the particular regions. Between-strain comparison was performed with t-test and corrected with false discovery rate (FDR). Amplitudes of low frequency fluctuations (ALFF) were reconstructed in AFNI for the frequency range of 0.01 Hz to 0.1 Hz, as a measure of the fluctuation amplitude from the signal profiles of each voxel. Average ALFF values were extracted from S1.
For CBV analysis, values were derived for CA-induced changes in (s−1) according to , with being the molar relaxivity, and being the steady-state concentration of CA in plasma. Relative CBVbsl was estimated for each voxel according to with Spre and S(0) being the steady-state signal intensities prior and 10 min after injection of Endorem.29 A t-test was used for comparison of CBVbsl (baseline (s−1)) values in S1 between the strains. For estimating induced CBV changes upon administration of acetazolamide (Diamox), the values were computed according to , with S(t) being the signal intensity at time t. Values for relative to baseline values were then derived according to . For the analyses, the ROIs were placed in S1 of both hemispheres. The slope of the induced CBV response (slope ΔCBV/Δt) was calculated per animal for the time window of 40 to 90 repetitions after acetazolamide administration serving as measure for vascular reactivity, while the maximum amplitude of the response (ΔCBVmax) was determined as measure of vascular reserve. A t-test served for comparison of slope and maximum amplitude values between strains.
For DTI data analysis, FA maps were reconstructed in FSL following eddy current correction. Images acquired with b = 0 s/mm2 were used to estimate the spatial transformation to a template and the transformations were applied to the FA maps. A ROI positioned on the anterior midline portion of the CC for C57BL/6 and BALB/c mice and in a corresponding region for I/LnJ mice was used to extract FA values from the individual maps. Additional ROIs analyzed were major forceps (mc), anterior commissure (ac), cingulum (cg), corpus callosum (cc), septum (se), external capsule (ec), internal capsule (ic), and hippocampal commissure (hc).
The systemic physiological parameter values for each animal were calculated by averaging all data points over the measurement period of 30 min and averaging all tested animals per strain.
The reflex activity upon electrical hindpaw stimulation was rated according to 0 = no response, 1 = whiskering, 2 = weak paw withdrawal reflex, and 3 = strong paw withdrawal reflex. The responses of all animals per strain were averaged and taken as an indicator of anesthesia depth.
Descriptive statistics are given as mean across animals ± standard error of the mean (SEM). Rs-fMRI analysis did not require operator-interaction using data-driven approaches. For ROI analysis, blinding was not possible due to obvious strain-specific image signatures. Some animals (I/LnJ = 4 and BALB/c = 2 mice) had to be excluded from analysis of cerebrovascular parameters due to technical issues (e.g. failure of CA administration, software crash, and instability of fMRI traces) or due to the fact that statistical analysis identified animals as outliers.
Statistical analysis of factors affecting fMRI signal
fMRI parameters representing interhemispheric interactions and local response amplitude were related in a linear model in R (3.0.1, The R Foundation for Statistical Computing, Vienna, Austria) to structural connectivity, vascular parameters extracted from the CBV measurements, and animal body weight. The ratio between ΔBOLDmax extracted from contra- and ipsilateral S1HL (ΔBOLDmax(il)/ΔBOLDmax(cl)) following electrical hindpaw stimulation and the FC between left and right S1 was used as metric for se- and rs-fMRI interhemispheric interaction, respectively. ΔBOLDmax(cl) for se-fMRI and ALFF in S1 for rs-fMRI were used as metric for local response amplitude. A model was devised including FA in the region of the CC as an indicator of interhemispheric structural connectivity, CBVbsl and reactive, i.e. acetazolamide-induced CBV change (slope ΔCBV/Δt and ΔCBVmax) in S1, and body weight. Pairwise interactions between the different factors were considered initially, but did not present a significant effect during model selection and thus were not included in the final model. The assumption of normal distribution of the residuals was tested and considered plausible for all models. Likelihood test ratio was used to test the hypotheses of the presence of an effect for each of the selected factors in the model. Pearson’s r values from the rs-fMRI analysis and FA were corrected with Fisher’s z transformation to account for bounded values.
Results
Bilateral se-fMRI response to unilateral paw stimulation also in I/LnJ mice
Electrical stimulation of the right hindpaw at 0.5 mA under 1.1% isoflurane yielded statistically significant BOLD signal changes in all three mouse strains. The response comprised large areas in both hemispheres in an almost symmetrical manner, with clusters not exclusively confined to regions known to be involved in somatosensory processing (Figure 1(a)). Even I/LnJ mice, which lack CC communication between the cerebral hemispheres, displayed bilateral BOLD responses. This result was further substantiated by the BOLD signal time courses for ROIs selected in the S1HL region contra- and ipsilateral to the stimulated paw revealing no significant differences in amplitudes of the signals in the two hemispheres. Similarly, the signal response in a control region (visual cortex, V1) yielded a signal pattern displaying a high degree of correlation with the stimulation paradigm (Figure 1(a)). Analysis of the physiological state revealed strain-specific differences in susceptibility to sensory stimuli when using the same anesthesia level of 1.1% isoflurane (Table 1). We therefore carried out additional experiments, by adjusting the isoflurane level to yield similar reflex responses, which was the case for isoflurane levels of 1.2% for C57BL/6 and 1.5% of I/LnJ mice. Using these isoflurane levels also significant widespread BOLD responses could be detected at 0.7 mA, similar to the previous observations. Again bilateral symmetry was observed (Figure 1(b)) analogous to the outcome at 1.1% isoflurane and 0.5 mA. Even a level of 1.5% isoflurane in C57BL/6 mice did not change the BOLD response to 0.7 mA with respect to laterality; the ratio between the maximum signal change amplitude to the first stimulation block of the contralateral and the ipsilateral S1HL was 1.06 ± 0.14 (Table 1; Figure 1(c)). The degree of bilaterality of the fMRI response in S1HL (Figure 1(a)) as revealed by the ratio ΔBOLDmax(il)/ΔBOLDmax(cl) did not depend on the residual reflex response (Table 1; Figure 1(c)) as measure of the degree of alertness of the mice.
