Abstract
Targeting uptake transporters such as organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood–brain barrier (BBB) can facilitate central nervous system (CNS) drug delivery. Effective blood-to-brain drug transport via this strategy requires characterization of mechanisms that modulate BBB transporter expression and/or activity. Here, we show that activation of activin receptor-like kinase (ALK)-1 using bone morphogenetic protein (BMP)-9 increases Oatp1a4 protein expression in rat brain microvessels in vivo. These data indicate that targeting ALK1 signaling with BMP-9 modulates BBB Oatp1a4 expression, presenting a unique opportunity to optimize drug delivery and improve pharmacotherapy for CNS diseases.
Keywords: Basic science, blood–brain barrier, endothelium, pharmacology, vascular biology
Introduction
Effective treatment of CNS diseases requires that drugs permeate the blood–brain barrier (BBB) and attain effective brain concentrations. Considerable research has focused on mechanisms that restrict BBB drug permeation such as ATP-binding cassette transporters (i.e. P-glycoprotein (P-gp), multidrug resistance proteins, breast cancer resistance protein).1,2 Clinical trials designed to pharmacologically target P-gp have been unsuccessful due to inhibitor toxicity and/or enhanced tissue penetration of xenobiotics.3,4 Our laboratory has proposed that effective CNS drug delivery can be achieved by targeting BBB uptake transporters such as organic anion transporting polypeptides (Oatps). Oatps are sodium-independent transporters from the solute carrier (SLC) superfamily. At the rodent BBB, the primary drug transporting Oatp isoform is Oatp1a4.5 Similar to its human orthologue OATP1A2, Oatp1a4 is localized to both the luminal and abluminal membranes of brain capillary endothelial cells.5–7 We have previously reported that Oatp1a4 can facilitate blood-to-brain transport of opioid peptides8 and atorvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor that has neuroprotective properties.9
In order to develop Oatps as molecular targets that can facilitate CNS drug delivery, it is paramount to characterize mechanisms that can modulate these transporters such as signaling mediated by transforming growth factor-beta (TGF-β) and related cytokines. At the BBB, TGF-β signaling pathways are mediated by two receptors (i.e. activin receptor-like kinase (ALK)-1 and ALK5), which exert opposite effects on the cerebral microvasculature.10,11 Specifically, the activation of ALK1 induces endothelial proliferation and increased vascular permeability, while ALK5-mediated signaling leads to vascular resolution and decreased permeability.5 Our laboratory has previously shown that Oatp1a4 functional expression is increased by pharmacological inhibition of TGF-β/ALK5 signaling.8,9 However, the role of ALK1 in regulation of BBB Oatp1a4 expression has not been elucidated. BMP-9 is an ALK1 agonist that modulates cellular processes including differentiation, proliferation, angiogenesis, and bone formation.12–14 The goal of the present study was to investigate, in vivo, the effect of targeting ALK1 signaling with BMP-9 on BBB Oatp1a4 expression.
Materials and methods
Animals and drug treatments
Animal experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and conform to both National Institutes of Health and Animal Research: Reporting in vivo Experiments (ARRIVE) guidelines. Female Sprague-Dawley rats (200–250 g; three months old; Envigo, Denver, CO) were purposely used for these studies to enable comparison with previous work.8–10 Rats were randomized and injected i.p. with BMP-9 (0–5 µg/kg (1.0 mL/kg) in 0.9% saline; R&D Systems, Minneapolis, MN). Inhibition experiments were performed using the established ALK1 antagonist LDN193189 (10 mg/kg (1.0 mL/kg) in 0.9% saline i.p.; Sigma-Aldrich, St. Louis, MO), which was administered 1 h prior to injection with BMP-9. Time-matched control animals were injected with vehicle (i.e. 0.9% saline). Following 6 h BMP-9 treatment, animals were anesthetized (100 mg/kg ketamine, 20 mg/kg xylazine, i.p.) and prepared for brain microvessel isolation.
