Abstract
In the recently published article, “Heterogeneous incidence and propagation of spreading depolarizations,” it is shown, in vivo and in vitro, how KCl-induced spreading depolarizations in mouse and rat brains can be highly variable, and that they are not limited, as once thought, to a concentric, isotropic, or homogenous depolarization wave in space or in time. The reported results serve as a link between the different species, and this paper contributes to changing the way in which SD expansion is viewed in the lissencephalic brain. Here, we discuss their results with our previous observations made in the gyrencephalic swine brain, in computer simulations, and in the human brain.
Keywords: Spreading depolarization, intrinsic optical signal imaging, lissencephalic brain, gyrencephalic brain, animal models
Spreading depolarizations (SDs) are propagating waves of massive neuronal and glial depolarization that can be accompanied by depression of the electrocorticographic activity. They are characterized by an abrupt failure of brain ion homeostasis, acidification, efflux of excitatory amino acids and an increase in energy metabolism.1,2 Over the past decade, SDs have been observed in the injured human brain. Indirect evidence suggests that they also occur during migraine aura. SD can be a relatively harmless, short-lasting depolarization, but prolonged variants can lead to neuronal degeneration and increase in infarcts associated with poor clinical outcome.3,4 Most of the knowledge of SD comes from studying the phenomenon in lissencephalic animal models. It is believed that there are important anatomical and physiological species-related differences that may impact SDs in larger gyrencephalic brains.5
In the recently published article entitled, “Heterogeneous incidence and propagation of spreading depolarizations,”6 Kauffman et al. studied in detail the propagation patterns of SDs in mice and rats in vivo, and in rat slices in vitro. They found novel results and also confirmed in the lissencephalic brain some of our previous observations made in a gyrencephalic swine brain model.7,8 This is of great importance, since the reproducibility and confirmation of pattern-forming principles in biology in different animal models cannot be taken for granted.
In the gyrencephalic swine brain, we studied the hemodynamic and propagation patterns of KCl-induced SDs, using intrinsic optical signal imaging in 14 male swine. We found in vivo different initiation and propagation patterns (Figure 1), including irregular patterns.8 We also described the development of spiral and re-entrant waves in the gyrencephalic brain (see Figure 1 and Videos 3–78). The generation of such patterns seemed to be exclusively attributed to anatomical features of the gyrencephalic brain.9 Nevertheless, mathematical models have suggested the existence of irregular patterns and propagating wave segments that remain stable in form, even in the absence of heterogeneities in the cortical substrate.10 Kaufmann et al.6 now report for the first time that non-uniform, irregular propagation of SDs is not the exception but rather the rule also in the lissencephalic brains of mice and rats. Although variability in spreading was reported in lissencephalic cerebral ischemia models,11,12 this is the first report of SDs elicited by KCl stimulation from a single point. Moreover, they report the presence of spiral and reverberating waves. This important observation in the lissencephalic brain may indicate that such patterns of SD dynamics do not require the structural complexity of the gyrencephalic brain as thought before. Accordingly, mathematical models of SD predicted that structural heterogeneities are not needed to generate irregular patterns. Tiny initial differences in a spatially perturbed state of extracellular ion concentrations, in an otherwise structurally homogeneous tissue, can either magnify and evolve into irregular SD patterns or rapidly die out and grow outwards as circular SD, depending on the strength of a spatial mean field inhibitory feedback that was hypothetically included into the mathematical SD model. This new kind of feedback was suggested to be provided by neurovascular coupling, which conveys information from neurons to neighboring vessels mediated by either direct interaction with inhibitory neurons, or indirect interaction via astrocytes.13
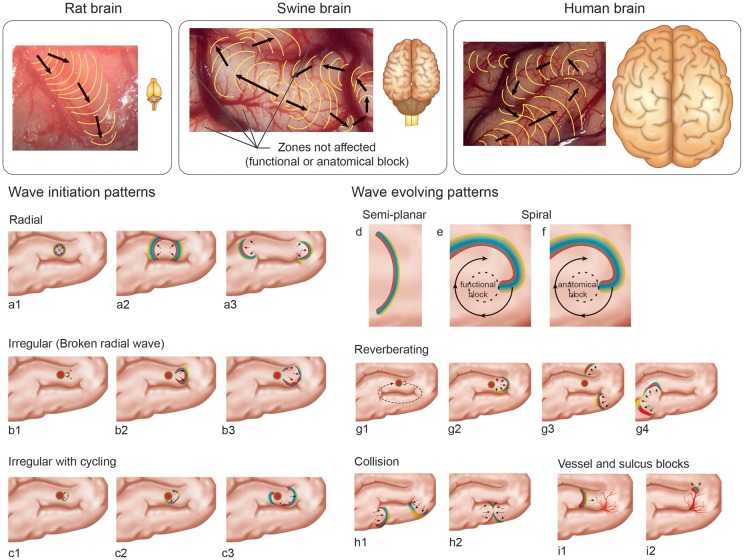
Figure 1.
