Abstract
Familial dysautonomia is an inherited autonomic disorder with afferent baroreflex failure. We questioned why despite low blood pressure standing, surprisingly few familial dysautonomia patients complain of symptomatic hypotension or have syncope. Using transcranial Doppler ultrasonography of the middle cerebral artery, we measured flow velocity (mean, peak systolic, and diastolic), area under the curve, pulsatility index, and height of the dictrotic notch in 25 patients with familial dysautonomia and 15 controls. In patients, changing from sitting to a standing position, decreased BP from 124 ± 4/64 ± 3 to 82 ± 3/37 ± 2 mmHg (p < 0.0001, for both). Despite low BP, all patients denied orthostatic symptoms. Middle cerebral artery velocity fell minimally, and the magnitude of the reductions were similar to those observed in healthy controls, in whom BP upright did not fall. While standing, patients had a greater fall in cerebrovascular resistance (p < 0.0001), an increase in pulsatility (p < 0.0001), and a deepening of the dicrotic notch (p = 0.0010), findings all consistent with low cerebrovascular resistance. No significant changes occurred in controls. Patients born with baroreflex deafferentation retain the ability to buffer wide fluctuations in BP and auto-regulate cerebral blood flow. This explains how they can tolerate extremely low BPs standing that would otherwise induce syncope.
Keywords: Familial dysautonomia, orthostatic hypotension, afferent baroreflex failure, cerebral blood flow, transcranial Doppler, cerebral hemodynamics
Introduction
Familial dysautonomia (FD, OMIM #223900) is a genetic disease that affects the development of the nervous system during embroygenesis.1,2 Particularly affected are the afferent fibers within the glossopharyngeal and vagus nerves, which relay information from the arterial baroreceptors to the brainstem.3 As a consequence, from birth, patients cannot regulate sympathetic vasomotor outflow and have extreme fluctuations in blood pressure.4–6
In affected patients, the increase in sympathetic activity that occurs on standing, when venous return to the heart falls, is absent. Thus, arterial resistance and heart rate fail to increase, and patients often have severe hypotension when standing.3,7 Yet, despite having very low blood pressure, they seldom complain of symptoms of hypotension.8 What is not immediately apparent is whether these patients fail to recognize the warning signs of systemic hypotension or they do not develop severe cerebral hypoperfusion due to an enhanced adaptive cerebrovascular auto-regulatory capacity.
Little is known about the long-term impact of congenital lesions of the autonomic nervous system on cerebrovascular control. Most studies in patients with FD were interpreted as indicating abnormal cerebrovascular auto-regulatory control,9–13 but this is at odds with the clinical phenotype. First, despite pronounced hypertension with systolic blood pressures frequently exceeding 200 mmHg, hemorrhagic stroke is rare;14 and second, despite severe orthostatic hypotension the incidence of syncope is surprisingly low.3,8 This lead us to hypothesize that patients with FD can buffer wide fluctuations in perfusion pressure thanks to an expanded cerebrovascular auto-regulatory capacity.
We set out to examine the control of cerebral blood flow during orthostatic hypotension in patients with FD and explore its relationship with symptoms.
Materials and methods
Participants
Between December 2014 and May 2016, we enrolled 25 patients with FD who had molecular confirmation of the FD founder splice mutation within the IKBKAP gene (IVS20 + 6T > C).2 All were recruited from our FD Patient Registry at New York University’s Dysautonomia Center. All patients had typical clinical histories and an autonomic phenotype consistent with baroreflex deafferentation, including labile blood pressure on ambulatory monitoring, excessive hypertensive reactions to mild anxiety, and orthostatic hypotension.7,15 We also enrolled 15 age- and sex-matched controls with no history of cardiovascular, neurological, or autonomic disorders. Controls were recruited from a pool of volunteers. All were free of systemic illness and were not undergoing autonomic testing for reasons other than this study. All procedures were approved by the New York University School of Medicine Institutional Review Board. Informed consent (or ascent) was obtained in all cases. The procedures were carried out in accordance with the Declaration of Helsinki. No participants received financial compensation for their involvement in the study.
Protocol and measurements
Participants were free of caffeine, nicotine, and alcohol from the previous evening. All measurements were non-invasive and carried out in the morning by trained investigators with medical staff supervision in a temperature-controlled dedicated autonomic laboratory.
Static blood pressure measurements were obtained at 1-min intervals on the left arm with an appropriate sized cuff (Colin PressMate 880P, USA). Continuous blood pressure was measured by finger plethsymography (Finometer, FMS, Netherlands) with the hand supported at heart level. RR intervals were measured with three chest wall electrodes. End-tidal CO2 levels were sampled breath-by-breath using a nasal cannula and measured with infrared analysis (Ohmeda 5200, BOC Healthcare, Inc., Manchester, UK). The middle cerebral artery (MCA) was visualized through the temporal window by an experienced sonographer using a 2 MHz transcranial Doppler ultrasound probe at an isonation depth of 45 mm or more (Multi-Dop T2 Digital, DWL, Singen, Germany). The angle between the ultrasound beam and the direction of flow was as close to 0° as possible. Once an optimal waveform was obtained, the probe angle was locked in place on a custom-built headband.