Microvessel isolation
Microvessel isolation was performed as previously described9 with few modifications. Following euthanasia by decapitation, brains were harvested and meninges and choroid plexus were removed. Cerebral hemispheres were homogenized in 5 mL microvessel buffer (300 mM mannitol, 5 mM EGTA, 12 mM Tris HCl, pH 7.4) containing protease inhibitor cocktail (Sigma-Aldrich). Homogenates were then mixed with 8 mL 26% dextran and centrifuged at 5000 g for 15 min at 4℃. The supernatant was then aspirated and capillary pellets were resuspended in 5 mL microvessel buffer and centrifuged at 150,000 g for 60 min at 4℃. Pellets containing total cellular membranes15 were resuspended in 500 µL of storage buffer (50% microvessel isolation buffer: 50% diH20, v/v) containing protease inhibitor cocktail. Samples were stored at −80℃.
Western blot analysis
Western blotting was performed as previously described.9 Isolated microvessel samples were quantified for total protein using Bradford reagent (Sigma-Aldrich) and heated at 37℃ for 30 min under reducing conditions (i.e. 2.5% (v/v) 2-mercaptoethanol (Sigma-Aldrich) in 1 × Laemmli sample buffer (Bio-Rad, Hercules, CA) for Oatp1a4 detection. For ALK1 detection, the samples were heated at 95℃ for 5 min under non-reducing conditions in 1 × Laemmli sample buffer. Following SDS-PAGE and transfer, PVDF membranes were incubated overnight at 4℃ with primary antibodies against Oatp1a4 (anti-Oatp2, Santa Cruz Biotechnology; 1:1000 dilution), ALK1 (anti-ALK1, Santa Cruz Biotechnology; 1:1500 dilution), tubulin (anti-α tubulin, Abcam; 1:20,000 dilution) Na+/K+-ATPase (anti-Na/K, Abcam, 1:100,000 dilution) or synaptophysin (anti-synaptophysin, Abcam, 1:800,000 dilution). Membranes were washed and incubated with horseradish peroxidase-conjugated anti-rabbit IgG (Jackson ImmunoResearch, 1:40,000 dilution) or anti-mouse IgG (Jackson ImmunoResearch, 1:50,000 dilution) for 60 min at room temperature. Membranes were developed using enhanced chemiluminescence (Super Signal West Pico, Thermo-Fisher). Bands were quantitated using ImageJ software (Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, MD) and normalized to either tubulin or Na+/K+-ATPase.
Immunofluorescence imaging
Isolated rat brain microvessels (50 µL) were smeared onto slides, heat fixed at 95℃ for 10 min, and permeabilized in ice-cold ethanol for 10 min. Slides were washed with PBS and incubated for 60 min with PBS containing 0.1% Triton-X100, 2% BSA, and 1% goat serum (v/v) at room temperature. Following blocking, slides were incubated with 200 µL of primary antibody (anti-platelet endothelial cell adhesion molecule (PECAM), Abcam, 1:100 dilution; anti-Oatp2, 1:10 dilution; anti-ALK1, 1:10 dilution) overnight at 4℃. Slides were washed with PBS 3 × 5 min and incubated with 200 µL of Alexa Fluor-conjugated secondary antibody (anti-mouse IgG or anti-rabbit IgG, Invitrogen; 1:300 dilution) for 60 min at room temperature, followed by PBS wash 3 × 5 min. Slides were then incubated with DAPI (Invitrogen, 1:40,000 dilution) for 5 min at room temperature and washed with PBS 3 × 5 min. Finally, slides were mounted with ProLong Gold anti-fade reagent (Life Technologies). Imaging was performed using a Leica DMI6000 inverted fluorescent microscope.
