Abstract
Adoptive transfer of T cells engineered to express a hepatitis B virus–specific (HBV-specific) T cell receptor (TCR) may supplement HBV-specific immune responses in chronic HBV patients and facilitate HBV control. However, the risk of triggering unrestrained proliferation of permanently engineered T cells raises safety concerns that have hampered testing of this approach in patients. The aim of the present study was to generate T cells that transiently express HBV-specific TCRs using mRNA electroporation and to assess their antiviral and pathogenetic activity in vitro and in HBV-infected human liver chimeric mice. We assessed virological and gene-expression changes using quantitative reverse-transcriptase PCR (qRT-PCR), immunofluorescence, and Luminex technology. HBV-specific T cells lysed HBV-producing hepatoma cells in vitro. In vivo, 3 injections of HBV-specific T cells caused progressive viremia reduction within 12 days of treatment in animals reconstituted with haplotype-matched hepatocytes, whereas viremia remained stable in mice receiving irrelevant T cells redirected toward hepatitis C virus–specific TCRs. Notably, increases in alanine aminotransferase levels, apoptotic markers, and human inflammatory cytokines returned to pretreatment levels within 9 days after the last injection. T cell transfer did not trigger inflammation in uninfected mice. These data support the feasibility of using mRNA electroporation to engineer HBV TCR–redirected T cells in patients with chronic HBV infection.
Keywords: Immunology, Infectious disease
Introduction
Adoptive T cell therapy with receptor-modified T cells is emerging as a promising strategy to treat cancers (1). T cells are, however, not only capable of killing cancer cells, but are also essential for the control of viral infection. T cells can be engineered to express virus-specific (human CMV [HCMV], EBV, HIV, hepatitis B virus [HBV], hepatitis C virus [HCV], SARS) T cell receptors (TCRs) (2–6). Virus-specific TCR-redirected T cells have shown protective capacity in animal models (7). In humans, the antiviral efficacy of virus-specific TCR-redirected T cells has not yet been demonstrated, even though adoptive transfer of in vitro–expanded autologous virus-specific T cells has shown remarkable efficacy in EBV and HCMV reactivation occurring in immunocompromised patients (8, 9).
Virus-specific T cells are known to also be protective in the setting of HBV infection. Deletion of HBV-specific CD8+ T cells in HBV-infected chimpanzees blocks HBV clearance (10), and in patients who resolve the infection (11), HBV control is associated with the presence of polyfunctional CD8+ and CD4+ T cell responses targeting multiple HBV antigens (11). Thus, HBV-specific T cells are determinant to clear the virus. On the other hand, patients with chronic HBV infection have severe quantitative and functional defects in HBV-specific T cell response (12, 13), and current treatments are unable to resolve chronic HBV infection. This is mostly due to the inability of treatments based on polymerase inhibitors to target the HBV persistent form, the so-called covalently closed circular DNA (cccDNA) in infected hepatocytes. Thus, despite efficient suppression of viral replication, cccDNA persists within the hepatocyte nucleus, causing viral relapse after treatment discontinuation. Complete elimination of the cccDNA remains very challenging, and it may even be an unrealistic goal, since low cccDNA amounts were shown to persist even after resolution of acute infection (14). However, substantial immune-mediated destruction of infected cells or the induction of substantial cccDNA destabilization and silencing appears mandatory for achieving immune control and hence functional cure (15).
The reconstitution of HBV-specific CD8+ T cell response through HBV-specific TCR transfer can constitute an effective therapy for functional HBV cure. Experimental evidence showing that an adoptive transfer of HBV immunity can lead to an HBV curative effect has already been reported in humans. Adoptive transfer of bone marrow, previously primed with HBV vaccine (16) or HBV infection (17), spawned HBV control in chronic HBV patients. Similarly, liver transplantation of an HBV-infected liver in an HBV-immune patient was followed by viral clearance (18).
Genetic modification of T cells to express HBV-specific receptors, either a chimeric antigen receptor (CAR) (19, 20) or TCR (6), may supplement the deleted or functionally exhausted HBV-specific T cells in chronic HBV patients and, after adoptive transfer, achieve HBV control. T cells obtained from lymphocytes derived from HBV chronically infected patients and engineered to express HBV-specific receptors (6) were shown to produce antiviral cytokines and selectively eliminate HBV-expressing cells in vitro and in animal models (6, 19, 20).
HBV-specific TCR redirected T cells have already been used in a patient with HBV-related hepatocellular carcinoma (HCC) (21), where they caused a substantial drop of HBsAg produced by DNA integrated in HCC cells (22). However, despite this use, toxicity concerns have hampered their implementation in the therapy of patients chronically infected with HBV. Of note, HBV-specific T cells cannot only selectively lyse HBV-infected hepatocytes, but can also trigger inflammatory events within the liver (23). Since T cells transduced by viral vectors stably express TCR, their unchecked expansion might lead to progressive liver toxicities difficult to clinically manage.
To bypass this problem, we engineered T cells that transiently express HBV-specific TCRs for 3 to 5 days through messenger RNA electroporation (24). These cells are not genetically modified, and because of their limited life span, they can be adoptively transferred in escalating doses and their potential toxicities more easily managed. We have demonstrated that such mRNA TCR–redirected T cells are still able, despite their transient expression of TCR, to control the expansion of HBV-expressing hepatoma cells in mice (24), though their potential antiviral activity was not tested.
In the present study, we aimed to test the antiviral and pathogenetic activity of human T cells that transiently express HBV-specific TCR in vitro and in HBV-infected human liver chimeric uPA/SCID/ILγR2 (USG) mice (25). We demonstrate that engineered T cells transiently expressing HBV-TCR are specifically recruited to the liver of HBV-infected mice, harboring human hepatocytes expressing HLA-A2–matched haplotype, and that repeated administrations of these T cells induce a progressive but timely controlled virus-specific immune-mediated reduction of serological and intrahepatic HBV viral loads.
Results
mRNA TCR–expressing T cells display antiviral efficacy in vitro.