Subjects rested for 20 min in the seated position while signals were acquired. They were then instructed to stand immobile for 5 min while measurements were obtained upright.
Symptoms of cerebral hypoperfusion were measured using the Orthostatic Hypotension Questionnaire (OH-Q), a validated patient-reported symptom assessment scale.16 Patients were asked to report symptoms of dizziness, light-headedness, or feeling like they might black out (OH-Q, item 1) at 60-s intervals throughout the standing test.
Data analysis
All data were de-identified. Mean blood pressure at brain level was estimated from mean blood pressure at the arm corrected for the hydrostatic pressure difference at the ultrasound probe using a standardized formula.17,18
The MCA waveform was sampled at a rate of 500 Hz (PowerLab 16SP hardware and LabChart 7 software; ADInstruments). Systolic, diastolic, and mean MCA velocities were measured from the waveform, as described.19 Area under the curve was calculated from the continuous mean MCA velocity over 5 min while sitting and standing. An index of cerebral vascular resistance (CVR) was calculated by dividing estimated mean pressure at brain level by mean MCA flow velocity.
Pulsatile components were determined from the MCA waveform. A total of five MCA beats were measured and averaged at the time that the brachial blood pressure values were acquired.17,20 Pulsatility index was calculated by subtracting the end diastolic velocity from the peak systolic velocity and dividing by mean velocity.19 Flow acceleration was calculated by subtracting peak systolic velocity from end systolic velocity and dividing by systolic upstroke time.21 The dicrotic notch was measured as the lowest velocity at the small downward deflection in the MCA waveform after the end of systole.22 The MCA waveform morphology was further examined by measuring flow velocity at six key inflection points; identifiable as the three distinctive peaks (P1, P2, and P3) and three troughs (T1, T2, and T3).23
To examine the influences of end-tidal CO2 on the cerebral circulation, we calculated the slope of the linear regression between MCA velocity and end-tidal CO2 and compared the steepness in patients and controls. As a measure of peripheral vascular responsiveness, we calculated the relationship between mean blood pressure and end-tidal CO2.
Data were first tested for normality using the Shapiro–Wilk and Kolmogorov–Smirnov tests. Descriptive statistics were used to summarize the data. Parametric and nonparametric evaluations were used as appropriate. Differences within groups (supine vs. standing) were compared with paired two-tailed t-tests. Differences between groups (patients vs. controls) were first expressed as percentage change and then compared using unpaired two-tailed t-tests and two-way analysis of variance (ANOVA). Linear regression analysis was used to assess the relationship between end-tidal CO2 vs. MCA blood flow velocity and end-tidal CO2 vs. mean blood pressure. Correlations between variables were determined using Pearson correlation coefficients. Data were analyzed using Prism (v6.0; GraphPad Software, San Diego, CA). All p values were two-tailed, and p < 0.05 was considered statistically significant. Data are expressed as mean ± standard error of the mean, unless otherwise stated.
Results
Baseline characteristics
The baseline characteristics of the patients and controls are listed in Table 1. There were no statistical differences between the groups in terms of age and sex. Compared with controls, patients with FD had a shorter stature (p = 0.0510) and a lower body weight (p = 0.0147), which are two well-characterized traits of the disease.4 Blood pressure in the seated position was higher in patients than in controls (p = 0.0403). The partial pressure of CO2 in expired air was also significantly higher in patients (42 ± 2 mmHg) compared with controls (34 ± 1 mmHg, p < 0.0001). This was not an unexpected finding, as neurogenic hypoventilation is also a characteristic of FD.8
Table 1.
Subject characteristics and response to standing.
