Abstract
Hyperglycemia is a common complication after ischemic stroke, but its link to worse outcome is not well understood. We hypothesized that hyperglycemia may reflect an impaired metabolic response that is associated with worse cytotoxic brain injury. We performed retrospective analysis of magnetic resonance imaging from a cohort of acute ischemic stroke patients prospectively collected from 2006 to 2010 with baseline demographic and laboratory data as well as three-month outcomes. The severity of cytotoxic injury was quantified in vivo using apparent diffusion coefficient imaging by measuring the signal intensity within the stroke relative to the normal signal intensity of the contralateral hemisphere. Both hyperglycemia and lower apparent diffusion coefficient signal were associated with worse outcome after ischemic stroke (OR 0.239, p = 0.017; OR 1.11, p < 0.0001, respectively). Hyperglycemia was also associated with lower apparent diffusion coefficient (r = −0.32, p < 0.001). In multivariate analysis, apparent diffusion coefficient but not hyperglycemia was associated with outcome, suggesting that cytotoxicity may mediate the effect of hyperglycemia. For interventions designed to target hyperglycemia in acute ischemic stroke, a concomitant effect on the evolution of apparent diffusion coefficient may provide insight into whether hyperglycemia leads to or reflects worse cytotoxic injury.
Keywords: Acute stroke, brain imaging, brain ischemia, diffusion weighted MRI, hyperglycemia
Introduction
The loss of blood flow in the initial hours after the onset of ischemic stroke leads to a failure in cellular energy metabolism and reduction in ATP, subsequent failure of the sodium potassium ATPase and then activation of intracellular pathways that ultimately result in cytotoxic cell death.1 This process can be monitored in vivo with apparent diffusion coefficient (ADC) magnetic resonance imaging.2 Our laboratory has previously shown that degree of ADC reduction on initial MRI is predictive of swelling on subsequent imaging.3 However, the relationship between degree of ADC reduction and outcome is unknown and risk factors for cytotoxicity are not well established.
Hyperglycemia is a common finding in ischemic stroke, being present in 37% to 68% of patients,4–6 a frequency that cannot be accounted for by incident diabetes alone. Patients who present with hyperglycemia have larger infarct size7,8 as well as a reduced salvage of perfusion-diffusion mismatch on MR imaging.9 Furthermore, hyperglycemia is correlated with poor outcome,10,11 particularly in those patients without a history of DM.7,12,13 Previous trials of tight glycemic control in stroke have thus far been inconclusive14–16 and a large multi-center trial of glucose control is ongoing.17 As a result, it is unknown if hyperglycemia plays a causative role in stroke outcome. Although some studies have suggested that hyperglycemia may be an epiphenomenon related to catecholamine release,7,18–20 other studies have suggested direct tissue damage through increased lactate production,9,11 or a broader disordered metabolic state that alters tissue reperfusion, calcium homeostasis, oxidative stress, and the inflammatory response.21,22
The goal of the current study was to use ADC imaging to evaluate the relationship between cytotoxic injury, hyperglycemia, and stroke outcome, in order to test the hypothesis that hyperglycemia represents an adverse metabolic response to ischemia that exacerbates tissue injury.
Methods
Patients
Subjects were enrolled in a prospective biomarker study of acute ischemic stroke as part of the Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) Network. The SPOTRIAS Biomarker study enrolled consecutive patients ≥ 18 years of age between January 2006 and April 2010 who presented within 12 h of symptom onset to Massachusetts General Hospital or Brigham & Women’s Hospital, with symptoms consistent with ischemic stroke (N = 522). The study protocol was reviewed and approved by the Institutional Review Board at Partners Healthcare (protocol #2011P002725) and written informed consent was obtained from all participants or their legal representatives prior to enrollment in the study. The study was carried out according to the guidelines set by the Partners Healthcare Human Research Committee.
