Abstract
Epidemiological studies have suggested a close relationship between cerebral ischemia and Alzheimer’s disease (AD). To clarify the pathological association of tau dynamics in both diseases, we performed comprehensive studies on the posttranslational modification of tau in cerebral ischemia and reperfusion (I/R) in rats. The present study suggests that both 4-repeat and 3-repeat tau isoforms are hyperphosphorylated in cerebral I/R, similar to the case in AD. The generation of a 60-kDa Asp421-truncated tau in cerebral I/R preceded the emergence of a 17-kDa 3-repeat tau fragment and a 25-kDa 4-repeat tau fragment. The regional redistribution of tau from the neuropil to neuronal perikarya in our stroke model is thought to share similarity with that occurring in AD. In addition, immunofluorescence staining revealed the formation of axonal varicosities in cerebral I/R. Altered tau distribution may influence microtubule stability, disturbances in axonal transport, and the resulting formation of axonal varicosities. The staining profiles of granules in the ischemic cortex that were immunopositive for RD3, RD4, and AT8 in neuronal perikarya and that were argyrophilic on Gallyas-Braak staining were similar to those in AD. These findings suggest that transient cerebral ischemia shares a common pathology with AD, in the modification of tau protein.
Keywords: Alzheimer’s disease, cerebral ischemia, cleavage, phosphorylation, tau
Introduction
Stroke and Alzheimer’s disease (AD) are common neurological disorders prevalent among the elderly. Epidemiological studies have suggested that the morbidity of cerebral ischemia increases the prevalence of subsequent dementia that is independent of direct cerebral damage.1–4 The risk factors for AD include aging, type 2 diabetes mellitus, ApoEɛ4, high serum cholesterol levels, and smoking.5–10 These AD risk factors involve vascular effects that predispose affected persons to cerebral ischemia.11 In addition, some studies regarding regional cerebral blood flow using single-photon emission computed tomography (SPECT) have demonstrated that mild cognitive impairment (MCI) patients who later converted to AD were distinguished from an age-matched non-MCI control group by the presence of temporoparietal hypoperfusion.12,13 The “ischemia-reperfusion theory” suggests that AD and cerebral ischemia share common pathological features; however, besides the aforementioned conditions, there are few research reports that compare the pathological alterations that occur in ischemic and AD brains.14
AD is characterized both by the gradual accumulation of extracellular amyloid-β (Aβ) in the form of senile plaques and by inclusions of intracellular hyperphosphorylated tau in the form of neuropil threads and neurofibrillary tangles (NFTs). In multivariate analyses, NFTs are closely associated with neuronal loss and memory deficits, while amyloid deposition is not strongly correlated, suggesting that the formation of NFTs composed of hyperphosphorylated tau is the most important event associated with neuronal loss in AD.15 Therefore, in the context of identifying a pathological association between cerebral ischemia and AD, the present study focused on tau dynamics in response to cerebral ischemia and reperfusion (I/R) injury.
The microtubule-binding protein, tau, concentrated mainly in the axonal compartment of neurons, plays an important role for neuronal morphogenesis, plasticity, and polarity.16,17 The inclusion of exon 10 from the gene encoding tau results in a tau isoform with all four microtubule-binding repeat regions, and the exclusion of exon 10 results in a tau isoform with three microtubule-binding repeat regions; these isoforms are known as 4-repeat tau and 3-repeat tau, respectively.18,19 Tauopathies have been biochemically characterized based on the isoform of aggregated tau protein present. In tauopathies, the distribution of hyperphosphorylated tau shifts from its normal position in the axon to the neuronal cell body and associated dendrites.20 In addition to the well-known effects of hyperphosphorylation on tau, proteolytic cleavage and conformational changes of tau have also been suggested to facilitate tau deposition and may play a pathological role in tauopathies.21–25 Cleavage of tau at aspartic acid 421 (Asp421) is an initial step of tau modification and is an important inducer of tau polymerization in the neurofibrillary pathology of AD.26 Furthermore, this truncation has been shown to be toxic and is capable of inducing morphologic and functional alterations in cell culture models.27,28
The effects of cerebral ischemia and/or reperfusion on the phosphorylation status of tau have been investigated using different stroke animal models but remain controversial.29–34 Posttranslational modifications of tau other than hyperphosphorylation, such as proteolytic cleavage and conformational changes, have scarcely been explored in relation to cerebral ischemia. Additionally, alterations in response to cerebral ischemia that involve the hyperphosphorylation and aggregation of 4-repeat and/or 3-repeat tau are not fully understood.35,36 To clarify the pathological association of tau dynamics in cerebral ischemia and AD, we applied the Phos-tag system37 to assess tau phosphorylation states and also performed comprehensive studies on the posttranslational modifications of tau protein in cerebral I/R in rats.
Materials and methods
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee at Hiroshima University and performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Six-week-old male Wistar rats (220–260 g) were purchased from Charles Rivers Laboratories (Wilmington, DE, USA) and maintained in our animal facility in a temperature-controlled room (22–26℃) with 12-h dark–light cycles in the Institute of Laboratory Animal Science of Hiroshima University. All rats were acclimatized for two to four weeks and had free access to laboratory chow and tap water before and after all procedures. The report was written in compliance with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments) (https://www.nc3rs.org.uk/arrive-guidelines).
Rat focal cerebral I/R model
Animals (8-to 10-weeks old, 313.3 ± 9.9 g) were anesthetized with halothane (5% for induction and 1.5–2% during experiments) in a 70% air and 30% O2 gas mixture and placed in a stereotaxic apparatus. To achieve a transient middle cerebral artery (MCA) occlusion based on the Koizumi technique,38 the common carotid artery (CCA) and internal carotid artery (ICA) were exposed, and the proximal part of the CCA and external carotid artery were permanently ligated with a 5–0 silk suture. The tip of a 4–0 monofilament nylon suture was rounded by heating. The suture was introduced into the CCA lumen through a puncture and was gently advanced approximately 200 mm to the distal ICA until the proper amount of resistance was felt. After 90 min, the suture was withdrawn from the CCA, and the CCA was immediately ligated. Sham controls received halothane anesthesia and exposure of the CCA without insertion of the suture or vessel occlusion. Body temperature was monitored by a rectal thermometer throughout the entire surgery period and maintained at 37 ± 0.5℃ with a heating pad.
Group assignment
The rats in the MCA occlusion group were assigned to five subgroups according to the time of sacrifice (6 h, 12 h, 24 h, 48 h, and 72 h after reperfusion). For Western blotting analysis, five animals were used from the 72 h reperfusion group, and three animals were used from each of the other reperfusion time groups (6 h, 12 h, 24 h, and 48 h). For Western blotting analysis with Phos-tag affinity electrophoresis, three animals were used from each reperfusion time point. For immunohistochemical staining and immunofluorescence staining, two animals were used from the 72 h reperfusion group, and one animal was used from each of the other reperfusion time groups. Samples from each animal assigned to the 6 h, 24 h and 72 h reperfusion groups were also subjected to modified Gallyas-Braak staining.
Assessment of neurological function
The Zea-Longa neurological deficit score39 was assessed prior to surgery, immediately following reperfusion and on the day of sacrifice, according to the following criterion: a score of 0 indicated no neurological deficits; a score of 1 (failure to fully extend the left forepaw) indicated a mild focal neurological deficit; a score of 2 (circling to the left) indicated a moderate focal neurological deficit; a score of 3 (falling to the left) indicated a severe focal deficit; a score of 4 (did not walk spontaneously) indicated rats that had a depressed level of consciousness; and a score of 5 indicated death.40 Rats with a score of 0, 4, or 5 following reperfusion were excluded from the study.
Antibodies
The anti-Ab-2 antibody (clone Tau5; Thermo Fisher Scientific, Waltham, MA, USA) reacts with both the non-phosphorylated and phosphorylated forms of tau. The anti-RD4 antibody (clone 1E1/A6; Merck Millipore, Darmstadt, Germany) reacts with its epitope in exon 10 of tau, resulting in three bands on Western blots of rodent brain tissue representing 0N4R, 1N4R, and 2N4R. The anti-RD3 antibody (clone 8E6/C11; Merck Millipore) reacts with its epitope in the vicinity of the exon 9/exon 11 junction of tau, resulting in three bands on Western blots representing 0N3R, 1N3R, and 2N3R. The anti-PHF-tau antibody (clone AT8; Innogenetics, Ghent, Belgium) recognizes tau phosphorylated at both serine 202 (Ser202) and threonine 205 (Thr205).41,42 This antibody recognizes PHF-tau and does not cross-react with normal tau.43 The anti-tau cleavage site 421/422 specific antibody (clone TauC3; Invitrogen, Waltham, MA, USA) reacts with tau truncated at Asp421. The anti-pan-axonal neurofilament marker antibody (clone SMI312; BioLegend, San Diego, CA, USA) specifically recognizes axons. The anti-cleaved caspase-3 antibody (clone 5A1E; Cell Signaling technology, Danvers, MA, USA) recognizes the large fragments (17 kDa and 19 kDa) of activated caspase-3 resulting from cleavage. The NeuN antibody (clone A60) was obtained from Merck Millipore. The anti-glial fibrillary acidic protein (GFAP) antibody (clone GA5) was obtained from Chemicon International (Billerica, MA, USA).