In situ brain perfusion
In situ brain perfusion experiments were performed as previously described.8,9 Animals were perfused for 10 min with an erythrocyte-free mammalian Ringer’s solution containing [3H]taurocholate (1.0 μCi/mL; 10 umol/L total concentration). Following perfusion, animals were decapitated and brains were harvested and solubilized in TS2 tissue solubilizer for two days at room temperature. Samples were measured on a model 1450 liquid scintillation counter (PerkinElmer, Boston, MA, USA). Results are expressed as picomoles of radiolabeled drug per gram of brain tissue (pmol/g tissue).
Statistical analysis
Western blot data are reported as mean ± SEM from at least three independent experiments, where each treatment group consisted of three individual animals (n = 3). In situ perfusion data are reported as mean ±SEM from five animals per treatment group. These sample sizes are based on the ability to detect a 35% difference between treatment groups with 20% variability. Statistical significance was determined using one-way ANOVA followed by post hoc multiple-comparison Bonferroni t-test. A value of p < 0.05 was accepted as statistically significant.
Results
Oatp1a4 and ALK1 protein expression and localization at the BBB
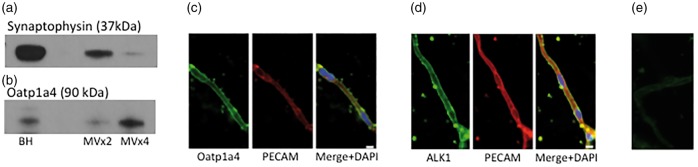
Western blot analysis showed Oatp1a4 and ALK1 protein expression in rat brain microvessels. Purity of our microvessels was demonstrated by reduced expression of synaptophysin, a neuronal marker protein (Figure 1(a)). We also show Oatp1a4 enrichment in the microvessel fraction (Figure 1(b)), suggesting preferential expression in brain microvasculature. Specificity of this band was confirmed using a second Oatp1a4 antibody that was provided by Dr. Ken-Ichi Hosoya (University of Toyama, Japan; data not shown).16 Similarly, immunofluorescence staining of rat brain microvessels demonstrated localization of Oatp1a4 (Figure 1(c)) and ALK1 (Figure 1(d)) at the microvascular endothelium as evidenced by co-localization with the endothelial marker protein PECAM.
Figure 1.
Oatp1a4 and ALK1 protein are expressed in and localized to rat brain microvessels. Western blot experiments demonstrated microvessel isolation purity by depletion of neuronal markers, i.e. synaptophysin (a) and enrichment of Oatp1a4 (b) as compared to brain homogenate fraction. Immunofluorescence staining showed localization of Oatp1a4 (c) and ALK1 (d) in rat brain microvessels. Staining with Alexa Fluor-conjugated secondary antibody alone showed minimal non-specific background fluorescence (e). Scale bar = 5 µm. BH = brain homogenate; MV × 2 = microvessel fraction post two 26% dextran spins; MVx4 = microvessel fraction post four 26% dextran spins. MV × 4 fractions were used in all drug treatment experiments.
Oatp1a4: organic anion transporting polypeptide 1a4; ALK: activin receptor-like kinase; PECAM: platelet endothelial cell adhesion molecule; DAPI: 4',6-diamidino-2-phenylindole.