In the current study, patients were eligible who had a baseline diffusion weighted imaging (DWI) available for analysis and either outcome data in the form of a three-month modified Rankin Scale (mRS) score or baseline research blood sample available (N = 318). Patients were excluded who had missing, motion degraded or poor quality imaging (N = 48), a cerebellar or brainstem infarct (N = 22), or bilateral infarcts (N = 20). Subjects were further excluded if stroke volume on ADC imaging was < 5 ml to avoid error due to volume averaging during image acquisition (N = 81). For outcome analysis, subjects were excluded if pre-stroke mRS was three or greater (N = 15). Modified Rankin Scores of 4 and 5 were combined for analysis given the small number of patients with mRS 5 (n = 3).
Imaging analysis
Region-of-interest (ROI) analysis was conducted using a semi-automated method in Analyze 11.0 (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN, USA), based on our prior methods.3 The stroke and normal contralateral hemisphere ROIs were initially defined on DWI and then transferred to the ADC map for removal of cerebrospinal fluid. ADCr was calculated by taking the ratio of the mean signal intensity in the stroke ROI to the mean signal intensity of the contralateral hemisphere and so is presented as a percent of baseline ADC signal. Normalization to the baseline was performed in order to adjust for variation among individuals and technical variation between scanner and ADC acquisition settings.23 Stroke volume (mL) and intensity ratios were calculated for all subjects. Imaging analyses were performed by trained readers (M.B.B. and N.H.V.) blinded to clinical and outcome data. The intraclass correlation coefficient was 0.996 for ADCr measurements between the two readers. For additional quality control, the ADC stroke volumes generated in this study were compared with prior DWI stroke volumes obtained as part of the original study analyses. The intraclass correlation coefficient was 0.92.
Laboratory analysis
Glucose and hemoglobin A1c values were obtained in stroke patients as part of standard of care and these values were abstracted from the medical record. Insulin was measured on research plasma samples using a commercially available method (R&D Human insulin Quantikine ELISA). Insulin resistance was calculated using the homeostatic model assessment, version 2 (HOMA2-IR)24 based on baseline clinical glucose and measured insulin concentrations.
Statistical analysis
Descriptive statistics of baseline variables and outcomes were performed, reported as mean ± standard deviation (for normally distributed continuous data), median with interquartile range ([IQR]; for non-normal or ordinal data), and proportions (for binary data). Comparisons between the complete cohort and subset used for outcome analysis were performed using t-test or chi-square as appropriate for the data type. Inter-rater agreement was assessed for stroke DWI volume using intraclass correlation coefficient and Bland–Altman analyses. Univariate logistic regression was performed to investigate the association of clinical and imaging variables with outcome. Multivariate logistic regression models were developed to test for independent effects. Linear regression and t-test were used to assess the relationship between time to MRI, IV tPA treatment status, blood glucose level, hemoglobin A1c, HOMA-IR, DM status, and ADCr. All tests were two-sided, with the threshold of significance set at p < 0.05. Results of comparison analyses are reported as mean ± standard error of the mean. Statistical analyses were performed using JMP Pro 12.0 (SAS Institute, Cary, NC, USA).
Results
Study cohort
The characteristics of the clinical cohort are shown in Table 1. Of the 522 patients in the cohort, 229 had acute DWI imaging showing unilateral supratentorial infarct. Of these, 141 had an infarct volume greater than or equal to 5 ml and baseline blood samples available, and thus constituted the study population. Ninety-nine subjects had three-month mRS scores available and were used for outcome analysis. The mean ADCr in the entire study population was 67.1 ± 8.2% based on an MRI obtained 5.6 ± 3.3 h after time last seen well, and was 61.7 ± 8.0% in the outcome subgroup, based on an MRI obtained 4.6 ± 3.3 h after time last seen well. There were no statistically significant differences in any of the baseline characteristics between the entire study population and the outcome subgroup.
Table 1.