| Controls | FD patients | |||||
|---|---|---|---|---|---|---|
| n | 15 | 25 | ||||
| Male:female | 5:10 | 13:12 | ||||
| Age (years) | 25 ± 2 | 23 ± 2 | ||||
| Height (m) | 168 ± 2 | 152 ± 3 | ||||
| Weight (kg) | 58 ± 4 | 44 ± 2 | ||||
| BMI | 20 ± 0.8 | 19 ± 0.5 | ||||
|
|
Sitting | Standing | p value | Sitting | Standing | p value |
| Cardiovascular data | ||||||
| Systolic BP (mmHg) | 111 ± 3 | 114 ± 3 | 0.0544 | 124 ± 4 | 82 ± 3 | <0.0001 |
| Diastolic BP (mmHg) | 59 ± 2 | 59 ± 2 | 0.9734 | 64 ± 3 | 37 ± 2 | <0.0001 |
| Mean BP (mmHg) | 76 ± 2 | 77 ± 2 | 0.5344 | 84 ± 4 | 52 ± 2 | <0.0001 |
| Heart rate (bpm) | 72 ± 4 | 83 ± 4 | <0.0001 | 84 ± 3 | 85 ± 3 | 0.1414 |
| End-tidal CO2 (mmHg) | 34 ± 1 | 31 ± 1 | <0.0001 | 42 ± 2 | 41 ± 1 | 0.3154 |
| MCA velocity | ||||||
| Mean (cm/s) | 60 ± 4 | 56 ± 4 | 0.0010 | 57 ± 3 | 51 ± 3 | 0.0002 |
| Systolic (cm/s) | 86 ± 7 | 83 ± 7 | 0.3039 | 86 ± 6 | 91 ± 6 | 0.1197 |
| Diastolic (cm/s) | 36 ± 2 | 36 ± 3 | 0.8123 | 34 ± 2 | 25 ± 2 | <0.0001 |
| Resistance index (cm/s) | 0.9 ± 01 | 1.0 ± 0.1 | 0.5442 | 1.1 ± 0.1 | 0.5 ± 0.1 | <0.0001 |
| MCA waveform characteristics | ||||||
| Dichrotic notch (cm/s) | 57 ± 5 | 54 ± 6 | 0.0815 | 47 ± 3 | 30 ± 2 | <0.0001 |
| Pulsatility index (units) | 0.8 ± 0.05 | 0.8 ± 0.06 | 0.8965 | 0.9 ± 0.1 | 1.3 ± 0.1 | <0.0001 |
| Flow acceleration (units) | 0.8 ± 0.07 | 0.8 ± 0.08 | 0.7948 | 0.8 ± 0.1 | 0.9 ± 0.1 | <0.0001 |
| Systolic upstroke (units) | 68 ± 4 | 66 ± 4 | 0.2747 | 71 ± 3 | 73 ± 4 | 0.7043 |
| Symptoms | ||||||
| Dizziness/lightheadedness | – | 0 | – | 0 | ||
| Syncope | – | None | – | None | ||
Data are mean ± SEM. The p values are for comparison of sitting and standing values in patients and controls.
BMI: body mass index; BP: blood pressure; MCA: middle cerebral artery.
Cardiovascular response to orthostatic stress
All healthy controls had a normal response to standing, with no significant change in systolic (p = 0.1462) or diastolic (p = 0.5339) blood pressure (Table 1). After 5 min of standing, heart rate increased significantly by + 11 ± 3 bpm (from 72 to 83 bpm, p < 0.0001). End-tidal CO2 levels declined significantly (from 34 ± 1 to 31 ± 1 mmHg standing, p < 0.0001). None of the controls reported symptoms of orthostatic dizziness/light-headedness while upright.
In contrast to controls, patients with FD did not have a normal response to standing. All met consensus criteria for orthostatic hypotension.24 As shown in Figure 1, systolic blood pressure fell markedly by 42 ± 3 mmHg (p < 0.0001) with absolute systolic values ranging from 65 to 105 mmHg. Diastolic pressure fell similarly by 26 ± 3 mmHg (p < 0.0001) and absolute values ranged from 17 to 65 mmHg. There was no significant increase in heart rate accompanying the fall in blood pressure. Indeed, 85% of patients with FD had slowing of the heart rate accompanying the fall in blood pressure, as previously described.3 In contrast to controls, patients did not decrease end-tidal CO2 levels when standing, and remained more hypercapnic (CO2 standing: FD 41 ± 1 vs. controls 31 ± 1 mmHg, p < 0.0001). Despite very low blood pressure standing, patients were asymptomatic, denied feeling the sensation of orthostatic dizziness/lightheadedness, and all were able to tolerate at least 5 min standing without syncope.
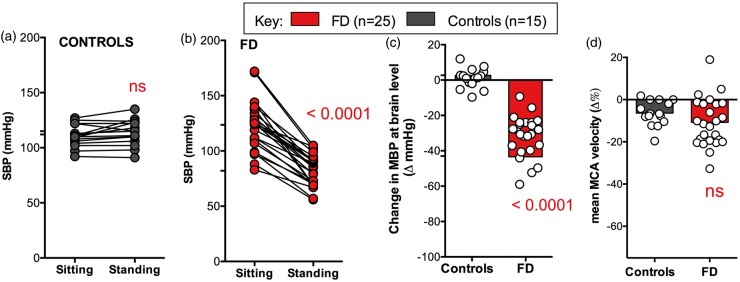
Figure 1.
Cardiovascular responses to orthostatic stress. (a) Shows the normal response to standing in healthy controls. (b) Shows the significant fall in systolic blood pressure on standing in all patients with FD. (c) Shows the significant decrease in estimated blood pressure at brain level induced by standing in patients with FD. (d) Shows that despite severe systemic hypotension while standing, patients with FD had only minimal changes in mean MCA velocity that were comparable with normal controls without hypotension. SBP: systolic blood pressure; FD: familial dysautonomia; MCA: middle cerebral artery. Data are mean ± s.e.m.
Cerebrovascular responses to orthostatic stress
To determine whether the lack of symptoms on standing in patients with FD was due to preserved cerebral blood flow, we examined the effects of orthostatic stress on the transcranial Doppler measurements.