Figure 1.
Se-fMRI analysis: Coronal MRI sections, statistical parametric maps, and the corresponding time courses of BOLD signal changes (ΔBOLD) elicited by electrical hindpaw stimulation at 0.5 and 0.7 mA, respectively, for C57BL/6, I/LnJ, and BALB/c mice anesthetized with isoflurane. (a) T2-weighted anatomical images with distances to Bregma and primary somatosensory hindlimb cortex contralateral to the stimulated paw (S1HL cl) indicated. Corresponding GE-EPI images displaying minimal geometrical distortions used for stimulus-evoked BOLD fMRI experiments under 1.1% isoflurane with statistical parametric maps (p ≤ 0.05 for N = 10 C57BL/6 mice, p ≤ 0.01 for N = 9 I/LnJ, and N = 9 BALB/c mice; cluster size > 10 voxels) showing widespread signal change during stimulation at 0.5 mA involving both contra- and ipsilateral (il) hemisphere. The color bar shows the respective β-values ranging from 0 to 1. Time courses of ΔBOLD in S1HL cl (blue line) and S1HL il (red line) during stimulation for the four stimulus periods of 20 s duration each (gray bar) visualize largest evoked signal changes in I/LnJ mice. Response patterns in the S1HL il and in a control region in the visual cortex V1 looked almost identical for all three strains indicating lack of specificity. Data are given as mean ± SEM. (b) Time courses of ΔBOLD in S1HL cl (blue line) and S1HL il (red line) during stimulation at 0.7 mA in N = 5 C57BL/6 mice under 1.5% isoflurane, respectively, and N = 4 I/LnJ mice under 1.5% isoflurane reflect bilateral symmetry analogous to the observations made at 1.1% isoflurane, and the BOLD responses were similarly widespread (not shown). Data are given as mean ± SEM. (c) Degree of bilaterality of the se-fMRI response in S1HL expressed by the ratio of the maximum amplitude of the BOLD response in the contra- and ipsilateral S1HL cortex (ΔBOLDmax(il)/ΔBOLDmax(cl)) did not depend on the residual reflex response to stimulation at 0.5 mA or 0.7 mA (green signs), respectively, as measure of the degree of alertness of the mice. Isoflurane levels used are noted next to the respective data points. Data are given as mean ± SEM.
Table 1.
Systemic physiological parameters recorded at rest and behavioral reaction to electrical hindpaw stimulation evaluated in bench-top experiments under different levels of isoflurane.
| Mouse strain | Isoflurane level | Physiological parameters at rest |
Reflex score upon electrical hindpaw stimulation |
|||
|---|---|---|---|---|---|---|
| Heart rate (bpm) | Pulse distention (µm) | O2 saturation (%) | 0.5 mA | 0.7 mA | ||
| C57BL/6 | 1.1% | 569.90 ± 5.31 | 15.08 ± 1.96 | 95.23 ± 1.53 | 1.33 ± 1.04 | – |
| C57BL/6 | 1.2% | 517.73 ± 7.99 | 11.39 ± 1.42 | 95.50 ± 0.29 | 0 | 0.40 ± 0.24 |
| C57BL/6 | 1.5% | 555.60 ± 32.90 | 14.80 ± 0.25 | 97.50 ± 0.36 | 0 | 0 |
| I/LnJ | 1.1% | 610.67 ± 9.67 | 19.22 ± 1.14 | 96.67 ± 0.67 | 2.00 ± 0.58 | – |
| I/LnJ | 1.5% | 560.22 ± 10.60 | 16.37 ± 0.73 | 94.73 ± 0.41 | 0 | 0.60 ± 0.24 |
| BALB/c | 1.1% | 596.19 ± 12.23 | 22.06 ± 1.39 | 94.90 ± 0.78 | 3.00 | – |
Heart rate, pulse distention and O2 saturation averaged over a period of 30 min at rest. Average reflex response to stimulation using 0.5 mA or 0.7 mA, respectively, with 0 = no response, 1 = whiskering, 2 = weak paw withdrawal reflex, 3 = strong paw withdrawal reflex. As animals under 1.1% isoflurane already were showing a reflex response at 0.5 mA, they were not further evaluated at 0.7 mA. Data from N = 3 mice per strain (1.1% isoflurane), N = 5 C57BL/6 mice (1.2% isoflurane), N = 2 C57BL/6 mice (1.5% isoflurane), and N = 5 I/LnJ mice (1.5% isoflurane) per experiment are given as mean across animals ± SEM.