Characteristics of the study cohort.
| Entire population n = 141 | Outcome subgroup n = 99 | p | |
|---|---|---|---|
| Age (years), mean ± SD | 70 ± 14 | 68 ± 15 | 0.19 |
| Sex, male, N (%) | 85 (60%) | 66 (67%) | 0.34 |
| Comorbidities, N (%) | |||
| Diabetes | 30 (21%) | 18 (18%) | 0.56 |
| Hypertension | 101 (72%) | 64 (65%) | 0.25 |
| Hyperlipidemia | 60 (43%) | 41 (41%) | 0.86 |
| Atrial fibrillation | 55 (39%) | 38 (38%) | 0.92 |
| Prior stroke | 28 (20%) | 16 (16%) | 0.47 |
| Treatment, N (%)a | |||
| IV tPA | 68 (58%) | 47 (57%) | 0.96 |
| Endovascular clot retrieval | 2 (1.7%) | 2 (2.4%) | 0.71 |
| Laterality (left), N (%) | 72 (54%) | 52 (53%) | 0.99 |
| Stroke category | 0.88 | ||
| Cardioembolic, N (%) | 78 (55%) | 53 (54%) | |
| Large vessel, N (%) | 29 (21%) | 24 (24%) | |
| Undetermined, N (%) | 25 (17%) | 15 (15%) | |
| Other, N (%) | 9 (6.4%) | 7 (7.0%) | |
| Admission NIHSS, median [IQR] | 12 [5, 17] | 9 [3, 15] | 0.083 |
| Time from LSW to MRI (h), mean ± SD | 5.6 ± 3.3 | 4.6 ± 3.3 | 0.71 |
| Time from LSW to glucose draw (h), mean ± SD | 4.8 ± 2.7 | 4.6 ± 2.6 | 0.67 |
| Baseline blood glucose (mg/dL), mean ± SD | 137 ± 56 | 134 ± 55 | 0.66 |
| Body mass index (kg/cm), mean ± SDb | 27.0 ± 5.2 | 26.8 ± 4.7 | 0.80 |
| Admission ADC volume (mL), median [IQR] | 21 [8, 59] | 18 [8, 45] | 0.13 |
| Admission ADC ratio, mean ± SD | 67.1 ± 8.2% | 67.1 ± 8.0% | 0.77 |
| 90-day mRS, median [IQR] | – | 2 [1, 4] | – |
tPA and endovascular intervention status were available for 118 patients, of whom 82 were in the outcome subgroup.
BMI data were available for 124 patients, of whom 91 were in the outcome subgroup.
Given the heterogeneity in MRI time in this cohort and the known decline in ADCr over 24 h following stroke, we compared ADCr to time of image acquisition using linear regression, finding no significant relationship (p = 0.14). A relatively high proportion of subjects in this cohort received IV tPA (58% overall, 57% in outcome subgroup). Given that reperfusion may alter cytotoxicity, we compared mean ADCr between those patients who did and did not receive tPA. Data on tPA status were missing for 23 patients, these were excluded from the comparison. There was no significant difference in mean ADCr between tPA treatment groups in either the overall cohort (68.0 ±1.0% tPA vs. 68.0 ± 1.2% no tPA, p = 0.99) or outcome subgroup (67.9 ± 1.2% tPA vs. 68.8 ± 1.4% no tPA, p = 0.63).
ADCr predicts 90-day outcome
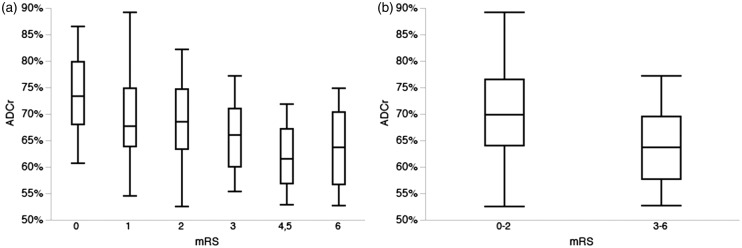
Lower ADCr on initial MRI was significantly associated with worse score on the mRS at 90 days both across the entire mRS scale (Figure 1(a); p < 0.0001) and when comparing dichotomized mRS 0–2 vs. 3–6 (Figure 1(b); 70.3 ± 1.1% vs. 64.0 ± 0.9%, p < 0.0001). Other univariate predictors of outcome across the entire mRS are summarized in Table 2 and included age, female sex, baseline stroke volume, admission glucose and presenting NIHSS. In a multivariate logistic regression model, ADCr remained an independent predictor of 90-day outcome across the mRS scale (p = 0.0079).
Figure 1.