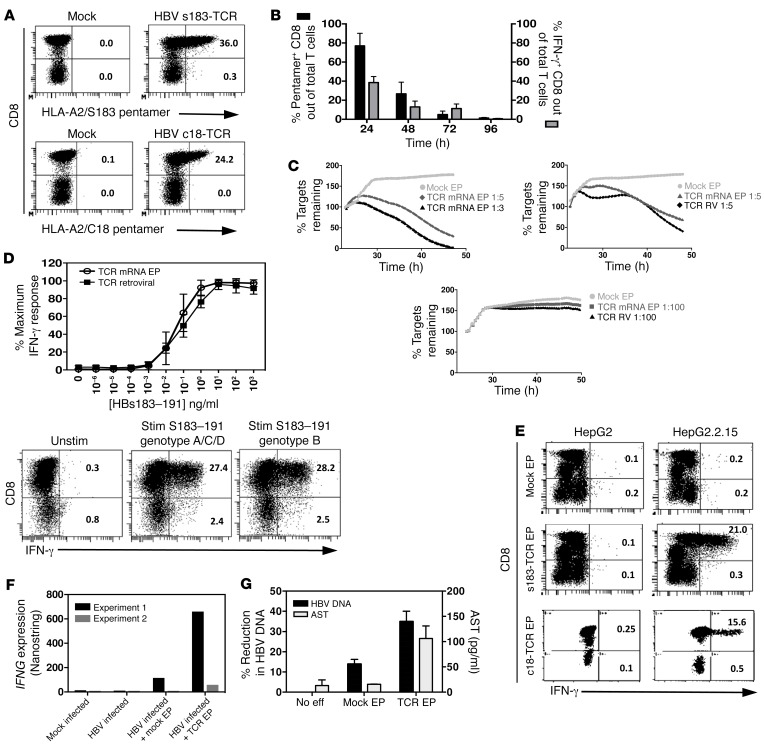
HBV-specific T cells transiently expressing HLA-A0201–restricted HBV-specific TCRs (HBV envelope s183-TCR and HBV core c18-TCR) can be engineered in vitro through direct electroporation of mRNA encoding for specific TCR variable α and β chains in activated human T cells (Figure 1, A and B). A detailed report of the method used has been previously published (24, 26). The HBV-specific TCR mRNA–electroporated T cells acquire HBV specificity and are activated only after incubation with target cells presenting the specific HLA-class I/HBV viral peptide complex (ref. 24 and Figure 1B). Since mRNA does not integrate into the host cell genome, the TCR expression and ability to be activated upon antigen recognition declined with time and was lost after 96 hours (Figure 1B). We then tested the cytotoxicity of HBV-specific TCR mRNA–electroporated T cells against target cells that express HBV proteins and compared their killing ability to that of retroviral transduced T cells that permanently expressed HBV-specific TCR. At high effector-to-target (E:T) ratios of 1:5 and 1:3, HBV-specific TCR mRNA–electroporated T cells efficiently lysed 70% and 98% of the target cells, respectively, within 24 hours and their killing ability was similar to that of T cells stably expressing TCR within 24 hours, but less cytotoxic beyond that, as TCR expression declined (Figure 1C). To mimic more closely the situation in vivo where few T cells could encounter their targets, we tested a low E:T ratio of 1:100. Our results showed that HBV-specific TCR mRNA–electroporated T cells, similar to T cells stably expressing TCR, could still recognize and kill few target cells even at a low E:T ratio (Figure 1C). To further characterize the TCR mRNA–electroporated T cells, we tested their sensitivity to being activated at various peptide concentrations compared with that of T cells stably expressing TCR. We found that the sensitivity of HBV s183–TCR mRNA–electroporated T cells was similar to that of stably transduced T cells and that activation could be observed at 1 pg/ml (Figure 1D). The ability of HBV s183–TCR mRNA–electroporated T cells to recognize different viral mutants was also tested by stimulating electroporated T cells with HBV s183–191 genotype B peptide (FLLTKILTI)– or HBV genotype A/C/D peptide (FLLTRILTI)–loaded T2 cells, and similar frequencies of IFN-γ–producing T cells were detected (Figure 1D). The sensitivity of HBV c18-TCR–engineered T cells (6) and the ability of HBV c18 CD8+ T cell lines to recognize variant epitopes (27) have been previously described.
Figure 1. Lytic and antiviral function of mRNA HBV–specific TCR–electroporated T cells in vitro.
(A) Activated T cells were electroporated with HBV s183–TCR or c18-TCR mRNA, and TCR expression was determined 24 hours after electroporation. Mock electroporated T cells served as negative control. Shown are representative plots. The percentages of HLA-A2/pentamer+ cells out of CD8+ or CD8− T cells are indicated. (B) TCR expression on electroporated cells was measured longitudinally from 24 hours to 96 hours. Electroporated T cells were cocultured with their respective peptide-pulsed T2 cells for 18 hours, and the frequencies of IFN-γ–producing CD8+ T cells out of total lymphocytes were quantified. (C) The ability of mRNA TCR–electroporated T cells to lyse HepG2.2.15 HBV–producing cells at 1:3, 1:5, and 1:100 E:T ratios within 24 hours after T cell addition was compared with that of retroviral transduced (TCR RV) T cells. (D) Sensitivity of T cell activation, displayed as percentage of maximum IFN-γ response using mRNA TCR–electroporated T cells compared with retroviral-transduced T cells (upper panel). MRNA HBV s183–TCR–electroporated T cells were cocultured with HBV s183–191 genotype B (FLLTKILTI) or genotype A/C/D (FLLTRILTI) peptide-loaded T2 cells. The percentages of CD8+ or CD8− T cells producing IFN-γ are indicated (lower panel). (E) Mock, mRNA HBV s183–TCR, or c18-TCR–electroporated T cells were cocultured with either HepG2 or HepG2.2.15 cells for 24 hours. The percentages of CD8+ or CD8− T cells producing IFN-γ are indicated. (F) mRNA HBV s183–TCR or c18-TCR–electroporated T cells were cocultured with mock or HBV-infected HepG2-NTCP for 24 hours, and IFNG gene expression was determined using NanoString analysis. (G) Mock or mRNA HBV s183–TCR–electroporated T cells were cocultured with HepG2.2.15 cells at a 1:3 E:T ratio for 24 hours, and intracellular HBV DNA was quantified by real-time quantitative PCR (qPCR). AST levels were determined in coculture media. Shown are means of percentage reduction in intracellular HBV DNA ± SD (black bars) and means of AST ± SD (gray bars) from 3 independent experiments (right panel).
We then investigated whether TCR mRNA–electroporated T cells can recognize not only HBV peptide–pulsed target cells, but hepatocyte-like cells producing HBV virions from stable HBV-DNA integrations (HepG2.2.15) and in HBV-infected cells (HepG2-NTCP), and whether these engineered T cells could suppress HBV replication in vitro. The expression of HBV s183–TCR allows activation of HBV s183–TCR T cells when cocultured with HepG2.2.15, while mock electroporated T cells were not activated and did not produce any IFN-γ (Figure 1E). Coculture of HBV s183–TCR T cells with HepG2 cells (non–HBV producing) did not cause any level of T cell activation (Figure 1E). This was further confirmed in an HBV-infected HepG2-NTCP system in which significant IFNG gene expression was measured in coculture of HBV s183–TCR T cells with HBV-infected HepG2-NTCP, but not with mock electroporated T cells (Figure 1F). Importantly, coculture of HBV s183–TCR T cells with HepG2.2.15 at a 1:3 E:T ratio for 18 hours caused direct lysis of about 69.5% and a 35% inhibition of HBV-DNA production in HepG2.2.15, accompanied by an increase in aspartate aminotransferase (AST) detected in the supernatant (Figure 1G). Similar results were obtained with T cells electroporated with a TCR specific for the HLA-A0201/core 18–27 complex (HBV c18-TCR T cells) (Figure 1, C, E–G). Taken together, these data show that electroporation of HBV TCR mRNA in T cells generates HBV-specific T cells able to recognize, inhibit, and lyse HepG2 cells producing HBV virions.
mRNA HBV–specific TCR–electroporated T cells display antiviral efficacy in vivo.