BMP-9 treatment increases Oatp1a4 and ALK1 expression at the BBB
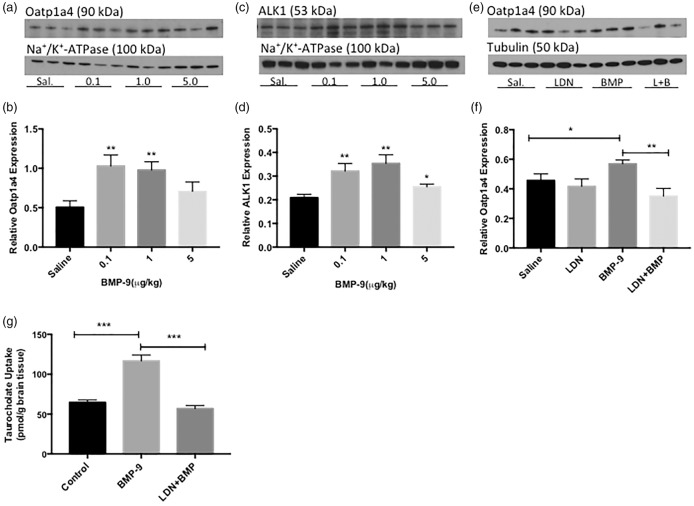
Western blot experiments showed increased (p < 0.01) Oatp1a4 protein expression in cerebral microvessels isolated from BMP-9-treated rats (Figure 2(a) and (b)). Specifically, treatment with 0.1 µg/kg and 1 µg/kg BMP-9 resulted in 103% and 93% increase in Oatp1a4 protein expression at the BBB, respectively. ALK1 protein expression was increased by up to 69% following BMP-9 administration (Figure 2(c) and (d)).
Figure 2.
Activation of the ALK1 receptor with BMP-9 increases functional expression of Oatp1a4 at the BBB. Western blot analysis of brain microvessels isolated from BMP-9 (0–5 µg/kg, i.p.) treated and LDN193189 (10 mg/kg, i.p.) pretreated rats. Animals receiving BMP-9 alone were treated for 6 h. Animals were dosed with LDN193189 for 1 h prior to administration of BMP-9. Isolated microvessels (10 µg) were resolved on a 4% to 12% SDS-polyacrylamide gel, transferred to PVDF membrane and analyzed for expression of Oatp1a4 ((a) and (b)) and ALK1 ((c) and (d)) and normalized to Na+/K+-ATPase. Microvessel samples from LDN193189 pretreated animals were analyzed for Oatp1a4 ((e) and (f)) and normalized to α-tubulin. Drug-treated groups were compared to control groups, i.e. 0.9% saline-injected animals. Western blot data are reported as mean ± SEM from at least three independent experiments, where each treatment group consisted of three individual animals (n = 3). In situ brain perfusion studies were performed to analyze Oatp-mediated uptake of [3H]taurocholate in rat brain (g). 6 h BMP-9 (1 µg/kg, i.p.)-treated animals were perfused with [3H]taurocholate (1.0 μCi/mL) for 10 min in the presence and absence of LDN193189 (10 mg/kg. i.p.; 1 h pretreatment). The results are expressed as mean ± SEM of five animals per treatment group. * p < 0.05; ** p < 0.01; *** p < 0.001.
Oatp1a4: organic anion transporting polypeptide 1a4; ALK: activin receptor-like kinase; BMP: bone morphogenetic protein.
LDN193189 attenuates BMP-9-induced upregulation of Oatp1a4 at the BBB
Pretreatment with LDN193189 (10 mg/kg i.p.) 1 h prior to BMP-9 (1 µg/kg i.p.) administration attenuated the upregulation of Oatp1a4 expression observed in the presence of BMP-9 alone (Figure 2(e) and (f)). Specifically, 6 h BMP-9 treatment resulted in a 25% increase in Oatp1a4 protein expression at the BBB; however, 1 h pre-treatment with LDN193189 followed by 6 h BMP-9 attenuated the BMP-9 mediated increase in Oatp1a4 expression by 38%.
BMP-9 treatment increases taurocholate brain accumulation
To determine whether the changes in microvascular Oatp1a4 expression following BMP-9 treatment corresponded to altered Oatp1a4 function at the BBB, the in situ perfusion technique was utilized. Brain uptake of [3H]taurocholate, an established Oatp substrate drug, was increased (p < 0.001) in animals treated for 6 h with BMP-9 (1 µg/kg). This increase in [3H]taurocholate uptake was attenuated in animals pretreated with LDN193189 (10 mg/kg) for 1 h prior to administration of BMP-9 (1 µg/kg). Taken together, these data provide evidence for increased functional expression of Oatp1a4 at the BBB due to targeting ALK1 signaling with BMP-9.