ADC ratio and outcome after ischemic stroke. (a) ADCr was associated with outcome across the entire modified Rankin Scale, with lower ADCr associated with worse outcome (p < 0.0001). Note that mRS 4 and 5 were combined for analysis given the low number of patients in this cohort with mRS 5. (b) Dichotomized mRS finds that lower ADCr was significantly associated with poor (mRS 3–6) outcome at 90 days after ischemic stroke (p < 0.0001).
Table 2.
Univariate and multivariate predictors of mRS across entire scale at 90 days.
| OR | 95% CI | p | Adjusted OR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Age | 0.95 | 0.93–0.98 | <0.0001 | 0.61 | 0.93–0.98 | 0.0005 |
| Sex (female) | 0.63 | 0.43–0.91 | 0.016 | 0.78 | 0.52–1.16 | 0.22 |
| Glucose (log) | 0.23 | 0.069–0.80 | 0.017 | 0.76 | 0.21–2.70 | 0.68 |
| NIHSS | 0.82 | 0.77–0.87 | <0.0001 | 0.87 | 0.81–0.94 | 0.0001 |
| ADC volume (log) | 0.33 | 0.22–0.50 | <0.0001 | 0.54 | 0.34–0.87 | 0.011 |
| ADCr | 1.11 | 1.06–1.16 | <0.0001 | 1.07 | 1.02–1.13 | 0.0079 |
Receiver operating curve analysis of ADCr showed an area under the curve of 0.714 for prediction of good (mRS 0–2) outcome (Figure 2). An ADCr cut off value of 64.0% predicts good outcome with a sensitivity of 0.81 and specificity of 0.53.
Figure 2.
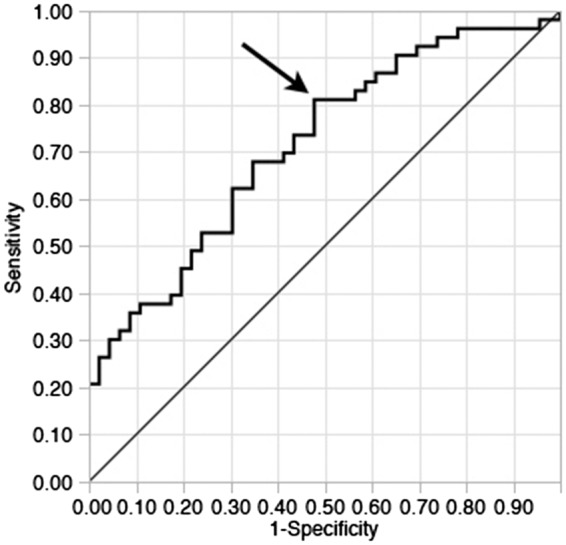
ROC curve for ADCr as a predictor of good (mRS 0–2) outcome. Receiver operating curve analysis for ADCr found an area under the curve of 0.714 for the prediction of mRS 0–2 at 90 days after ischemic stroke. Using a cutoff value of 64.0% for ADCr results in a sensitivity of 0.81 and specificity of 0.53 for prediction of good outcome.
ADCr is associated with hyperglycemic traits
To investigate the relationship between hyperglycemia and cytotoxicity, we compared blood glucose values measured on presentation with ADCr determined on baseline MRI. We found that lower ADCr was associated with higher baseline glucose (n = 141, r = –0.32, p < 0.001). Furthermore, if the cohort was divided into those with and without hyperglycemia (defined as glucose > 130 mg/dl13), ADCr was significantly lower in hyperglycemic subjects (63.8% vs. 68.9%, p = 0.0002).
Elevated glucose after stroke may reflect a stress response7,18,19 or elicit an inducible form of insulin resistance.10,11,25 We therefore assessed whether ADCr was associated with markers of insulin resistance and diabetes and found that ADCr was associated with higher hemoglobin A1c (n = 130, r = −0.34, p < 0.0001), a greater degree of insulin resistance (n = 116, r = −0.26, p = 0.0086) and a history of diabetes (n = 141, p < 0.0001). We also compared initial glucose level to ADCr in those patients without a known history of DM (n = 111). ADCr remained significantly associated with hyperglycemia in this subset (r =−0.19, p = 0.027), while insulin resistance was not associated with ADCr.