Typical examples of the MCA responses from a patient and a control are shown in Figure 2. Seated systolic (p = 0.3549), diastolic (p = 0.4689), and mean (p = 0.8890) flow velocities were similar at baseline. In response to standing, estimated blood pressure at brain level fell significantly in patients (Figure 1, p < 0.0001), but not in controls. To compensate for the severe orthostatic hypotension, the decrease in cerebrovascular resistance was significantly greater in patients than in controls (−50 ± 2 vs. 10 ± 3 Δ%, p < 0.0001). As a result, changes in mean MCA velocity were minimal (−6.4 ± 1.5 Δ%) and comparable with controls (vs. −10.8 ± 1.9 Δ%, p = 0.1756). Changes in MCA area under the curve were also not different (Figure 3, p = 0.9407).
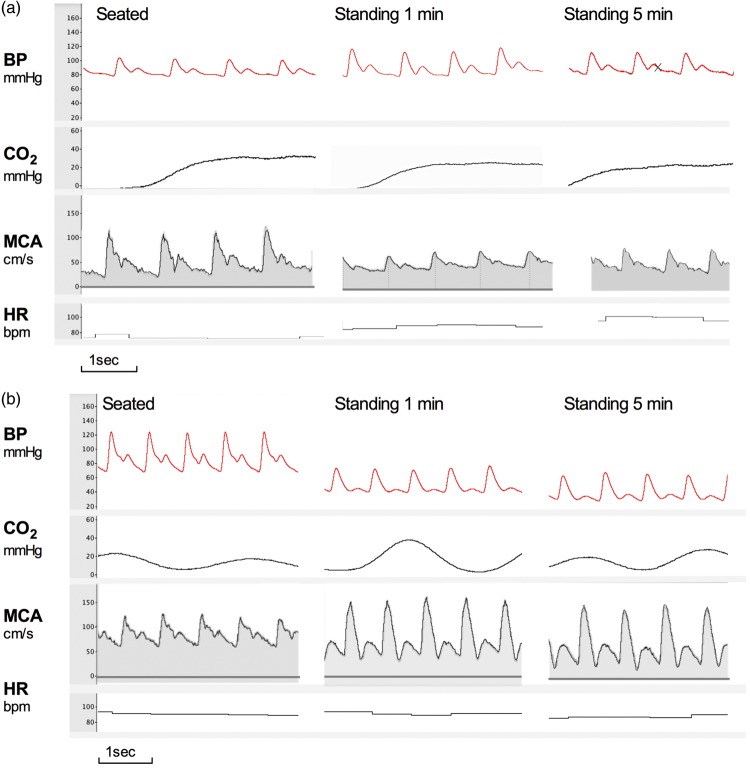
Figure 2.
Typical examples continuous blood pressure and MCA velocity. (a) Top panel shows tracings of beat-to-beat blood pressure, heart rate, and MCA velocity with accompanying end-tidal CO2 measurements in a 22-year-old healthy female control sitting and standing. (b) Bottom panel shows the same measurements obtained in a 20-year-old female patient with familial dysautonomia. Note the severe progressive orthostatic blood pressure fall, without compensatory tachycardia in the patient. Despite blood pressure being 60/30 mmHg, she did not complain of symptoms of cerebral hypoperfusion. Systolic and mean MCA flow velocity remained well preserved. The MCA velocity waveform shows increased pulsatility and deeper dichrotic notch, indicating low resistance in the cerebral arterioles to compensate for the decline in stroke volume. BP: blood pressure; CO2: end-tidal CO2 levels; MCA: middle cerebral artery; HR: heart rate. Images were obtained from raw tracings recorded with PowerLab and processed with LabChart 7 (AD Instruments, Dunedin, New Zealand).
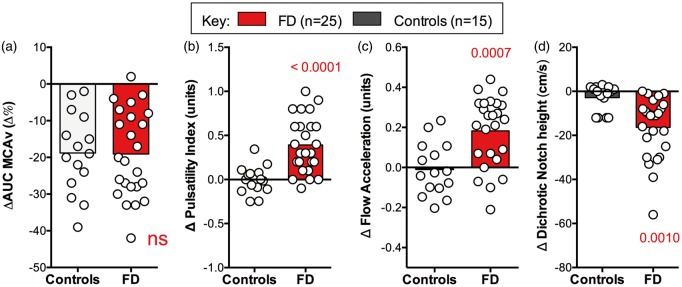
Figure 3.
Cerebrovascular responses to orthostatic stress. (a) Shows percentage change in MCA area under the curve induced by standing, which were minimal and similar in patients and controls. (b) Shows significant increase in pulsatility index induced by orthostatic stress in patients with FD, which was not observed in healthy controls. (c) Shows the significant increase in flow acceleration upright in patients with FD. (d) Shows the significant decline in the height of the dichrotic notch in patients with FD while upright. AUC: area under the curve; MCAv: middle cerebral artery velocity (transcranial Doppler); FD: familial dysautonomia. Data are mean ± s.e.m. See text for details.
Regression analysis in patients did not show a relationship between the severity of hypotension and the decline in mean MCA velocity (R2 = 0.0618, p = 0.2307) or area under the curve (R2 = 0.0168, p = 0.5466). There was no relationship between the changes in MCA velocity and how fast the blood pressure fell within the first minute of standing (R2 = 0.0035, p = 0.7764).