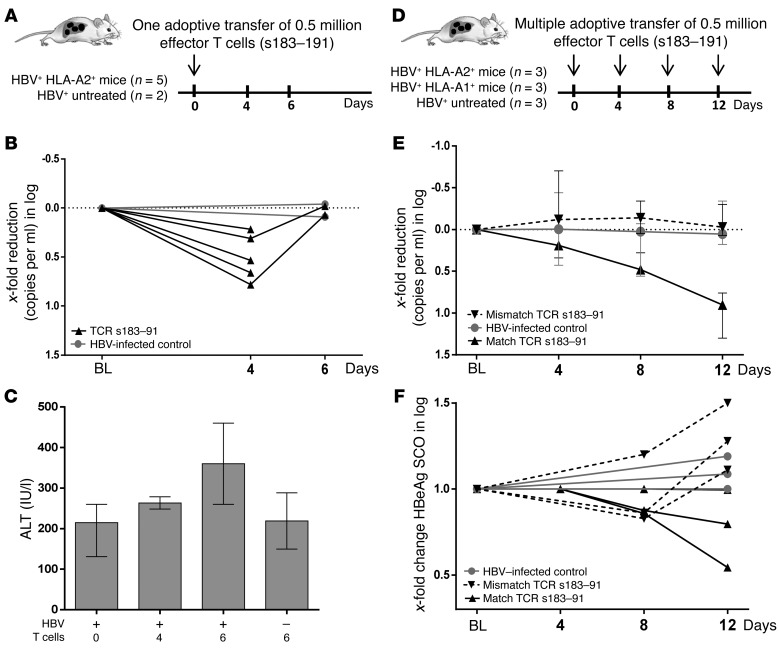
To assess the in vivo antiviral effects of adoptively transferred human T cells transiently expressing an HBV-specific TCR, in a first set of experiments, peripheral blood mononuclear cells (PBMCs) of an HLA-A201+ healthy subject were used. Note that human T cells and human hepatocytes were allogenic, but shared HLA-A0201 expression. After being cultured for 1 week in the presence of IL-2 and anti-CD3 to enrich the fraction of T cells, cells were electroporated with HBV s183–TCR as described in Methods. After 24 hours, HLA-tetramer staining showed that the frequency of pentamer-positive CD8+ T cells ranged between 20% and 25% (data not shown) and that 0.5 million effector HBV s183–TCR T cells were adoptively transferred in each viremic mouse (≥109 HBV-DNA copies/ml) reconstituted with HLA-A2+ hepatocytes (HBV+A2+ mice). As shown in Figure 2, A and B, already 1 single i.p. injection of mRNA HBV s183–TCR T cells caused a drop of viremia in all 5 mice (median Δ0.5 log), which was detected at day 4. However, HBV-DNA values returned to the levels determined in the same animals before treatment at day 6. No decrease of viremia was determined in untreated controls (n = 2). Of note, although relatively high and variable levels of alanine aminotransferase (ALT) are present in this model of liver regeneration, ALT levels appeared exclusively elevated in HBV-infected mice receiving the activated T cells (Figure 2C), since ALT measured in uninfected animals also receiving haplotype-matched T cells remained comparable to levels determined in untreated controls.
Figure 2. mRNA HBV–specific TCR–electroporated T cells show antiviral efficacy in vivo.
(A) Schematic representation of the experiment performed to assess the effect of 1 single injection of electroporated effector T cells in high viremic mice reconstituted with haplotype-matched hepatocytes. (B) Viremia changes relative to baseline levels determined after 4 and 6 days in individual mice upon 1 injection of mRNA HBV s183–TCR T cells (n = 5) and in untreated controls (n = 2). (C) ALT levels determined in HBV-infected and uninfected mice receiving a single injection of effector T cells. (D) Schematic representation of the experiment performed to assess the antiviral effect of multiple injections of electroporated effector T cells in high viremic mice reconstituted either with haplotype-matched (n = 3) or -mismatched (n = 3) human hepatocytes and in comparison with mice that were left untreated (n = 3). (E) Median viremia changes determined within each group depicted in D and relative to baseline levels determined in individual mice upon 3 injections of mRNA HBV s183–TCR T cells. Blood was taken 4, 8, and 12 days after the first T cell injection. (F) Median changes in levels of circulating HBeAg were determined by ELISA in all animal groups. BL, baseline.
To confirm the specificity of HBV s183–TCR T cell treatment and to assess whether multiple adoptive transfer of mRNA TCR–T cells might have an additive antiviral effect, we designed a 3-dose (4-day interval) treatment (Figure 2D). HBV s183–TCR T cells were transferred to HBV-infected mice (0.5 million effector cells for each injection) previously reconstituted with either HLA-A2+ hepatocytes (A2+HBV+) or with HLA-A1+ hepatocytes (A1+HBV+).
As shown in Figure 2E, multiple transfers of HBV s183–TCR T cells caused a progressive and substantial decrease of serum HBV-DNA (median 1 log reduction) in A2+HBV+ mice (n = 3) in only 12 days. No reduction of viremia was detected in mismatched A1+/HBV+ mice (n = 3) treated in parallel with the same effector T cells. A light reduction of circulating hepatitis B viral protein HBeAg levels could already be determined in two-thirds of mice exclusively upon transfer of matched HBV s183–TCR T cells (Figure 2F), whereas HBeAg concentrations remained comparable or even further increased in mismatched A1+HBV+ mice and in infected untreated controls. Conceivably, the rather long half-life (28) and the high amounts of circulating HBsAg present in these animals (median 2.2 × 104 IU/ml; range from 1.1 × 104 to 4.7 × 104 IU/ml) did not enable us to note clear HBsAg changes in only 12 days of treatment (data not shown). Lack of antiviral effects in mice reconstituted with HLA-A1+ hepatocytes that were injected with the same electroporated HBV s183–TCR T cells proved that recognition of infected hepatocytes was essential for activating HBV-specific TCRs and promoting their antiviral activity.
Analysis of intrahepatic events.
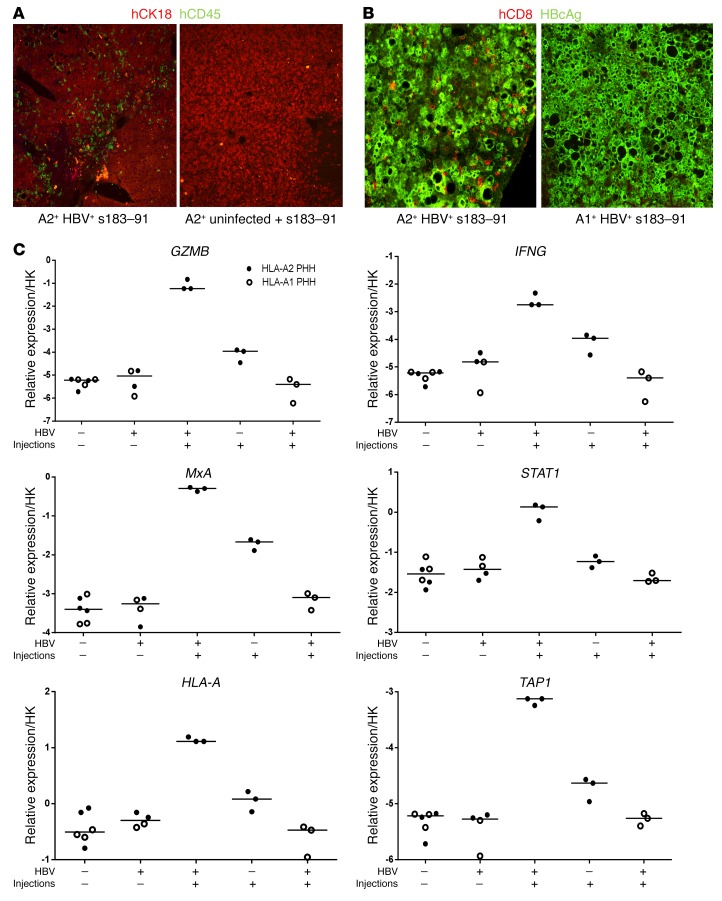
To analyze the intrahepatic immunological and virological events occurring in mice receiving multiple infusions of activated effector T cells, animals were sacrificed 4 days after the third T cell injection. A clear engraftment and enrichment of human CD45+CD8+ T cells could be determined in the livers of A2+HBV+ mice (Figure 3, A and B), whereas very few T cells were detected in HBV-infected mice reconstituted with mismatched hepatocytes (HLA-A1) or in uninfected animals repopulated with HLA-A2+ matched hepatocytes. The specific recruitment of human redirected T cells was accompanied by the increase of serum protein levels of migration, maturation, and differentiation factors exclusively in HBV-infected mice receiving haplotype-matched HBV-specific TCRs, as shown in Supplemental Figure 1 (supplemental material available online with this article; https://doi.org/10.1172/JCI93024DS1 for IL-5; granulocyte-macrophage CSF [GM-CSF] and MDC). Briefly, a strong increase in GM-CSF was determined only in the serum of treated A2+HBV+ mice (>3 log), while the IL-5 increase appeared mild (to 50 ng/ml) and was comparable to the increase also determined in vitro by culturing activated T cells with hepatocyte-like cells infected with HBV (65–71 pg/ml IL-5).