Histopathology
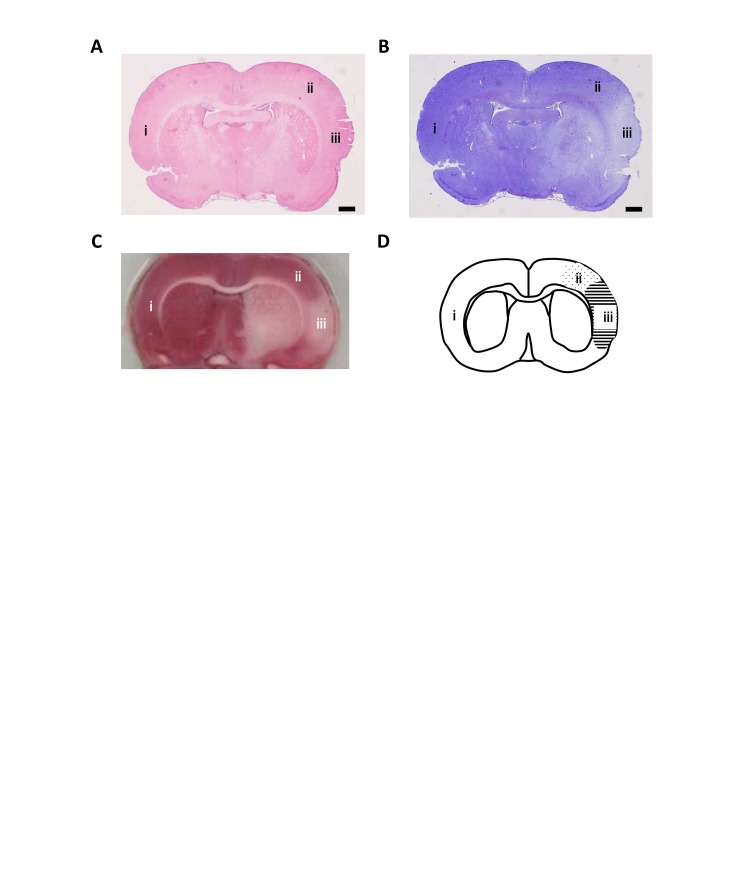
Ischemic lesions were assessed via hematoxylin-eosin staining (H&E), Nissl staining, and TTC staining. For histopathological analyses, animals were perfused transcardially with 4% paraformaldehyde in sodium phosphate buffer (pH 7.4). Frozen brains were sliced into 8-µm-thick coronal sections by using a cryostat vibratome. The sections were stained with H&E or 0.1% cresyl violet for Nissl staining (Supplementary Figure 1(a) and (b)). For TTC staining, animals were briefly anaesthetized and immediately sacrificed by decapitation. Fresh brain tissues were cut into five coronal slices (2-mm thick) using a slicer matrix (1.0 mm-slice intervals, RBM-A1-C, Muromachi Kikai Corporation, Tokyo, Japan) and a razor (FA-10, Feather Safety Razor Corporation, Osaka, Japan). The sections were stained for 30 min at 37℃ with 2% TTC (Nacalai Tesque, Kyoto, Japan) in the dark. The stained brain sections were captured with a scanner (ES-10000 G, Epson, Nagano, Japan). TTC and Nissl staining showed an area of infarction that included the striatum and the cerebral cortex (Supplementary Figure 1(c)).
Immunohistochemistry
For immunohistochemical analysis, animals were perfused transcardially with 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4. Frozen brains were sliced into 10-µm-thick coronal sections by using a cryostat vibratome. In accordance with a rat brain atlas by Paxinos and Watson,43 the sections between 0.96 and −0.36 mm from the bregma were used for this study. After blocking with 10% normal horse serum in PBS for 30 min, each section was incubated with primary antibodies containing Ab-2 (at a dilution of 1:400), RD3 (1:400), and RD4 (1:400) overnight at 4℃. After washing, the sections were incubated with the secondary antibody, biotinylated anti-mouse horse IgG preadsorbed against rat serum (Vector Laboratories, Burlingame, CA, USA), for 30 min. The sections were incubated with avidin-biotin complex reagents (VECTASTAIN Elite ABC Kit, Vector Laboratories) for 30 min. After washing, the sections were treated with 0.3% H2O2 in PBS for 30 min to quench endogenous peroxidase activity. After washing, the sections were stained by exposing them to a substrate composed of 0.04% 3.3′ diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich, St. Louis, MO, USA) and then sealed with cover slips for microscopy (Eclipse E1000, Nikon, Tokyo, Japan). As a negative control, sections were incubated in the absence of primary antibodies. Three areas of the contralateral cortex (i) peri-ischemic area (ii) and ischemic core (iii) were used for immunohistochemistry analyses (Supplementary Figure 1(d)).
Double fluorescence immunohistochemistry using a mouse IgG-labeling kit
To observe the spatial relationship between total-tau, 4-repeat tau, 3-repeat tau, PHF-tau and axons in detail, double fluorolabeling to differentiate each tau isoform is necessary but difficult to achieve because the antibodies against Ab-2, RD4, RD3, AT8, and SMI312 are all mouse IgG1 antibodies. To perform double immunofluorolabeling with two antibodies of the same class from the same species, we used the Zenon mouse IgG-labeling kit (Thermo Fisher Scientific).
The sections were permeabilized in PBS containing 0.2% Triton X-100 for 20 min. Each section was blocked with PBS containing 0.2% BSA, 5% normal goat serum, and 5% normal horse serum for 30 min. Zenon Alexa 488 mouse IgG-labeling reagent was added to the Ab-2 antibody solution. Zenon Alexa 594 mouse IgG-labeling reagent was added to the antibody solution containing AT8, RD3, RD4, SMI312, NeuN, and GFAP. Each mixture was incubated for 5 min. The blocking reagent was added to the reaction mixture and incubated for 5 min. The sections were incubated with the complex solution for 2 h. After washing, a second fixation of the tissue sections was performed for 15 min in 4% paraformaldehyde solution in PBS. After washing, the sections were counterstained with Hoechst 33342 and washed with PBS. The slides were mounted with Vecta-shield media (Vector Laboratories), and the fluorescence was observed by microscopy (BZ-9000, Keyence, Osaka, Japan). As a negative control, sections were incubated in the absence of primary antibodies.
Tissue homogenates
After reperfusion, animals were anesthetized by the intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight, Somnopentyl, Kyoritsu Seiyaku Corporation, Tokyo, Japan) and immediately decapitated. The brains were removed within 2 min and placed in an ice-cold saline solution. The cortex in the ischemic area or the corresponding contralateral area was dissected out on a cooled glass surface. Protein extracts for immunoblot experiments were prepared by homogenizing rat brains that had been frozen immediately at the time of dissection. The brains were homogenized in RIPA buffer [50 mM Tris-HCl (pH 7.4), 140 mM KCl, 3 mM EDTA and 0.5% Triton X-100] supplemented with protease inhibitors (10 µg/ml aprotinin, 10 µg/ml leupeptin, and 1 mM PMSF). The homogenate was centrifuged at 10,000 g for 10 min at 4℃. The resulting supernatant was boiled for 5 min and frozen at −80℃ for later analysis.
Electrophoresis and western blotting
Equal amounts of protein from each sample were separated on 12% SDS-polyacrylamide gels and electrophoretically transferred to ClearTrans SP PVDF Membrane (Wako Pure Chemical Industries, Osaka, Japan). Nonspecific binding sites on the PVDF membranes were blocked by incubation with blocking buffer [4% skim milk in TBST (Tris-buffered saline (TBS) and 0.1% Tween 20] for 1 h. After washing with PBS with 0.1% Tween 20 (PBST), the PVDF membranes were incubated overnight at 4℃ with the appropriate primary antibody in blocking buffer (Ab-2, AT8, and TauC3 at a 1:1000 dilution, RD3 and RD4 at a 1:5000 dilution). After washing, the membrane was incubated with species-specific HRP-conjugated secondary antibodies for 1 h, followed by another wash in TBST. The immunoreactive bands were visualized with an enhanced chemiluminescent solution and a luminescent image analyzer (FPM100, Fuji film, Tokyo, Japan). Densitometric data were obtained in the linear range of blot exposure. The background optical density of each blot was determined in an empty lane, and the obtained value was subtracted from each specific signal. Protein expression was quantified from the band density using ImageJ software and normalized to the expression level of α-tubulin as an internal control.