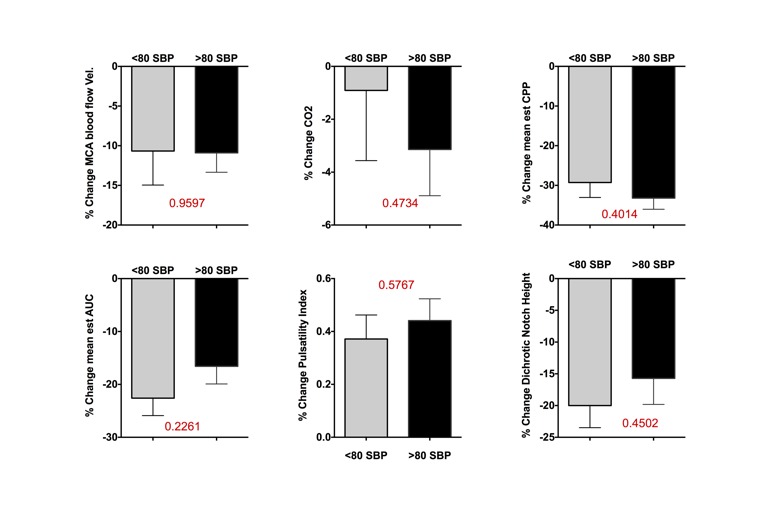
Furthermore, we divided patients into two groups: (a) those with less severe orthostatic hypotension (systolic blood pressures standing > 80 mmHg) and (b) those with more severe orthostatic hypotension (systolic blood pressures standing < 80 mmHg). Yet, we found no difference in change in MCA blood flow velocity (p = 0.9597) or MCA AUC (p = 0.226, supplemental figure). Taken together, the findings indicate that with a congenital autonomic lesion, cerebral auto-regulatory capacity can be expanded to regulate blood flow.
To further explore the adaptive mechanisms of the cerebral vessels, we examined the morphology of the MCA waveform. As shown in Figures 2 and 4, when perfusion pressure was low on standing in patients with FD, MCA flow velocity during systole was preserved (p = 0.7563), but during diastole was reduced by −32.9 ± 8.4 Δ% (p < 0.0001). During hypotension, the MCA waveform became more pulsatile, with a significant increase in pulsatility index from sitting to standing (p = 0.0041, Figure 4). There was also a noticeable deepening of the dicrotic notch and a decrease in the height of the dicrotic wave (Table 1, Figures 2 and 4). These finding are all consistent with low resistance in the downstream cerebral arterioles.
Figure 4.
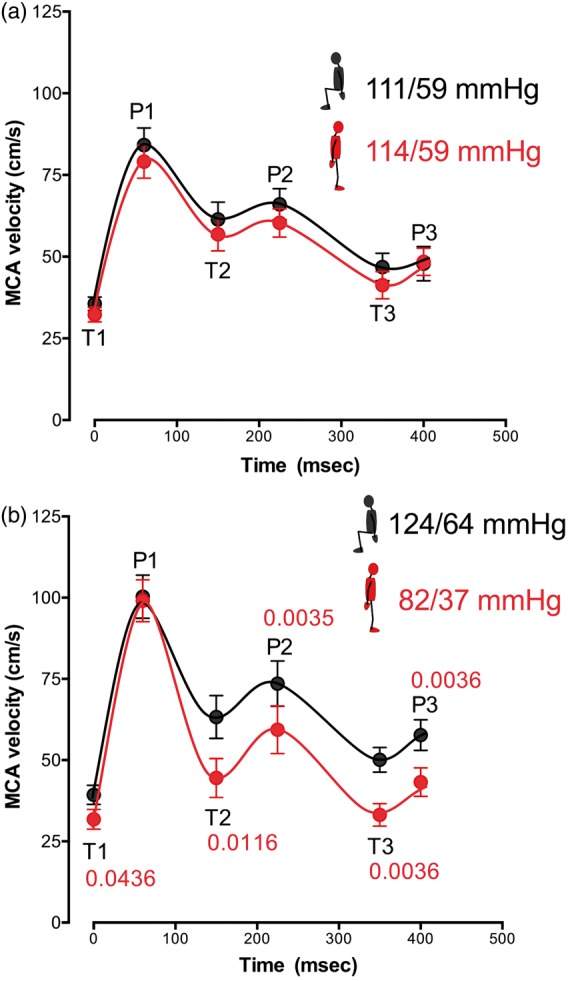
MCA velocity. Key inflection points were identified at three peaks (P1: systolic peak; P2: reflected peak; and P3: following peak) and three troughs (T1: systolic velocity; T2: following trough; and T3: dichrotic notch). Data are averaged for 15 controls (upper panel, (a)) and 25 FD patients (lower panel, (b)), sitting (black lines), and standing (red lines). Average brachial blood pressure sitting (black) and standing (red) at the time of analysis are included. When perfusion pressure was low on standing in patients with FD, systolic flow was maintained while diastolic flow was reduced (panel (b)). Note also the deepening of the dichrotic notch in patients with FD when standing with hypotension. MCA: middle cerebral artery; MCA: middle cerebral artery blood flow velocity; BP: blood pressure. Data are mean ± s.e.m.