Reduced interhemispheric rs-fMRI derived FC in I/LnJ as compared with C57BL/6 and BALB/c mice
For evaluating the interhemispheric cortical FC in the three mouse lines, a seed (2 × 2 voxels) was positioned in the left aPc (S1) to extract temporal series, which were used as a regressor to which temporal profiles of all other voxels of the rs-fMRI data set were compared. Group analysis indicated the presence of significantly shared temporal information between the seed and regions in the contralateral hemisphere in both C57BL/6 and BALB/c mice. However, for I/LnJ mice only isolated voxels in the contralateral hemisphere correlated with the seed (Figure 2(a)). This was also the case for a seed positioned caudally in the visual cortex V1 (Suppl. Fig. S3).
Figure 2.
Rs-fMRI analysis: Statistical parametric maps of seed-based analysis and cortical network representation for C57BL/6 (N = 10), I/LnJ (N = 9), and BALB/c (N = 9) mice. (a) Average seed-based maps for a seed positioned in the right aPc (S1). Significant temporal association with the signal from the seed is observed in homotopic areas on the contralateral side in C57BL/6 and also in BALB/c mice, whereas in I/LnJ mice only few voxels reached significance. The color bar shows t-statistics with a threshold set at p ≤ 0.05. (b) Cortical network analysis based on ICA parcellation of the brain derived from Grandjean et al.22 Average network maps of both C57BL/6 and BALB/c mice show highest FC between interhemispheric homotopic regions (diagonal elements), which was not the case for I/LnJ mice, with the exception of the cingulate/retrosplenial and prefrontal cortex. The color bar shows Fisher’s z transformed Pearson’s r correlation coefficient. (c) Statistical comparison highlights the differences in cortical FC between the strains. The comparisons between C57BL/6 and I/LnJ mice, and between BALB/c and I/LnJ mice reveal significant differences in homotopic interhemispheric correlations, which denotes reduced interhemispheric FC in I/LnJ mice compared with the other strains. The color bar shows FDR corrected p-value. Labels on network maps are: aPc: anterior parietal cortex; mPc: medial parietal cortex; pPc: posterior parietal cortex; Mc: motor cortex; Cg: cingulate cortex; PFc: prefrontal cortex.
To visualize the effect across the extended cortical regions, the correlations of the time course of selected cortical regions are represented as group average network maps. For better representation, correlation matrices have been split into matrices representing intra- and interhemispheric correlations only. In all strains, we observed highest intrahemispheric correlations between adjacent ROIs (Figure 2(b)), while the values for longer distance interactions were significantly lower. A major feature of the network maps of both C57BL/6 and BALB/c mice is the presence of homotopic interhemispheric correlations (Figure 2(b)). For example, for a ROI in the aPc (S1) values for the interhemispheric FC were z = 1.08 ± 0.33 for C57BL/6 and z = 0.75 ± 0.32 for BALB/c mice, while the corresponding value was significantly reduced for I/LnJ mice with z = 0.08 ± 0.16 (C57BL/6 ≠ I/LnJ: p ≤ 0.001, BALB/c ≠ I/LnJ: p ≤ 0.01). A similar reduction in I/LnJ mice compared with the other strains has been obtained for the other ROIs in the parietal and motor cortex. However, not all cortical regions were equally affected by the absence of the CC. Acallosal I/LnJ mice displayed interhemispheric FC for cingulate/retrosplenial cortex and for prefrontal cortex (Figure 2(b)). Both for intra- and interhemispheric correlations C57BL/6 mice displayed the highest values. Experiments under the other anesthetic regimens yielded the same result: I/LnJ mice did not display homotopic parietal cortical FC (Suppl. Fig. S1). The same FC patterns were observed, albeit with generalized decreased correlation strength, when analyzing fluctuations in CBV (Suppl. Fig. S2).
To investigate the amplitude of the rs-fMRI signal locally, we estimated ALFF for a frequency range of 0.01 Hz to 0.1 Hz (Suppl. Fig. S4). All three strains presented highest ALFF values not only in the frontal sensory cortex regions but also in the regions overlapping with large arteries and veins such as the anterior cerebral artery and the sagittal sinuses. Voxel-wise analysis could not reveal significant differences between groups.
FA of white matter structures reveals difference in all three mouse strains tested
As expected, both the group average FA and fiber orientation map of I/LnJ mice reveal a lack of callosal fibers crossing the cerebral midline (Figure 3(a) and (b)). FA values for I/LnJ mice (FA = 0.17 ± 0.004) quantified in a midline ROI corresponding to the CC in C57BL/6 and BALB/c mice were lowest and close to FA values found in cortical gray matter (FA = 0.13 ± 0.001). Moreover, the orientation of the largest eigenvalue of the diffusion tensor in I/LnJ mice was found to point along the rostral-caudal direction. In contrast, FA values in this ROI were significantly larger in C57BL/6 (FA = 0.38 ± 0.01, p ≤ 0.001) and BALB/c mice (FA = 0.30 ± 0.02, p ≤ 0.001) with the largest eigenvalue pointing in the lateral direction. Interestingly, FA values in that region were found to be significantly lower in BALB/c (though we did not observe a complete absence of callosal fibers in the sample investigated) as compared with C57BL/6 mice (p ≤ 0.01; Figure 3(c)). In addition to the differences found for the FA in the CC, values for I/LnJ mice also differed significantly (p ≤ 0.001) from the other strains in the regions of the hippocampal commissure, the septum, and the internal capsule (Suppl. Fig. S5). Otherwise, the general appearance of the white matter tracts was comparable between mice of the three strains (Figure 3(a) and (b)).
Figure 3.