Figure 3. mRNA HBV–specific TCR–electroporated T cells are specifically recruited and activated in livers of haplotype-matched HBV-infected mice.
(A) Liver tissues of humanized HBV-infected mice and uninfected mice that underwent 3 injections of HBV s183–TCR T cells and were sacrificed 4 days after the third T cell transfer were used for immunofluorescence. Human hepatocytes were identified using human-specific CK18 Abs (red). Transferred human immune cells were visualized using human-specific CD45 Abs (green). (B) Liver tissues of HBV-infected mice adoptively transferred with either HLA-A2– or HLA-A1–presenting human hepatocytes were costained with HBV core–specific Ab (green) and human CD45-specific Ab (red). (C) Transcript levels of human T cell response–related genes (GZMB and IFNG) and human ISGs (MxA, STAT1, TAP1, and HLA-A) were measured by quantitative reverse-transcriptase PCR (qRT-PCR) and normalized against human housekeeping transcripts. Statistical analysis was performed with GraphPad Prism 6 software.
As shown in Figure 3C, multiple injections of activated HBV-restricted T cells led to a clear enhancement of T cell activity markers, such as IFNG (IFN-γ), which appeared to be the major cytokine produced, and granzyme B (GZMB), in the liver of haplotype-matched HBV-infected mice. Either much weaker or no enhancement of expression of the same genes was determined in uninfected mice that had also received multiple injections of haplotype-matched activated T cells and in HBV-infected mice repopulated with mismatched human hepatocytes, respectively. A similar pattern of induction of human IFN-stimulated genes (ISGs), such as MX dynamin like GTPase 1 (MxA) and STAT1, and of genes involved in antigen presentation (HLA-I and TAP1) could be determined in A2+HBV+ mice that received HBV-specific T cells (Figure 3C). Enhancement of HLA-I could also be confirmed at the protein level by immunofluorescence (data not shown).
Gene expression was also analyzed in animals that received a single injection of T cells and were sacrificed after either 4 or 6 days as well as in control mice (which did not receive T cells) and compared with levels induced after 3 T cell injections. As shown in Supplemental Figure 2, human genes related to T cell activity (GZMB, IFNG), antigen presentation (HLA-A, TAP1), and ISGs (MxA, STAT1) appeared increased already 4 days after 1 single T cell transfer, while levels remained in the same range or slightly decreased 2 days later (day 6) if no further T cells were injected. However, enhancement of genes related to T cell responses was highest after multiple T cell injections. These analyses support the notion that RNA-electroporated T cells trigger a transient induction of immune markers in the infected livers and that repeated injections are needed to maintain and further enhance such induction.
Of note, in line with the reduction of viremia, lower levels of intrahepatic HBV DNA loads (median –0.8 log relaxed circular DNA [rcDNA]/cell and –0.85 log cccDNA/primary human hepatocyte [PHH]) (Supplemental Figure 3A) and of viral transcripts (median –0.6 log total HBV RNA and –0.9 log pgRNA/human GAPDH, respectively) (Supplemental Figure 3B) were determined in mice that had received multiple injections of haplotype-matched activated T cells in comparison with the haplotype- and viremia-matched animals that had been infected in parallel and mock treated. Intrahepatic viral loads remained comparable between mice that were repopulated with mismatched human hepatocytes, had been infected in parallel (A1+HBV+), and received either 3 injections of A2 effector T cells or remained untreated (Supplemental Figure 3, A and B). Individual changes of HSA levels correlated with ALT induction and HBeAg reduction in A2+HBV+ mice (n = 3) receiving s183 redirected T cells (Supplemental Figure 3C), indicating that the most pronounced HBeAg decrease (46%) was determined in the animal displaying the highest ALT level (2555 IU/l) and the strongest HSA reduction (54%).
Together, these results indicate that multiple injections of T cells temporarily expressing HBV-specific TCR via mRNA electroporation are able to lower both the amount of intrahepatic cccDNA loads and to transiently suppress HBV activity in vivo.
In vivo antiviral effect of human T cells redirected with different HBV-specific TCRs.
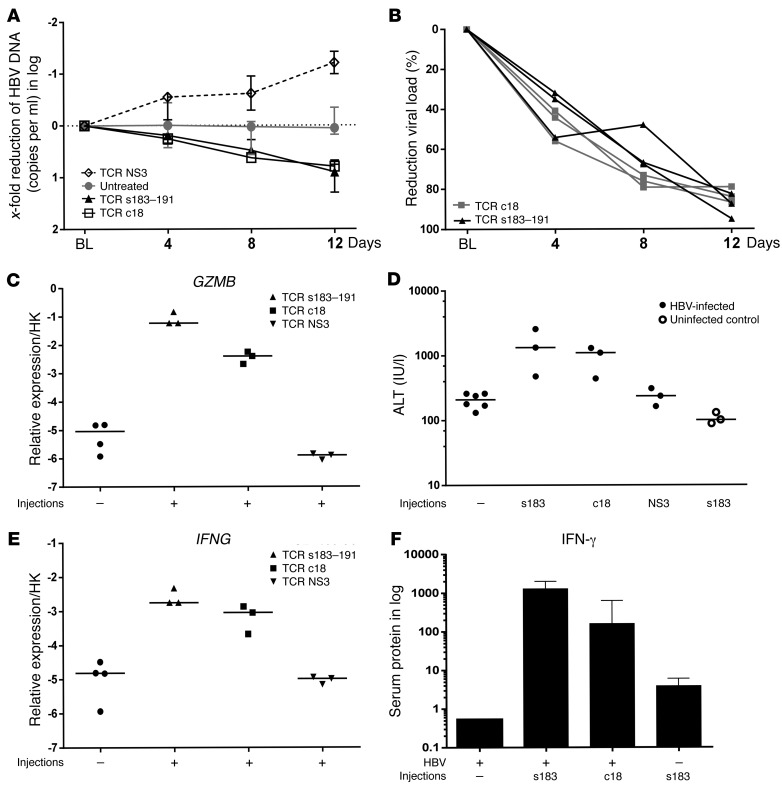
In a different set of experiments, we also tested the in vivo antiviral efficacy of T cells electroporated with a different HBV-specific TCR expressing the HLA-A2/core 18–27 complex (HBV c18-TCR T cells) (n = 3). Furthermore, as a negative control for functional analysis, a similar quantity of human T cells was also electroporated with mRNA coding for an irrelevant HCV-specific HLA-A2–restricted TCR (NS3) and injected in A2+HBV+ mice (n = 3). As shown in Figure 4, A and B, 3 sequential injections of 0.5 million effector T cells temporarily expressing either the HBV-specific envelope or core TCR complex provoked 80% to 90% viremia reduction in only 12 days, while viral titers remained unchanged or even increased further in mice that had been infected in parallel and left untreated or received the irrelevant NS3 TCR.
Figure 4. Antiviral and inflammatory events after in vivo multiple injections of different mRNA HBV–specific TCR–electroporated T cells.
(A) Median viremia changes relative to baseline levels were determined as indicated after 4, 8, and 12 days upon multiple injections of mRNA HBV s183–TCR T cells (n = 3), mRNA c18-TCR T cells (n = 3), and mRNA mock TCR T cells (n = 3) as well as in untreated controls (n = 6). (B) Individual reduction (shown as percentages) of viremia relative to baseline levels determined on days 4, 8, and 12 upon transfer of mRNA s183–191 T cells and mRNA c18 T cells. Transcriptional changes of human T cell response–related genes (C, GZMB; E, IFNG) were measured by qRT-PCR and normalized against human housekeeping transcripts. (D) ALT levels were determined in uninfected (n = 3) and HBV-infected mice receiving multiple injections of effector T cells presenting s183 (n = 3), c18 (n = 3), or HCV NS3 as mock control (n = 3) in comparison with HBV-infected control mice (n = 6). (F) Median changes in human IFN-γ serum protein levels were determined by multiplex measurement in HBV-infected (s183 median = 1280 ng/ml or c18 median = 160 ng/ml) as well as uninfected (s183 median = 3.6 ng/ml) mice that received 3 injections of HBV-specific effector T cells relative to HBV-infected control mice (n = 4; median = 0.53 ng/ml).