Western blot analysis using Phos-tag affinity electrophoresis
For detection of phosphorylated forms of tau protein, we used the Phos-tag system. The Phos-tag SDS-PAGE technique is suitable for the separation and detection of a phosphorylated protein and its non-phosphorylated counterpart by the decreased migration speed of phosphorylated proteins that are bound by Phos-tag in the gel.37 After electrophoresis using the SuperSep Phos-tag precast gel which are commercially available from Wako Pure Chemical Industries, the gels were soaked in a solution containing transfer buffer and 1.0 mM EDTA for 10 min and then soaked in transfer buffer for 10 min. The resolved proteins were transferred electrophoretically to a PVDF membrane. After electrophoretic transfer, the membranes were soaked in blocking buffer (4% skim milk in TBST) for 1 h at room temperature followed by overnight incubation at 4℃ with the appropriate primary antibody in blocking buffer (Ab-2 at a 1:1000 dilution, RD3 and RD4 at a 1:5000 dilution). After washing, the membrane was incubated with species-specific HRP-conjugated secondary antibodies for 1 h at room temperature, followed by another wash in TBST. The target proteins were detected using enhanced chemiluminescent solution and a FPM100 image analyzer.
Modified Gallyas-Braak staining
Animals were perfused transcardially with 4% paraformaldehyde in PBS. The sections were embedded in paraffin, and 10 µm-thick coronal sections were stained with a modified Gallyas-Braak staining method.44
Statistical analyses
The data are expressed as the mean ± standard error (SE). The changes in the band density were analyzed by one-way ANOVA followed by Dunnett’s test. Differences were considered significant at p < 0.05. The degree of colocalization for the double fluorescence experiments was analyzed using Pearson’s correlation coefficient and quantified using the WCIF plugin of Image J software (National Institutes of Health, Bethesda, MD, USA).
Results
Tau protein is hyperphosphorylated and cleaved in response to cerebral I/R
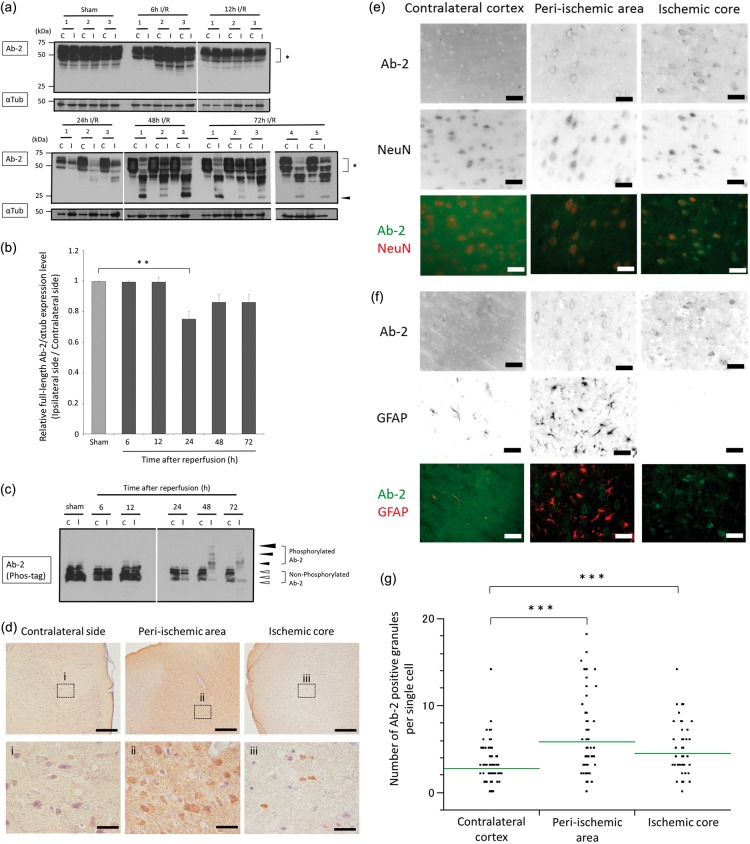
We evaluated the chronological posttranslational modification of tau protein in cerebral I/R. The tau protein detected by anti-Ab-2 antibody on immunoblots presented as multiple bands ranging from 45 to 65 kDa, indicating the presence of multiple isoforms, similar to what is observed in human brain extracts (Figure 1(a)). Compared with tau expression in sham-operated rats and/or in the unaffected contralateral cortex, the expression of full-length total tau (detected by Ab-2) was unchanged at 6 h and 12 h after reperfusion in the ipsilateral cortex but was significantly decreased at 24 h (24.6 ± 6.7%, p = 0.01) and showed a downward trend at 48 h (13.7 ± 6.7%, p = 0.197) and 72 h (13.7 ± 6.0%, p = 0.133) (Figure 1(a) and (b)). A 25-kDa band, thought to be a tau fragment, was not detected in the contralateral cortex, but emerged in the ipsilateral cortex at 48 h and 72 h (Figure 1(a)). These results demonstrate that the full-length form of tau was cleaved, and a 25-kDa tau fragment emerged in response to cerebral I/R.
Figure 1.
Posttranslational modifications and alterations in the distribution of total tau in response to cerebral ischemia and reperfusion.
(a) Total tau protein expression was assessed by Western blot analysis using the anti-Ab-2 antibody and the α-tubulin loading control antibody. Contralateral and ipsilateral cortical tissues were homogenized from rats subjected to a sham operation or 6–72 h of reperfusion following 90 min of ischemia. Full-length tau is indicated by an asterisk, and the 25-kDa tau cleavage fragments are indicated by a black arrowhead. Each lane contains 40 µg of protein. (b) Quantification results are shown for the immunoblot data presented in (a). The optical densities were determined for full-length tau and α-tubulin in the ipsilateral and contralateral cortex, and the tau densitometry values were normalized to those of α-tubulin. The graph shows the ratio of full-length tau in the ipsilateral cortex compared with that in the contralateral cortex at each time point. The data are expressed as the mean ± SE and were analyzed by one-way ANOVA followed by Dunnett’s test. **p < 0.01, indicates a significant difference compared with sham-operated rats; n = 3 to 5 for each time point. (c) Homogenates from the contralateral and ipsilateral cortex tissues of rats that were subjected to a sham operation or 6–72 h of reperfusion following 90 min of ischemia were assessed for tau phosphorylation using the Phos-tag SDS-PAGE Western blot technique. Each lane contains 50 µg of protein. Phosphorylated tau is indicated with black arrowheads and migrated slower than non-phosphorylated tau, indicated by white arrowheads. The size of the black arrowheads indicates the degree of tau phosphorylation. (d) Tau immunohistochemistry staining using the anti-Ab-2 antibody is shown in the contralateral cortex, peri-ischemic area, and ischemic core of rats subjected to 72 h of reperfusion following 90 min of ischemia. Insets (i–iii) show a magnified view of the boxed region. The scale bars represent 500 µm (in the upper row of images) and 50 µm (in the lower row of images). (e, f) Representative immunofluorescence staining is shown using Ab-2 and NeuN or GFAP antibodies in the contralateral cortex (first column), peri-ischemic area (second column), and ischemic core (third column) of rats subjected to 72 h of reperfusion following 90 min of ischemia. The first row shows the inverse signal of Ab-2 immunofluorescence. The second row shows the inverse signal of NeuN immunofluorescence (in E) or GFAP immunofluorescence (in F). The third row shows the overlay of images for Ab-2 immunofluorescence (in green) to that of either NeuN or GFAP immunofluorescence (in red) as indicated. The scale bars represent 40 µm. (g) The number of Ab-2-positive granules in the neuronal perikarya per single cell in the peri-ischemic area and ischemic core compared with the contralateral cortex of rats subjected to 72 h of reperfusion following 90 min of ischemia. Three fields of view at 60-fold magnification were randomly selected from each area, and the mean value for each field of view was calculated. The data were analyzed using one-way ANOVA followed by Dunnett’s test. ***p < 0.001 indicates a significant difference compared with the contralateral cortex.
We further measured the electrophoretic mobility of tau using the Phos-tag SDS-PAGE technique. Non-phosphorylated forms of tau were observed as multiple bands (Figure 1(c)). The slower migrating bands, corresponding to phosphorylated tau, were unchanged from 6 to 24 h after reperfusion. However, the levels of phosphorylated tau increased in the ipsilateral cortex at 48 h and 72 h after reperfusion compared with the levels of phosphorylated tau in the contralateral cortex and in the cortex of sham-operated rats observed at the same time points (Figure 1(c)). These results demonstrate that tau protein is hyperphosphorylated in response to cerebral I/R.