Discussion
Ischemic injury to brain tissue results in energy failure and subsequent cytotoxic cell death, a process that is reflected by ADC imaging.2 Our data indicate that the degree of reduction in ADC signal, acting as an imaging marker of cytotoxicity, is predictive of outcome following ischemic stroke. This association is independent of other known predictors of stroke outcome, including infarct size, initial clinical stroke severity (as measured by NIHSS), age and sex. This finding is consistent with animal studies, which have shown that degree of ADC reduction is associated with other markers of tissue injury. In a rat middle cerebral artery occlusion model, regions with ADC values of approximately 90% of baseline show reduced tissue pH, while regions with greater ADC reduction, approximately 77%, show depletion of ATP.26,27 In a separate study, even greater reduction in ADC to 58% of baseline was associated with elevated lactate and histologic evidence of cell death.28 The association of ADCr and outcome in clinical subjects demonstrated in the current study further supports ADCr as an imaging biomarker of for worsened cytotoxic injury.
Our data also identify a relationship between hyperglycemia and the degree of cytotoxicity, which is most prominent in those with a history of diabetes mellitus. Our findings suggest that worse cytotoxic injury may be a mechanistic link between diabetes and its known association with poor outcome after stroke.29 We also observed an association between hyperglycemia and ADCr in those patients without a documented history of DM. This parallels an animal study which found that hyperglycemia increased lesion volume following middle cerebral artery occlusion in both normal rats and those that model the metabolic syndrome.30 Our findings are also consistent with earlier reports in humans showing larger infarct size and more severe hemiparesis,7 increased disability and mortality,12 and greater stroke severity and worse functional outcome13 in those hyperglycemic patients without a prior history of DM when compared to those with known DM.
The recognition of ADCr as an imaging biomarker for cytotoxicity will allow for future studies attempting to better characterize any broader metabolic disturbance signified by acute post-stroke hyperglycemia. The association of ADCr and outcome allows it to serve as an imaging biomarker of cytotoxicity, which can be used to both gauge the effect of interventions targeting ischemic cell death as well as to identify risk factors for worsened cytotoxic injury. In particular, ADCr may provide a tool to select those patients who could stand to benefit from tight glucose control. Several studies have looked at the effect of strictly controlling glucose in the immediate post-stroke period. The Glucose-Insulin in Stroke Trial (GIST-UK) compared continuous infusion of glucose-potassium-insulin to placebo with a primary outcome of mortality at 90 days.15 The trial showed no difference in outcome, but was underpowered to definitively determine a clinical effect. Additional insulin trials found a non-significant but favorable trend in exploratory analyses.14,16 Our data suggest that hyperglycemia is linked to early change in cytotoxic injury, raising the possibility that the timing of initiation of glucose therapy may have an important role in clinical outcome, and may explain apparent discrepancies in prior studies. This is consistent with a prior study that did a voxel-based analysis of ADC values following MCA stroke to identify regions of lower ADC in patients with poor outcome and without arterial recanalization.31 This study also showed that ADC values were lower in hyperglycemic patients. Completion of an ongoing trial of intravenous insulin after acute stroke may provide further opportunity to evaluate an interaction between time of treatment and potential efficacy of glucose control.17 Finally, our data suggest that ADCr and subsequent evolution of ADC may be a useful intermediate efficacy endpoint in future trials of glucose control after stroke.