Enhancement of human GZMB and IFNG expression in the liver (Figure 4, C and E) as well as the increase of ALT and IFN-γ protein concentrations in serum (Figure 4, D and F) was also exclusively determined in the 2 animal groups receiving multiple injections of T cells expressing HBV-specific TCRs, thus demonstrating the specificity of the antiviral effect induced by redirected T cells. In agreement with the increase of serological markers of liver damage, an increase of caspase 3–positive hepatocytes (2.7%–3%) was determined in the liver of infected mice treated with HBV-specific T cells (Supplemental Figure 4, A and C), while in liver tissues of the control groups, less than 1% of the cells appeared positive for active caspase 3. The immune-mediated destruction of HBV-infected cells was accompanied by human hepatocyte proliferation that increased to 1.8% in mice receiving 3 injections of c18 T cells and to 2.3% in animals similarly treated with the s183-presenting T cells. In comparison, 0.5% of the PHH appeared Ki67 positive in mock-treated and untreated HBV-infected mice (Supplemental Figure 4, B and D).
To test whether even stronger antiviral effects could be achieved in mice with lower levels of infection, 2 mice displaying baseline viremia levels (107 HBV DNA/ml) and slightly lower amounts of human hepatocytes (baseline HSA = 0.8 mg/ml) received T cells (3 times in 12 days) electroporated with HBV-specific TCRs expressing the HLA-A2/s183–191 and core 18–27 complex (s183–TCR + c18–TCR) and were treated in parallel with mice (n = 2) displaying higher levels of viremia and human hepatocyte repopulation (n = 2; >1 × 109 HBV DNA/ml; HSA = 2.6 mg/ml). As shown in Supplemental Figure 5, A and B, a more profound reduction of viremia and HBeAg was determined in mice displaying lower baseline viremia in comparison with mice displaying higher baseline infection loads (up to 1.5 log vs. Δ0.7 log viremia reduction, respectively). Similar effects were also determined at the intrahepatic level (Δ0.5 log vs. Δ0.25 log median cccDNA, respectively) (Supplemental Figure 5C), while ALT elevation was similar in both groups (Supplemental Figure 5D).
Transient expression of HBV-specific TCRs leads to short-lasting liver inflammation and cell damage.
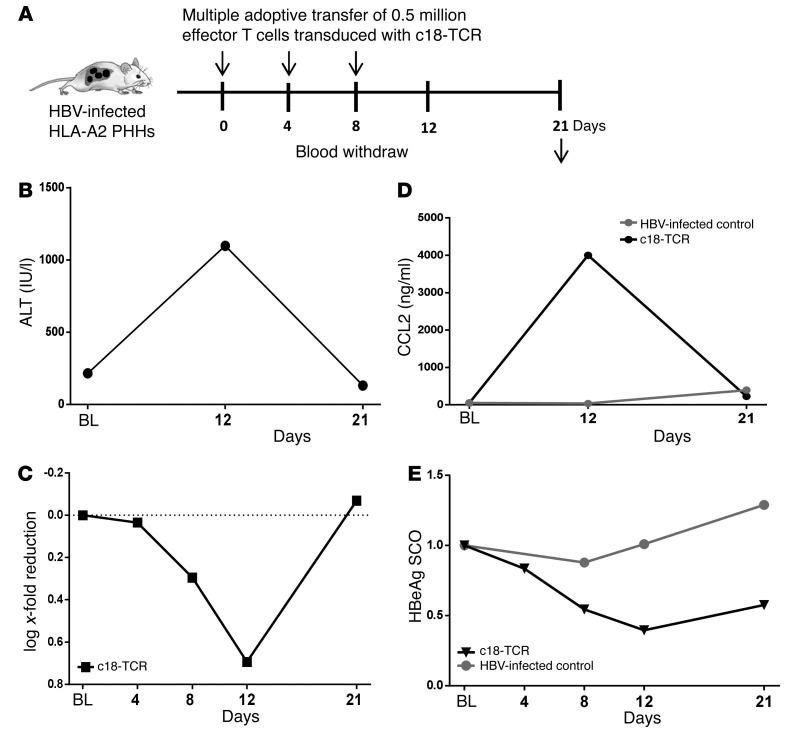
Prolonged T cell response can result in uncontrolled liver damage. Thus, having confirmed that T cells expressing TCRs specific for HLA-2–restricted envelope and core epitopes are both able to recognize HBV-infected PHHs in vivo and to produce proinflammatory cytokines and specific cell killing, we wanted to assess whether their antiviral and cytotoxic activity were also timely limited. Mice that had received 3 injections of T cells expressing the HBV-specific TCRs were sacrificed 9 days after the last T cell infusion (day 21 after treatment begin) to assess the effect of immune cell therapy at a time when HBV-specific TCRs were no longer expressed (Figure 5A).
Figure 5. Adoptive transfer of mRNA HBV–specific TCR–electroporated T cells leads to temporary limited liver inflammation and cell damage.
(A) Schematic representation of the experiment performed to assess the effect of multiple injections of electroporated effector T cells after treatment cessation both on inflammatory and virological parameters. (B) ALT levels were determined in 1 HBV-infected animal shortly before T cell injection (baseline) after receiving 3 injections of HBV-specific c18-TCR T cells (day 12) and 9 days after the last T cell injection (day 21). (C) Longitudinal changes in viremia relative to baseline were determined at 4, 8, 12, and 21 days after the first T cell transfer as depicted in A. (D) Serum protein levels of CCL2 were determined in both 1 HBV-infected control mouse (gray) and 1 mouse receiving multiple T cell injections (c18-TCR), and that was monitored for 21 days. (E) Longitudinal changes in levels of circulating HBeAg were determined by ELISA in the same mice described in D.
Despite the clear induction of transaminases and inflammatory cytokines determined during therapy, serological analyses performed 9 days after treatment cessation revealed that liver damage occurred only transiently. As shown in Figure 5, B and C, both levels of ALT and chemokines (i.e., CCL2) returned to baseline. Accordingly, gene expression levels of granzyme β, human caspase 1, IFNG, and caspase 8 also returned to baseline in mice after long-term treatment (data not shown).
Because of the transient antiviral effect induced by the HBV-specific T cells and the high amount of infected human hepatocytes still present in this immune-deficient chimeric mouse model, virological parameters also rebounded after treatment cessation (Figure 5, D and E).
Discussion
Immunotherapy using T cells with redirected specificities has favored strategies that lead to the production of cells with stable expression of the introduced TCR. Such engineered T cells can persist in the host and establish a memory-like T cell immunity with therapeutic advantages. The expansion and long-term presence of antigen-specific T cells can, however, result in severe side effects due to off-target activation of T cells or to the massive uncontrolled lysis of the targeted cells and severe liver damage.
To reduce such safety concerns, we have previously shown that direct electroporation of mRNA coding HBV-specific TCR allows the production of large quantities of TCR-redirected T cells expressing the introduced TCR only transiently (24). Although the functional efficiency of such cells is limited to 72 to 96 hours, mRNA TCR–redirected T cells were shown to possess antitumor activity in a xenograft model of HCC (24).