DTI analysis: Maps of FA and fiber orientation, and comparison of FA in the region of the CC for C57BL/6 (N = 10), I/LnJ (N = 9), and BALB/c (N = 9) mice. (a) Averaged FA maps highlight lack of CC in I/LnJ mice. The red frame indicates the ROI for quantification of FA in (c). The gray bar shows FA values. (b) Fiber orientation map in a representative animal of each strain reflecting the preferred water diffusion direction (first eigenvector). The colors represent water diffusion in the following directions: blue: rostral-caudal, green: superior-inferior, red: lateral. (c) Quantification of FA from the ROI shown in (a) revealed significant differences (***p ≤ 0.001) between values of all three strains with I/LnJ mice presenting the lowest values. All data are given as mean across animals ± SEM.
Higher CBVbsl values are associated with lower values of vascular reactivity and reserve
As fMRI signals reflect local changes in cerebral hemodynamics, information on cerebrovascular parameters such as baseline CBV and dilatory capacity of cerebral vessels becomes relevant. We therefore estimated relative CBVbsl values for the three strains from T2* changes elicited by the administration of Endorem (Figure 4(b)). Values are given in (s−1) assuming a linear relationship between CBV and . Comparisons of CBVbsl values between homotopic cortical areas did not show significant differences in any of the strains. Therefore, average signals extracted from the two hemispheres were used in the analysis. CBVbsl in S1 of C57BL/6 mice was found to be highest with values of (bsl) = 89.8 ± 3.30 s−1. The corresponding values in BALB/c mice were significantly lower with (bsl) = 56.23 ± 4.70 s−1 (p ≤ 0.001). I/LnJ mice ranged in-between the other two strains with respect to the CBVbsl values and showed a higher degree of variability with (bsl) = 75.6 ± 10.0 s−1.
Figure 4.
Cerebrovascular characterization: Time courses of average CBV changes (ΔCBV) elicited by i.v. infusion of 15 mg/kg acetazolamide (Diamox), comparison of cerebrovascular parameters, and correlation of CBVbsl with vascular reactivity and reserve, respectively, for C57BL/6 (N = 10), I/LnJ (N = 5), and BALB/c (N = 7) mice. (a) Time courses of ΔCBV extracted from S1 upon injection of acetazolamide (gray vertical line) reveal increasing CBV responses in the order C57BL/6 < I/LnJ < BALB/c, respectively. Variability in CBV response was largest in I/LnJ mice. (b) Contrast agent (CA)-induced changes in baseline relaxivity as marker for relative CBVbsl values in S1. C57BL/6 mice displayed the highest CBVbsl, and BALB/c mice the lowest. (c,d) Quantification of the slope ΔCBV/Δt (c) of the CBV response elicited in S1 by the injection of acetazolamide as a measure of vascular reactivity and the mean value of individual ΔCBVmax in S1 (d) as a measure for vascular reserve. All data are given as mean across animals ± SEM. Significant differences between regions are indicated (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001). (e,f) Negative correlation between individual CBVbsl values and slope ΔCBV/Δt (e) and ΔCBVmax (f) of the acetazolamide-induced fMRI response in S1 for the three strains. I/LnJ mice display high interindividual variability.
Apart from CBVbsl values, the ability of cerebral vessels to respond on demand might influence the fMRI signals. We therefore analyzed the mean CBV signal changes in percent of baseline values (ΔCBV) elicited by acetazolamide (Diamox; Figure 4(a)). As a measure of vascular reactivity, we determined the slope of the induced CBV changes in the time interval 40 to 90 s after onset of drug administration (Figure 4(c)). C57BL/6 mice showed the lowest vascular reactivity values in S1 (slope ΔCBV/Δt = 0.09 ± 0.02) differing significantly from those of the other two strains (I/LnJ: slope ΔCBV/Δt = 0.19 ± 0.04, p ≤ 0.05; BALB/c: slope ΔCBV/Δt = 0.20 ± 0.03, p ≤ 0.01). Similarly, the vascular reserve reflected by the maximum amplitude of the CBV response was smallest in C57BL/6 mice (ΔCBVmax = 13.28 ± 1.54) and differed significantly from the vascular reserve determined for BALB/c mice (ΔCBVmax = 21.10 ± 2.75, p ≤ 0.05). The variability was highest for I/LnJ mice (ΔCBVmax = 17.09 ± 4.41; Figure 4(d)). A strong negative linear correlation between acetazolamide-induced CBV change and CBVbsl has been found for all three strains both regarding the vascular reactivity measures (R = 0.71; Figure 4(e)) and the vascular reserve (R = 0.73; Figure 4(f)).
Physiological parameters at rest and reflex test indicate higher alertness in I/LnJ and BALB/c as compared with C57BL/6 mice
Measurements of heart rate, pulse distention, and O2 saturation under resting conditions in mice anesthetized with isoflurane are summarized in Table 1. Acquisition of data over a period of 30 min indicated that physiological variables were more stable over time for C57BL/6 mice compared with the other two strains displaying more fluctuations in measured values (data not shown). Compared with C57BL/6, I/LnJ mice displayed a higher heart rate, which however did not differ profoundly from the mean heart rate measured in BALB/c mice. Anesthesia depth was estimated by testing the residual reflexes in response to electrical hindpaw stimulation in bench-top experiments (Table 1). The three mouse lines showed different susceptibility to sensory stimulation. Compared with C57BL/6 mice, the other strains seemed more alert, i.e. the depth of anesthesia reached under 1.1% isoflurane appeared lower in BALB/c and I/LnJ mice. Under higher levels of isoflurane, i.e. 1.2% and 1.5% for C57BL/6 mice, and 1.5% for I/LnJ mice, no reaction could be detected at 0.5 mA stimulus amplitude; at 0.7 mA, the reflex response was very mild, scoring below 1 on average.
