Abstract
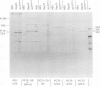
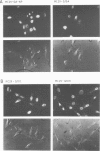
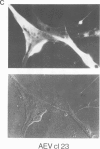
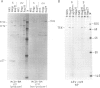
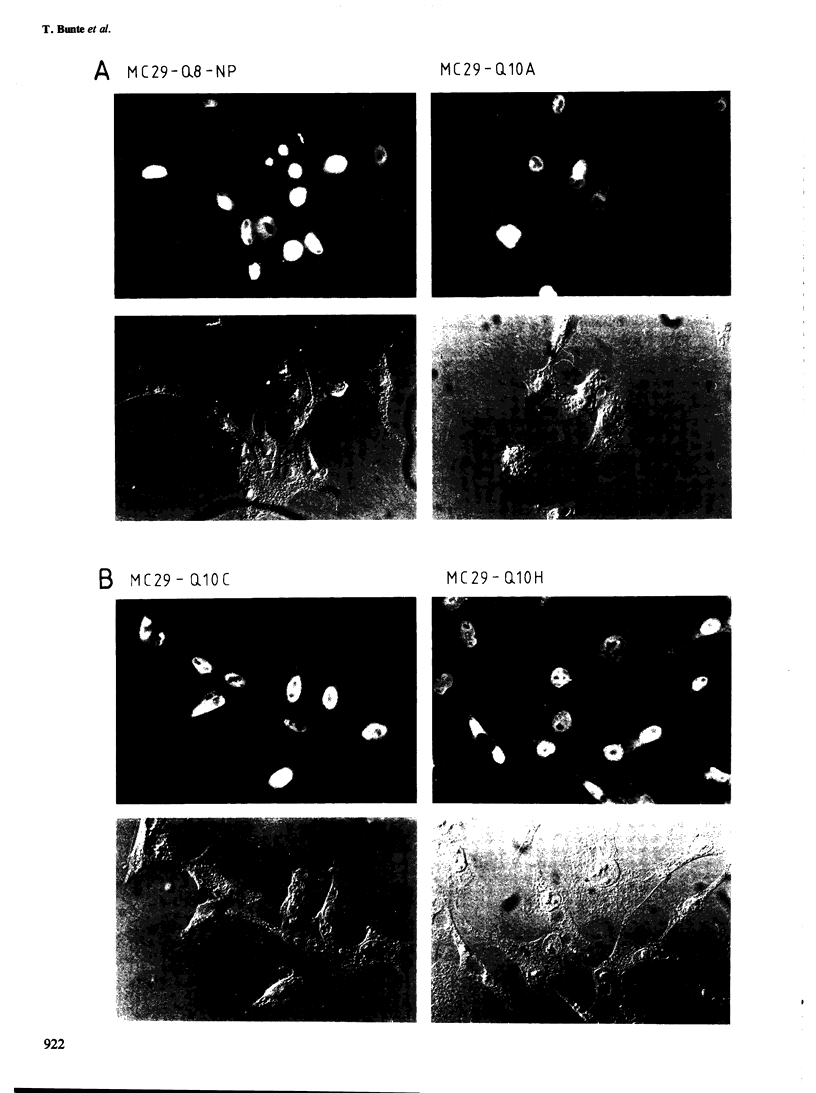
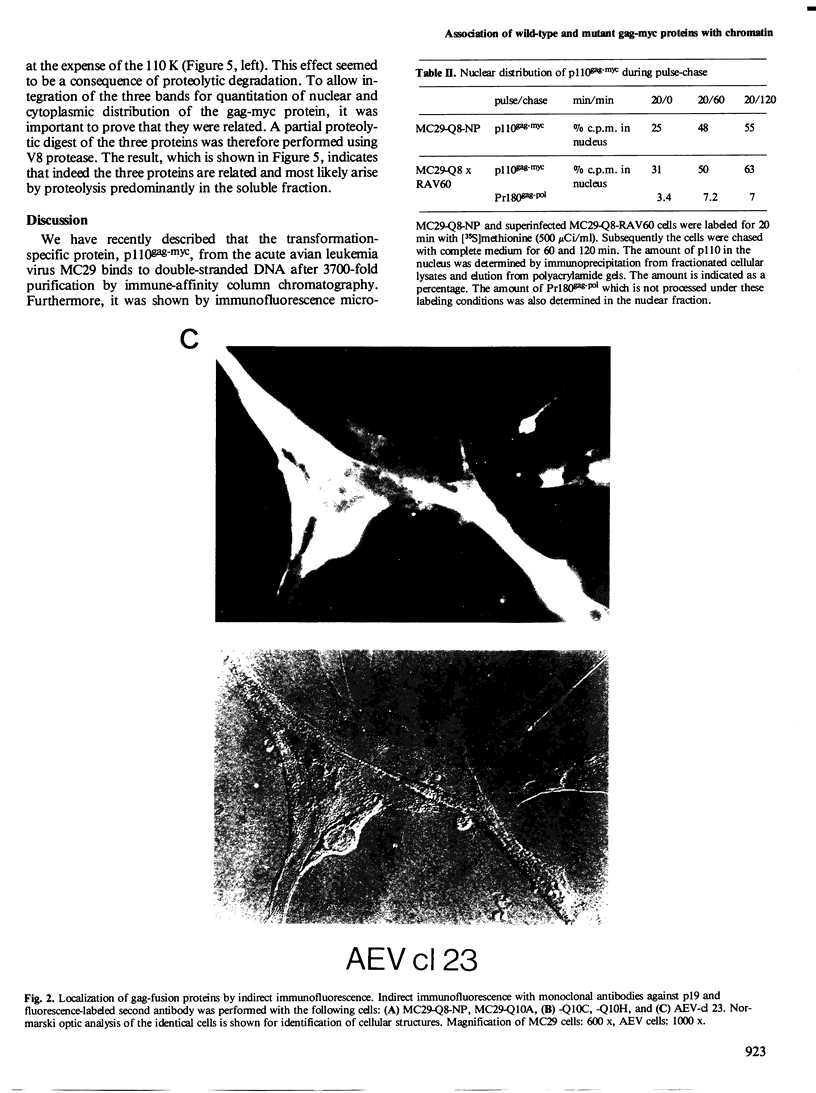
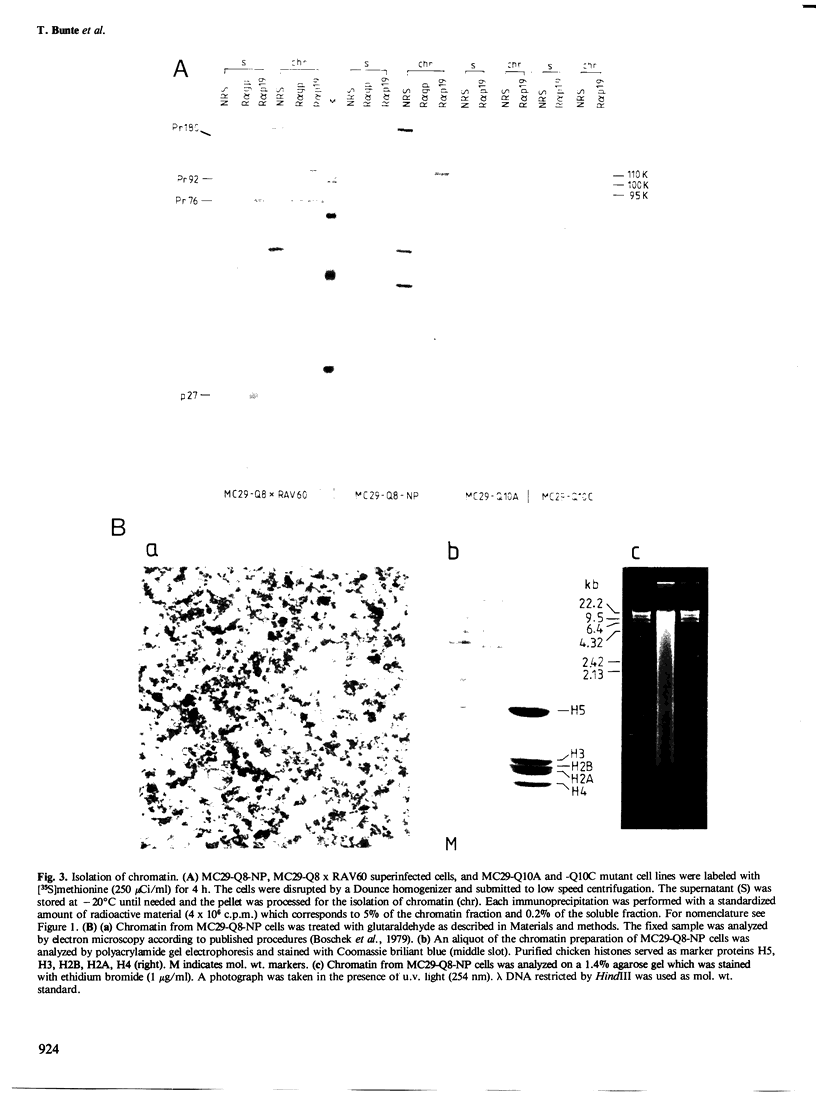
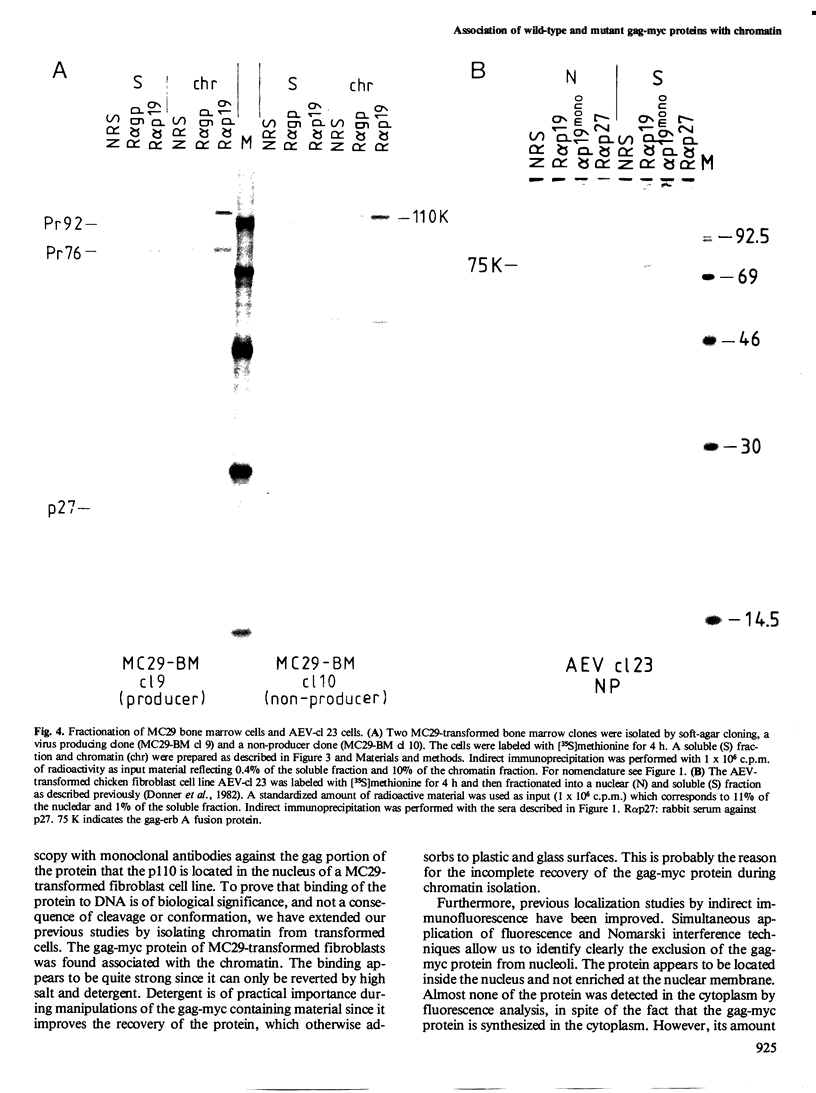
The localization of the transformation-specific proteins was analyzed in quail embryo fibroblast cell lines transformed by wild-type avian myelocytomatosis virus MC29 and by three of its deletion mutants, Q10A , Q10C , and Q10H , with altered transforming capacities, and in a chicken fibroblast cell line transformed by the avian erythroblastosis virus (AEV). These viruses code for polyproteins consisting of part of the gag gene and of a transformation-specific region, myc for MC29 and erb A for AEV. Analysis by indirect immunofluorescence using monoclonal antibodies against p19, the N-terminal region of the polyprotein, showed that the gag-myc proteins in cells transformed by the wild-type MC29 as well as by the three deletion mutants are located in the nucleus. In contrast, cells transformed by AEV, which express the gag-erb A protein, give rise to cytoplasmic fluorescence. Fractionation of cells