The current study has limitations. It is based on a retrospective analysis, involving primarily mild to moderate strokes with a relatively small median infarct size. It is unknown if the findings can be generalized to larger size strokes. We measured mean ADC signal intensity, and it is possible, particularly in larger strokes, that sub-regions that exhibit ADC heterogeneity may have a differing relationship with outcome. This study also looked at a single imaging time point, which occurred prior to the nadir of ADC signal.3 Reperfusion and collateral assessments were not available in this cohort. This will be particularly important to address in future studies given prior findings that tight control of hyperglycemia limited lactate as measured by MR spectroscopy, but was associated with greater infarct growth in those patients with persistent vessel occlusion.32
The initial study from which we drew subjects was not designed to assess hyperglycemia, so we have limited data on blood glucose and interventions to manage it. Our categorization of patients as hyperglycemic relies on the single glucose measurement available in our cohort. This may be relevant given prior data that sustained hyperglycemia over 72 h is associated with expanded infarct size and worsened outcome.8 However, the more limited glucose measurements in the current study cohort would tend to bias towards the null hypothesis and limit the detection of a difference in outcome or degree of cytotoxicity. Similarly, we do not have data on prior-to-admission medications or on any interventions undertaken to manage hyperglycemia. Patients at our institution are typically kept NPO in the initial hours after stroke until swallow screening can be performed. Oral anti-hyperglycemics are held while inpatient, and elevated glucose is covered with sliding scale insulin. Our lack of data on specific interventions may also contribute to uncertainty about effects of transient versus prolonged hyperglycemia.
Finally, while we found an association between hyperglycemia and reduced ADC signal intensity, this study does not address the causality of elevated blood glucose as it relates to cytotoxicity. Glucose values and ADC images in this study were obtained approximately 5 h after the last seen normal time, so it is possible that hyperglycemia was a response to, rather than a cause of worsened cytotoxic injury. This, and other limitations identified here, will be important to address in future prospective studies.
In summary, this study identifies a link between hyperglycemia and cytotoxic injury and highlights the potential role for ADC ratio as an imaging biomarker of cytotoxicity.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (Grant #NS076597) and the American Heart Association (Grant #14GRNT19060044).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Kimberly receives research support from Remedy Pharmaceuticals for the GAMES-RP clinical trial which is outside the scope of the submitted work. All other authors have no conflicts to disclose.
Authors’ contributions
Dr. Bevers and Dr. Kimberly had full access to all of the data for the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: MBB, WTK.
Acquisition, analysis or interpretation of data: MBB, NHV, LP, TWKB, WTK.
Drafting of the manuscript: MBB, WTK.
Critical revision of the manuscript for important intellectual content: MBB, NHV, LP, TWKB, WTK.
Study supervision: MBB, WTK.
References
- 1.Lipton P. Ischemic cell death in brain neurons. Physiol Rev 1999; 79: 1431–1568. [DOI] [PubMed] [Google Scholar]
- 2.Neumann-Haefelin T, Moseley ME, Albers GW. New magnetic resonance imaging methods for cerebrovascular disease: emerging clinical applications. Ann Neurol 2000; 47: 559–570. [PubMed] [Google Scholar]
- 3.Battey TW, Karki M, Singhal AB, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke 2014; 45: 3643–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott JF, Robinson GM, French JM, et al. Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet 1999; 353: 376–377. [DOI] [PubMed] [Google Scholar]
- 5.McCormick MT, Muir KW, Gray CS, et al. Management of hyperglycemia in acute stroke: How, when, and for whom? Stroke 2008; 39: 2177–2185. [DOI] [PubMed] [Google Scholar]
- 6.Fuentes B, Castillo J, San Jose B, et al. The prognostic value of capillary glucose levels in acute stroke: the glycemia in acute stroke (GLIAS) study. Stroke 2008; 40: 562–568. [DOI] [PubMed] [Google Scholar]
- 7.Murros K, Fogelholm R, Kettunen S, et al. Blood-Glucose, Glycosylated Hemoglobin, and Outcome of Ischemic Brain Infarction. J Neurol Sci 1992; 111: 59–64. [DOI] [PubMed] [Google Scholar]