Anatomical differences between gyrencephalic brains and a lissencephalic rat brain (upper). Typical propagation patters reported by Kaufmann et al.6 in rats; example of irregular propagation pattern seen in the swine model8 and a hypothetical irregular propagation pattern in a human brain. In the lower section, wave initiation and evolving SD patterns in a gyrencephalic brain are presented by type, presented by letters over time (represented by numbers). Initiation patterns a, b and c correspond to KCl stimulations. The typical initiation is a radial wave, but early breaking can originate irregular patterns. Evolving patterns (d to i) are described according to findings in swine experiments.8 The spirals (e and f) were predicted even before my mathematical models.
An important observation made in the gyrencephalic brain was that the patterns of SD expansion and propagation are affected by the anatomical configuration of the brain, as well as by vessels. In particular, the vascular modulation in the swine brain was quite unpredictable, as seen in Videos 8 and 9.8 This would be expected, since gyrencephalic brains have more pronounced anatomical differences due to gyri and sulci and thicker vessels. In their work, Kaufmann et al.6 also observed that the SD propagation is affected by vascular and cytoarchitectonic modulation, and homologous to our findings they also report a lack of predictability with regard to how vessels modulate SDs. These similarities may contribute to changing how SDs are considered in the lissencephalic brain and what they should look like in humans. In addition, the authors comment that, apparently, the vessels affecting propagation are mostly veins. We also studied the effect of SDs on pial arteries in a recent paper,14 and we found that arteries react to SDs, but they seem not to affect the SD propagation, as was previously observed in veins.8 Additionally, we suggested that anatomical blocking on the tissue, where SDs cannot propagate, promote the existence of irregular and re-entrance patterns (Figure 1). Those anatomical blocks are determined mainly by anatomical structures, such as vessels and sulci, or by hypoperfused or dead tissue, such as after middle cerebral artery occlusion.15 Moreover, functional blocks can exist. They are determined by temporary refractory periods.
The first SD seems to be different from the subsequent SDs in the lissencephalic brain, according to Kauffmann et al. This confirms previous observations.16,17 In the supplementary material of our recent paper,18 we also report differences between the first and later SDs. Again, the mathematical principles of pattern formation can explain a wide range of behaviors. For instance, the speed of subsequent waves following at certain time intervals may be slower or faster, depending on the time interval – a phenomenon called dispersion. A medium that carries waves is said to have normal dispersion when the subsequent wave becomes slower as the time interval after the preceding waves becomes shorter.16,17 Regimes of anomalous dispersion behave the opposite way. Subsequent waves are speeding up when they come closer to the preceding wave, a phenomenon that gives rise to merging of wave trains into a single wave or stacking of waves in rapid succession. The conditions for normal and anomalous dispersion in the cortex as the medium of SDs remain unclear but are likely related to the kinetics of the CBF.