Tau protein redistributes from its usual position in the axon into the neuronal perikarya in cerebral I/R
We evaluated the alterations in the distribution of tau protein in cerebral I/R by immunohistochemistry. Immunofluorescence staining with anti-Ab-2 antibody revealed homogenous Ab-2 immunoreactivity in the neuropil and perikarya of the ipsilateral cortex of sham-operated rats. In the ipsilateral cortex of rats subjected to 24 h of reperfusion, Ab-2-positive granules were noted in neuronal perikarya. In the ipsilateral cortex of rats subjected to 72 h of reperfusion, there were increased amounts of Ab-2-positive granules in neuronal perikarya (data not shown). Because this preliminary experiment showed that there was a greater change in Ab-2 immunoreactivity in rats subjected to 72 h of reperfusion, the sections of rats subjected to 72 h of reperfusion were used for the following pathological examination. Immunostaining with anti-Ab-2 antibody of rats subjected to 72 h of reperfusion revealed that Ab-2 immunoreactivity homogeneously stained in the neuropil and faintly stained perikarya in the contralateral cortex. In the peri-ischemic area, there was increased Ab-2 staining in the neuropil and perikarya. In the ischemic core, Ab-2 immunoreactive aggregates were noted only in some perikarya (Figure 1(d)). Immunofluorescence staining of rats subjected to 72 h of reperfusion revealed that tau protein detected by anti-Ab-2 antibody homogeneously stained in the neuropil and perikarya. In the peri-ischemic area and ischemic core, the relative staining intensity of anti-Ab-2 antibody was increased in perikarya (Supplementary Figure 2). These results indicate that the redistribution of tau protein shifted from the neuropil to perikarya in response to cerebral I/R. To investigate the cell types in which Ab-2 was localized, double-staining with different antibodies against cell-type-specific antigens was performed. The cells labeled with anti-Ab-2 antibodies were also labeled with anti-NeuN antibodies. GFAP immunostaining revealed marked astrocytic proliferation in the peri-ischemic area, but these astrocytes were immunonegative for Ab-2, suggesting that the Ab-2-expressing cells are neurons (Figure 1(e) and (f)). These results indicate a redistribution of tau protein from axons to neuronal perikarya in response to cerebral I/R, similar to that observed in AD. The number of Ab-2-positive granules in the neuronal perikarya per single neuron was significantly increased in the peri-ischemic area (mean ± SE: 5.8 ± 0.3, p < 0.001) and in the ischemic core (4.4 ± 0.3, p < 0.001) compared with the contralateral cortex (2.8 ± 0.2) (Figure 1(g)).
Both the 4-repeat and 3-repeat tau isoforms are hyperphosphorylated and cleaved in cerebral I/R
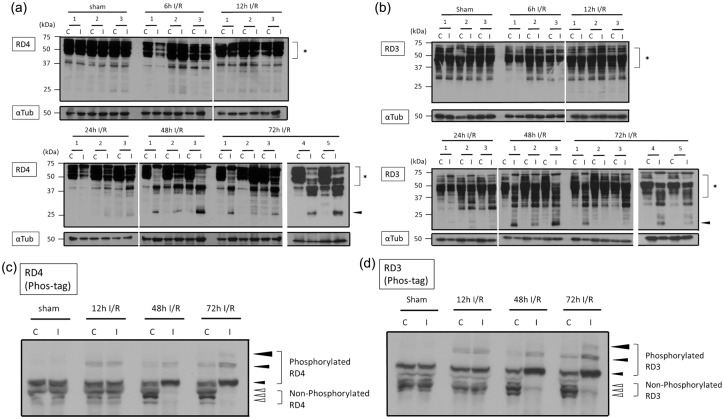
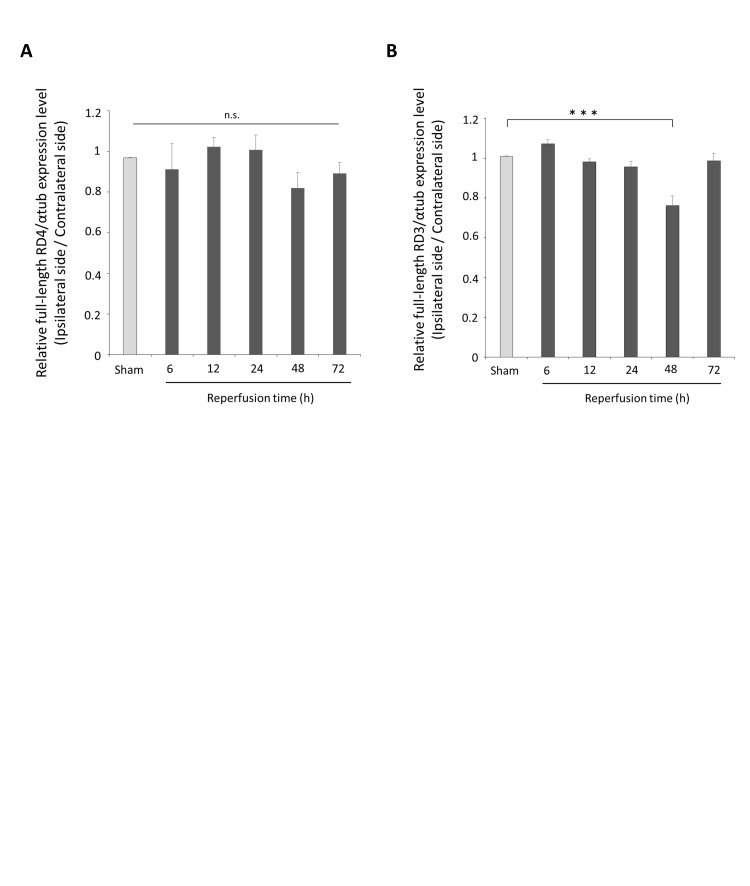
We evaluated the posttranslational modifications of 4-repeat and 3-repeat tau in cerebral I/R. Densitometry quantitation of Western blot band signal intensity showed that the levels of full-length 4-repeat tau, corresponding to a band at 45-65 kDa, decreased in the ipsilateral cortex compared with the contralateral cortex at 48 h after reperfusion, but the changes were not significant (Supplementary Figure 3(a)). A 25-kDa band, suggestive of the 4-repeat tau fragment, was detected faintly in the ischemic cortex at 6 h and 12 h after reperfusion; however, the intensity of the band increased at 48 h and 72 h after reperfusion (Figure 2(a)). The levels of full-length 3-repeat tau, corresponding to a 45–65 kDa band, were significantly decreased in the ischemic cortex at 48 h after reperfusion compared with the levels in the cortex of sham-operated rats and/or the contralateral cortex (3-repeat tau levels decreased by 24.7 ± 4.5% compared with the sham-operated control levels at 48 h after reperfusion, p < 0.001; Supplementary Figure 3(b)). A 17-kDa band, assumed to be a fragment of 3-repeat tau, was barely detectable at 6 h and 12 h after reperfusion; however, the 17-kDa band was expressed to varying degrees in the ipsilateral cortex of all three rats from the 48 h post-reperfusion group and in three of the five rats from the 72 h post-reperfusion group (Figure 2(b)). These results demonstrate that both 4-repeat tau and 3-repeat tau were cleaved, and a 25-kDa 4-repeat tau fragment and 17-kDa 3-repeat tau fragment emerged in response to cerebral I/R.
Figure 2.
Phosphorylation and cleavage of 4-repeat and 3-repeat tau in response to cerebral ischemia and reperfusion.(a) The expression of 4-repeat tau was assessed by Western blot using the anti-RD4 antibody and the α-tubulin loading control antibody. Contralateral and ipsilateral cortical tissues were homogenized from rats subjected to a sham-operation or 6–72 h of reperfusion following 90 min of ischemia. Full-length 4-repeat tau is indicated by an asterisk, and 25-kDa 4-repeat tau fragments are indicated by a black arrowhead. Each lane contains 40 µg of protein. (b) The expression of 3-repeat tau was assessed by Western blot using the anti-RD3 antibody and the α-tubulin antibody loading control. Contralateral and ipsilateral cortical tissues were homogenized from rats subjected to a sham operation or 6–72 h of reperfusion following 90 min of ischemia. Full-length 3-repeat tau is indicated by an asterisk, and 25-kDa 3-repeat tau fragments are indicated by a black arrowhead. (c, d) Homogenates from the contralateral and ipsilateral cortex tissues of rats that were subjected to a sham operation or 6–72 h of reperfusion following 90 min of ischemia were assessed for 4-repeat tau phosphorylation (in C) or 3-repeat tau phosphorylation (in D) using the Phos-tag SDS-PAGE Western blot technique. Each lane contains 50 µg of protein. Phosphorylated 4-repeat tau (in C) and 3-repeat tau (in D) is indicated with black arrowheads and migrated slower than non-phosphorylated 4-repeat tau (in C) and non-phosphorylated 3-repeat tau (in D), indicated by white arrowheads. The size of the black arrowheads indicates the degree of phosphorylation.
Next, we employed the Phos-tag SPS-PAGE technique to separate phosphorylated forms of 4-repeat and 3-repeat tau, respectively. As expected, both phosphorylated RD4 and RD3 migrated slower than the corresponding non-phosphorylated RD4 and RD3 tau isoforms. Both phosphorylated and non-phosphorylated RD4 and RD3 tau isoforms were observed as three bands (Figure 2(c) and (d)). The slower migrating bands, corresponding to phosphorylated 4-repeat and 3-repeat tau, were increased in the ipsilateral cortex at 48 h and 72 h after reperfusion compared with the control cortex of sham-operated rats and/or the contralateral cortex (Figure 2(c) and (d)). This result suggests that cerebral I/R leads to the hyperphosphorylation of both isoforms of tau protein.