Discussion
Our laboratory has shown effects of ALK5 signaling on BBB Oatp1a4 expression.8,9 However, the role of ALK1 signaling on regulation of Oatp1a4 functional expression needs to be studied. Here, we demonstrate for the first time that BMP-9, an established ALK1 agonist, increased the expression and activity of Oatp1a4 at the BBB in vivo. We administered a pharmacological dose of BMP-9 that ranged from 0.1 to 5 µg/kg. This dose range was greater than the reported EC50 for BMP-9 activation of ALK1 signaling (i.e. 50 pg/ml).13 BMP-9 treatment (0.1 µg/kg and 1.0 µg/kg) increased the expression of both Oatp1a4 and ALK1 protein (p < 0.01). Interestingly, the highest BMP-9 dose used in our studies (i.e. 5 µg/kg) caused a lower magnitude of increase in Oatp1a4 and ALK1 expression as well as a significant decrease in actin and α-tubulin protein expression (data not shown), observations that suggest cellular toxicity. This was an important finding as it demonstrated an upper limit on BMP-9 concentrations that can be used to target the BBB in vivo. Specificity of the BMP-9 effect on Oatp1a4 functional expression was determined using LDN193189, an established ALK1 antagonist. Since ALK1 and ALK5 receptors exert opposing effects, it is essential to determine the net effect of these pathways on BBB Oatp1a4 functional expression. This is particularly apparent because we have shown that Oatp1a4 expression and transport activity are increased via inhibition of ALK5 signaling.8,9
Oatp1a4 is an attractive target for drug delivery due to its substrate profile, which includes HMG-CoA reductase inhibitors (i.e. statins).5,17 Brain delivery of statins offers an opportunity to improve treatment of CNS diseases. Indeed, clinical studies have shown that statins are associated with neurological improvement following ischemic stroke.18 While these observations may be related to extension of the therapeutic window for recombinant tissue plasminogen activator,19 it has been suggested that statins directly exert neuroprotective effects.20 Preclinical studies have also demonstrated that atorvastatin can reduce CNS oxidative stress by acting as a reactive oxygen species scavenger in the brain.21,22 In an in vivo model of cerebral hypoxia, our laboratory has shown reduced expression of neuronal apoptosis markers following CNS delivery of atorvastatin via Oatp1a4.9 Clearly, Oatps can facilitate brain uptake of currently marketed drugs for the treatment of CNS diseases; however, the utility of Oatps in drug delivery requires knowledge on regulatory mechanisms that control this transporter. Our data implicate ALK1 receptors as a molecular target that can be exploited to achieve this critical goal. Current research in our laboratory is aimed at achieving a greater understanding of ALK1 signaling in an effort to develop strategies for treatment of CNS diseases. Studies will also be performed in male rats to increase translational potential of our ongoing work.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Institute of Health (R01-NS084941, R01-DA11271). WA is supported by a pre-doctoral appointment to a National Institutes of Health Training Grant (T32-HL07244).
Acknowledgements
The authors would like to thank Dr. Ken-ichi Hosoya (University of Toyama, Japan) for kindly providing the Oatp1a4 antibody used to confirm our western blot and immunofluorescence results. The authors adhere to the NIH Grants Policy Statement on Sharing of Biomedical Research Resources, including the “Principles and Guidelines for Recipients of NIH Research Grants and Contracts on Obtaining and Disseminating Biomedical Research Resources: Final Notice” (64FR 72090, 23 December 1999; and described at http://ott.od.nih.gov/NewPages/RTguide/final.html).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
WA planned and executed the experiments, analyzed data, and prepared the manuscript; HB performed drug treatments, planned, and conducted experiments. KI assisted with experimental work. TPD critically reviewed the manuscript, provided scientific guidance on this project, and obtained funding via R01-DA11271. PTR participated in experimental design, data analysis, preparation of the manuscript, and obtained funding via R01-NS084941.