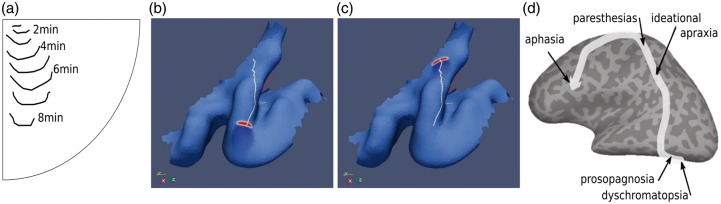
Irregular and transient SD waves pose challenging problems, both experimentally and even more so with regard to clinical observability. One fundamental question in migraine research, for example, is whether the characteristics of different forms of propagating SD waves are related to the major subforms of migraine without aura and migraine with aura and, if so, how. Computer simulations show SD waves that, for example, break away from an initial focus, initially spreading out both radially and in irregular forms, but eventually breaking into segments after no later than a few minutes and then these segments propagate further in only one direction. The arc length (width) of the SD frontline was estimated using this computer model to be of between a few millimeters up to several centimeters. Such a propagating focal wave segment pattern might explain why symptoms remain focal instead of becoming more generalized, which would be the natural assumption if a circular SD wave engulfs large parts of the cerebral hemisphere. Mathematical models that were designed to simulate an aura causing the self-reported visual field defects during migraine (Figure 2(a)) and possibly further subsequent aura symptoms (Figure 2(b)) were implemented on the cortical surface obtained by magnetic resonance imaging scanner readings from the reporting migraine sufferer (Figure 2(c) and (d)).19 The only “obstacle” that could change the direction of the SD wave was, in this simulation, the space-varying convolution, which had a significant influence.
Figure 2.
Personalized computer simulation of SD causing a propagating visual field defect in support of SD as a localized small wave segment. (a) Propagation pattern of a self-reported visual field defect during migraine with aura in the lower quadrant of the right visual hemifield. The position of the propagating visual field defect is marked by a line every minute for 8 min resulting in eight lines starting near the center of the visual field and passing roughly parallel to the right of the vertical meridian. (b) 3D form (blue) of the left primary visual cortex obtained by magnetic resonance imaging scanner readings from a migraine sufferer who draw his visual field defect in (a). The occipital pole is facing backwards. The computer simulation of SD (red wave segment) reproduced the path causing the visual field defect shown in (a). The wave segment was initiated near the occipital pole in such a way that the direction of SD propagation points toward the cunes. (c) Same as (b) showing the end of the computer simulation where the SD wave segment propagated for about 8 min a distance of about 24 mm on a particular path (white line) on the gyral crown on the cuneus. (d) Cortical areas successively affected reported by other patient with complex aura, which provides further evidence of a specified cortical path of a wave segment rather than an all-engulfing circular SD that spreads concentrically in all directions (modified from Vincent et al.19).
In addition, the noxious signature of irregular SD waves would significantly vary with the convolution, which would further influence not only the size of the affected cortical surface area but also the location of this area with respect to gyral and sulcal position. Hence, it has been suggested that such irregular waves support the controversial2 idea that SDs have a causal relationship with the headache phase in migraine and, therefore, occur not only in migraine with aura but also in migraine without aura, i.e. in the two major migraine subtypes. The new results suggest that the relation between SD wave patterns and noxious signatures transmitted into the meninges should be an objective of future research. With respect to stroke and brain trauma, it would be particularly interesting to learn how disturbances in metabolism and neurovascular responses to SD modify the propagation patterns and refractory periods between subsequent SDs. On the other hand, the blocking effect of brain veins would also promote the limitation of SD expansion to the whole hemisphere, remaining a focal event, but the blocking effect would be dependent on the condition of the brain (migraine patient) or damaged brain. At the moment, the only SDs seen through imaging in humans are those from nine patients suffering from malignant stroke during decompressive craniectomy recorded through laser speckle, where those hemodynamic changes detected were localized in small areas of the brain.20 Those single observations fit in the concept that expansion can be reduced by repetitive events and each of the multiple SDs remain local.
The results also support seeking the factors that shape the apparent modulation of SD propagation now seen in lissencephalic animals. We encourage further research to clarify the open questions even beyond the mammalian brain. In fact, SDs are even observed in insects,21 which lack blood circulation and it would be interesting to reveal which physiological and anatomical changes during evolution are associated with changes in propagation patterns and refractory periods.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Edgar Santos is supported by the Physician Scientist Program of the Faculty of Medicine, University of Heidelberg.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and publication of this article.
Authors’ contributions
ES: Wrote the manuscript. RSP, OWS and JPD: literature research, editing and reviewing. MAD: Wrote the manuscript.