Rs-fMRI derived FC correlates with FA values in CC, while dependence of fMRI readouts on reactive CBV was not significant
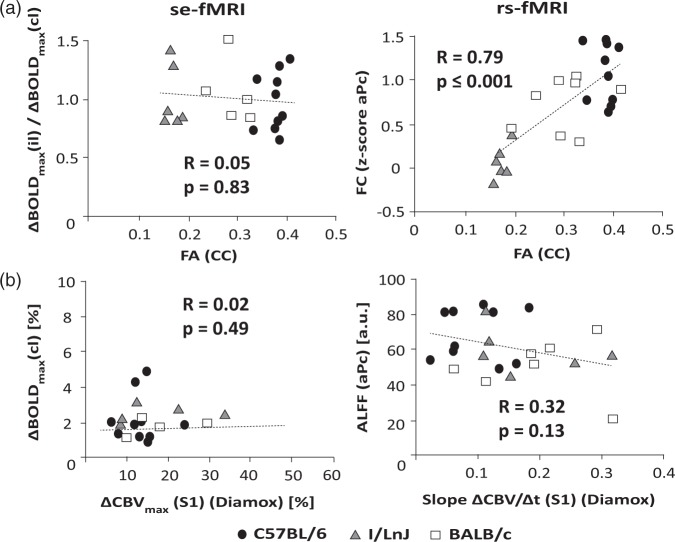
Correlating functional readouts representing the bilaterality of BOLD responses in S1HL evoked by hindpaw stimulation (ΔBOLDmax(il)/ΔBOLDmax(cl)) and rs-fMRI derived FC measures (z-score in aPc (S1)) with FA values in the CC reflecting structural connectivity indicates a strong positive correlation between FC and FA (R = 0.79) under resting-state conditions. In contrast, fMRI signal changes in S1HL upon stimulation of the animals did not show any influence of a missing CC reflected by the ratio ΔBOLDmax(il)/ΔBOLDmax(cl) versus FA value (R = 0.05; Figure 5(a)). None of the fMRI readouts (ΔBOLDmax or ALFF) showed a significant dependency on cerebrovascular parameters (vascular reserve and reactivity). Statistical analysis confirmed a significant association between FC and FA (p ≤ 0.01; Table 2), and between ΔBOLDmax(cl) and ΔCBVmax (p ≤ 0.05), whereas no further associations between fMRI readouts and other structural or vascular factors could be inferred from the analysis. CBV-fMRI analysis derived results yielded an inferior correlation between the z-scores for aPc (S1) FC and callosal FA values as compared with the correlation to the product FA ċ(ΔCBV/Δt)α, which yielded R = 0.67 for α = 0.34. Average α values have been determined for the four cortical regions (anterior, medial, and posterior parietal cortex, motor cortex) yielding α = 0.25 ± 0.07. However, this interaction effect between FA and reactive CBV was not statistically significant neither for BOLD nor CBV rs-fMRI derived FC values.
Figure 5.
Correlation of functional readouts from se- and rs-fMRI experiments with structural connectivity and cerebrovascular parameters for individual C57BL/6 (N = 10), I/LnJ (N = 5), and BALB/c (N = 7) mice. (a) Correlation of interhemispheric functional interaction with structural connectivity (FA (CC)). In se-fMRI, interhemispheric interaction is expressed by the ratio of the maximum amplitude of the BOLD response in the contra- and ipsilateral S1HL cortex (ΔBOLDmax(il)/ΔBOLDmax(cl)) following electrical hindpaw stimulation, while in rs-fMRI, it is given by the z-score for the FC in aPc (S1). Analysis reveals a strong positive correlation for FC and FA (R = 0.79) under resting-state conditions; however, no influence of a missing CC was found reflected by the correlation of ΔBOLDmax(il)/ΔBOLDmax(cl) versus FA (R = 0.05). (b) Correlation of local fMRI signal amplitudes, i.e. ΔBOLDmax(cl) derived from the se-fMRI and ALFF in aPc (S1) of the rs-fMRI signal with vascular parameters extracted from the CBV response in S1 elicited by acetazolamide (Diamox) reflecting the vascular reactivity (slope ΔCBV/Δt) and reserve (ΔCBVmax). Neither ΔBOLDmax(cl) nor ALFF in aPc (S1) measured in rs-fMRI showed any dependence on vascular reserve or vascular reactivity, respectively.
Table 2.
Correlation of fMRI readouts with structural connectivity and vascular parameters.