Alterations in the distribution of 4-repeat and 3-repeat tau isoforms in cerebral I/R
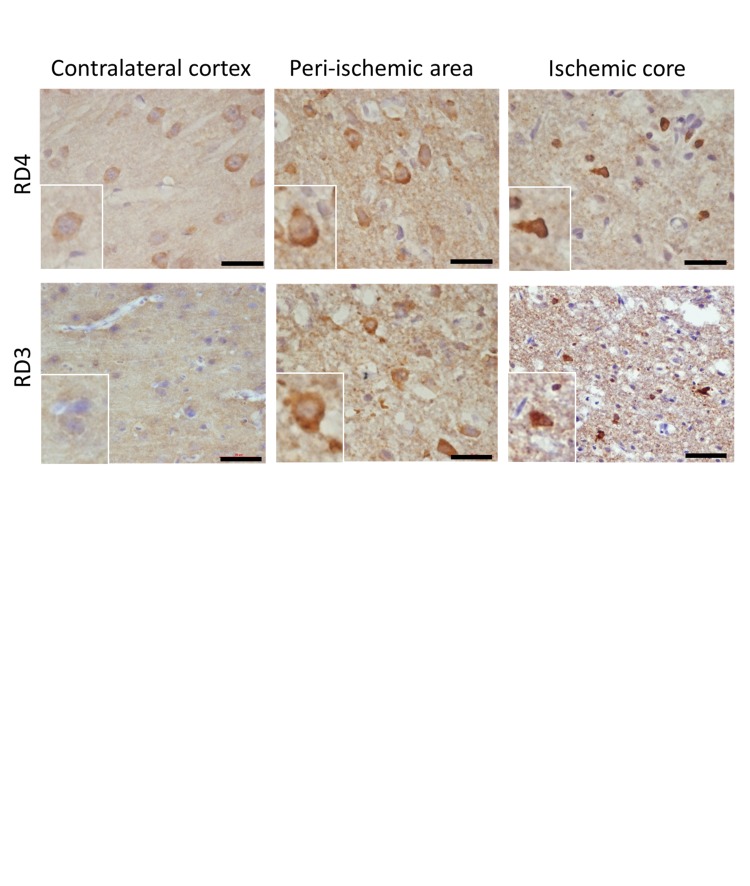
We investigated the alterations in the distribution of each tau isoform in cerebral I/R by immunohistochemistry. Similar to staining results for anti-Ab-2 antibody, immunostaining with anti-RD4 antibody and anti-RD3 antibody in the cortex of rats subjected to 72 h of reperfusion revealed that an elevated immunoreactivity for RD4 and RD3 was observed in the perikarya and part of the neuropil of the peri-ischemic area and ischemic core (Supplementary Figure 4).
Double-labeling fluorescence by combination of RD4 and RD3 antibodies showed homogeneous staining in neuropil on the contralateral cortex. Additionally, co-localization of RD4 and RD3 with granule-like protein inclusions was detected within neuronal perikarya on the contralateral cortex (Figure 3(a)). Homogeneous or granular staining of neuronal perikarya was RD4-dominant, RD3-dominant or positive for both RD4 and RD3 in the peri-ischemic area and ischemic core (Figure 3(a)). The degree of colocalization between 3-repeat and 4-repeat tau was analyzed using Pearson’s correlation coefficient (r) and quantified using WCIF Image J software. The Pearson’s correlation coefficients for RD3 and RD4 in the peri-ischemic area (r = 0.551, p < 0.001) and ischemic core (r = 0.737, p < 0.001) were significantly lower than in the contralateral cortex (r = 0.870) (Figure 3(b)).
Figure 3.
Alterations in the distribution of 4-repeat and 3-repeat tau isoforms in response to cerebral ischemia and reperfusion. (a) Representative double immunofluorescence staining is shown using RD3 and RD4 antibodies in the contralateral cortex (first column), peri-ischemic area (second column), and ischemic core (third column) of rats subjected to 72 h of reperfusion following 90 min of ischemia. The first row shows an overlay of images for RD3 (green) and RD4 immunofluorescence (red). The second row shows magnified views of the square insets from the first row. The scale bars represent 40 µm. (b) A scatter plot showing the relationship between the intensity of RD3 and RD4 immunofluorescence in the contralateral cortex, peri-ischemic area and ischemic core. The intensities in the Red-Green correlation plot represent the actual color of the pixels in the image (x-axis: red, y-axis: green). The green plots represent RD3-dominant staining, the red plots represent RD4-dominant staining, and the yellow plots represent positive staining for both RD3 and RD4. The data were analyzed in randomly selected 60-fold-magnification fields of view from the contralateral cortex, peri-ischemic area and ischemic core.
Tau mislocalized to neuronal perikarya is hyperphosphorylated at Ser202 and Thr205 and shows conformational changes similar to PHF-tau in cerebral I/R
To further characterize the phosphorylation and conformational status of tau in cerebral I/R, we examined the presence of PHF-tau phosphorylated at Ser202 and Thr205 using an anti-AT8 antibody. Western blotting showed that the levels of the anti-AT8 immunoreactive tau protein were significantly increased in the ischemic cortex at 48 h and 72 h of reperfusion compared with the levels in the cortex of sham-operated rats and/or the levels on the contralateral side (p = 0.001 at 48 h, p = 0.004 at 72 h after reperfusion; Figure 4(a) and (b)). Co-immunofluorescence of tissue sections using a combination of Ab-2 and AT8 antibodies revealed a colocalization of AT8 and Ab-2 immunostaining in pyknotic neurons in the peri-ischemic area. Some Ab-2-positive pyramidal-like neurons exhibited a partial overlap in AT8 expression in the peri-ischemic area. In the ischemic core, co-localization of AT8 and Ab-2 was observed in granules that were detected in a few neuronal cell bodies. The neuropil in the peri-ischemic area and ischemic core was faintly stained with the AT8 antibody (Figure 4(c)). Taken together, these results suggest that cerebral I/R induces PHF-like phosphorylation as well as conformational changes of tau protein in neuronal perikarya but not in neuropil. The number of AT8-positive granules in the neuronal perikarya per single neuron was significantly increased in the peri-ischemic area (mean ± SE: 4.9 ± 0.3, p = 0.0026) and ischemic core (5.1 ± 0.3, p < 0.001) compared with the contralateral cortex (3.3 ± 0.3) (Figure 4(d)).
Figure 4.
Expression and immunofluorescence profile of phosphorylated PHF-tau in response to cerebral ischemia and reperfusion. (a) The expression of phosphorylated PHF-tau was assessed by Western blot using the anti-AT8 antibody and the α-tubulin loading control antibody. Contralateral and ipsilateral cortical tissues were homogenized from rats subjected to a sham operation or 6–72 h of reperfusion following 90 min of ischemia. Each lane contains 40 µg of protein. (b) Quantification results are shown for the immunoblot data presented in (a). The optical densities were determined for phosphorylated PHF-tau and α-tubulin in the ipsilateral and contralateral cortex, and the phosphorylated PHF-tau densitometry values were normalized to those of α-tubulin. The graph shows the ratio of phosphorylated PHF-tau in the ipsilateral cortex compared with that in the contralateral cortex at each time point. The data are expressed as the mean ± SE and were analyzed by one-way ANOVA followed by Dunnett’s test. **p < 0.01 indicates a significant difference compared with sham-operated rats; n = 3 to 5 for each time point. (c) Representative double immunofluorescence staining is shown using Ab-2 and AT8 antibodies in the contralateral cortex (first column), peri-ischemic area (second column), and ischemic core (third column) of rats subjected to 72 h of reperfusion following 90 min of ischemia. The first row shows an overlay of images for Ab-2 (green) and AT8 immunofluorescence (red). The second row shows magnified views of the square insets from the second row. The scale bars represent 40 µm. (d) The number of AT8-positive granules in the neuronal perikarya per single cell in the peri-ischemic area and ischemic core compared with the contralateral cortex. Three 60-fold-magnification fields of view were randomly selected from each area, and the mean value for each field of view was calculated. The data were analyzed using one-way ANOVA followed by Dunnett’s test. ***p < 0.001 indicates a significant difference compared with the contralateral cortex.