- 8.Baird TA, Parsons MW, Phan T, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 2003; 34: 2208–2214. [DOI] [PubMed] [Google Scholar]
- 9.Parsons MW, Barber PA, Desmond PM, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol 2002; 52: 20–28. [DOI] [PubMed] [Google Scholar]
- 10.Bruno A, Biller J, Adams HP, et al. Acute blood glucose level and outcome from ischemic stroke. Neurology 1999; 52: 280. [DOI] [PubMed] [Google Scholar]
- 11.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.[see comment]. Stroke 2001; 32: 2426–2432. [DOI] [PubMed] [Google Scholar]
- 12.Weir CJ, Murray GD, Dyker AG, et al. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long term follow up study. BMJ 1997; 314: 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stead LG, Gilmore RM, Bellolio MF, et al. Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care 2009; 10: 181–186. [DOI] [PubMed] [Google Scholar]
- 14.Bruno A, Kent TA, Coull BM, et al. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke 2008; 39: 384–389. [DOI] [PubMed] [Google Scholar]
- 15.Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol 2007; 6: 397–406. [DOI] [PubMed] [Google Scholar]
- 16.Johnston KC, Hall CE, Kissela BM, et al. Glucose regulation in acute stroke patients (grasp) trial: A randomized pilot trial. Stroke 2009; 40: 3804–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruno A, Durkalski VL, Hall CE, et al. The stroke hyperglycemia insulin network effort (SHINE) trial protocol: a randomized, blinded, efficacy trial of standard vs. intensive hyperglycemia management in acute stroke. Int J Stroke 2014; 9: 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murros K, Fogelholm R, Kettunen S, et al. Serum cortisol and outcome of ischemic brain infarction. J Neurol Sci 1993; 116: 12–17. [DOI] [PubMed] [Google Scholar]
- 19.Tenerz A, Nilsson G, Forberg R, et al. Basal glucometabolic status has an impact on long-term prognosis following an acute myocardial infarction in non-diabetic patients. J Intern Med 2003; 254: 494–503. [DOI] [PubMed] [Google Scholar]
- 20.Winder K, Seifert F, Ohnemus T, et al. Neuroanatomic correlates of poststroke hyperglycemia. Ann Neurol 2015; 77: 262–268. [DOI] [PubMed] [Google Scholar]
- 21.Garg R, Chaudhuri A, Munschauer F, et al. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke 2006; 37: 267–273. [DOI] [PubMed] [Google Scholar]
- 22.Kruyt ND, Biessels GJ, Devries JH, et al. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol 2010; 6: 145–155. [DOI] [PubMed] [Google Scholar]
- 23.Tsujita N, Kai N, Fujita Y, et al. Interimager variability in ADC measurement of the human brain. Magn Reson Med Sci 2014; 13: 81–87. [DOI] [PubMed] [Google Scholar]
- 24.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diab Care 1998; 21: 2191–2192. [DOI] [PubMed] [Google Scholar]
- 25.Dungan KM, Braithwaite SS, Preiser J-C. Stress hyperglycaemia. Lancet 2009; 373: 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoehn-Berlage M, Norris DG, Kohno K, et al. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab 1995; 15: 1002–1011. [DOI] [PubMed] [Google Scholar]
- 27.Olah L, Wecker S, Hoehn M. Relation of apparent diffusion coefficient changes and metabolic disturbances after 1 hour of focal cerebral ischemia and at different reperfusion phases in rats. J Cereb Blood Flow Metab 2001; 21: 430–439. [DOI] [PubMed] [Google Scholar]
- 28.Back T, Hoehn-Berlage M, Kohno K, et al. Diffusion nuclear magnetic resonance imaging in experimental stroke. Correlation with cerebral metabolites. Stroke 1994; 25: 494–500. [DOI] [PubMed] [Google Scholar]
- 29.Kaarisalo MM, Räihä I, Sivenius J, et al. Diabetes worsens the outcome of acute ischemic stroke. Diabetes Res Clin Pract 2005; 69: 293–298. [DOI] [PubMed] [Google Scholar]
- 30.Tarr D, Graham D, Roy LA, et al. Hyperglycemia accelerates apparent diffusion coefficient-defined lesion growth after focal cerebral ischemia in rats with and without features of metabolic syndrome. J Cereb Blood Flow Metab 2013; 33: 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosso C, Pires C, Corvol JC, et al. Hyperglycaemia, insulin therapy and critical penumbral regions for prognosis in acute stroke: further insights from the INSULINFARCT trial. PLoS One 2015; 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormick M, Hadley D, McLean JR, et al. Randomized, controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol 2010; 67: 570–578. [DOI] [PubMed] [Google Scholar]