References
- 1.Ronaldson PT, Davis PT. Targeting transporters: promoting blood-brain barrier repair in response to oxidative stress injury. Brain Res 2015; 1623: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharm Exp Ther 2002; 301: 7–14. [DOI] [PubMed] [Google Scholar]
- 3.Potschka H. Modulating P-glycoprotein regulation: future perspective for pharmacoresistant epilepsies? Epilepsia 2010; 51: 1333–1347. [DOI] [PubMed] [Google Scholar]
- 4.Palmeira A, Sousa E, Vasconcelos MH, et al. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Curr Med Chem 2012; 19: 1946–2025. [DOI] [PubMed] [Google Scholar]
- 5.Ronaldson PT, Davis PT. Targeted drug delivery to treat pain and cerebral hypoxia. Pharmacol Rev 2013; 65: 291–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Eur J Physiol 2004; 447: 653–665. [DOI] [PubMed] [Google Scholar]
- 7.Gao B, Stieger B, Noe B, et al. Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem 1999; 47: 1255–1263. [DOI] [PubMed] [Google Scholar]
- 8.Ronaldson PT, Finch JD, DeMarco KM, et al. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharm Exp Ther 2011; 336: 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson BJ, Sanchez-Covarrubias, Slosky LM, et al. Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood-brain barrier: relevance to CNS drug delivery. J Cereb Blood Flow Metab 2014; 34: 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronaldson PT, DeMarco KM, Sanchez-Covarrubias L, et al. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab 2009; 29: 1084–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curado F, Spuul P, Egana I, et al. ALK5 and ALK1 play antagonistic roles in transforming growth factor-β-induced podosome formation in aortic endothelial cells. Mol Cell Biol 2014; 34: 4389–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown MA, Zhao Q, Baker KA, et al. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem 2005; 280: 25111–25118. [DOI] [PubMed] [Google Scholar]
- 13.Herrera B, Dooley S, Breitkopf-Heinlein K. Potential roles of bone morphogenetic protein (BMP)-9 in human liver diseases. Int J Mol Sci 2014; 15: 5199–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki Y, Ohga N, Morishita Y, et al. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci 2010; 123: 1684–1692. [DOI] [PubMed] [Google Scholar]
- 15.Brzica H, Breljak D, Krick W, et al. The liver and kidney expression of sulfate anion transporter sat-1 in rats exhibits male-dominant gender differences. Eur J Physiol 2009; 457: 1381–1392. [DOI] [PubMed] [Google Scholar]
- 16.Akanuma S, Hirose S, Tachikawa M, et al. Localization of organic anion transporting polypeptide (Oatp) 1a4 and Oatp1c1 at the rat blood-retinal barrier. Fluids Barriers CNS 2013; 10: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ose A, Kusuhara H, Endo C, et al. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos 2010; 38: 168–176. [DOI] [PubMed] [Google Scholar]
- 18.Montecucco F, Quercioli A, Mirabelli-Badenier M, et al. Statins in the treatment of acute ischemic stroke. Curr Pharm Biotechnol 2012; 13: 68–76. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Zhang ZG, Chopp M. The neurovascular unit and combination treatment strategies for stoke. Trends Pharmacol Sci 2012; 33: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland BA, Minnerup J, Balami JS, et al. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke 2012; 7: 407–418. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield DA, Barone E, Di Domenico F, et al. Atorvastatin treatment in a dog preclinical model of Alzheimer’s disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int J Neuropsychopharmacol 2012; 15: 981–987. [DOI] [PubMed] [Google Scholar]
- 22.Barone E, Mancuso C, Di Domenico F, et al. Biliverdin reductase-A: a novel drug target for atorvastatin in a dog pre-clinical model of Alzheimer disease. J Neurochem 2012; 120: 135–146. [DOI] [PubMed] [Google Scholar]