Transient cerebral ischemia induces the appearance of neurotoxic Asp421-truncated tau fragments that compose NFTs in AD
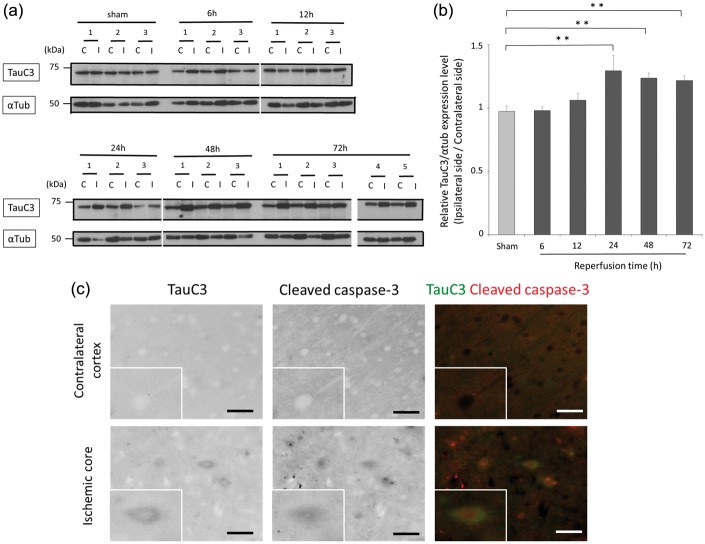
Following cerebral I/R, the expression level of tau truncated at Asp421 was investigated by immunoblot with the TauC3 antibody. The levels of Asp421-truncated tau, detected at 60 kDa, were similar when comparing between the ipsilateral and contralateral cortex of ischemia treated rats at 6 h and 12 h after reperfusion. However, from 24 h to 72 h after reperfusion, the levels of Asp421-truncated tau were significantly increased in the ipsilateral cortex compared with the levels in the cortex of sham-operated rats and the levels in the contralateral cortex (p = 0.009 at 24 h, p = 0.033 at 48 h, p = 0.026 at 72 h after reperfusion; Figure 5(a) and (b)). TauC3 is a protein of a theoretical molecular weight of 79 kDa, but an apparent molecular mass of 46–80 kDa is consistent with a previous study.45 Therefore, this result suggests that the 60-kDa Asp421-truncated tau fragment differs from the 25-kDa 4-repeat tau fragment and 17-kDa 3-repeat tau fragment. These results demonstrate that an Asp421-truncated tau fragment emerges in response to cerebral I/R. Co-immunofluorescence staining of tissue sections with a combination of TauC3 and cleaved caspase-3 antibodies revealed that Asp421-truncated tau was colocalized with cleaved caspase-3 in some neurons in the ischemic core; however, TauC3 immunoreactivity was barely detectable in the contralateral cortex (Figure 5(c)).
Figure 5.
Expression of Asp421-truncated tau in response to cerebral ischemia and reperfusion.(a) The expression of Asp421-truncated tau was assessed by Western blot using the anti-TauC3 antibody and the α-tubulin loading control antibody. Contralateral and ipsilateral cortical tissues were homogenized from rats subjected to a sham operation or 6–72 h of reperfusion following 90 min of ischemia. Each lane contains 40 µg of protein. (b) Quantification results are shown for the immunoblot data presented in (a). The optical densities were determined for Asp421-truncated tau and α-tubulin in the ipsilateral and contralateral cortex, and the Asp421-truncated tau densitometry values were normalized to those of α-tubulin. The graph shows the ratio of Asp421-truncated tau in the ipsilateral cortex compared with that in the contralateral cortex at each time point. The data are expressed as the mean ± SE and were analyzed by one-way ANOVA followed by Dunnett’s test. **p < 0.01 indicates a significant difference compared to sham-operated rats; n = 3 to 5 for each time point. (c) Representative double immunofluorescence staining is shown using TauC3 and cleaved caspase-3 antibodies in the contralateral cortex (in the first row) and ischemic core (in the second row) of rats subjected to 72 h of reperfusion following 90 min of ischemia. The first column shows the inverse signal of TauC3 immunofluorescence. The second column shows the inverse signal of cleaved caspase-3 immunofluorescence. The third column shows the overlay of images for TauC3 immunofluorescence (in green) to that of cleaved caspase-3 immunofluorescence (in red). The scale bars represent 40 µm.
Axonal varicosity formation in cerebral I/R
We investigated the effects of cerebral I/R on axon morphology by performing immunofluorescence staining with the anti-SMI312 antibody. Following cerebral I/R, SMI312 immunofluorescence revealed several axonal abnormalities in the peri-ischemic area and ischemic core, including axonal loss, axonal transection, and multiple serial swellings distributed along the axon with a “beads on a string” appearance, indicating the formation of axonal spheroids and varicosities (Figure 6(a)). The number of varicosities was significantly increased in the peri-ischemic area (mean ± SE: 15.6 ± 0.6, p < 0.007) and showed a positive trend in the ischemic core (8.3 ± 2.9, p < 0.173) compared with the contralateral cortex (3.0 ± 1.5) (Figure 6(b)).
Figure 6.
Axon structural abnormalities, including spheroid and varicosity formation, and axonal loss in response to cerebral ischemia and reperfusion. (a) A representative immunofluorescence staining is shown using the SMI312 antibody in the contralateral cortex (first column), peri-ischemic area (second column), and ischemic core (third column) of rats subjected to 72 h of reperfusion following 90 min of ischemia. The first row shows the inverse signal for SMI312 immunofluorescence. The second row shows magnified views of the square insets in the first row. The third row shows the schema of the varicosities in the second row. The scale bars represent 40 µm. (b) The number of varicosities in the contralateral cortex, peri-ischemic area, and ischemic core. Three 60-fold-magnification fields of view were randomly selected from each area, and the mean value for each field of view was calculated. The data were analyzed using one-way ANOVA followed by Dunnett’s test. **p < 0.01 indicates a significant difference compared with the contralateral cortex.
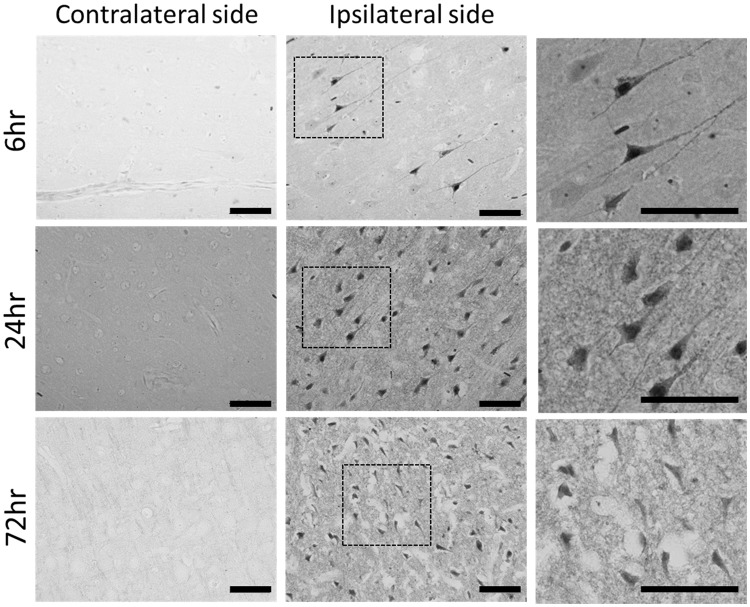
Cerebral I/R induce Gallyas-Braak silver deposition in cell bodies
Silver staining was evident in cell bodies and neurite-like structures in the ipsilateral cortex after 6 h of reperfusion and was even more prominent in all ipsilateral cortical layers after 24 h of reperfusion. In comparison to the 24-h time point, silver deposition within neurite-like structures of the ipsilateral cortex became less prominent after 72 h of reperfusion. For all time points observed after reperfusion, Gallyas-positive neurons were absent in the cortical region contralateral to the ischemic insult (Figure 7).
Figure 7.
Modified Gallyas-Braak staining in cerebral ischemia and reperfusion. Modified Gallyas staining was performed on cortical tissue sections that were taken from rats at 6 h (in the first row), 24 h (in the second row), and 72 h (in the third row) of reperfusion following 90 min of ischemia. The first and second column shows Gallyas staining in the contralateral cortex and ipsilateral cortex, respectively, at each time point. The third column shows magnified views of the square insets in the second column. The scale bars represent 50 µm.
Discussion
To better understand the pathological association of tau dynamics in cerebral ischemia and AD, we investigated the effects of cerebral I/R in rats on tau phosphorylation. Our study showed that cerebral I/R induced hyperphosphorylation and conformational changes of tau protein. Increased expression of phosphorylated tau was observed by Western blots using the Phos-tag SDS-PAGE technique and the phosphorylation-specific tau antibody AT8. The phosphorylation status of tau protein appears to be dynamically regulated in response to cerebral ischemia and/or reperfusion. In previous studies, tau dephosphorylation has been reported in permanent MCA occlusion of rats.29 Additionally, global ischemia has been shown to induce tau dephosphorylation in rats30 and canines,31 with rephosphorylation occurring toward control levels following reperfusion. Hyperphosphorylation of tau has been reported in response to reperfusion after transient MCA occlusion in rats32 and bilateral CCA occlusion in mongolian gerbils.33,34 The different tau phosphorylation states that are observed in animal models are thought to reflect dynamic changes in the balance of tau protein phosphatases and kinases that occur during cerebral I/R.