into nuclear and cytoplasmic fractions and analysis by immunoprecipitation and gel electrophoresis confirmed these results. About 60% of the gag-myc proteins of wild-type as well as of mutant origin were found in the nucleus, while 90% of the gag-erb A protein was present in the cytoplasm. Also, pulse-chase analysis indicated that the gag-myc protein rapidly accumulates in the nucleus in just 30 min. Further, it was shown that the wild-type and also mutant gag-myc proteins are associated with isolated chromatin. Association to chromatin was also observed for the gag-myc protein from MC29-transformed bone marrow cells, which are believed to be the target cells for MC29 virus in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams H. D., Rohrschneider L. R., Eisenman R. N. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell. 1982 Jun;29(2):427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Structure and specific sequences of avian erythroblastosis virus RNA: evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bister K., Ramsay G. M., Hayman M. J. Deletions within the transformation-specific RNA sequences of acute leukemia virus MC29 give rise to partially transformation-defective mutants. J Virol. 1982 Mar;41(3):754–766. doi: 10.1128/jvi.41.3.754-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Purchio A. F., Erikson E., Collett M. S., Brugge J. S. Molecular events in cells transformed by Rous Sarcoma virus. J Cell Biol. 1980 Nov;87(2 Pt 1):319–325. doi: 10.1083/jcb.87.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Fanshier L., Bishop J. M., Moscovici C., Moscovici M. G. The genome and the intracellular RNAs of avian myeloblastosis virus. Cell. 1981 Jan;23(1):279–290. doi: 10.1016/0092-8674(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Greiser-Wilke I., Owada K. M., Moelling K. Isolation of monoclonal antibodies against avian oncornaviral protein p19. J Virol. 1981 Jul;39(1):325–329. doi: 10.1128/jvi.39.1.325-329.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Hayman M. J. Transforming proteins of avian retroviruses. J Gen Virol. 1981 Jan;52(Pt 1):1–14. doi: 10.1099/0022-1317-52-1-1. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Khalaf R. M., Bainbridge D. R. Nomarski differential interference contrast studies of murine lymphocytes. Eur J Immunol. 1981 Oct;11(10):816–824. doi: 10.1002/eji.1830111014. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Neil J. C., Vogt P. K. Cell-free translation of avian erythroblastosis virus RNA yields two specific and distinct proteins with molecular weights of 75,000 and 40,000. Virology. 1980 Jan 30;100(2):475–483. doi: 10.1016/0042-6822(80)90537-1. [DOI] [PubMed] [Google Scholar]
- Lapeyre J. N., Bekhoe I. Effects of 5-bromo-2'-deoxyuridine and dimethyl sulfoxide on properties and structure of chromatin. J Mol Biol. 1974 Oct 15;89(1):137–162. doi: 10.1016/0022-2836(74)90167-3. [DOI] [PubMed] [Google Scholar]
- Pawson T., Martin G. S. Cell-free translation of avian erythroblastosis virus RNA. J Virol. 1980 Apr;34(1):280–284. doi: 10.1128/jvi.34.1.280-284.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G. M., Hayman M. J. Isolation and biochemical characterization of partially transformation-defective mutants of avian myelocytomatosis virus strain MC29: localization of the mutation to the myc domain of the 110,000-dalton gag-myc polyprotein. J Virol. 1982 Mar;41(3):745–753. doi: 10.1128/jvi.41.3.745-753.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G., Graf T., Hayman M. J. Mutants of avian myelocytomatosis virus with smaller gag gene-related proteins have an altered transforming ability. Nature. 1980 Nov 13;288(5787):170–172. doi: 10.1038/288170a0. [DOI] [PubMed] [Google Scholar]