In this study, we tested the antiviral efficacy of mRNA HBV–TCR–electroporated human T cells in vivo, using mice harboring livers reconstituted with PHHs. Despite the high levels of human chimerism achieved in these immune-deficient animals, where HBV inoculation leads to infection persistence in nearly all human hepatocytes and production of high levels of viremia (>109 HBV DNA/ml serum) and circulating viral antigens, we demonstrate here that transient expression of TCRs did not affect the anti-HBV efficacy of our engineered T cells. The effect induced already after 1 single i.p. injection of effector T cells is remarkable, considering the low ratio of E:T cells (approximately 1:100) used in our experimental setting, where 0.5 million effector T cells were inoculated i.p. in mice harboring almost 50 million HBV-infected human hepatocytes (median 4.7 × 107 hepatocytes per liver).
Adoptive transfer of mRNA HBV–specific TCR redirected T cells resulted in an intrahepatic accumulation of inflammatory cells, increased expression of genes related to T cell activation (IFNG, GZMB), and a clearly quantifiable decrease of HBV viremia (median 1 log reduction) and HBeAg as well as lower intrahepatic viral loads, including cccDNA and viral transcripts in comparison with matched controls. Furthermore, an even stronger viremia decrease (up to 1.5 log) was achieved in only 12 days when mice displaying lower baseline viremia received 3 injections of HBV-specific T cells. Moreover, the recruitment of HBV-specific TCR-redirected T cells and inflammatory modifications were observed only in HBV-infected mice receiving HBV-specific TCR-redirected T cells, but not in uninfected mice also reconstituted with hepatocytes matched for the HLA-A2 haplotype. In addition, no viremia decrease could be determined in similarly treated HBV-infected animals harboring HLA-mismatched hepatocytes. Adoptive transfer of T cells engineered with an irrelevant TCR, in this case specifically recognizing HCV-infected cells, also did not provoke T cell recruitment and reduction of HBV viremia. In sum, these in vivo experiments show that off-target cytotoxic effects related to HBV-specific redirected T cells did not occur in any of the control experiments, supporting the hypothesis that this approach should not trigger unspecific liver damage. Of note, the antiviral and inflammatory events were temporally limited, since viremia levels and inflammatory events returned to baseline levels 9 days after treatment cessation. Together, these results demonstrate the ability of human T cells transiently expressing HBV-specific TCRs to reach the intrahepatic environment, to recognize the infected human hepatocytes, and to initiate a pathway of viral control exclusively mediated by antigen-specific T cell recognition.
The antiviral efficacy of the adoptively transferred TCR-redirected T cells in these chimeric mice was already detectable 4 days after 1 single infusion. Single injection of about 0.5 × 106 cells per mouse achieved about a 0.5 log reduction of HBV viremia, while multiple injections (n = 3) were able to induce progressive decrease of viremia (up to 1.5 log) in only 12 days. Such fast reduction of viremia is quite remarkable and comparable to the kinetic of viremia reduction often achieved in this animal model using approved antiviral drugs such as polymerase inhibitors (nucleotide analogue [NA] therapy) (29–31). Note that a central point of this work was to test the ability of mRNA TCR–redirected T cells to recognize infected human hepatocytes in vivo and to provoke only temporary immune-mediated killing of the infected cells. The reversion of ALT values detected 9 days after the last T cell infusion shows that the bulk of intrahepatic inflammatory reaction induced by HBV-TCR–redirected T cells activated in vivo vanished with the loss of the TCR from the activated T cells. Thus, mRNA TCR–redirected T cells exerted a finite therapeutic and inflammatory effect in vivo. Previous studies based on a xenograft model of HBV-HCC (24) indicated that viral vector–transduced T cells eliminated all the HBV-expressing targets, while the mRNA-electroporated T cells had a more limited effect. Thus, it may be that T cells stably expressing HBV-TCR would wipe out HBV-infected hepatocytes with higher efficiency. However, such an approach would bear the risk of causing severe and difficult to control viral hepatitis. Because of the absence of genetic modification, the transient TCR expression, and the subsequent short lifetime of such redirected T cells, mRNA-electroporated T cells are more likely to be used in patients with chronic hepatitis B. This has important implications for the possible clinical translation of T cell therapies, since it will allow a safe implementation of dose-escalating regimens of HBV-TCR T cell transfer, an approach that cannot be performed with viral vector–transduced T cells.
Our chimeric mice received about 0.5 million effector T cells per injection, which corresponds to approximately 15 million HBV-TCR T cells per kg. This is a quantity of HBV-specific T cells that is achievable using our mRNA TCR T cell strategy. However, it may be possible to lower the quantity of HBV-specific T cells in patients receiving NA therapy or that are expected to harbor low amounts of infected cells (i.e., HBeAg negative, refs. 32, 33). In this regard, treatment with redirected HBV-TCR T cells appeared even more effective in mice harboring slightly lower levels of infection. The flexibility of using repeated injections of autologous T cells transiently expressing virus-specific mRNA TCRs might allow the optimization of dose and therapy length required to achieve substantial reduction of the pool of HBV-infected hepatocytes. Furthermore, it could be envisaged that the implementation of strategies aiming at preventing viral rebound either by blocking hepatocyte reinfection and intrahepatic HBV spreading (Myrcludex B) (34, 35) and/or by suppressing viral replication (NA therapy) could contribute substantially to achieving control of the infection and hence functional HBV cure.
The lower amounts of HBV transcripts, rcDNA, and even cccDNA determined per human hepatocyte were accompanied by an increased rate of cell death and human hepatocyte proliferation as well as transient production of cytokines, in particular, IFN-γ. Together, these events also suggest that antiviral effects occurring beyond the direct elimination of infected cells took place in the setting of adoptive T cell transfer. Since both cytolytic and noncytolytic immune-mediated antiviral effects, such as suppression of cccDNA transcription (36) and destabilization due to compensatory cell growth (37) as well as cytokine-mediated events (38) may be essential to achieving control of HBV infection, future studies need to be designed to dissect the contribution of these different mechanisms.
A note of caution about the efficacy of adoptive T cell therapy in the real-world clinical setting should, however, be pointed out, since the in vivo antiviral activity of HBV-specific CD8+ T cells (murine or HBV–TCR–human T cells) has so far been assessed in models that are devoid of chronic liver inflammatory events and fibrosis. Unfortunately, chronic liver inflammation can reduce the accessibility of HBV-specific CD8+ T cells to HBV-infected hepatocytes through the modification of the sinusoidal fenestration that allows T cells to directly recognize HBV-infected hepatocytes without proper extravasation (22). As such, the antiviral effect of TCR-redirected T cell therapy could be compromised in patients with marked chronic liver inflammatory events. Such a possibility needs to be taken into account by the selection of chronic HBV patients that might be more likely to respond to such immunological therapies. Thus, patient populations with chronic active liver inflammation might not necessarily be the ones in which immunological therapies should be applied. Nevertheless, one other possibility could be to engineer T cells through introduction of functional modifications that allow a better migratory capacity in the fibrotic microenvironment.
On the other hand, it should also be pointed out that this study shows that TCR-redirected human T cells are also able to recognize HBV-infected allogenic human hepatocytes in a milieu in which very high levels of HBV and circulating viral antigens are produced. Moreover, keeping also in mind that these immune cells acted in a very peculiar immune-deficient murine environment with limited cytokine crosstalk, it is probable that we may have underestimated the in vivo antiviral efficacy of autologous TCR-redirected T cells if applied in humans.
It seems clear that new analysis in animal models and patients will be necessary to carefully design experimental and clinical trials in which such variables should be taken into account. Despite all the odds, we think that the demonstration of the antiviral effect of mRNA-HBV-TCR T cells constitutes a first important step in the development of therapies designed to overcome the deficient HBV-specific immunity status of chronic hepatitis B–infected (CHB-infected) patients.
Methods
Preparation of engineered T cells via electroporation.