Tauopathies have been biochemically characterized based on which tau isoforms are found as tau aggregates. In Pick’s disease, tau aggregates are predominantly composed of 3-repeat tau. In contrast, in progressive supranuclear palsy and corticobasal degeneration, 4-repeat tau is predominant.45–47 AD contains a mixture of both tau isoforms.48 The present study showed that cerebral I/R leads to the hyperphosphorylation of both tau isoforms, which was observed by Western blot using the Phos-tag SDS-PAGE technique. In previous studies, the selective appearance of 4-repeat tau has been reported in neurons following brain autopsies of patients with cerebral infarction.49 However, the induction of 3-repeat tau has been reported in cortical neurons following transient MCA occlusion.50 The current study provides evidence supporting a similarity between cerebral I/R and AD with respect to tau hyperphosphorylation and isoform expression.
In addition to tau hyperphoshorylation, proteolytic cleavage of tau has also been suggested to facilitate tau deposition and to play a role in neurodegeneration.21–25,51–53 In the present study, a 17-kDa 3-repeat tau fragment and a 25-kDa 4-repeat tau fragment were observed in response to cerebral I/R. The 25-kDa 4-repeat tau fragment and the 17-kDa 3-repeat tau fragment both involve a tau microtubule-binding domain; therefore, cleavage at these sites could potentially decrease the binding affinity of both tau isoforms for microtubules. Increased levels of a 60-kDa Asp421-truncated tau fragment were also observed in the current study following cerebral I/R. Tau fragments truncated at Asp421 by caspase show a higher propensity to aggregate than full-length tau in vitro,54 and the generation of these fragments is suggested as a relatively early event in formation of NFTs in the AD brain.55,56 The current study showed that Asp421-truncated tau, stained with the TauC3 antibody, was co-expressed with cleaved caspase-3 in some neurons in the ischemic core. Generation of Asp421-truncated tau fragments has been thought to produce neuronal toxicity and may contribute to synaptic deficits and cellular demise in AD.27,28 This possibility suggests that, in addition to decreasing the tau-binding affinity for microtubules by modulating tau hyperphosphorylation and cleavage, cerebral I/R may also generate neurotoxic tau fragments that have both a loss of function and a toxic gain of function. Herein, we observed that the Asp421-truncated tau fragment generated in response to cerebral I/R preceded the emergence of the 17-kDa 3-repeat tau fragment, the 25-kDa 4-repeat tau fragment, and the hyperphosphorylated forms of tau. These results suggest that the generation of the Asp421-truncated tau fragment occurs as a relatively early event in the modification of tau protein by cerebral I/R.
In AD and other tauopathies, the hyperphosphorylation of tau protein leads to a decreased binding affinity of tau for microtubules and redistributes tau from its normal position in the axon into the neuronal cell body and associated dendrite.20 Herein, we observed the redistribution of tau protein from axons to neuronal cell bodies in response to cerebral I/R, similar to that observed in AD. Hyperphosphorylation of tau in response to cerebral I/R may impair the ability of tau to bind to microtubules, possibly leading to such redistribution. Following cerebral I/R, the levels of total tau, the 4-repeat tau isoform, and the 3-repeat tau isoform were unchanged between the contralateral and ipsilateral cortex examined by immunoblot with Ab-2, RD4, and RD3 antibodies, respectively. However, an elevated immunoreactivity for Ab-2, RD4, and RD3 was observed in the perikarya and part of the neuropil of the peri-ischemic area and ischemic core, and this finding may represent an intracellular redistribution of tau, changes in tau protein conformation and/or alterations of tau antibody recognition sites.29,32,36,57–59
We also investigated the effects of cerebral I/R on axon morphology using immunofluorescence with the SMI312 antibody and discovered structural abnormalities, including the formation of axonal spheroids and varicosities and axonal loss in the peri-ischemic area and ischemic core. Spheroids and varicosities have been previously thought to begin on unbroken axons in a variety of neurological disorders.60 Spheroids frequently appear as tandemly repeated swellings on individual axons, which suggests a multifocal block of axonal transport.60 Focal breakage of microtubules has been reported to be observed within the swollen regions of axons compared with adjacent, non-swollen regions of axons with a normal linear morphology.61 It is therefore concluded that alterations of tau distribution in response to cerebral I/R may influence microtubule stability and may contribute to disturbances in axonal transport and the resulting formation of axonal varicosities.
To further elucidate the pathological association between cerebral ischemia and AD, we performed modified Gallyas-Braak staining. Argyrophilic neurons with Gallyas-Braak staining have been reported in and around ischemic foci on human brains with cerebral infarction.49 In the present study, modified Gallyas-Braak staining revealed silver impregnation of neuronal cell bodies and neurite-like structures in cortical tissues ipsilateral to the cerebral ischemic insult. This staining pattern is similar to that observed in human brains with cerebral infarction, although it was necessary to reduce artifacts, such as the dark neuron artifact.49 The current study has shown that cerebral I/R induces the localization of hyperphosphorylated tau, 4-repeat tau isoform, and 3-repeat tau isoform to granules found in neuronal perikarya and also results in argyrophilic Gallyas-Braak staining in neurons of the ischemic cortex and peri-infarct area. These staining profiles are consistent with the deposition of tau in AD.
Conclusions
The present study shows that 3-repeat and 4-repeat tau isoforms are hyperphosphorylated in cerebral I/R in a manner similar to that observed in AD. Our study also shows that cerebral I/R generates neurotoxic 60-kDa Asp421-truncated tau and subsequently forms 17-kDa 3-repeat tau fragments, 25-kDa 4-repeat tau fragments, and various hyperphosphorylated tau isoforms. These results suggest that the generation of Asp421-truncated tau is a relatively early tau modification that is induced by cerebral I/R. Tau was also redistributed from axons to neuronal cell bodies in our stroke model and is known to follow a similar pattern in AD. Further, we show that cerebral I/R induced the formation of axonal spheroids and varicosities and also led to axonal loss. It is possible that the altered tau distribution that we observed may influence microtubule stability and subsequently disturb axonal transport, resulting in the formation of axonal varicosities and other axonal abnormalities. Although it is possible that different models, experimental approaches, and time points analyzed may show different tau dynamics, we believe that this study will help to clarify the underlying mechanisms of the onset and progression of AD that involve cerebral ischemia. Future studies to understand the effects of tau protein modifications on pathology formation, especially as it relates to cerebral ischemia, may provide insights into AD pathogenesis.