Influence of CO2
There was a significant positive correlation between end-tidal CO2 and MCA blood flow velocity in patients with FD (R = 0.820 ± 0.025, p = 0.003) and controls (R = 0.831 ± 0.045, p = 0.014). The slope of the relationship was similar in both groups (FD 0.988 ± 0.247 vs. 1.804 ± 0.580 cm.s/mmHg, p = 0.156). This suggests that patients have a normal responsiveness of the cerebral circulation to changes in end-tidal CO2.
A significant positive correlation between end-tidal CO2 and blood pressure was found in 67% of patients with FD (16/24, data excluded in one subject), but in only 13% of controls (2/15). This would suggest that in patients with FD, because of the absence of arterial baroreceptor buffering, changes in end-tidal CO2 have a greater influence on peripheral vasculature tone and blood pressure.
Discussion
This study shows, for the first time, that patients born with baroreflex deafferentation retain the ability to buffer wide fluctuations in arterial pressure and auto-regulate cerebral blood flow. Our data show that the ability of patients with FD to tolerate very low blood pressure is, at least in part, due to intact vasodilatation of the downstream cerebral arterioles and an expanded auto-regulatory range. This compensatory response allows patients with FD to remain asymptomatic with standing blood pressures that, in the general population, would typically result in severe orthostatic symptoms or syncope.17,20,25
Previous studies of cerebral blood flow regulation in patients with FD are limited to small case series. All have postulated abnormalities in cerebrovascular control and auto-regulatory failure.9–13 Early studies using Xenon-133 inhalation found resting regional cerebral blood flow to be increased in five patients10 and areas of focal hyperperfusion were visualized with perfusion SPECT imaging during a hypertensive vomiting crisis in two patients.11 Both studies were interpreted as indicating low cerebral vasoconstrictor tone due to sympathetic denervation. Transcranial Doppler measurements contradict this and were interpreted as showing increased cerebrovascular tone in the supine and upright positions, and during the Valsalva maneuver,12,13 which lead to the speculation that the cerebral vessels were more rigid.9
The changes in the MCA waveform documented in patients with FD at the time of low blood pressure are similar to those observed during pre-syncope just before an impending vasovagal syncope,22 and are indicative of low cerebrovascular tone.26 In both cases, the cerebral vessels appear to be maximally dilated to compensate for the low driving force owing to reduced stroke volume on standing.22,27 On the rare instance that syncope does occur in patients with FD, it usually happens in the setting of a gastrointestinal bleed, severe dehydration, or severe hypoxia. This suggests that patients may have little remaining autoregulatory reserve to compensate for additional aggravating factors that reduce intravascular volume or decrease blood oxygen levels.
Earlier transcranial Doppler studies performed in patients with FD showed that the direct metabolic effect of CO2 on cerebral vessels is intact.13 In this study, we were also able to demonstrate that the cerebral vessels responded appropriately to changes in end-tidal CO2. It appears likely that CO2-induced cerebrovascular dilatation is playing a role in preserving blood flow when standing. Unlike patients with efferent autonomic failure28 or those with recurrent vasovagal syncope,17 patients with FD do not develop hypocapnia on standing. This may allow the cerebral arterioles to remain relaxed, maintaining low resistance to preserve flow in the setting of hypotension.17 Higher levels of end-tidal CO2 should also help maintain critical closing pressure low to preserve diastolic blood flow in the setting of hypotension.29
Perfusion of the brain depends on the pressure gradient between the arteries and the veins (CPP). Cerebral venous pressure is difficult to measure but it approximates to the more easily measured intracranial pressure (ICP). Cerebral perfusion pressure (CPP) is estimated by subtracting ICP from mean arterial pressure (CPP = MAP − ICP). On standing, pressure within the cerebral veins and sinuses in patients with FD is likely to be very low.30 The fall in ICP may act as a further driving force, creating a negative pressure gradient “suctioning” blood through the cerebral arterioles. As recent work indicates that ICP falls on standing more than previously estimated negative ICP and may be an important contributing mechanism to maintain CPP during orthostasis even in normal subjects.31 Unfortunately, ICP cannot be estimated reliably non-invasively.32,33
Unlike more common autonomic disorders, FD is a hereditary sensory and autonomic neuropathy (HSAN), expressed at birth.34 To put our results into perspective, despite having an expanded autoregulatory capacity,35 elderly patients with Parkinson disease and orthostatic hypotension develop symptoms of cerebral hypoperfusion when their mean blood pressure at the heart level falls below 75 mmHg,25 whereas patients with FD remain asymptomatic at this level of blood pressure. Healthy controls experience near syncope when systolic blood pressure at the heart level reaches 80 mmHg and cerebral blood flow declines by 50%.36 Patients with FD remain asymptomatic at systolic pressures at the heart level well below 80 mmHg and have only a 10% decline in MCA velocity. Moreover, patients with FD who have systolic blood pressures below 80 mmHg do not have a greater fall in MCA velocity than those that maintained their systolic blood pressure above 80 mmHg. This further emphasizes the ability of patients with HSANs to compensate for deficiencies in sympathetic activity on standing.37 Whether the cerebrovascular auto-regulatory capacity of patients with FD differs to that of other autonomic disorders is something that should be explored.