HBV envelope s183–191 TCR, HBV core 18–27 TCR mRNA, and HCV NS3 1073–1081 TCR were produced using in vitro transcription, as previously described (24, 26). A detailed report describing selection of HBV-specific T cells and cloning of their corresponding TCRs has been previously described (3). The HCV NS3 1073–1081 TCR cloned in pVAX1 was a gift from Margaret Sällberg Chen (Karolinska Institutet, Department of Dental Medicine, Huddinge, Sweden). To produce activated T cells for electroporation, PBMCs were stimulated with 600 IU/ml IL-2 (rIL-2; R&D Systems) and 50 ng/ml anti-CD3 (OKT-3; eBioscience) in AIM-V plus 2% human AB serum for 8 days, and rIL-2 was increased to 1000 IU/ml 1 day before electroporation. For electroporation with the nucleofector device II (Lonza), 1 × 107 activated T cells were suspended in 100 μl of Cell Line Nucleofector Solution V (Lonza) and TCR mRNA was added at 200 μg/ml. The mixture was placed in a certified cuvette (Lonza) and electroporated using program X-001. After electroporation, cells were resuspended in AIM-V 10% human AB serum plus 100 IU/ml rIL-2, and cultured at 37°C and 5% CO2 overnight until analysis. T cells expressing the introduced TCR were quantified by flow cytometry using the specific HLA class I pentamers (HLA-A201–HBV envelope 183–91–PE pentamer, HLA-A201–HBV core 18–27–PE pentamer, HLA-A201–HCV NS3 1073–1081–PE pentamer) from ProImmune.
In vitro function of TCR mRNA–electroporated T cells.
To test the functionality of TCR mRNA–electroporated T cells, HLA-A2+ T2 cells were pulsed with 1 μg/ml of HBV envelope 183–191 peptide or HBV core 18–27 peptide for 1 hour at 106 cells/ml and then washed twice. TCR mRNA–electroporated T cells were cocultured with peptide-loaded T2 cells overnight in the presence of 2 μg/ml brefeldin A and stained for CD8 and IFN-γ as previously described.
Coculture with HBV-producing hepatoma cell line.
1 × 105 HBV-producing HepG2.2.15 hepatoma cells per well were seeded overnight in a 96-well plate to permit adherence. TCR mRNA–electroporated T cells were added at a 1:3 E:T ratio for 24 hours. Supernatants from coculture experiments were collected for measurement of ALT after 24 hours. RLT buffer with β-mercaptoethanol was added to lyse the HepG2.2.15 cells for isolation of intracellular viral nucleic acids using the QIAamp MinElute Virus Spin Kit (QIAGEN), and HBV DNA was quantified by real-time PCR. Real-time PCR was performed using the artus HBV RG PCR kit following the manufacturer’s instructions in a Rotor-Gene Q 2-plex instrument (QIAGEN).
xCELLigence real-time cell analysis of target cell lysis by TCR-expressing T cells.
1 × 105 HBV-producing HepG2.2.15 hepatoma cells per well were seeded overnight in 96-well electronic microtiter plates (ACEA Biosciences). TCR mRNA–electroporated T cells were added at a 1:3, 1:5, or 1:100 E:T ratio at 24 hours after electroporation, and target cell lysis was monitored in real time for up to 24 hours following the addition of T cells. The impedance, which reflects adherence of the target cells to the bottom of the plate, was measured every 15 minutes using an XCELLigence RTCA MP (ACEA Biosciences).
Generation of humanized mice, infection, and T cell administration.
Human liver chimeric uPA/SCID/ILγR2 (USG) mice were generated by transplanting 1 million thawed human hepatocytes in 3-week-old mice anesthetized with isofluoran. Generation of uninfected and stably HBV-infected human liver chimeric mice was performed as previously reported (29). Human hepatocytes obtained from 2 donors, one with haplotype HLA-A2 and one with HLA-A1, were used as indicated in Results.
Levels of human liver chimerism were determined by measuring HSA in mouse serum using the HSA ELISA Quantitation Kit (Bethyl Laboratories, Biomol GmbH). To establish HBV infection, animals received a single i.p. injection of HBV-infectious serum (1 × 107 HBV DNA copies/mouse; genotype D). Both naive and HBV-infected mice displaying high levels of viremia (≥1 × 109 HBV DNA copies/ml) were used here for adoptive transfer of engineered human T cells. Human T cells isolated from PBMCs were expanded in vitro and electroporated following the same procedure as described for the in vitro experiments. 0.5 × 106 activated effector T cells, corresponding to an approximately 1:40 E:T ratio, were injected per mouse. Blood was withdrawn at indicated times. Liver specimens removed at sacrifice were snap-frozen in 2-methylbutane for histological and molecular analyses.
Virological and human gene expression analyses.
DNA was extracted from liver specimens using the Master Pure DNA Purification Kit (Epicentre), and cccDNA was isolated by Hirt extraction (37). RNA was extracted from liver using the RNeasy RNA Purification Kit (QIAGEN) (29). Intrahepatic total viral loads were quantified using primers and probes specific for total HBV DNA and cccDNA (39). Primers and probes specific for total HBV RNA and pgRNA were used for reverse transcription and amplification, while the expression of the human housekeeping gene GAPDH was used for normalization (39).
HBsAg and HBeAg quantification were performed on the Abbott Architect platforms (Quantitative HBsAg Kit and HBeAg Kit, Abbott, Diagnostic Division) after diluting the mouse serum (1:40 for HBsAg and HBeAg) in the manual dilution serum (Abbott) as recommended by the manufacturer. HBeAg results were displayed as signal versus noise (SC/O).
To determine gene expression levels, human- and mouse-specific primers from the TaqMan Gene Expression Assay System were used and samples analyzed in the ViiA 7 Real-Time PCR System (both Life Technologies). The mean of the human housekeeping genes GAPDH and ribosomal protein L30 was used to normalize human gene expression levels.
Protein analysis by immunofluorescence and Luminex technologies.
Human hepatocytes were identified in frozen mouse liver sections using a human cytokeratin-18 monoclonal mouse Ab (Dako Diagnostika). HBcAg staining was detected with a polyclonal rabbit anti-HBcAg Ab (Dako), and specific signals were visualized with Alexa Fluor 488– or 546–labeled secondary Abs (Invitrogen) or the TSA Fluorescein System (PerkinElmer). Nuclear staining was achieved by Hoechst 33258 (Invitrogen). Human CD8 staining was performed using a mouse monoclonal anti-CD8 Ab (Dako). Proliferation of human hepatocytes and immune cells was determined using Abs to human Ki-67 (Dako). To detect active caspase 3, mouse livers were costained with mouse monoclonal anti-human cytokeratin 18 Ab and rabbit anti-human active caspase 3 (BD Transduction Laboratories). Stained sections were analyzed by fluorescence microscopy (Biorevo BZ-9000, Keyence).
Levels of human cytokines in the serum of humanized mice were determined using the MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel according to the manufacturer’s instructions (Merck Millipore).
Study approval.
PHHs were isolated from rejected explant livers using protocols approved by the ethical committee of the city and state of Hamburg (OB-042/06) and according to the principles of the Declaration of Helsinki. Animals were housed under specific pathogen–free conditions according to institutional guidelines under authorized protocols by the Ethical Committee of the city and state of Hamburg. All animal experiments were conducted in accordance with the European Communities Council Directive (86/EEC) and were approved by the city of Hamburg, Germany.
Author contributions
AB and MD initiated and supervised the research study. JK, SK, MD, and AB designed the experiments. JK, SK, TV, and EC conducted experiments and acquired data. JK, SK, EC, TV, LA, and ML analyzed data. LA and TV also generated the chimeric mice. JK, SK, MD, and AB wrote the manuscript. All authors discussed the data and corrected the manuscript.