Supplementary Material
Acknowledgments
The authors also thank Ms. Y. Furuno and Ms. M. Sasanishi for their excellent technical assistance.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
HF and TT designed the study. ST and NS provided advice on producing animal models of ischemic stroke. HF and TM performed experiments. HF, TT, NH, HM, and MM analyzed the data. HF wrote the first draft of the manuscript. MM supervised the study. All authors contributed to drafting and/or revising the article, and all authors approved the final version intended for publication.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Tatemichi TK, Paik M, Bagiella E, et al. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology 1994; 44: 1885–1891. [DOI] [PubMed] [Google Scholar]
- 2.Henon H, Durieu I, Guerouaou D, et al. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology 2001; 57: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 3.Tatemichi TK, Desmond DW, Mayeux R, et al. Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology 1992; 42: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 4.Loeb C, Gandolfo C, Croce R, et al. Dementia associated with lacunar infarction. Stroke 1992; 23: 1225–1229. [DOI] [PubMed] [Google Scholar]
- 5.Ott A, Stolk RP, Hofman A, et al. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia 1996; 39: 1392–1397. [DOI] [PubMed] [Google Scholar]
- 6.Kalmijn S, Launer LJ, Lindemans J, et al. Total homocysteine and cognitive decline in a community based sample of elderly subjects: the Rotterdam Study. Am J Epidemiol 1999; 150: 283–289. [DOI] [PubMed] [Google Scholar]
- 7.Ott A, Slooter AJ, Hofman A, et al. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study. Lancet 1998; 351: 1840–1843. [DOI] [PubMed] [Google Scholar]
- 8.Notkola IL, Sulkava R, Pekkanen J, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology 1998; 17: 14–20. [DOI] [PubMed] [Google Scholar]
- 9.Martin AJ, Friston KJ, Colebatch JG, et al. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 1991; 11: 684–689. [DOI] [PubMed] [Google Scholar]
- 10.Graves AB, van Duijn CM, Chandra V, et al. Alcohol and tobacco consumption as risk factors for Alzheimer’s disease: a collaborative re-analysis of case-controlled studies. EURODEM risk factors research group. Int J Epidemiol 1991; 20: 48–57. [DOI] [PubMed] [Google Scholar]
- 11.de la Torre JC. Hemodynamic consequences of deformed microvessels in the brain in Alzheimer’s disease. Ann N Y Acad Sci 1997; 826: 75–91. [DOI] [PubMed] [Google Scholar]
- 12.Kogure D, Matsuda H, Ohnishi T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med 2000; 41: 1155–1162. [PubMed] [Google Scholar]
- 13.Rodriguez G, Vitali P, Calvini P, et al. Hippocampal perfusion in mild Alzheimer’s disease. Psychiatry Res 2000; 100: 65–74. [DOI] [PubMed] [Google Scholar]
- 14.Pluta R. Alzheimer lesions after ischemia-reperfusion brain injury. Folia Neuropathol 2004; 42: 181–186. [PubMed] [Google Scholar]
- 15.Weingarten MD, Lockwood AH, Hwo SY, et al. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 1975; 72: 1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drubin DG, Feinstein SC, Shooter EM, et al. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol 1985; 101: 1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caceres A, Kosik KS. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature 1990; 343: 461–463. [DOI] [PubMed] [Google Scholar]
- 18.Goedert M, Wischik CM, Crowther RA, et al. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci USA 1988; 85: 4051–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neve RL, Harris P, Kosik KS, et al. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res 1986; 387: 271–280. [DOI] [PubMed] [Google Scholar]
- 20.Andorfer C, Kress Y, Espinoza M, et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 2003; 86: 582–590. [DOI] [PubMed] [Google Scholar]
- 21.García-Sierra F, Wischik CM, Harrington CR, et al. Accumulation of C-terminally truncated tau protein associated with vulnerability of the performant pathway in early stages of neurofibrillary pathology in Alzheimer’s disease. J Chem Neuroanat 2001; 22: 65–77. [DOI] [PubMed] [Google Scholar]
- 22.Ghoshal N, García-Sierra F, Fu Y, et al. Tau-66: evidence for a novel tau conformation in Alzheimer’s disease. J Neurochem 2001; 77: 1372–1385. [DOI] [PubMed] [Google Scholar]
- 23.Gamblin TC, Chen F, Zambrano A, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 2003; 100: 10032–10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, et al. Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging 2005; 26: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 25.Luna-Muñoz J, García-Sierra F, Falcón V, et al. Regional conformational change involving phosphorylation of tau protein at the Thr231, precedes the structural change detected by Alz-50 antibody in Alzheimer’s disease. J Alzheimers Dis 2005; 8: 29–41. [DOI] [PubMed] [Google Scholar]
- 26.Guillozet-Bongaarts AL, Cahill ME, Cryns VL, et al. Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem 2006; 97: 1005–1014. [DOI] [PubMed] [Google Scholar]
- 27.Chung CW, Hong YM, Song S, et al. Atypical role of proximal caspase-8 in truncated Tau-induced neurite regression and neuron cell death. Neurobiol Dis 2003; 14: 557–566. [DOI] [PubMed] [Google Scholar]
- 28.Rissman RA, Poon WW, Blurton-Jones M, et al. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 2004; 114: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewar D, Dawson D. Tau protein is altered by focal cerebral ischaemia in the rat: An immunohistochemical and immunoblotting study. Brain Res 1995; 684: 70–78. [DOI] [PubMed] [Google Scholar]
- 30.Shackelford DA, Yeh RY. Dephosphorylation of tau during transient forebrain ischemia in the rat. Mol Chem Neuropathol 1998; 34: 103–120. [DOI] [PubMed] [Google Scholar]
- 31.Mailliot C, Podevin-Dimster V, Rosenthal RE, et al. Rapid tau protein dephosphorylation and differential rephosphorylation during cardiac arrest-induced cerebral ischemia and reperfusion. J Cereb Blood Flow Metab 2000; 20: 543–549. [DOI] [PubMed] [Google Scholar]
- 32.Wen Y, Yang S, Liu R, et al. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res 2004; 1022: 30–38. [DOI] [PubMed] [Google Scholar]
- 33.Morioka M, Kawano T, Yano S, et al. Hyperphosphorylation at serine 199/202 of tau factor in the gerbil hippocampus after transient forebrain ischemia. Biochem Biophys Res Commun 2006; 347: 273–278. [DOI] [PubMed] [Google Scholar]
- 34.Gordon-Krajcer W, Kozniewska E, Lazarewicz JW, et al. Differential changes in phosphorylation of tau at PHF-1 and 12E8 epitopes during brain ischemia and reperfusion in gerbils. Neurochem Res 2007; 32: 729–737. [DOI] [PubMed] [Google Scholar]
- 35.Ichihara K, Uchihara T, Nakamura A, et al. Selective deposition of 4-repeat tau in cerebral infarcts. J Neuropathol Exp Neurol 2009; 68: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 36.Suh J, Im DS, Moon GJ, et al. Hypoxic ischemia and proteasome dysfunction alter tau isoform ratio by inhibiting exon 10 splicing. J Neurochem 2010; 114: 160–170. [DOI] [PubMed] [Google Scholar]
- 37.Kinoshita E, Kinoshita-Kikuta E, Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat Protoc 2009; 4: 1513–1521. [DOI] [PubMed] [Google Scholar]
- 38.Koizumi J, Yoshida Y, Nakazawa T, et al. Experimental studies of ischemic brain edema. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke 1986; 8: 1–8. [Google Scholar]
- 39.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20: 84–91. [DOI] [PubMed] [Google Scholar]
- 40.Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 1995; 189: 167–169. [DOI] [PubMed] [Google Scholar]
- 41.Goedert M, Jakes R, Crowther RA, et al. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci USA 1993; 90: 5066–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porzig R, Singer D, Hoffmann R. Epitope mapping of mAbs AT8 and Tau5 directed against hyperphosphorylated regions of the human tau protein. Biochem Biophys Res Commun 2007; 358: 644–649. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, San Diego, CA: Academic Press, 1998. [Google Scholar]
- 44.Uchihara T, Kondo H, Kosaka K, et al. Selective loss of nigral neurons in Alzheimer’s disease: a morphometric study. Acta Neuropathol 1992; 83: 271–276. [DOI] [PubMed] [Google Scholar]
- 45.Dickson DW. Neuropathology of Pick’s disease. Neurology 2001; 56: 16–20. [DOI] [PubMed] [Google Scholar]
- 46.Delacourte A, Robitaille Y, Sergeant N, et al. Specific pathological Tau protein variants characterize Pick’s disease. J Neuropathol Exp Neurol 1996; 55: 159–168. [DOI] [PubMed] [Google Scholar]
- 47.Rizzini C, Goedert M, Hodges JR, et al. Tau gene mutation K257T causes a tauopathy similar to Pick’s disease. J Neuropathol Exp Neurol 2000; 59: 990–1001. [DOI] [PubMed] [Google Scholar]
- 48.Uchihara T. Silver diagnosis in neuropathology: Principles, practice and revised interpretation. Acta Neuropathol 2007; 113: 483–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ichihara K, Uchihara T, Nakamura A, et al. Selective deposition of 4-repeat tau in cerebral infarcts. J Neuropathol Exp Neurol 2009; 68: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 50.Suh J, Im DS, Moon GJ, et al. Hypoxic ischemia and proteasome dysfunction alter tau isoform ratio by inhibiting exon 10 splicing. J Neurochem 2010; 114: 160–170. [DOI] [PubMed] [Google Scholar]
- 51.Zemlan FP, Mulchahey JJ, Gudelsky GA. Quantification and localization of kainic acid-induced neurotoxicity employing a new biomarker of cell death: cleaved microtubule-associated protein-tau (C-tau). Neuroscience 2003; 121: 399–409. [DOI] [PubMed] [Google Scholar]
- 52.Gabbita SP, Scheff SW, Menard RM, et al. Cleaved-tau: a biomarker of neuronal damage after traumatic brain injury. J Neurotrauma 2005; 22: 83–94. [DOI] [PubMed] [Google Scholar]
- 53.Arnaud LT, Myeku N, Figueiredo-Pereira ME. Proteasome-caspasecathepsin sequence leading to tau pathology induced by prostaglandin J2 in neuronal cells. J Neurochem 2009; 110: 328–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gamblin TC, Chen F, Zambrano A, et al. Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 2003; 100: 10032–10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotman CW, Poon WW, Rissman RA, et al. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol 2005; 64: 104–112. [DOI] [PubMed] [Google Scholar]
- 56.Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, et al. Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging 2005; 26: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 57.Dewar D, Dawson D. Tau protein is altered by focal cerebral ischaemia in the rat: an immunohistochemical and immunoblotting study. Brain Res 1995; 684: 70–78. [DOI] [PubMed] [Google Scholar]
- 58.Wen Y, Yang S, Liu R, et al. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res 2004; 1022: 30–38. [DOI] [PubMed] [Google Scholar]
- 59.Suh J, Im DS, Moon GJ, et al. Hypoxic ischemia and proteasome dysfunction alter tau isoform ratio by inhibiting exon 10 splicing. J Neurochem 2010; 114: 160–170. [DOI] [PubMed] [Google Scholar]
- 60.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci 2005; 6: 889–898. [DOI] [PubMed] [Google Scholar]
- 61.Tang-Schomer MD, Johnson VE, Baas PW, et al. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol 2012; 233: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.