References
- 1.Ayata C, Lauritzen M. spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev 2015; 95: 953–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron 2015; 86: 902–922. [DOI] [PubMed] [Google Scholar]
- 3.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011; 17: 439–447. [DOI] [PubMed] [Google Scholar]
- 4.Hartings JA, Shuttleworth CW, Kirov SA, et al. The continuum of spreading depolarizations in acute cortical lesion development: examining Leão’s legacy. J Cereb Blood Flow Metab. Metab 2017; 37(5): 1571–1594. [DOI] [PMC free article] [PubMed]
- 5.Ayata C. Pearls and pitfalls in experimental models of spreading depression. Cephalalgia Int J Headache 2013; 33: 604–613. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann D, Theriot JJ, Zyuzin J, et al. Heterogeneous incidence and propagation of spreading depolarizations. J Cereb Blood Flow Metab. 2017; 37(5): 1748–1762. [DOI] [PMC free article] [PubMed]
- 7.Santos E, Schöll M, Sanchez-Porras R, et al. Cortical spreading depression dynamics can be studied using intrinsic optical signal imaging in gyrencephalic animal cortex. Acta Neurochir Suppl 2013; 118: 93–97. [DOI] [PubMed] [Google Scholar]
- 8.Santos E, Schöll M, Sánchez-Porras R, et al. Radial, spiral and reverberating waves of spreading depolarization occur in the gyrencephalic brain. NeuroImage 2014; 99: 244–255. [DOI] [PubMed] [Google Scholar]
- 9.Dahlem MA, Müller SC. Self-induced splitting of spiral-shaped spreading depression waves in chicken retina. Exp Brain Res Exp Hirnforsch Expérimentation Cérébrale 1997; 115: 319–324. [DOI] [PubMed] [Google Scholar]
- 10.Dahlem MA, Graf R, Strong AJ, et al. Two-dimensional wave patterns of spreading depolarization: retracting, re-entrant, and stationary waves. Phys Nonlinear Phenom 2010; 239: 889–903. [Google Scholar]
- 11.Clark D, Institoris A, Kozák G, et al. Impact of aging on spreading depolarizations induced by focal brain ischemia in rats. Neurobiol Aging 2014; 35: 2803–2811. [DOI] [PubMed] [Google Scholar]
- 12.Bere Z, Obrenovitch TP, Kozák G, et al. Imaging reveals the focal area of spreading depolarizations and a variety of hemodynamic responses in a rat microembolic stroke model. J Cereb Blood Flow Metab 2014; 34: 1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zandt B, ten Haken B, van Putten MJAM, et al. How does spreading depression spread? Physiology and modeling. Rev Neurosci 2015; 26: 183–198. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Porras R, Santos E, Scholl M, et al. Ketamine modulation of the haemodynamic response to spreading depolarization in the gyrencephalic swine brain. J Cereb Blood Flow Metab. 2017; 37(5): 1720–1734. [DOI] [PMC free article] [PubMed]
- 15.Schöll MJ, Santos E, Sanchez-Porras R, et al. Large field-of-view movement-compensated intrinsic optical signal imaging for the characterization of the haemodynamic response to spreading depolarizations in large gyrencephalic brains. J Cereb Blood Flow Metab. 2017; 37(5): 1706–1719. [DOI] [PMC free article] [PubMed]
- 16.Verhaegen M, Todd MM, Warner DS. The influence of different concentrations of volatile anesthetics on the threshold for cortical spreading depression in rats. Brain Res 1992; 581: 153–155. [DOI] [PubMed] [Google Scholar]
- 17.Menyhárt Á, Makra P, Szepes BÉ, et al. High incidence of adverse cerebral blood flow responses to spreading depolarization in the aged ischemic rat brain. Neurobiol Aging 2015; 36: 3269–3277. [DOI] [PubMed] [Google Scholar]
- 18.Santos E, León F, Silos H, et al. Incidence, hemodynamic, and electrical characteristics of spreading depolarization in a swine model are affected by local but not by intravenous application of magnesium. J Cereb Blood Flow Metab 2016; 36: 2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent MB, Hadjikhani N. Migraine aura and related phenomena: beyond scotomata and scintillations. Cephalalgia Int J Headache 2007; 27: 1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woitzik J, Hecht N, Pinczolits A, et al. Propagation of cortical spreading depolarization in the human cortex after malignant stroke. Neurology 2013; 80: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 21.Spong KE, Andrew RD, Robertson RM. Mechanisms of spreading depolarization in vertebrate and insect central nervous systems. J Neurophysiol 2016; 116: 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]