Our study has limitations. It is possible that patients with FD may have had subtle signs of cerebral hypoperfusion, which might have been revealed by testing cognitive performance. However, pressor responses to CNS arousal are markedly exaggerated in afferent baroreflex failure, and formally testing cognitive performance would have instantly resulted in increased sympathetic outflow and blood pressure.3 We did not use a tilt table test as a means to administer orthostatic stress in our patients with FD, as they are known to have undue hypertensive reactions to medical procedures. We considered the active standing test closer to the real-life stress encountered by the patients on a daily basis.
Unlike previous studies in patients with FD, we did not find evidence of cerebrovascular stiffening or abnormalities in the capacity of the cerebral vessels to dilate or auto-regulate.9,12 These past assumptions were in part based on changes in MCA velocity during a Valsalva maneuver, a procedure that these patients cannot perform due to poor respiratory coordination and when attempted produces anxiety-induced sympathetic activation.3,38 Moreover, at the time that these previous studies were performed, patients were being treated empirically with high doses of fludrocortisone, which is known to affect vascular responsiveness.39 Fludrocortisone is now only used sparingly in these patients. An important factor to also emphasize is that these are relative young patients, in whom we have demonstrated to have normal vascular endothelial function.40
In conclusion, our findings underscore the remarkable ability of the cerebral vessels to auto-regulate blood flow in a severe congenital autonomic disease. Whether older adults with acquired afferent baroreflex failure have a similarly efficient auto-regulatory capacity is unknown.
Supplementary Material
Acknowledgments
We would like to thank all the participants in this study and the Dysautonomia Foundation for their support. We would also like to thank all staff at the Dysautonomia Center.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: LJNK, HK, and J-AP receive research support from the National Institutes of Health (U54 NS065736), the Food and Drug Administration’s Office of Orphan Product Development (R01-FD004772). All authors receive research support from the Dysautonomia Foundation, Inc.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
CFM contributed to the collection of data, analysis and provided the initial draft of the manuscript text. J-AP contributed to the collection of data and revising the manuscript for important intellectual content. HK contributed to the interpretation of the data and revising the manuscript for important intellectual content. LJNK contributed to the study design, collection of data, analysis and interpretation of data and revising the manuscript. All authors have seen and approved the final version of the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.George L, Chaverra M, Wolfe L, et al. Familial dysautonomia model reveals Ikbkap deletion causes apoptosis of Pax3+ progenitors and peripheral neurons. Proc Natl Acad Sci USA 2013; 110: 18698–18703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slaugenhaupt SA, Blumenfeld A, Gill SP, et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet 2001; 68: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology 2010; 75: 1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norcliffe-Kaufmann L, Axelrod FB, Kaufmann H. Developmental abnormalities, blood pressure variability and renal disease in Riley Day syndrome. J Hum Hypertens 2011; 27: 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macefield VG, Norcliffe-Kaufmann LJ, Axelrod F, et al. Cardiac-locked bursts of muscle sympathetic nerve activity are absent in familial dysautonomia. J Physiol 2013; 591(3): 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norcliffe-Kaufmann LJ, Axelrod FB, Kaufmann H. Cyclic vomiting associated with excessive dopamine in Riley-day syndrome. J Clin Gastroenterol 2013; 47: 136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norcliffe-Kaufmann L, Kaufmann H. Familial dysautonomia (Riley-Day syndrome): when baroreceptor feedback fails. Auton Neurosci 2012; 172: 26–30. [DOI] [PubMed] [Google Scholar]
- 8.Palma JA, Norcliffe-Kaufmann L, Fuente-Mora C, et al. Current treatments in familial dysautonomia. Expert Opin Pharmacother 2014; 15: 2653–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilz MJ, Axelrod FB, Steingrueber M, et al. Valsalva maneuver suggests increased rigidity of cerebral resistance vessels in familial dysautonomia. Clin Auton Res 2002; 12: 385–392. [DOI] [PubMed] [Google Scholar]
- 10.Gadoth N, Abramovitch D, Melamed E. The regional cerebral blood flow in familial dysautonomia. Brain Dev 1989; 11: 179–182. [DOI] [PubMed] [Google Scholar]
- 11.Axelrod FB, Zupanc M, Hilz MJ, et al. Ictal SPECT during autonomic crisis in familial dysautonomia. Neurology 2000; 55: 122–125. [DOI] [PubMed] [Google Scholar]