Supplementary Material
Acknowledgments
We are grateful to Anne Groth and Roswitha Reusch for their excellent work with the mouse colony and to Claudia Dettmer for her great technical help. The study was supported by the German Research Foundation (DFG) by a grant (Collaborative Research Centre SFB 841 A5 to MD), by a Heisenberg Professorship (DA1063/3-2 to MD) and by a Singapore Translational Research (STaR) Investigator Award (NMRC/STaR/013/2012 to AB). MD also received funding from the German Center for Infection Research (DZIF; TTU-Hepatitis 05.806).
Footnotes
Conflict of interest: S. Koh is the Director of Research at Lion TCR Pte. Ltd., a biotech company developing T cell receptors for treatment of virus-related cancers and chronic viral diseases. A. Bertoletti is a cofounder of Lion TCR Pte. Ltd.
Reference information: J Clin Invest. 2017;127(8):3177–3188.https://doi.org/10.1172/JCI93024.
Contributor Information
Janine Kah, Email: j.kah@uke.de.
Sarene Koh, Email: sarene_koh@sics.a-star.edu.sg.
Tassilo Volz, Email: t.volz@uke.de.
Erica Ceccarello, Email: ceccarelloe@student.imcb.a-star.edu.sg.
Lena Allweiss, Email: lenaallweiss@hotmail.com.
Marc Lütgehetmann, Email: m.luetgehetmann@uke.de.
Antonio Bertoletti, Email: antonio@sics.a-star.edu.sg.
Maura Dandri, Email: m.dandri@uke.de.
References
- 1.Feldman SA, Assadipour Y, Kriley I, Goff SL, Rosenberg SA. Adoptive cell therapy–tumor-infiltrating lymphocytes, T-cell receptors, and chimeric antigen receptors. Semin Oncol. 2015;42(4):626–639. doi: 10.1053/j.seminoncol.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schub A, Schuster IG, Hammerschmidt W, Moosmann A. CMV-specific TCR-transgenic T cells for immunotherapy. J Immunol. 2009;183(10):6819–6830. doi: 10.4049/jimmunol.0902233. [DOI] [PubMed] [Google Scholar]
- 3.Banu N, et al. Building and optimizing a virus-specific T cell receptor library for targeted immunotherapy in viral infections. Sci Rep. 2014;4:4166. doi: 10.1038/srep04166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh HL, et al. Engineering T cells specific for a dominant severe acute respiratory syndrome coronavirus CD8 T cell epitope. J Virol. 2011;85(20):10464–10471. doi: 10.1128/JVI.05039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. Transduction of human T cells with a novel T-cell receptor confers anti-HCV reactivity. PLoS Pathog. 2010;6(7):e1001018. doi: 10.1371/journal.ppat.1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gehring AJ, et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol. 2011;55(1):103–110. doi: 10.1016/j.jhep.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Mueller K, et al. Protective capacity of virus-specific T cell receptor-transduced CD8 T cells in vivo. J Virol. 2012;86(19):10866–10869. doi: 10.1128/JVI.01472-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stemberger C, et al. Lowest numbers of primary CD8(+) T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood. 2014;124(4):628–637. doi: 10.1182/blood-2013-12-547349. [DOI] [PubMed] [Google Scholar]
- 9.Leen AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thimme R, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77(1):68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64(1 Suppl):S71–S83. doi: 10.1016/j.jhep.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Boni C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster GJ, et al. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol. 2004;78(11):5707–5719. doi: 10.1128/JVI.78.11.5707-5719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2(10):1104–1108. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 15.Revill P, Testoni B, Locarnini S, Zoulim F. Global strategies are required to cure and eliminate HBV infection. Nat Rev Gastroenterol Hepatol. 2016;13(4):239–248. doi: 10.1038/nrgastro.2016.7. [DOI] [PubMed] [Google Scholar]
- 16.Ilan Y, et al. Adoptive transfer of immunity to hepatitis B virus after T cell-depleted allogeneic bone marrow transplantation. Hepatology. 1993;18(2):246–252. [PubMed] [Google Scholar]
- 17.Lau GK, et al. Clearance of hepatitis B surface antigen after bone marrow transplantation: role of adoptive immunity transfer. Hepatology. 1997;25(6):1497–1501. doi: 10.1002/hep.510250631. [DOI] [PubMed] [Google Scholar]
- 18.Loggi E, et al. Anti-HBs re-seroconversion after liver transplantation in a patient with past HBV infection receiving a HBsAg positive graft. J Hepatol. 2009;50(3):625–630. doi: 10.1016/j.jhep.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Bohne F, et al. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology. 2008;134(1):239–247. doi: 10.1053/j.gastro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Krebs K, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology. 2013;145(2):456–465. doi: 10.1053/j.gastro.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 21.Qasim W, et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62(2):486–491. doi: 10.1016/j.jhep.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Guidotti LG, et al. Immunosurveillance of the liver by intravascular effector CD8(+) T cells. Cell. 2015;161(3):486–500. doi: 10.1016/j.cell.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sitia G, Isogawa M, Iannacone M, Campbell IL, Chisari FV, Guidotti LG. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J Clin Invest. 2004;113(8):1158–1167. doi: 10.1172/JCI21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh S, et al. A practical approach to immunotherapy of hepatocellular carcinoma using T cells redirected against hepatitis B virus. Mol Ther Nucleic Acids. 2013;2:e114. doi: 10.1038/mtna.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dandri M, Petersen J. Chimeric mouse model of hepatitis B virus infection. J Hepatol. 2012;56(2):493–495. doi: 10.1016/j.jhep.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 26.Koh S, Bertoletti A. Circumventing failed antiviral immunity in chronic hepatitis B virus infection: triggering virus-specific or innate-like T cell response? Med Microbiol Immunol. 2015;204(1):87–94. doi: 10.1007/s00430-014-0377-7. [DOI] [PubMed] [Google Scholar]
- 27.Tan AT, et al. Host ethnicity and virus genotype shape the hepatitis B virus-specific T-cell repertoire. J Virol. 2008;82(22):10986–10997. doi: 10.1128/JVI.01124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chulanov VP, Shipulin GA, Schaefer S, Gerlich WH. Kinetics of HBV DNA and HBsAg in acute hepatitis B patients with and without coinfection by other hepatitis viruses. J Med Virol. 2003;69(3):313–323. doi: 10.1002/jmv.10291. [DOI] [PubMed] [Google Scholar]
- 29.Allweiss L, et al. Immune cell responses are not required to induce substantial hepatitis B virus antigen decline during pegylated interferon-alpha administration. J Hepatol. 2014;60(3):500–507. doi: 10.1016/j.jhep.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Bissig KD, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120(3):924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa S, et al. Targeted induction of interferon-λ in humanized chimeric mouse liver abrogates hepatotropic virus infection. PLoS One. 2013;8(3):e59611. doi: 10.1371/journal.pone.0059611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werle-Lapostolle B, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126(7):1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut. 2012;61(Suppl 1):i6–i17. doi: 10.1136/gutjnl-2012-302056. [DOI] [PubMed] [Google Scholar]
- 34.Volz T, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58(5):861–867. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Petersen J, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26(3):335–341. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- 36.Belloni L, et al. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest. 2012;122(2):529–537. doi: 10.1172/JCI58847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allweiss L, et al. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut. doi: 10.1136/gutjnl-2016-312162. [published online ahead of print on April 20, 2017]. https://doi.org/10.1136/gutjnl-2016-312162. [DOI] [PubMed] [Google Scholar]
- 38.Lucifora J, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343(6176):1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giersch K, et al. Hepatitis Delta co-infection in humanized mice leads to pronounced induction of innate immune responses in comparison to HBV mono-infection. J Hepatol. 2015;63(2):346–353. doi: 10.1016/j.jhep.2015.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.