| (a) | Interhemispheric interaction |
|||
|---|---|---|---|---|
| se-fMRI: ΔBOLDmax(il)/ΔBOLDmax(cl) |
rs-fMRI: FC (z-score aPc (S1)) |
|||
| Factor | Variance explained (%) | p-value | Variance explained (%) | p-value |
| FA (CC) (structural connectivity) | 0.27 | 0.47 | 62.51 | 0.003 (**) |
| CBVbsl (S1) | 0.06 | 0.21 | 0.01 | 0.98 |
| Slope ΔCBV/Δt (S1) (vascular reactivity) | 0.31 | 0.96 | 0.96 | 0.59 |
| ΔCBVmax (S1) (vascular reserve) | 6.69 | 0.19 | 0.57 | 0.67 |
| Body weight | 8.11 | 0.24 | 1.12 | 0.45 |
| Residuals | 84.54 | 34.81 | ||
| (b) | Local signal amplitude |
|||
| se-fMRI: ΔBOLDmax(cl) |
rs-fMRI: ALFF in aPc (S1) | |||
| Factor |
Variance explained (%) | p-value | Variance explained (%) | p-value |
| FA (CC) (structural connectivity) | 4.52 | 0.97 | 5.41 | 0.94 |
| CBVbsl (S1) | 7.87 | 0.98 | 4.53 | 0.63 |
| Slope ΔCBV/Δt (S1) (vascular reactivity) | 3.18 | 0.33 | 9.98 | 0.20 |
| ΔCBVmax (S1) (vascular reserve) | 27.23 | 0.02 (*) | 0.04 | 0.96 |
| Body weight | 0.14 | 0.84 | 2.6 | 0.46 |
| Residuals | 57.06 | 77.44 | ||
Statistical analysis with linear models for interhemispheric interactions and local amplitudes for both se-fMRI and rs-fMRI signals as a function of structural connectivity, vascular parameters, and body weight, measured under 1.1% isoflurane using a stimulus amplitude of 0.5 mA. (a) The ratio of the maximal amplitude of the BOLD response in the contra- and ipsilateral S1HL cortex (ΔBOLDmax(il)/ΔBOLDmax(cl)) following electrical hindpaw stimulation and FC in aPc were used as metrics to represent interhemispheric interactions for se-fMRI and rs-fMRI, respectively. (b) ΔBOLDmax(cl) following hindpaw stimulation in the se-fMRI experiment and resting-state ALFF values in (aPc) S1 were selected as metrics to representing local amplitude of the fMRI signal. The following factors were selected as explanatory variables: interhemispheric structural connectivity represented by FA of the CC, CBVbsl, vascular reactivity represented by the slope ΔCBV/Δt of the CBV response elicited by the injection of acetazolamide, vascular reserve represented by the ΔCBVmax of the acetazolamide response in S1, and the animal body weights. Structural connectivity (FA (CC)) was found to have a significant association with interhemispheric FC between the aPc regions, whereas vascular reserve was significantly associated with the BOLD response evoked by sensory paw stimulation. Variance explained of each factor in the model is expressed as percentage of the total sum of square. The p-values are obtained with likelihood-test ratio, *p ≤ 0.05, **p ≤ 0.01. Significant values are highlighted in bold case.
Discussion
The purpose of the study was to investigate the influence of transcallosal communication and vascular factors on fMRI responses to peripheral stimulation and FC in three mouse strains. Electrical hindpaw stimulation using a conventional block stimulation paradigm led to widespread BOLD fMRI responses even in acallosal I/LnJ mice. Yet, the patterns of FC derived from rs-fMRI displayed a unilateral organization in the cortex of I/LnJ as compared with BALB/c and C57BL/6 mice showing bilateral homotopic FC. We found a dominant statistical association between bilateral cortical FC and structural connectivity (FA) via the CC. Combining these results, we conclude that the contribution of transcallosal communication to bilateral responses in S1 to unilateral sensory input is negligible. The lack of topological specificity indicates a dominating contribution by unspecific vascular signals due to generalized neural activity and systemic cardiovascular changes caused by a stimulus-elicited arousal response. Contributions of cerebrovascular parameters (vascular reactivity and reserve) on both the maximal BOLD response amplitude and the resting-state FC measures z-score and ALFF remained marginal.
Voltage sensitive dye imaging experiments in acallosal I/LnJ mice17 showed that—in contrast to normal callosal mice—resting-state cortical activity was less synchronous indicating that synchrony predominantly depends on the integrity of transcallosal communication. Residual temporal correlation between cortical hemispheres was suggested to arise from communication via subcortical regions, such as anterior commissure or interhemispheric thalamic connections. This is in line with our observation, which clearly indicated unilateral cortical functional patterns in I/LnJ but not in BALB/c and C57BL/6 mice. Similar results have been obtained for BTBR mice, another mouse line lacking a CC, for which unilateral cortical FC has been reported,21 albeit, contrary to our results, the BTBR mice presented bilateral FC in caudal ROIs, such as in the visual cortex. Our results also present similarities to those from a study in rats following CC sectioning,30 and two studies in humans with agenesis of the CC31 or following sectioning of the CC.32 Further, during unilateral sensory stimulation under healthy conditions voltage sensitive dye imaging did not reveal involvement of the ipsilateral hemisphere in I/LnJ mice18 in contrast to our se-fMRI findings, which excludes a neuronal basis for the ipsilateral fMRI responses. This clearly supports the hypothesis that the bilateral and widespread BOLD response to unilateral sensory stimuli in I/LnJ mice arises from arousal-associated vascular responses. In fact, neither the maximal amplitude of the BOLD response in the contralateral S1HL cortex nor the ratio of the response between contra- and ipsilateral cortices could be associated with FA in the CC region as measure of interhemispheric structural connectivity.
Because of its hemodynamic nature changes in baseline CBV and reactive CBV are likely to modulate the shape and amplitude of BOLD responses and FC metrics.33 In view of this, it has been proposed to use measures of these vascular parameters for normalizing the BOLD response.34,35 Yet we did not observe a dependence of the stimulus-evoked BOLD amplitude on vascular reactivity and/or reserve. We speculate that the BOLD signal change is not primarily mitigated by the neurovascular coupling mechanism but rather reflects a passive vasodilation imposed by peripheral changes in perfusion pressure overruling cerebral autoregulation. In the case of rs-fMRI, ALFF represents a likely candidate to reflect vascular characteristics because of their direct effect on fluctuation amplitudes. In fact, group average maps indicate higher ALFF values in regions co-localizing with major arteries (Suppl. Fig. S4). However, cortical ALFF extracted from different ROIs could not be statistically associated to any of the vascular parameters considered in this study. Interhemispheric FC, however, showed a weak dependence on vascular parameters. Including a vascular reactivity term assuming α = 0.25 ± 0.07, a value in the range of the Grubb constant of the power law relating CBV and cerebral blood flow (CBF),36,37 improved the correlation between CBV-based interhemispheric FC values and structural connectivity. This might capture effects of neurovascular coupling on FC values, though the result did not reach statistical significance. This observation echoes our previous work, which concluded that baseline CBV could not explain the different FC patterns associated with different anesthetic regimens.22
When analyzing intra- and interhemispheric FC in the three mouse lines, we observed differences, with C57BL/6 mice yielding the significantly higher values for correlation coefficients as compared with I/LnJ mice, and BALB/c mice ranging in-between. Several factors might account for this: (a) Differences in nature/strength of FC might be due to the already discussed differences in structural connectivity with FA values decreasing in the order C57BL/6, BALB/c, and I/LnJ mice. (b) FC analysis measures the temporal correlation of hemodynamic signal fluctuations as surrogate of fluctuations in neural activity. Correspondingly, intrinsic or anesthesia-induced differences in the efficiency of neurovascular coupling across the strains might affect the results. (c) The pattern of spontaneous neural activity might differ across mouse strains due to intrinsic differences or due to different susceptibility to the anesthetic used. Despite identical experimental conditions, we observed I/LnJ and BALB/c mice to react more strongly to mild electrical stimulation compared with C57BL/6 mice indicative of a reduced level of anesthesia reached. We have not directly recorded anesthesia depth, however, received an indication by analyzing the reflex responses to hindpaw stimulation in bench-top experiments. Being aware of potential issues arising from different susceptibility of the strains to anesthesia, we evaluated se-fMRI responses for different concentrations of isoflurane. Anesthesia levels did not affect the qualitative features of the fMRI responses across the three strains in both se- and rs-fMRI. Anesthesia depth only had an effect on the quantitative aspects of the signals, i.e. amplitudes of evoked responses and spontaneous fluctuations as well as on the degree of interindividual variability.
In summary, we investigated the effect of structural connectivity and cerebrovascular parameters on the stimulus-evoked BOLD responses and cortical FC at rest in mice. Whereas the influence of baseline and reactive hemodynamic parameters on any of the fMRI readouts was minimal, FA reflecting structural connectivity was found to correlate significantly with FC values. The observation of widespread and almost symmetric bilateral BOLD signal changes in acallosal I/LnJ mice confirms that the contribution of specific neuronal processing of peripheral input to the se-fMRI response is negligible. In contrast to rs-fMRI measurements, during which variations in physiological state stay within a range that can be handled by cerebrovascular autoregulation warranting the integrity of neurovascular coupling of the association of BOLD signals and neural activity, se-fMRI measurements in mice are contaminated by stress-induced changes in cerebral hemodynamics that outweigh the effects elicited via neurovascular coupling. Consequently, changes in BOLD signal in se-fMRI measurements in mice using conventional block paradigms for stimulation cannot be directly related to changes in neural activity associated to stimulus processing, whereas FC appears to reflect the underlying neuroarchitecture.
Supplementary Material
Acknowledgements
None of the material contained in this manuscript has been published or presented previously, except in abstract form at international conferences. This paper has not been submitted for publication elsewhere, and it has been reviewed and approved by all the authors.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Swiss National Science Foundation (Grant number SNF 310030_160310).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
A.S. designed the study; A.S., J.G., and B.J.S. performed the experiments; A.S., J.G., and F.S. analyzed the data; A.S., J.G., and M.R. wrote the manuscript; all authors contributed to and approved the final manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Hyder F, Behar KL, Martin MA, et al. Dynamic magnetic resonance imaging of the rat brain during forepaw stimulation. J Cereb Blood Flow Metab 1994; 14: 649–655. [DOI] [PubMed] [Google Scholar]
- 2.Peeters RR, Verhoye M, Vos BP, et al. A patchy horizontal organization of the somatosensory activation of the rat cerebellum demonstrated by functional MRI. Eur J Neurosci 1999; 11: 2720–2730. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Ramos-Cabrer P, Wiedermann D, et al. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage 2006; 29: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 4.Masamoto K, Kim T, Fukuda M, et al. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex 2007; 17: 942–950. [DOI] [PubMed] [Google Scholar]
- 5.Lowe AS, Beech JS, Williams SCR. Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation. Neuroimage 2007; 35: 719–728. [DOI] [PubMed] [Google Scholar]
- 6.Huttunen JK, Gröhn O, Penttonen M. Coupling between simultaneously recorded BOLD response and neuronal activity in the rat somatosensory cortex. Neuroimage 2008; 39: 775–785. [DOI] [PubMed] [Google Scholar]
- 7.Zhao F, Zhao T, Zhou L, et al. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage 2008; 39: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sydekum E, Baltes C, Ghosh A, et al. Functional reorganization in rat somatosensory cortex assessed by fMRI: elastic image registration based on structural landmarks in fMRI images and application to spinal cord injured rats. Neuroimage 2009; 44: 1345–1354. [DOI] [PubMed] [Google Scholar]
- 9.Ahrens ET, Dubowitz DJ. Peripheral somatosensory fMRI in mouse at 11.7 T. NMR Biomed 2001; 14: 318–324. [DOI] [PubMed] [Google Scholar]
- 10.Adamczak JM, Farr TD, Seehafer JU, et al. High field BOLD response to forepaw stimulation in the mouse. Neuroimage 2010; 51: 704–712. [DOI] [PubMed] [Google Scholar]
- 11.Nasrallah FA, Tay H-C, Chuang K-H. Detection of functional connectivity in the resting mouse brain. Neuroimage 2014; 86: 417–424. [DOI] [PubMed] [Google Scholar]
- 12.Bosshard SC, Baltes C, Wyss MT, et al. Assessment of brain responses to innocuous and noxious electrical forepaw stimulation in mice using BOLD fMRI. Pain 2010; 151: 655–663. [DOI] [PubMed] [Google Scholar]
- 13.Bosshard SC, Stuker F, von Deuster C, et al. BOLD fMRI of C-fiber mediated nociceptive processing in mouse brain in response to thermal stimulation of the forepaws. PLoS One 2015; 10: e0126513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeter A, Schlegel F, Seuwen A, et al. Specificity of stimulus-evoked fMRI responses in the mouse: the influence of systemic physiological changes associated with innocuous stimulation under four different anesthetics. Neuroimage 2014; 94: 372–384. [DOI] [PubMed] [Google Scholar]
- 15.Schlegel F, Schroeter A, Rudin M. The hemodynamic response to somatosensory stimulation in mice depends on the anesthetic used: Implications on analysis of mouse fMRI data. Neuroimage 2015; 116: 40–49. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland MT. The hand and the ipsilateral primary somatosensory cortex. J Neurosci 2006; 26: 8217–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohajerani MH, McVea DA, Fingas M, et al. Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J Neurosci 2010; 30: 3745–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohajerani MH, Aminoltejari K, Murphy TH. Targeted mini-strokes produce changes in interhemispheric sensory signal processing that are indicative of disinhibition within minutes. Proc Natl Acad Sci USA 2011; 108: E183–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995; 34: 537–541. [DOI] [PubMed] [Google Scholar]
- 20.Kalthoff D, Po C, Wiedermann D, et al. Reliability and spatial specificity of rat brain sensorimotor functional connectivity networks are superior under sedation compared with general anesthesia. NMR Biomed 2013; 26: 638–650. [DOI] [PubMed] [Google Scholar]
- 21.Sforazzini F, Bertero A, Dodero L, et al. Altered functional connectivity networks in acallosal and socially impaired BTBR mice. Brain Struct Funct 2016; 221: 941–954. [DOI] [PubMed] [Google Scholar]
- 22.Grandjean J, Schroeter A, Batata I, et al. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage 2014; 102 (Pt 2): 838–847. [DOI] [PubMed] [Google Scholar]
- 23.Zerbi V, Grandjean J, Rudin M, et al. Mapping the mouse brain with rs-fMRI: An optimized pipeline for functional network identification. Neuroimage 2015; 123: 11–21. [DOI] [PubMed] [Google Scholar]
- 24.Chan KC, Fan S-J, Chan RW, et al. In vivo visuotopic brain mapping with manganese-enhanced MRI and resting-state functional connectivity MRI. Neuroimage 2014; 90: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandjean J, Schroeter A, He P, et al. Early alterations in functional connectivity and white matter structure in a transgenic mouse model of cerebral amyloidosis. J Neurosci 2014; 34: 13780–13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahlsten D. Heritable aspects of anomalous myelinated fibre tracts in the forebrain of the laboratory mouse. Brain Res 1974; 68: 1–18. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G and Franklin KB. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press/Elsevier Science, 2004.
- 28.Mueggler T, Baumann D, Rausch M, et al. Bicuculline-induced brain activation in mice detected by functional magnetic resonance imaging. Magn Reson Med 2001; 46: 292–298. [DOI] [PubMed] [Google Scholar]
- 29.Kim S-G, Harel N, Jin T, et al. Cerebral blood volume MRI with intravascular superparamagnetic iron oxide nanoparticles. NMR Biomed 2013; 26: 949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnuson ME, Thompson GJ, Pan W-J, et al. Effects of severing the corpus callosum on electrical and BOLD functional connectivity and spontaneous dynamic activity in the rat brain. Brain Connect 2014; 4: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quigley M, Cordes D, Turski P, et al. Role of the corpus callosum in functional connectivity. AJNR Am J Neuroradiol 2003; 24: 208–212. [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston JM, Vaishnavi SN, Smyth MD, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci 2008; 28: 6453–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu TT. Neurovascular factors in resting-state functional MRI. Neuroimage 2013; 80: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen ER, Rostrup E, Sidaros K, et al. Hypercapnic normalization of BOLD fMRI: comparison across field strengths and pulse sequences. Neuroimage 2004; 23: 613–624. [DOI] [PubMed] [Google Scholar]
- 35.Thomason ME, Foland LC, Glover GH. Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Hum Brain Mapp 2007; 28: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen JJ, Pike GB. BOLD-specific cerebral blood volume and blood flow changes during neuronal activation in humans. NMR Biomed 2009; 22: 1054–1062. [DOI] [PubMed] [Google Scholar]
- 37.Grubb RL, Raichle ME, Eichling JO, et al. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 1974; 5: 630–639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.