- 12.Hilz MJ, Axelrod FB, Haertl U, et al. Transcranial Doppler sonography during head up tilt suggests preserved central sympathetic activation in familial dysautonomia. J Neurol Neurosurg Psychiatry 2002; 72: 657–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardi L, Hilz M, Stemper B, et al. Respiratory and cerebrovascular responses to hypoxia and hypercapnia in familial dysautonomia. Am J Respir Crit Care Med 2003; 167: 141–149. [DOI] [PubMed] [Google Scholar]
- 14.Axelrod FB, Goldberg JD, Ye XY, et al. Survival in familial dysautonomia: Impact of early intervention. J Pediatr 2002; 141: 518–523. [DOI] [PubMed] [Google Scholar]
- 15.Norcliffe-Kaufmann L, Palma JA, Kaufmann H. Mother-induced hypertension in familial dysautonomia. Clin Auton Res 2016; 26: 79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, et al. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res 2012; 22: 79–90. [DOI] [PubMed] [Google Scholar]
- 17.Norcliffe-Kaufmann LJ, Kaufmann H, Hainsworth R. Enhanced vascular responses to hypocapnia in neurally mediated syncope. Ann Neurol 2008; 63: 288–294. [DOI] [PubMed] [Google Scholar]
- 18.Claydon VE, Schroeder C, Norcliffe LJ, et al. Water drinking improves orthostatic tolerance in patients with posturally related syncope. Clin Sci (London, England) 2006; 110: 343–352. [DOI] [PubMed] [Google Scholar]
- 19.Nicoletto HA, Burkman MH. Transcranial Doppler series part III: interpretation. Am J Electroneurodiagn Technol 2009; 49: 244–259. [PubMed] [Google Scholar]
- 20.Schroeder C, Bush VE, Norcliffe LJ, et al. Water drinking acutely improves orthostatic tolerance in healthy subjects. Circulation 2002; 106: 2806–2811. [DOI] [PubMed] [Google Scholar]
- 21.Wilterdink JL, Feldmann E, Furie KL, et al. Transcranial Doppler ultrasound battery reliably identifies severe internal carotid artery stenosis. Stroke 1997; 28: 133–136. [DOI] [PubMed] [Google Scholar]
- 22.Albina G, Fernandez Cisneros L, Laino R, et al. Transcranial Doppler monitoring during head upright tilt table testing in patients with suspected neurocardiogenic syncope. Europace 2004; 6: 63–69. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal S, Brooks DM, Kang Y, et al. Noninvasive monitoring of cerebral perfusion pressure in patients with acute liver failure using transcranial doppler ultrasonography. Liver Transpl 2008; 14: 1048–1057. [DOI] [PubMed] [Google Scholar]
- 24.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011; 21: 69–72. [DOI] [PubMed] [Google Scholar]
- 25.Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord 2015; 30: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Riva N, Budohoski KP, Smielewski P, et al. Transcranial Doppler pulsatility index: what it is and what it isn't. Neurocrit Care 2012; 17: 58–66. [DOI] [PubMed] [Google Scholar]
- 27.Michel E, Zernikow B. Gosling's Doppler pulsatility index revisited. Ultrasound Med Biol 1998; 24: 597–599. [DOI] [PubMed] [Google Scholar]
- 28.Gibbons CH, Freeman R. Orthostatic dyspnea: a neglected symptom of orthostatic hypotension. Clin Auton Res 2005; 15: 40–44. [DOI] [PubMed] [Google Scholar]
- 29.Carey BJ, Eames PJ, Panerai RB, et al. Carbon dioxide, critical closing pressure and cerebral haemodynamics prior to vasovagal syncope in humans. Clin Sci (Lond) 2001; 101: 351–358. [PubMed] [Google Scholar]
- 30.Tasker RC. Brain vascular and hydrodynamic physiology. Semin Pediatr Surg 2013; 22: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andresen M, Juhler M. Intracranial pressure following complete removal of a small demarcated brain tumor: a model for normal intracranial pressure in humans. J Neurosurg 2014; 121: 797–801. [DOI] [PubMed] [Google Scholar]
- 32.Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res 2009; 19: 197–211. [DOI] [PubMed] [Google Scholar]
- 33.Robba C, Bacigaluppi S, Cardim D, et al. Non-invasive assessment of intracranial pressure. Acta Neurol Scand 2016; 134(1): 4–21. [DOI] [PubMed] [Google Scholar]
- 34.Norcliffe-Kaufmann L, Slaugenhaupt SA, Kaufmann H. Familial dysautonomia: History, genotype, phenotype and translational research. Prog Neurobiol 2017; 152: 131–148. [DOI] [PubMed] [Google Scholar]
- 35.Novak V, Novak P, Spies JM, et al. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke 1998; 29: 104–111. [DOI] [PubMed] [Google Scholar]
- 36.Hainsworth R. Pathophysiology of syncope. Clin Auton Res 2004; 14(Suppl 1): 18–24. [DOI] [PubMed] [Google Scholar]
- 37.Norcliffe-Kaufmann L, Katz SD, Axelrod F, et al. Norepinephrine deficiency with normal blood pressure control in congenital insensitivity to pain with anhidrosis. Ann Neurol 2015; 77: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuente Mora C, Norcliffe-Kaufmann L, Palma JA, et al. Chewing-induced hypertension in afferent baroreflex failure: a sympathetic response? Exp Physiol 2015; 100(11): 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mion D, Jr, Rea RF, Anderson EA, et al. Effects of fludrocortisone on sympathetic nerve activity in humans. Hypertension 1994; 23: 123–130. [DOI] [PubMed] [Google Scholar]
- 40.Jelani QU, Norcliffe-Kaufmann L, Kaufmann H, et al. Vascular endothelial function and blood pressure regulation in afferent autonomic failure. Am J Hypertens 2015; 28: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials