Abstract
Most of the thermal tolerance studies on fish have been performed on juveniles and adults, whereas limited information is available for larvae, a stage which may have a particularly narrow range in tolerable temperatures. Moreover, previous studies on thermal limits for marine and freshwater fish larvae (53 studies reviewed here) applied a wide range of methodologies (e.g. the static or dynamic method, different exposure times), making it challenging to compare across taxa. We measured the Critical Thermal Maximum (CTmax) of Atlantic herring (Clupea harengus) and European seabass (Dicentrarchus labrax) larvae using the dynamic method (ramping assay) and assessed the effect of warming rate (0.5 to 9°C h-1) and acclimation temperature. The larvae of herring had a lower CTmax (lowest and highest values among 222 individual larvae, 13.1–27.0°C) than seabass (lowest and highest values among 90 individual larvae, 24.2–34.3°C). At faster rates of warming, larval CTmax significantly increased in herring, whereas no effect was observed in seabass. Higher acclimation temperatures led to higher CTmax in herring larvae (2.7 ± 0.9°C increase) with increases more pronounced at lower warming rates. Pre-trials testing the effects of warming rate are recommended. Our results for these two temperate marine fishes suggest using a warming rate of 3–6°C h-1: CTmax is highest in trials of relatively short duration, as has been suggested for larger fish. Additionally, time-dependent thermal tolerance was observed in herring larvae, where a difference of up to 8°C was observed in the upper thermal limit between a 0.5- or 24-h exposure to temperatures >18°C. The present study constitutes a first step towards a standard protocol for measuring thermal tolerance in larval fish.
Introduction
Performance in fish and other ectotherms is highly controlled by temperature, which sets the pace of physiological processes [1,2]. For that reason, temperature is believed to be largely responsible for the geographical patterns in distribution and abundance of most species [3]. Climate-driven changes, especially global warming, have been correlated to changes in phenology, distribution and abundance of some temperate species [4] and warming may be particularly deleterious for stenothermic animals inhabiting low and high latitudes [5,6]. Despite the importance of understanding thermal physiology to disentangling the mechanisms behind climate-driven changes in populations, basic information on thermal limits is lacking for a large number of marine fish species. Such information is important given the (re-) emphasis of integrating physiological thresholds within models projecting climate impacts [7–9].
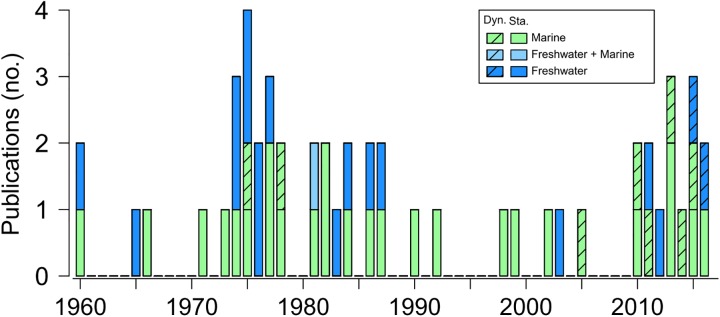
In fish and other ectotherms, limits to thermal tolerance and the impact of temperature on physiological processes can be stage-specific and larvae are assumed to be a more sensitive life stage (i.e. displaying relatively narrow ranges in tolerable temperatures) compared to juveniles or adults [10]. For instance, the lower latitudinal limit of Arctic cod (Boreogadus saida) is unlikely due to adult thermal tolerance and more likely controlled by summer temperatures beyond the tolerable range of larvae [11]. Hence, understanding ontogenetic changes in thermal tolerance is highly relevant in order to identify potential population bottlenecks in future warming scenarios [12]. Unfortunately, relatively few data are available on the thermal tolerance of fish larvae (38 freshwater and 19 marine species, Fig 1, Table 1) compared to juvenile and adult fish (>110 marine species, [9]). Standard protocols are available for juveniles and adults [13,14] but not for larvae which may explain, in part, why far fewer thermal tolerance estimates are available for larvae compared to later life stages.
Fig 1. Summary of studies reporting thermal limits for the larvae of freshwater, brackish and marine fish species.
Shaded and filled bars are studies using the static (Sta.) and dynamic (Dyn.) method, respectively. See text for further details on both methods.
Table 1. Compilation of published studies on thermal limits of marine and freshwater larvae.
| Order & Family | Species | Common Name | Larval Habitat | Larval Age / Size | Method | Rearing T (°C) | Thermal limit | Study | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor (LT or CT) | Ind. / Groups | Time (h) | Change Rate (°C h-1) | End-point | Lower | Upper | |||||||
| Ord. Acipenseriformes | |||||||||||||
| Fam. Acipenseridae | |||||||||||||
| Scaphirhynchus albus | Pallid sturgeon | FW | 6–10 mm TL | LT (S) | G | 0.1 | - | D + LOE | 22 | - | 32.0 | [15] | |
| Ord. Atheriniformes | |||||||||||||
| Fam. Atherinidae | |||||||||||||
| Leurestes sardina | Gulf grunion | SW | 0–30 dph | LT (S) | G | 0.5–72.0 | - | D | 20–30 | 7.0–8.0 | 31.0–36.0 | [16] | |
| Leurestes tenuis | Californian grunion | SW | 0–30 dph | LT (S) | G | 0.5–72.0 | - | D | 20–30 | 3.0–8.0 | 32.0–40.0 | [16] | |
| Ord. Beloniformes | |||||||||||||
| Fam. Adrianichthyidae | |||||||||||||
| Oryzias melastigma | Marine medaka | SW | 5 mm | CT (D) | I | 168.0 | 18 | LOE | 12–32 | 6.3–12.3 | 39.9–42.8 | [17] | |
| Ord. Clupeiformes | |||||||||||||
| Fam. Clupeidae | |||||||||||||
| Alosa pseudoharengus | Alewife | FW | 1 dph | LT (S) | G | 24.0 | abrupt | D | 14–15 | - | 31.0 | [18] | |
| Brevoortia tyrannus | Atlantic menhaden | SW | - | LT (S) | G | > 12.0 | - | D | 7–15 | 1.5–4.0 | - | [19] | |
| Clupea harengus | Atlantic herring | SW | YS | LT (S) | G | 24.0 | - | D | 7, 13 | 20.5–23.5 | [20] | ||
| SW | - | LT (S) | G | 0.1–1.0 | - | D | 8 | - | 25.0–31.0 | [21] | |||
| SW | 6–8 mm | LT (S) | G | 24.0 | - | D | 7–15 | -2.0–-0.35 | 22.0–23.5 | [22] | |||
| Fam. Engraulidae | |||||||||||||
| Engraulis australis | Australian anchovy | SW | E to YS | LT (S) | G | 0.5–24.0 | 15 | D | 25–27 | - | 35.1 | [23] | |
| Ord. Cypriniformes | |||||||||||||
| Fam. Catostomidae | |||||||||||||
| Catostomus commersonii | White sucker | FW | YS | LT (S) | G | 24.0–168.0 | - | D | 9–21 | 3.0–6.1 | 28.0–32.0 | [24] | |
| Chasmistes brevirostris | Shortnose sucker | FW | 35 dph | LT (S) | G | 96.0 | - | D | 20 | - | 31.7–32.0 | [25] | |
| Chasmistes liorus | June sucker | FW | 7 dph | LT + ILT (S) | G | 0.1–720.0 | abrupt | D | 16 | - | 21.0–33.0 | [26] | |
| Deltistes luxatus | Lost River sucker | FW | 35 dph | LT (S) | G | 96.0 | - | D | 20 | - | 31.5–32.0 | [25] | |
| Fam. Cyprinidae | |||||||||||||
| Agosia chrysogaster | Longfin dace | FW | 22 dph | CT (D) | I | - | 42 | LOE | 18–30 | - | 33.5–39.7 | [27] | |
| Labeo rohita | Rohu carp | FW | CT (D) | I | - | 18 | LOE | 26–36 | 12.0–14.4 | 42.3–45.6 | [28] | ||
| Pimephales promelas | Fathead minnow | FW | 3 dph | CT (D) | G | - | 18 | LOE | 22–23 | 3.4–9.9 | 31.4–35.9 | [29] | |
| Ord. Cyprinodontiformes | |||||||||||||
| Fam. Cyprinodontidae | |||||||||||||
| Cyprinodon nevadensis | Amargosa pupfish | FW | 60 dph | CT (D) | G | - | 18 | LOE | 20–36 | - | 38.0–44.0 | [30] | |
| Fam. Fundulidae | |||||||||||||
| Fundulus grandis | Gulf killifish | SW | <9 mm SL | CT (D) | I | - | 18 | LOE | 29 | - | 42.6–43.6 | [31] | |
| Fundulus heteroclitus | Mummichog | SW | <9 mm SL | CT (D) | I | - | 18 | LOE | 29 | - | 42.8–44.5 | [31] | |
| Ord. Esociformes | |||||||||||||
| Fam. Esocidae | |||||||||||||
| Esox lucius | Northern pike | FW | YS | LT (S) | G | 24.0–168.0 | abrupt change | D | 6–18 | - | 20.4–28.9 | [32] | |
| FW | YS | LT (S) | - | 240.0 | 3 | D | 3–24 | 4.2 | > 25.0 | [33] | |||
| Esox masquinongy | Muskellunge | FW | 1–53 dph | CT (D) | G | 1272.0 | 60 | S | 17–23 | - | 29.0–35.0 | [34] | |
| Ord. Gadiformes | |||||||||||||
| Fam. Gadidae | |||||||||||||
| Gadus morhua | Atlantic cod | SW | E to YS | LT (S) | G | 24.0–600.0 | - | D | 6 | - | >12 | [35] | |
| Ord. Mugiliformes | |||||||||||||
| Fam. Mugilidae | |||||||||||||
| Mugil cephalus | Hawaiian striped mullet | SW | YS | LT (S) | G | 168.0 | 2 | 3–33 | 14.2a | 30.1 | [36] | ||
| Ord. Osmeriformes | |||||||||||||
| Fam. Osmeridae | |||||||||||||
| Hypomesus transpacificus | Delta smelt | FW | 30–64 dph | CT (D) | I | - | 18 | LOE | 16 | - | 29.0–30.0 | [37] | |
| Mallotus villosus | Capelin | SW | 2–4 dph | LT (S) | G | 24.0 (+ 0.3) | - | D | 5 | -2.0–-3.0 | > 20.0 | [38] | |
| Osmerus mordax | Rainbow smelt | FW | - | LT (S) | G | 0.1–1.0 | - | D | 13 | - | 29.0–32.0 | [21] | |
| Ord. Perciformes | |||||||||||||
| Fam. Carangidae | |||||||||||||
| Atule mate | Yellowtail scad | SW | 0–6 dph | LT + ILT (S) | G | 0.5–72.0 | - | D | 24 | - | 26.0–37.0 | [39] | |
| Fam. Centrarchidae | |||||||||||||
| Micropterus salmoides | Largemouth bass | FW | 0–12 dph | LT (S) | G | 24.0 | - | D | 20–30 | - | 31.2–33.7 | [40] | |
| FW | YS, FL | LT (S) | G | 1.0–96.0 | 0–5 | D | 18–38 | - | 32.8–34.1 | [41] | |||
| Fam. Cichlidae | |||||||||||||
| Oreochromis mossambicus | Mozambique tilapia | FW | E to YS | LT (S) | G | 240.0 | - | D | 11–40 | 20.0 | > 34.0 | [42] | |
| Oreochromis niloticus | Nile tilapia | FW | YS | LT (S) | G | YS to swim-up larvae | - | D | 11–40 | 21.8 | 32.1 | [43] | |
| FW | 13–32 mm TL | CT (D) | - | 24.0–60.0 | 0.04 | D | 25–37 | - | 38.0–39.0 | [44] | |||
| Fam. Moronidae | |||||||||||||
| Morone chrysops | White bass | FW | 1 dph | LT (S) | G | 24.0 | - | D | 14–26 | - | 30.8–32.0 | [45] | |
| Morone saxatilis | Striped bass | FW | 3–14 mm TL | LT (S) | G | 24.0 | abrupt | D | 15–23 | - | 31.7–36.7 | [46] | |
| Fam. Percidae | |||||||||||||
| Etheostoma fonticola | Fountain darter | FW | 24–72 hph | LT (S) | G | 24.0 | - | D | 23 | 3.8 | - | [47] | |
| Perca flavescens | Yellow perch | FW | E to YS | LT (S) | - | YS to swim-up larvae | - | D | 12 | 9.3–9.8 | 18.8–22.5 | [48] | |
| FW | YS | LT (S) | - | 24.0 | - | D | 18 | 3.0 | 28.0 | [49] | |||
| Sander lucioperca | Pikeperch | FW | 4–6 mm | LT (S) | D | 6.0–6.5 | 30.0–32.0 | [50] in [49] | |||||
| Fam. Sciaenidae | |||||||||||||
| Bairdiella icistia | Bairdiella | SW | 2 mm | LT (S) | G | 1.0–72.0 | abrupt | D | 21–30 | - | 29.0–36.0 | [51] | |
| Fam. Scombridae | |||||||||||||
| Thunnus albacares | Yellowfin tunna | SW | YS | LT + ILT (S) | G | E to YS | 0.04 | M + D | 19–36 | 19.5 | 35.2 | [52] | |
| Fam. Sparidae | |||||||||||||
| Pagrus major | Red sea bream | SW | 0–42 dph | LT (S) | - | 24.0 | - | D | 20 | 9.5–12.0 | 26.5–30.5 | [53] | |
| Sparus aurata | Gilt-head bream | SW | 12 dph | CT (D) | G | - | 1 | SWI | 18 | - | 22.0–30.0 | [54] | |
| Ord. Petromyzontiformes | |||||||||||||
| Fam. Petromyzontidae | |||||||||||||
| Ichthyomyzon fossor | Northern brook lamprey | FW | AMM | LT (S) | - | 336.0 | - | D | 15 | - | 30.5 | [55] | |
| Lampetra lamottenii | Brook lamprey | FW | AMM | LT (S) | - | 336.0 | - | D | 15 | - | 28.5 | [55] | |
| Lampetra planeri | European brook lamprey | FW | AMM | LT (S) | - | 336.0 | - | D | 5–25 | - | 27.0–29.0 | [55] | |
| Petromyzon marinus | Sea lamprey | FW | AMM | LT (S) | - | 336.0 | - | D | 5–25 | - | 29.5–31.0 | [55] | |
| Ord. Pleuronectiformes | |||||||||||||
| Fam. Pleuronectidae | |||||||||||||
| Pleuronectes putnami | Smooth flounder | SW | - | LT (S) | G | 0.1–1.0 | - | D | 4 | - | 27.0–32.0 | [21] | |
| Pseudopleuronectes americanus | Winter flounder | SW | 5 dph | LT (S) | G | 0.1–1.0 (+ 24.0) | - | D | 5 | - | 29.0–33.0 | [56] | |
| Fam. Scophthalmidae | |||||||||||||
| Scophthalmus maximus | Turbot | SW | 0–25 dph | LT (S) | G | 2.0 (+ 60.0–84.0) | - | D | 17 | - | 22.0–29.0 | [57] | |
| Fam. Soleidae | |||||||||||||
| Solea solea | Dover sole | SW | 1–12 mg WW | LT (S) | G | 96.0 | - | D | 8–15 | 5.0–8.7 | 23.0–28.1 | [58] | |
| Ord. Salmoniformes | |||||||||||||
| Fam. Salmonidae | |||||||||||||
| Coregonus artedi | Cisco | FW | - | LT (S) | G | 24.0 | - | D | 3 | - | 19.8 | [59] | |
| Oncorhynchus clarkii virginalis | Cutthroat trout | FW | 7–14 dph | LT + ILT (S) | G | 720.0–1440.0 | 0.04 | D | 10–26 | - | 22.6–25.7 | [60] | |
| Oncorhynchus gilae apache | Apache trout | FW | E to YS | LT (S) | G | 336.0 | 0.25 | D | 15–27 | - | 17.1–17.9 | [61] | |
| Oncorhynchus kisutch | Coho salmon | FW | E to YS | LT (S) | G | > 1400.0 | - | D | 1–17 | - | 12.5 | [62] | |
|
Prosopium williamsoni |
Mountain whitefish |
FW | 30–300 mg WW | LT + ILT (S) | I | 0.1–792.0 | 0.04 | D | 10 | - | 22.6–23.6 | [63] | |
| FW | 870–3700 mg WW | CT (D) | I | - | 30 | LOE | 13 | - | 26.7 | [63] | |||
| Salmo salar | Atlantic salmon | FW | - | LT (S) | G | 120.0–168.0 | 1 | D | 5–6 | - | 22.0–28.0 | [64] | |
| Salmo trutta fario | Brown trout | FW | - | LT (S) | G | 120.0–168.0 | 1 | D | 5–6 | - | 23.0–28.0 | [64] | |
| Salmo trutta trutta | Sea trout | FW | - | LT (S) | G | 120.0–168.0 | 1 | D | 5–6 | - | 22.0–28.0 | [64] | |
| Salvelinus alpinus | Arctic charr | FW | 13–15 mm | LT + ILT (S) | G | 72.0 (+ 0.2–168.0) | 2 | D | 0–20 | - | 19.3–26.2 | [65] | |
| Ord. Scorpaeniformes | |||||||||||||
| Fam. Sebastidae | |||||||||||||
| Sebastes thompsoni | Rockfish | SW | - | LT (S) | - | 24.0 | - | D | 10–25 | 25.6–28.8 | [66] | ||
| Ord. Siluriformes | |||||||||||||
| Fam. Clariidae | |||||||||||||
| Clarias gariepinus | African sharp-tooth catfish | FW | E to YS | LT (S) | G | >193.0 | - | D | 17–36 | 18.9 | 33.2 | [67] | |
Abbreviations: FW, freshwater; SW, seawater; LT, lethal temperature; ILT, incipient lethal temperature; CT, critical temperature; S, static method; D, dynamic method; dph, days post-hatch; E, eggs; YS, yolk sac larvae; FL, feeding larvae; AMM, ammocoetes; TL, total length; SL, standard length; WW, wet weight; I, individuals; G, groups; D, death; LOE, loss of equilibrium; S, onset of spasms; M, malformations; SWI, swimming ceases.
Upper thermal limits in ectotherms have been estimated using either static or dynamic methods [14,68]. The former exposes groups of fish to different, constant temperatures (exposure time varies) to estimate the temperature at which 50% of the individuals in the group die which, depending on exposure time, has been referred to as the Upper Lethal Temperature (LT50max) [20,22] or Upper Incipient Lethal Temperature (UILT, sensu Fry, [69]) [39]. On the other hand, the dynamic method exposes individuals or groups of fish to a constant increase in temperature (starting at the ambient temperature) until physiological failure is noted (e.g. muscular spasms, loss of equilibrium, motor function stops) [14]. The Critical Thermal Maximum (CTmax) is estimated using the dynamic method and it is defined as “the thermal point at which locomotory activity becomes disorganized and the animal loses its ability to escape from conditions that will promptly lead to its death” [14, p.1562]. Since the 1980s, CTmax has been estimated more frequently than LT50max, which is likely due to the fact that the former is easier to apply, it requires fewer animals and takes less time compared to the latter [14]. Also, CTmax is considered to be more ecologically relevant than LT50max, as it sets the upper limit in thermal reaction norms (or performance curves), and uses (or can use) warming rates observed in situ [70,71]. Nevertheless, lethal (LT50min / LT50max) as opposed to critical (CTmin / CTmax) thermal limit protocols have been and continue to be used in most studies performed on larval fish [15,61], although the number of studies using the dynamic method has markedly increased since 2010 (Fig 1).
Small differences in the protocol used may have large impacts on CTmax [70,72,73], as occurs with other time-dependent tolerance measurements (e.g critical swimming speed, hypoxia tolerance) [74,75]. Warming rate is likely the most sensitive parameter in the CTmax protocol. Previous protocols have used warming rates between ~0.1°C min-1 to 0.1°C h-1 and the choice is not trivial: faster rates than those generally experienced by the organism in the wild (e.g. diurnal differences in temperature) may overestimate CTmax due to an impairment of physiological processes acting on the organism, whereas slower rates may underestimate CTmax due to longer exposures to warm temperatures and accumulation of heat damage [70,73,76]. Other studies have pointed out, however, that very slow warming rates may allow animals to acclimate to warmer temperatures, which would lead to an overestimation of CTmax [77]. Such slow heating rates (e.g. 1°C h-1, 1°C d-1, 2.5°C week-1) have been employed to explore the adaptive capacity of thermal tolerance such as work by Morley et al. [78] comparing invertebrates from different latitudes. Therefore, the choice of the CTmax protocol and the corresponding interpretation of the results and application to field conditions needs to be done with care.
Most studies of CTmax (or CTmin) in fish larvae have used a warming or cooling rate of 0.3°C min-1 [27,37,63], a rate recommended in protocols established for juvenile and adult fish by Becker and Genoway [13]. However, the impact of different warming rates on CTmax of marine fish larvae has never been assessed. Understanding the impact of methodology on the estimation of critical thermal limits is essential to develop methods that take into account the specific traits of early stages of fish (e.g. higher surface-to-volume ratio, handling sensitivity, greater sensitivity to starvation).
We investigated the upper thermal tolerance (CTmax) of larvae of Atlantic herring (Clupea harengus) and European seabass (Dicentrarchus labrax). We explored how CTmax was influenced by developmental stage, acclimation temperature as well as warming rate. To our knowledge, this is the first study to examine how methods used in protocols affect estimates of CTmax in fish larvae. Recommendations for developing protocols to estimate critical thermal maxima and minima in larval fish are also provided.
Materials and methods
Ethics
Experiments on Atlantic herring were performed under the German law on experimental animals and were approved by the Ethics Committee of the Hamburg Authority for Health and Consumer Protection (Application nr. 95/11). Those on European seabass were performed under French national regulations and approved by the Comité d’Éthique Finistérien en Expérimentation Animale (CEFEA- registering code C2EA–74) (Autorisation APAFIS 4341# 2016030214474531).
Herring larval rearing
Adult herring were obtained from a commercial fisherman in the Kiel Fjord (54.36°N, 10.13°E) in March 2014, and transported on ice to the Elbe Aquarium of the Institute of Hydrobiology and Fisheries Science, University of Hamburg. Eggs were strip-spawned from 21 females (mean (± SD) length, 24.7 (± 1.1) cm; mean (± SD) wet weight, 167.1 (± 30.2) g) and fertilized using the milt from 10 males (mean (± SD) length, 24.6 (± 1.0) cm; mean (± SD) wet weight, 168.1 (± 27.9) g). The large number of females and males helped avoid any parental effects on offspring quality. Egg and larval rearing conditions were similar to those described in Moyano et al. [79], except for the rearing temperatures. Briefly, dark green, circular 90-L tanks containing filtered seawater (0.5 μm, Reiser Filtertechnik GmbH, Seligenstadt am Main, Germany) renewed at 50% d-1 were used to incubate eggs and rear larvae. Temperature was measured every 10 minutes (TLog64-USB, Hygrosens, Donaueschingen, Germany) and salinity (WTW cond3110 probe, Weilheim, Germany) and ammonium (Tetra NH3/NH4+ kit, Spectrum Brands, VA, USA) were measured daily. The light regime was 14 L: 10 D. Eggs were incubated at a mean (± SD) temperature of 9.0 (± 0.4°C, and salinity of 16.2 (± 0.5). After hatching, ca. 1600 larvae were transferred to the new rearing tanks, and temperature was adjusted 0.5°C d-1 to a rearing temperature of either 7 or 13°C, with two replicate tanks (A and B) at each temperature. The final temperature was reached at a larval age of 9 days post-hatch (dph), after which the mean (± SD) temperature was 7.6 (± 0.4), 7.5 (± 0.4), 12.7 (± 0.2), and 12.8 (± 0.3) °C in tank 7A, 7B, 13A and 13B, respectively. The mean salinity of each tank was between 16.3 (± 0.4) and 16.6 (± 0.5). Larvae were reared in the presence of algae (Rhodomonas baltica, 10,000 cell mL-1) and dinoflagellates (Oxyrrhis marina, 1,000 cells mL-1) and fed natural prey (different stages of the copepod Acartia tonsa), supplemented with small amounts of brine shrimp (Artemia sp.) nauplii.
Each week, 20 larvae were taken out from each rearing tank to obtain length and weight estimates for growth rate calculations. They were anesthetized with metomidate (10 mg L-1, Aquacalm, Syndel Laboratories, BC, Canada), digitally photographed under a stereomicroscope (Leica MZ 16, Wetzlar, Germany), euthanized with an anesthetic overdose and stored at -80°C. Body length (measured as notochord length for preflexion larvae and standard length for flexion and postflexion larvae) was measured using ImageJ [80]. Finally, larvae were freeze-dried (0.200 mbar; >16 h, Christ Alpha 1–4 LSC, Osterode am Harz, Germany) and weighed (± 0.1 μg, Sartorius Genius SE2 microbalance, Göttingen, Germany).
Seabass larval rearing
Three-day old seabass larvae were obtained in January 2016 from Aquastream, a commercial hatchery in Ploemeur (France). These larvae were the progeny of wild spawners (Morbihan, France) including four females (mean weight 4.5 kg) and ten males (2.4 kg). Spawners were maintained at 13°C, with a light regime of 8.75 L: 15.25 D, and a water salinity of 35.
After larvae were transported to Ifremer-Centre de Bretagne, ca. 5000 were distributed to each of three grey, 35-L tanks located in a temperature-controlled room. Water temperature, salinity, pH and dissolved oxygen concentration were monitored daily (WTW Multi3410, Weilheim, Germany). Mean (± SD) temperature and salinity was 20 (± 0.1) °C and 33 (± 0.2), respectively. A light regime of 15 L: 9 D was used, and the light intensity gradually increased from 0 lux (3 dph) to 96 lux (44 dph). Larvae were fed brine shrimp (Artemia sp) nauplii ad libitum using automatic feeders.
Every 8–10 days, 20 larvae were collected from each tank, anesthetized with MS-222 (Tricaine Methane Sulfonate 1000 mg g-1, PHARMAQ, Hampshire, UK), photographed and stored at -80°C to measure body length and dry weight, using the same equipment and procedures as for herring larvae.
CTmax trials
A total of eight CTmax trials was conducted on herring larvae and two on seabass larvae (Table 2). Each CTmax trial used four thermal control units (Fisherbrand FBC 30, Fisher Scientific GmbH, Schwerte, Germany) which contained nine 250-mL beakers. One unit was used as a control and maintained at the starting temperature throughout the trial (max. 40 h for herring, 10 h for seabass). Warming rate treatments were randomly assigned to a thermal control unit in each trial. At the start of each trial, the water temperature and salinity and the light regime were the same as those experienced by larva in their rearing tank. Each beaker was aerated using a small pump (Tetra APS400, Spectrum Brands, VA, USA) with small bubbles produced using a fine glass pipette. Individual larvae were randomly collected from replicate rearing tanks at the test temperature and gently transferred to a beaker. Beakers were randomly assigned to a treatment group (warming rate). For seabass, 3 larvae were randomly collected from each of the three replicate tanks for each warming rate (9 individuals in total). After a 15-min acclimation period, the CTmax protocol was started. In total, five different warming rates were examined for herring (0.5, 1, 2, 4, and 8°C h-1) and seabass (1.5, 3, 6, 6 + 1.5, and 9°C h-1). The heating rate “6 + 1.5” for seabass consisted of a quick start (6°C h-1) up to a point close to the expected CTmax (i.e. ~ 26°C), followed by a slower heating rate (1.5°C h-1). Laboratory access regulations required that all work on seabass be completed in <9h. For all slow warming treatments (or controls) which lasted >14h (i.e. 0.5, 1°C h-1 in herring), constant light (24 L: 0 D) was used. No prey was added to the beakers at any point during a trial.
Table 2. Details of the Critical Thermal maxima (CTmax) trials conducted with Atlantic herring and European seabass larvae.
Note “age” refers to the days-post hatch at the start of the CTmax trial, and “size” is the mean larval size of all the larvae used in each CTmax trial.
| Species | Trial Nr. | Age (dph) | Age (dd) | Size (mm, mean ± SE) | Rearing T (°C) | Warming rate (°C h-1) | n* |
|---|---|---|---|---|---|---|---|
| Herring | 1 | 1 | 9 | 7.8 ± 0.1 | 9 | 0.5, 1, 2, 4, 8 | 9 |
| 2 | 20 | 140 | 10.3 ± 0.2 | 7 | 0.5, 1, 2, 4, 8 | 9 | |
| 3 | 45 | 327 | 14.4 ± 0.5 | 7 | 1, 2 | 9 | |
| 4 | 55 | 402 | 17.1 ± 0.3 | 7 | 0.5, 1, 2, 4, 8 | 9 | |
| 5 | 66 | 491 | 20.9 ± 0.3 | 7 | 1, 2 | 9 | |
| 6 | 14 | 130 | 12.8 ± 0.2 | 13 | 0.5, 1, 2, 4, 8 | 9 | |
| 7 | 34 | 387 | 21.2 ± 0.3 | 13 | 0.5, 1, 2, 4, 8 | 9 | |
| 8 | 43 | 499 | 21.2 ± 0.3 | 13 | 1, 2 | 9 | |
| Seabass | 9 | 15–16 | 283 | 6.5 ± 0.1 | 20 | 1.5, 3, 6, 6+1.5, 9 | 9 |
| 10 | 43–44 | 863 | 14.1 ± 0.2 | 20 | 1.5, 3, 6, 6+1.5, 9 | 9 |
Abbreviations: dph, days post-hatch; dd, degree-days; T, temperature; SE, standard error.
* n per trial (warming rate).
Once the warming protocol started, larvae were checked every 15 min (only every 30 min between the hrs of 23:00 and 06:00 for the 0.5 and 1°C h-1 rates used in herring), and the state of the larva and the temperature was recorded (P700, ±0.1°C, Dostmann electronic, Wertheim-Reicholzheim, Germany). The CTmax endpoint was considered to be the loss of equilibrium. Once a larva had lost its equilibrium, it was taken out of the beaker, anesthetized with metomidate (10 mg L-1), digitally photographed under a stereomicroscope, euthanized by an anesthetic overdose and stored at -80°C. Body length and dry weight were measured using methods previously described.
Statistical analysis
Herring specific growth rates (dry weight, % SGRDW) were calculated between 15–66 dph for rearing tanks 7A and 7B, and 14–45 dph for 13A and 13B. For seabass, SGRDW was calculated between the ages of 17 and 46 dph.
The effect of warming rate and body length on CTmax was assessed using generalized linear models [81]. Three different models were used, one for yolk-sac herring larvae, one for exogenously feeding herring larvae and one for exogenously feeding seabass larvae. GLMs included warming rate, body length and acclimation temperature (if present) as fixed effects for herring and seabass exogenously feeding larvae. In order to avoid heteroscedasticity, different variances were allowed depending on body length per warming rate treatment. In the case of herring yolk sac larvae, body length was not included as a fixed effect (all larvae were similar in size). A backward model selection procedure starting with the most complex (i.e. interactive effect between all fixed factors) and ending with all non-significant factors removed [82] helped identify which variables influenced CTmax. The residuals of each final model were plotted against all significant predictors to identify any remaining heteroscedasticity and check that no relationships between predictors and CTmax were ignored. In addition, the model residuals were also checked for a potential effect of rearing tank by analysis of variance (ANOVA).
Previously published estimates of LT50max for herring larvae were combined with the CTmax data collected in this study to explore the time dependency of upper thermal limits. The arithmetic mean value of CTmax for each treatment group in each trial was calculated and the upper thermal limits were plotted against the total time larvae were exposed to temperatures > 18°C. This temperature threshold was selected as a proxy for pejus temperature (Tpej) in the light of temperature-dependent growth rates of herring larvae [83].
All analyses were carried out using the R statistical software [84] with the nlme package [81].
Results
Herring larvae hatched 12 days after fertilization with a mean (± SE) body length of 7.9 (± 0.2) mm. The mean SGRDW after yolk sac absorption was 4.7, 3.5, 9.3 and 14.0% d-1 for larvae in tanks 7A, 7B, 13A and 13B, respectively.
Seabass larvae reared at 20°C had a mean SGRDW between 10.3 and 13.9% d-1 in the different rearing tanks.
CTmax in herring larvae
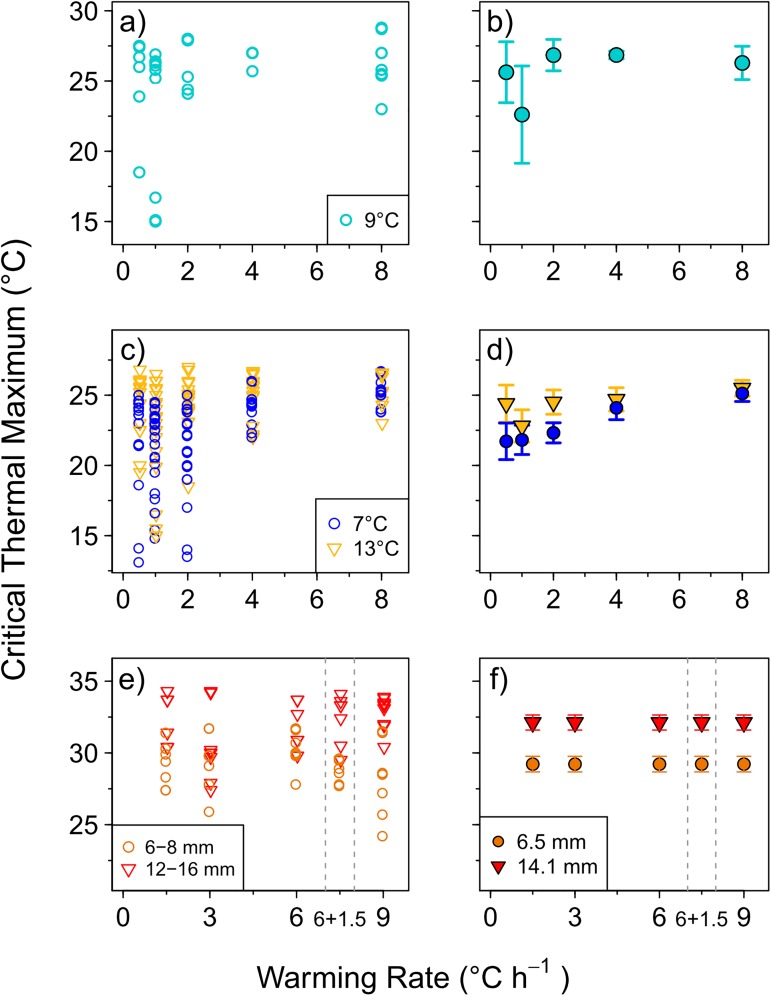
Larval survival in the controls was generally 89–100% (a maximum of 1 larva died during a trial) except in trial 2 (7°C, 140 degree-days) where survival was 67% (Table 2). The CTmax value varied markedly among individuals (range 13°C), especially among larvae experiencing slower warming rates (≤ 2°C h-1). In yolk sac larvae, CTmax ranged from 15.0 to 28.8°C and treatment means were between 22.6 and 26.8°C (Fig 2A). Warming rate had no significant effect on CTmax of yolk sac larvae (GLM, not shown). For exogenously feeding larvae, CTmax values ranged from 13.1 to 27.0°C (Fig 2C) and faster warming rates (4 and 8°C h-1) led to significantly higher CTmax values compared to slower rates (0.5, 1, and 2°C h-1) (p< 0.05) (Fig 2D, S1 Table). Overall, CTmax was significantly warmer for larvae reared at 13°C compared to 7°C (increase of 2.7 ± 0.9°C, S1 Table) but the differences in CTmax between acclimation temperatures were relatively minor (~0.7 and 0.4°C) for larvae in faster (4 and 8°C h-1) warming rate treatments. Body length had no significant effect on CTmax in exogenously feeding herring larvae (S1 Fig) nor did rearing tank (ANOVA, p>0.05).
Fig 2.
Critical thermal maxima (CTmax) estimates of Atlantic herring yolk sac larvae (a-b) and exogenously feeding larvae (c-d), and European seabass exogenously feeding larvae (e-f) at different warming rates. Left-hand panels show CTmax of individual larvae. Right-hand panels show the mean treatment values (± 95% CI) from Generalized Linear Model (see S1 Table), except for yolk sac larvae (panel b) in which mean (±95% CI) CTmax values are shown (as no model was fitted to this dataset).
CTmax in seabass larvae
The CTmax of individual larvae ranged from 24.2 to 34.3°C with treatment-specific means between 27.8 and 32.8°C (Fig 2E). Warming rate had no significant effect on CTmax, (p = 0.505) but body length had a highly significant effect (p < 0.01) (Fig 2F, S1 Table). Overall, larger larvae (14.1 mm mean size) had a significantly higher CTmax than smaller larvae (6.5 mm mean size). Rearing tank had no effect on CTmax (ANOVA, p>0.05).
Time dependency of thermal tolerance
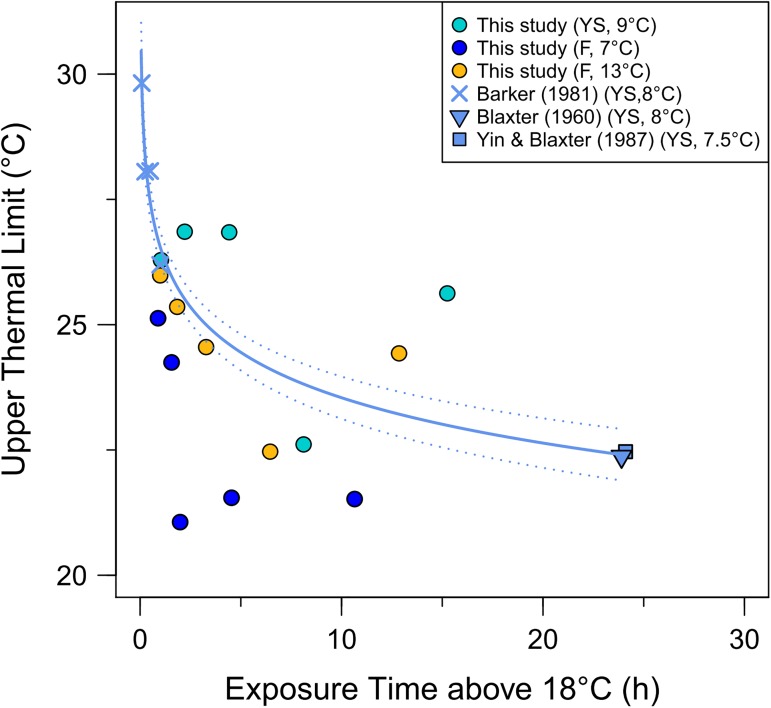
The data from this and previous studies on herring yolk sac larvae suggest that survival time decreases with increasing exposure time above Tpej (18°C) according to a negative, logistical model (Fig 3). When exposed to temperatures > Tpej for 30min, LT50max was ~28–30°C whereas LT50max declined to 22.5°C after a 24-h exposure period. Similarly, for exogenously feeding larvae reared at both 7 and 13°C, shorter exposure times (in faster warming rate treatments) were associated with higher mean CTmax (Fig 3). However, this time-dependency of CTmax was not evident for yolk sac larvae from this study.
Fig 3. Time dependency of upper thermal limits in Atlantic herring larvae.
Values for upper thermal limit (UTL, °C) including both LT50max and CTmax estimates (see text) versus exposure time (t, h) beyond temperatures favorable for growth (>18°C). The LTmax and CTmax estimates for yolk-sac (YS), and feeding larvae (F) at two temperatures (7 and 13°C) are also shown. For the LT50max data of YS larvae, the best fit regression equation (solid line) is UTL = 26.55(± 0.16 SE)—1.31(±0.08 SE) * Ln(t), (p<0.001), 95% CI of the curve are included as a dotted line.
Discussion
Obtaining robust estimates of thermal tolerance in ectotherms is fundamental if we hope to make projections of species performance under future climate scenarios. Early life stages of fish are expected to have a narrower thermal tolerance than juveniles and adults [9,12]. However, relatively few studies have been published on thermal limits of fish larvae, especially for marine species (Table 1). The lack of a standard methodology for larvae may be slowing our progress to compile data which can be compared across life stages and species, as has been done with other larval traits (e.g. critical swimming speed, [85]). Here we explored the impact of, arguably, the most important methodological factor (warming rate) on the upper thermal limit of the larvae of two temperate marine species. Using these results, together with a compilation from previous studies on thermal tolerance in fish larvae, we make recommendations for protocols to be used to estimate thermal limits in temperate marine larvae. Further tests in other groups (e.g. polar, tropical species) might enable “a universal protocol” to be developed for larval fish, which would facilitate intra- and interspecific comparisons.
Upper thermal limits in Atlantic herring and European seabass
The CTmax values measured here for yolk sac larvae of herring (treatment means 22–27°C) are in the range of the lethal temperatures previously estimated for this species and life stage [20–22]. The CTmax was slightly lower (treatment means 21–26°C) in exogenously feeding larvae, and a similar decline in LT50max was observed by Yin & Blaxter [20] when comparing larvae prior to and after yolk sac absorption. However, CTmax did not significantly differ across body sizes in exogenously feeding larvae. These CTmax values agree well with the life cycle scheduling of Atlantic herring in the southwest Baltic Sea, where herring start spawning in coastal areas in early spring after temperatures increase above 5°C. Waves of spawning occur producing larvae that inhabit coastal nurseries until early summer when temperatures are 15 to 20°C [86]. Then large larvae (> 20 mm) migrate to deeper, colder offshore waters which represent the feeding grounds of juveniles and adults. Although far below CTmax, chronic (long-term) exposure to temperatures above upper Tpej (~18°C) may impact growth performance and, ultimately, survival. It should also be noted that cold snaps (5 to 2.5°C) in early spring can lead to massive mortalities as reported for larval herring in the Vistula Lagoon in the southern Baltic Sea [87].
European seabass larvae could tolerate warmer temperatures (CTmax treatment means 28–33°C) than Atlantic herring larvae. In contrast to herring, thermal tolerance of seabass appeared to increase with increasing size and this may continue into the juvenile stage where CTmax values of ~ 28–35°C have been reported [88]. This increase in thermal tolerance with size/age matches the migratory life cycle for this species [89]. In the North Atlantic and Mediterranean Sea, spawning occurs offshore in winter (8.5–15°C for the southern North Sea, [90]). Post-larvae enter shallow, sheltered coastal estuaries or lagoons in spring, where they remain as juveniles during the warm summer period when they are likely exposed to warm snaps of >28°C [91].
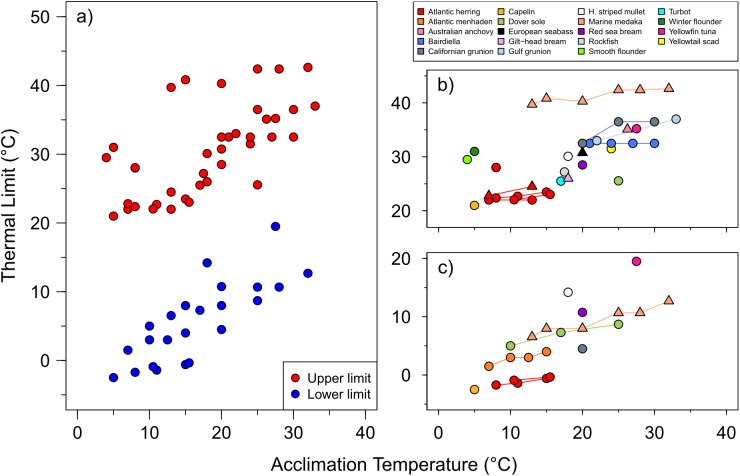
The CTmax of herring larvae is in the range of the CTmax or LT50max of larvae from other cold-temperate marine species such as capelin (Mallotus villosus) or turbot (Scophthalmus maximus) acclimated to similar temperatures (Fig 4). The CTmax values for seabass larvae are warmer and closer to LT50max estimates for red sea bream (Pagrus major) and Hawaiian striped mullet (Mugil cephalus). But these upper thermal limits for herring and seabass are much colder than, for example, those estimated for medaka (Oryzias melagstima) (LT50max > 40°C) reared at the same acclimation temperature. The available estimates of both the lower and upper thermal limits of a small number of species suggest that larvae of some species (e.g. herring or capelin) have a wider thermal tolerance than others (e.g. Dover sole Solea solea). Unfortunately, these comparisons need to be taken cum grano salis, as different methods were applied. In studies of lethal limits, a variety of exposure times (from minutes to weeks) and transfer rates to new temperatures (acute change or acclimation allowed) were used. In studies of CTmax, different rates of warming (or cooling) have been applied (Table 1). With the present data compilation and our measurements of the effect of warming rate on thermal limits, we hope to stimulate the community to create a standard protocol for early life stages of fish.
Fig 4. Upper and lower thermal limits of marine fish larvae.
a) Average upper (red) and lower thermal limits (blue) of marine fish larvae at different acclimation temperatures. b) Detail of the upper (LT50max, CTmax), and c) lower limits (LT50min, CTmin), color-coded by species and shape-coded by method (static, circles; dynamic, triangles). Lines connect estimates from the same study. Study details are provided in Table 1.
Impact of methodology on CTmax estimates
Although estimating CTmax is one of the most common methods used to assess upper thermal limits in ectotherms such as fish, surprisingly, no general consensus exists on the protocol. The same is true for protocols developed to measure other physiological traits such as critical swimming speed [74] and hypoxia tolerance [75]. Hence, the community is often faced with a broad range of values obtained with a large variety of methodologies. Thus, a disjunctive situation arises in which some employ a standard protocol (for example, to make intra- or interspecific comparisons), while others use protocols adjusted to a specific research objective or to a particular environmental event of condition being examined. The first CTmax protocols developed for juvenile and adult fish employed fast, arguably unrealistically fast, rates of temperature change, 18°C h-1 [13], 60°C h-1 [14]. On the other hand, more recent CTmax protocols have been tailored to the research question addressed, e.g. tolerance to heat waves in enclosed bays (1°C h-1, [92]), long-term adaptation of heat tolerance in relation to global warming using much slower “ecologically relevant” rates (from 1°C d-1 to 1°C month-1, [93]) or CTmax as a measurement of fish health in challenge tests (7 + 0.5°C h-1, [94]). Recently published CTmax studies on fish larvae have used warming rates ≥ 0.3°C min-1 (18°C h-1) [31,37] (Table 1), regardless of whether the aim was to estimate upper thermal tolerance for aquaculture or biogeographical distribution. Surprisingly, the present study is the first (to the best knowledge) to try to reconcile the different options available (e.g. standardization vs tailoring) by examining the impact of warming rate on CTmax in larval fish. Our results show clear, species-specific effects of warming rate on larval CTmax as suggested for adult fish and other taxa (e.g. Crustacea) [72,95,96].
In this study, Atlantic herring exposed to warming rates ≤ 2°C h-1 had lower CTmax values than those exposed to faster rates, likely due to oxygen limitation and accumulation of anaerobic end products [97]. At the slowest warming rates, thus, no acclimation potential was observed, suggesting that even slower rates would be needed to explore thermal acclimation (e.g. 1°C d-1) [78]. Within the slow warming rate treatments applied here, CTmax was much more variable among individuals, especially for 7°C-reared larvae, probably due to the relatively long exposure time to warmer temperatures (i.e. using a warming rate of 1°C h-1, reaching a CTmax of 27°C takes 20h from 7°C, and 14h from 13°C). Interestingly, patterns of inter-individual variation suggest that slower warming rates may help one better distinguish larvae with different traits or condition compared to faster warming rates. The pattern of change in CTmax versus warming rate observed here agrees well with the typical trends observed in other ectotherms [70,73,76]. Additionally, no effect of rearing tank, and thus growth rate, was observed. In the case of herring yolk sac larvae, warming rate had no effect on CTmax. This discrepancy in the trends observed for yolk sac larvae and for later larval stages may be related to the low number of yolk sac larvae tested (i.e. only one trial was conducted, n = 9 per warming rate), and the high inter-individual variability in CTmax observed at the slow warming rates. Given the variation observed here, a minimum sampling size of 15–20 individuals per trial and/or warming rate would be recommended. Moreover, it is also more challenging to assess the loss of equilibrium in very small, slow-moving larvae, thus, some authors have used cessation of swimming as a CTmax endpoint [54].
In seabass larvae, warming rate had no effect on CTmax for a given larval size. This pattern could be due to some degree of adaptation potential in heat tolerance at the slowest warming rates, as was suggested for other ectothermic species [76]. Alternatively, it could be due to the relatively narrow range of warming rates tested (1.5 to 9.0°C day-1), since, in other species, CTmax was only significantly reduced at warming rates <1°C h-1 [96 and references herein]. Additionally, no significant differences were found (in the mean or variance in CTmax) between constant warming rates (e.g. 6°C h-1) and variable rates that combine a fast start (6°C h-1) with a slower rate afterwards (1.5°C h-1). This latter method has been used for adult fish, and is thought to better resolve potential inter-individual differences in CTmax [98]. Combining fast and slow heating rates appears to be a good method to use because it decreases the duration of CTmax trials but still makes it practical to ascertain the exact endpoint temperature.
Estimating thermal tolerance in early life stages of fish presents unique challenges, e.g. larvae can have high growth rates (ca. 10–30% d-1) and have, in general, a low resistance to starvation. Long trials using very slow warming rates (e.g. 1°C d-1) could yield CTmax estimates, which may be difficult to interpret as larvae pass through different developmental stages (leading to an “integrated CTmax”), or may be biased by other time-dependent processes such as starvation [20,99]. If one aims to identify differences across developmental stages, we argue that a measurement of an “instantaneous CTmax”, estimated over one day, is most appropriate. For example, estimates from such rapid methods can be compared across life stages to identify bottlenecks in the persistence of populations in warmer waters in the future (e.g. [11,37]). Since one cannot predict, a priori, how the warming rate will impact on the CTmax of the larvae of different fish species using one standard heating rate across species is likely unwarranted [95,96]. In this context, we recommend that different heating rates (e.g. from 1 to 10°C h-1) be tested in pre-trials and that one chooses a rate associated with the highest CTmax, as has been proposed for adult fish [96]. For other contexts, such as using CTmax as an indicator of fish health against several stressors, one could choose a rate that is more practical (logistically) for the study and compare the estimates across treatments.
Several studies have highlighted the relationship between CTmax and the geographic range of a species and/or population [9,100], and the importance of quantifying the difference between CTmax and habitat temperature in order to explore the likelihood that this thermal buffer is exceeded during warm episodes [101,102]. In the light of this research, one could argue that the generation of a standard protocol for estimating what we have termed the “instantaneous CTmax” would allow intra- and interspecific comparisons and parameterization of numerical, physiological models exploring climate impacts [7,9]. Such standard protocols have been successfully applied for decades for other physiological traits, such as critical swimming speed [74,103] or hypoxia tolerance [75]. Additional research is needed on the CTmax of larvae and adults of more stenothermal (e.g. tropical or high latitude) species, which live much closer to their CTmax, before developing any “universal CTmax protocol”. Moreover, the most suitable endpoint for these trials (e.g. loss of equilibrium, spasms, cessation of swimming) also needs to be carefully considered, especially for larvae. Finally, we wish to emphasize the importance of combining estimates of CTmax with measurements of thermal limits of other physiological processes (e.g. growth, metabolism) to account for acclimation potential and short- versus long-term thermal sensitivity to build a full picture of the thermal tolerance and performance curves of a species.
Impact of acclimation temperature on CTmax
Thermal acclimation is a type of phenotypic plasticity that occurs in many ectotherms [100]. This acclimation can be reversible (e.g. in response to diel or seasonal changes) or irreversible (in response to temperatures experienced during ontogeny). The plasticity of thermal tolerance due to acclimation has been examined by rearing at different, constant temperatures as well as at fluctuating (daily in situ) temperatures [104,105]. At either constant or fluctuating temperatures, and when experiencing increased temperature for either a short or long period of time (developmental temperature or heat pulses), exposure to warm temperatures subsequently increases tolerance to warmer temperatures (i.e. higher CTmax). Hence, the increase in CTmax observed here for herring larvae reared (after the exogenous feeding stage) at 13°C compared to 7°C is not unexpected. Young herring larvae and eggs were reared at the same temperature to avoid any potential carry-over effects [106]. Therefore, one could suggest that the acclimation observed here is reversible (if larvae experienced prolonged, colder temperatures). However, this remains to be tested. In future studies, it will also be important to assess not only acclimation mechanisms [102] but also adaptive capacity since rapid increases in warming tolerance have been reported to occur within as little time as one generation in some species [107,108], although other reviews on ectotherms argue that plasticity in thermal tolerance is limited [109].
Time dependency of thermal limits
Upper thermal limits of any species are largely time dependent [93,110]. One well-accepted concept to explain this time-limitation of thermal limits is the Oxygen- and Capacity-Limited Thermal Tolerance [110]. Beyond Tpej, species start to experience the adverse effects of oxidative and thermal stress at the molecular level, which activates a suite of protective mechanisms (e.g. antioxidant and heat-shock responses, anaerobic metabolism). But these protective mechanisms are time-limited. Therefore, temperature tolerance is higher for shorter exposures and vice versa. A curvilinear relationship is expected between upper thermal limits and exposure time above Tpej [9], and this was observed in the data compiled on studies of the lethal limits (LT50max) of yolk sac larvae of Atlantic herring. The time-dependency was also evident for CTmax estimates of exogenously feeding herring larvae collected in the present study. It is unclear why CTmax estimates of yolk sac larvae in this study are similar to LT50max values reported in other studies. Given the protocols and endpoints, one would expect the former to be higher than the latter. It could be that the temperatures of both endpoints are very similar or that it is simply difficult to make precise assessment of the loss of equilibrium in small larvae. Considering the present compilation, it is clear that much study is still needed on the time-limitation of thermal limits for different ontogenetic stages of fish if we wish to assess the impact of extreme events (e.g. heat waves) on populations.
Conclusions
In the present study, we contribute to the growing body of literature on thermal limits of marine fish early life stages by examining the CTmax of Atlantic herring and European seabass and testing the effect of warming rate, a critical parameter of the dynamic method (i.e. ramping assay). In agreement with differences in the field distribution of these species, the larvae of herring had a lower CTmax (13.1–27.0– °C) than seabass (24.2–34.3°C). Importantly, warming rate had a species-specific impact on CTmax, suggesting that future work on other species should first conduct pre-trials and then choose warming rates relevant for the context of the study. From a practical standpoint, the time dependency of survival at different, suboptimal cold or warm temperatures requires much additional study in order to understand the impact of extreme events (e.g. cold snaps, heat waves) on populations. The ultimate goal would be to compare this basic information on thermal limits (gained from a relatively rapid assay) with thermal performance curves for different traits (e.g. growth, swimming, feeding) which are more time consuming to obtain; clear relationships between longer-term thermal performance and short-term limits would improve confidence in making meaningful intra- (life stage-) and inter-specific comparisons of thermal sensitivity. It will be important to examine how each physiological trait is impacted by additional, interacting factors (e.g. pCO2, hypoxia). This information can be integrated within physiology-based models to make more robust (mechanistic) projections of how climate change will impact on the suitability of aquatic (marine and freshwater) habitats [111].
Supporting information
The y-axis represents the residuals from the Generalized Linear Model (GLM) presented in Fig 1. No significant effect of body size on CTmax was observed (see text). Symbols are shape-coded by acclimation temperature (circles, 7°C, triangles, 13°C).
(TIF)
(DOCX)
Acknowledgments
The authors are indebted to Martina Wichmann, Johanna Thoms and Katrin Engler for help rearing the Atlantic herring larvae, and to Louise Cominassi, Sarah Howald and Patrick Quazuguel for rearing the seabass larvae. Thanks also to Björn Illing for helping with the literature compilation.
Data Availability
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.t5453.
Funding Statement
This work was partially funded by the FITNESS project (Deutsche Forschungemeinschaft, http://www.dfg.de/en/, PE 1157/8-1). This work also received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 678193 (CERES, Climate Change and European Aquatic Resources, contribution nr. 6), and contributes to the ERANet program CLIMAR "Climate-driven changes in the Habitat Suitability of Marine Organisms" (BMBF DLR 01DN17019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fry FEJ. Effect of the environment on animal activity. Univ Tor Stud Biol Ser. 1947;55: 1–62. [Google Scholar]
- 2.Andrewartha HG, Birch LC. The distribution and abundance of animals. Chicago, IL: University of Chicago Press; 1954. [Google Scholar]
- 3.Burrows MT, Schoeman DS, Buckley LB, Moore P, Poloczanska ES, Brander KM, et al. The pace of shifting climate in marine and terrestrial ecosystems. Science. 2011;334: 652–655. doi: 10.1126/science.1210288 [DOI] [PubMed] [Google Scholar]
- 4.Poloczanska ES, Burrows MT, Brown CJ, García Molinos J, Halpern BS, Hoegh-Guldberg O, et al. Responses of marine organisms to climate change across oceans. Front Mar Sci. 2016;3 doi: 10.3389/fmars.2016.00062 [Google Scholar]
- 5.Enzor LA, Zippay ML, Place SP. High latitude fish in a high CO2 world: Synergistic effects of elevated temperature and carbon dioxide on the metabolic rates of Antarctic notothenioids. Comp Biochem Physiol A Mol Integr Physiol. 2013;164: 154–161. doi: 10.1016/j.cbpa.2012.07.016 [DOI] [PubMed] [Google Scholar]
- 6.Rummer JL, Couturier CS, Stecyk JAW, Gardiner NM, Kinch JP, Nilsson GE, et al. Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob Change Biol. 2014;20: 1055–1066. doi: 10.1111/gcb.12455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenigstein S, Mark FC, Gößling-Reisemann S, Reuter H, Poertner H- O. Modelling climate change impacts on marine fish populations: process-based integration of ocean warming, acidification and other environmental drivers. Fish Fish. 2016;17: 972–1004. doi: 10.1111/faf.12155 [Google Scholar]
- 8.Lefevre S. Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO 2 and their interaction. Conserv Physiol. 2016;4: cow009 doi: 10.1093/conphys/cow009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pörtner HO, Peck MA. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol. 2010;77: 1745–1779. doi: 10.1111/j.1095-8649.2010.02783.x [DOI] [PubMed] [Google Scholar]
- 10.Pörtner HO, Farrell AP. Ecology. Physiology and climate change. Science. 2008;322: 690–2. doi: 10.1126/science.1163156 [DOI] [PubMed] [Google Scholar]
- 11.Drost HE, Fisher J, Randall F, Kent D, Carmack EC, Farrell AP. Upper thermal limits of the hearts of Arctic cod Boreogadus saida: adults compared with larvae. J Fish Biol. 2015;88: 718–726. doi: 10.1111/jfb.12807 [DOI] [PubMed] [Google Scholar]
- 12.Rijnsdorp AD, Peck MA, Engelhard GH, Mollmann C, Pinnegar JK. Resolving the effect of climate change on fish populations. ICES J Mar Sci. 2009;66: 1570–1583. doi: 10.1093/icesjms/fsp056 [Google Scholar]
- 13.Becker CD, Genoway RG. Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ Biol Fishes. 1979;4: 245–256. doi: 10.1007/BF00005481 [Google Scholar]
- 14.Lutterschmidt WI, Hutchison VH. The critical thermal maximum: history and critique. Can J Zool. 1997;75: 1561–1574. doi: 10.1139/z97-783 [Google Scholar]
- 15.Miller IR, Kappenman KM, Talbott MJ. Upper lethal temperature of larval pallid sturgeon Scaphirhynchus albus (Forbes and Richardson). J Appl Ichthyol. 2016;32: 272–276. doi: 10.1111/jai.12994 [Google Scholar]
- 16.Reynolds WW, Thomson DA, Casterlin ME. Temperature and salinity tolerances of larval Californian grunion, Leuresthes tenuis (Ayres): A comparison with gulf grunion, L. sardina (Jenkins & Evermann). J Exp Mar Biol Ecol. 1976;24: 73–82. doi: 10.1016/0022-0981(76)90044-7 [Google Scholar]
- 17.Li AJ, Leung PTY, Bao VWW, Lui GCS, Leung KMY. Temperature-dependent physiological and biochemical responses of the marine medaka Oryzias melastigma with consideration of both low and high thermal extremes. J Therm Biol. 2015;54: 98–105. doi: 10.1016/j.jtherbio.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 18.Kellogg RL. Temperature requirements for the survival and early development of the anadromous alewife. Progress Fish-Cult. 1982;44: 63–73. doi: 10.1577/1548-8659(1982)44[63:TRFTSA]2.0.CO;2 [Google Scholar]
- 19.Lewis RM. The effect of minimum temperature on the survival of larval Atlantic menhaden, Brevoortia tyrannus. Trans Am Fish Soc. 1965;94: 409–412. doi: 10.1577/1548-8659(1965)94[409:TEOMTO]2.0.CO;2 [Google Scholar]
- 20.Yin MC, Blaxter JHS. Temperature, salinity tolerance, and buoyancy during early development and starvation of Clyde and North Sea herring, cod, and flounder larvae. J Exp Mar Biol Ecol. 1987;107: 279–290. [Google Scholar]
- 21.Barker SL. Mortalities of Atlantic herring, Clupea harengus, smooth flounder, Liopsetta putnami, and rainbow smelt, Osmerus mordax, larvae exposed to acute thermal shock. Fish Bull. 1981;79: 198–200. [Google Scholar]
- 22.Blaxter JHS. The effect of extremes of temperature on herring larvae. J Mar Biol Assoc U K. 1960;39: 605 doi: 10.1017/S0025315400013576 [Google Scholar]
- 23.Powles PM. Survival of Australian anchovy (Engraulis australis) eggs and larvae in a heat trap In: Blaxter JHS, editor. The Early Life History of Fish. Berlin, Heidelberg: Springer Berlin Heidelberg; 1974. pp. 373–381. Available: http://www.springerlink.com/index/10.1007/978-3-642-65852-5_31 [Google Scholar]
- 24.McCormick JH, Jones BR, Hokanson KEF. White sucker (Catostomus commersoni) embryo development, and early growth and survival at different temperatures. J Fish Res Board Can. 1977;34: 1019–1025. doi: 10.1139/f77-154 [Google Scholar]
- 25.Saiki M., Monda D., Bellerud B. Lethal levels of selected water quality variables to larval and juvenile Lost River and shortnose suckers. Environ Pollut. 1999;105: 37–44. doi: 10.1016/S0269-7491(98)00212-7 [Google Scholar]
- 26.Kappenman KM, Fraser WC, Toner M, Dean J, Webb MAH. Effect of temperature on growth, condition, and survival of juvenile shovelnose sturgeon. Trans Am Fish Soc. 2009;138: 927–937. doi: 10.1577/T07-265.1 [Google Scholar]
- 27.Troia MJ, Whitney JE, Gido KB. Thermal performance of larval longfin dace (Agosia chrysogaster), with implications for climate change. Environ Biol Fishes. 2015;98: 395–404. doi: 10.1007/s10641-014-0270-7 [Google Scholar]
- 28.Das T, Pal AK, Chakraborty SK, Manush SM, Sahu NP, Mukherjee SC. Thermal tolerance, growth and oxygen consumption of Labeo rohita fry (Hamilton, 1822) acclimated to four temperatures. J Therm Biol. 2005;30: 378–383. doi: 10.1016/j.jtherbio.2005.03.001 [Google Scholar]
- 29.Heath S, Bennett WA, Kennedy J, Beitinger TL. Heat and cold tolerance of the fathead minnow, Pimephales promelas, exposed to the synthetic pyrethroid cyfluthrin. Can J Fish Aquat Sci. 1994;51: 437–440. doi: 10.1139/f94-045 [Google Scholar]
- 30.Shrode JB. Developmental temperature tolerance of a Death Valley pupfish (Cyprinodon nevadensis). Physiol Zool. 1975;48: 378–389. [Google Scholar]
- 31.Galleher SN, Gilg MR, Smith KJ. Comparison of larval thermal maxima between Fundulus heteroclitus and F. grandis. Fish Physiol Biochem. 2010;36: 731–740. doi: 10.1007/s10695-009-9347-1 [DOI] [PubMed] [Google Scholar]
- 32.Hokanson KEF, Mccormick JH, Jones BR. Temperature requirements for embryos and larvae of the northern pike, Esox lucius (Linnaeus). Trans Am Fish Soc. 1973;102: 89–100. doi: 10.1577/1548-8659(1973)102<89:TRFEAL>2.0.CO;2 [Google Scholar]
- 33.Hassler TJ. Effect of temperature on survival of northern pike embryos and yolk-sac larvae. Progress Fish-Cult. 1982;44: 174–178. doi: 10.1577/1548-8659(1982)44[174:EOTOSO]2.0.CO;2 [Google Scholar]
- 34.Bonin JD, Spotila JR. Temperature tolerance of larval muskellunge (Esox masquinongy mitchill) and F1 hybrids reared under hatchery conditions. Comp Biochem Physiol A Physiol. 1978;59: 245–248. doi: 10.1016/0300-9629(78)90154-8 [Google Scholar]
- 35.Iversen SA, Danielssen DS. Development and mortality of cod (Gadus morhua L.) eggs and larvae in different temperatures. Flodevigen Rapp. 1984;1: 49–65. [Google Scholar]
- 36.Sylvester JR, Nash CE. Thermal tolerance of eggs and larvae of Hawaiian striped mullet, Mugil cephalus L. Trans Am Fish Soc. 1975;104: 144–147. doi: 10.1577/1548-8659(1975)104<144:TTOEAL>2.0.CO;2 [Google Scholar]
- 37.Komoroske LM, Connon RE, Lindberg J, Cheng BS, Castillo G, Hasenbein M, et al. Ontogeny influences sensitivity to climate change stressors in an endangered fish. Conserv Physiol. 2014;2: cou008–cou008. doi: 10.1093/conphys/cou008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davenport J, Stene A. Freezing resistance, temperature and salinity tolerance in eggs, larvae and adults of capelin, Mallotus villosus, from Balsfjord. J Mar Biol Assoc U K. 1986;66: 145 doi: 10.1017/S0025315400039710 [Google Scholar]
- 39.Santerre MT. Effects of the temperature and salinity on the eggs and early larvae of Caranx mate (Cuv. & Valenc.) (Pisces: Carangidae) in Hawaii. J Exp Mar Biol Ecol. 1976;21: 51–68. [Google Scholar]
- 40.McCormick JH, Wegner JA. Responses of largemouth bass from different latitudes to elevated water temperatures. Trans Am Fish Soc. 1981;110: 417–429. doi: 10.1577/1548-8659(1981)110<417:ROLBFD>2.0.CO;2 [Google Scholar]
- 41.Storms KG, Foltz JW, Wilde EW. Upper thermal tolerance of early fish stages of South Carolina and Florida largemouth bass. Proc Annu Conf Southeast Assoc Fish Wildl Agencies. 1986;40: 57–64. [Google Scholar]
- 42.Subasinghe RP, Sommerville C. Effects of temperature on hatchability, development and growth of eggs and yolksac fry of Oreochromis mossambicus (Peters) under artificial incubation. Aquac Res. 1992;23: 31–39. doi: 10.1111/j.1365-2109.1992.tb00593.x [Google Scholar]
- 43.Rana KJ. Influence of incubation temperature on Oreochromis niloticus (L.) eggs and fry. Aquaculture. 1990;87: 165–181. doi: 10.1016/0044-8486(90)90273-P [Google Scholar]
- 44.Bishai HM. Resistance of Tilapia nilotica L. to high temperatures. Hydrobiologia. 1965;25: 473–488. doi: 10.1007/BF00838508 [Google Scholar]
- 45.McCormick JH. Effects of temperature on hatching success and survival of larvae in the white bass. Progress Fish-Cult. 1978;40: 133–137. doi: 10.1577/1548-8659(1978)40[133:EOTOHS]2.0.CO;2 [Google Scholar]
- 46.Kellogg RL, Ligotino RJ, Jinks SM. Thermal mortality prediction equations for entrainable striped Bass. Trans Am Fish Soc. 1984;113: 794–802. doi: 10.1577/1548-8659(1984)113<794:TMPEFE>2.0.CO;2 [Google Scholar]
- 47.Bonner TH, Brandt TM, Fries JN, Whiteside BG. Effects of temperature on egg production and early life stages of the fountain darter. Trans Am Fish Soc. 1998;127: 971–978. doi: 10.1577/1548-8659(1998)127<0971:EOTOEP>2.0.CO;2 [Google Scholar]
- 48.Hokanson KEF, Kleiner CF. Effects of constant and rising temperatures on survival and developmental rates of embryonic and larval yellow perch, Perca flavescens (Mitchill) In: Blaxter JHS, editor. The Early Life History of Fish. Berlin, Heidelberg: Springer Berlin Heidelberg; 1974. pp. 437–448. Available: http://www.springerlink.com/index/10.1007/978-3-642-65852-5_36 [Google Scholar]
- 49.Hokanson KEF. Temperature requirements of some Percids and adaptations to the seasonal temperature cycle. J Fish Res Board Can. 1977;34: 1524–1550. doi: 10.1139/f77-217 [Google Scholar]
- 50.Zhdanova NN. Lethal temperature limit, and depths required for the artificial rearing of young pike-perch. Tr Azov Nauch Issled Inst Rybn Khoz. 1966;8: 79–88. [Google Scholar]
- 51.May RC. Effects of acclimation on the temperature and salinity tolerance of the yolk-sac larvae of Bardiella icistia (Pisces: Sciaenidae). Fish Bull. 1975;73: 249–255. [Google Scholar]
- 52.Wexler JB, Margulies D, Scholey VP. Temperature and dissolved oxygen requirements for survival of yellowfin tuna, Thunnus albacares, larvae. J Exp Mar Biol Ecol. 2011;404: 63–72. doi: 10.1016/j.jembe.2011.05.002 [Google Scholar]
- 53.Ishibashi Y, Ozawa M, Hirata H, Kumai H. Ontogenic changes in various stress tolerances of larval and juvenile red sea bream Pagrus major. Nippon Suisan Gakkaishi. 2003;69: 36–43. doi: 10.2331/suisan.69.36 [Google Scholar]
- 54.Madeira D, Costa PM, Vinagre C, Diniz MS. When warming hits harder: survival, cellular stress and thermal limits of Sparus aurata larvae under global change. Mar Biol. 2016;163: 91 doi: 10.1007/s00227-016-2856-4 [Google Scholar]
- 55.Potter IC, Beamish FWH. Lethal temperatures in ammocoetes of four species of lampreys. Acta Zool. 1975;56: 85–91. doi: 10.1111/j.1463-6395.1975.tb00084.x [Google Scholar]
- 56.Itzkowitz N, Schubel JR. Tolerance of five-day-old winter flounder, Pseudopleuronectes americanus, larvae to thermal shock. Fish Bull. 1983;81: 913–916. [Google Scholar]
- 57.Guan J, Zheng Y, Liu H, Lei J, Zhang Q, Guan S. Study on the thermal tolerance of embryos and larvae of turbot Scophthalmus maximus. Prog Fish Sci. 2012;1: 34–39. [Google Scholar]
- 58.Irvin DN. Temperature tolerance of early developmental stages of Dover sole, Solea solea (L.) In: Blaxter JHS, editor. The Early Life History of Fish. Berlin, Heidelberg: Springer Berlin Heidelberg; 1974. pp. 449–463. Available: http://www.springerlink.com/index/10.1007/978-3-642-65852-5_37 [Google Scholar]
- 59.McCormick JH, Jones BR, Syrett RF. Temperature requirements for growth and survival of larval Ciscos (Coregonus artedii). J Fish Res Board Can. 1971;28: 924–927. doi: 10.1139/f71-134 [Google Scholar]
- 60.Zeigler MP, Brinkman SF, Caldwell CA, Todd AS, Recsetar MS, Bonar SA. Upper thermal tolerances of Rio Grande cutthroat trout under constant and fluctuating temperatures. Trans Am Fish Soc. 2013;142: 1395–1405. doi: 10.1080/00028487.2013.811104 [Google Scholar]
- 61.Recsetar MS, Bonar SA. Survival of apache trout eggs and alevins under static and fluctuating temperature regimes. Trans Am Fish Soc. 2013;142: 373–379. doi: 10.1080/00028487.2012.741551 [Google Scholar]
- 62.Tang J, Bryant MD, Brannon EL. Effect of temperature extremes on the mortality and development rates of coho salmon embryos and alevins. Progress Fish-Cult. 1987;49: 167–174. doi: 10.1577/1548-8640(1987)49<167:EOTEOT>2.0.CO;2 [Google Scholar]
- 63.Brinkman SF, Crockett HJ, Rogers KB. Upper thermal tolerance of mountain whitefish eggs and fry. Trans Am Fish Soc. 2013;142: 824–831. doi: 10.1080/00028487.2013.765503 [Google Scholar]
- 64.Bishai HM. The effect of water currents on the survival and distribution of fish larvae. J Cons Int Explor Mer. 1960;25: 134–146. [Google Scholar]
- 65.Elliott J, Klemetsen A. The upper critical thermal limits for alevins of Arctic charr from a Norwegian lake north of the Arctic circle. J Fish Biol. 2002;60: 1338–1341. doi: 10.1006/jfbi.2002.1934 [Google Scholar]
- 66.Ouchi K. Temperature tolerance of young rockfish, Sebastes thompsoni (Jordan et Hubbs). Bull Jap Sea Reg Res Lab. 1977;28: 1–8. [Google Scholar]
- 67.Prokešová M, Drozd B, Kouřil J, Stejskal V, Matoušek J. Effect of water temperature on early life history of African sharp-tooth catfish, Clarias gariepinus (Burchell, 1822). J Appl Ichthyol. 2015;31: 18–29. doi: 10.1111/jai.12849 [Google Scholar]
- 68.Terblanche JS, Deere JA, Clusella-Trullas S, Janion C, Chown SL. Critical thermal limits depend on methodological context. Proc R Soc B Biol Sci. 2007;274: 2935–2943. doi: 10.1098/rspb.2007.0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fry FEJ, Hart JS, Walker KF. Lethal temperature relations for a sample of young speckled trout. University of Toronto Studies, Biological Series; 1946. pp. 9–35. [Google Scholar]
- 70.Terblanche JS, Hoffmann AA, Mitchell KA, Rako L, le Roux PC, Chown SL. Ecologically relevant measures of tolerance to potentially lethal temperatures. J Exp Biol. 2011;214: 3713–3725. doi: 10.1242/jeb.061283 [DOI] [PubMed] [Google Scholar]
- 71.Mora C, Ospina AF. Tolerance to high temperatures and potential impact of sea warming on reef fishes of Gorgona Island (tropical eastern Pacific). Mar Biol. 2001;139: 765–769. doi: 10.1007/s002270100626 [Google Scholar]
- 72.Vinagre C, Leal I, Mendonça V, Flores AAV. Effect of warming rate on the critical thermal maxima of crabs, shrimp and fish. J Therm Biol. 2015;47: 19–25. doi: 10.1016/j.jtherbio.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 73.Overgaard J, Kristensen TN, Sørensen JG. Validity of thermal ramping assays used to assess thermal tolerance in Arthropods. Dornhaus A, editor. PLoS ONE. 2012;7: e32758 doi: 10.1371/journal.pone.0032758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farrell AP. Comparisons of swimming performance in rainbow trout using constant acceleration and critical swimming speed tests. J Fish Biol. 2008;72: 693–710. doi: 10.1111/j.1095-8649.2007.01759.x [Google Scholar]
- 75.Claireaux G, Chabot D. Responses by fishes to environmental hypoxia: integration through Fry’s concept of aerobic metabolic scope: hypoxia and fry’s paradigm of aerobic scope. J Fish Biol. 2016;88: 232–251. doi: 10.1111/jfb.12833 [DOI] [PubMed] [Google Scholar]
- 76.Chown SL, Jumbam KR, Sørensen JG, Terblanche JS. Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct Ecol. 2009;23: 133–140. doi: 10.1111/j.1365-2435.2008.01481.x [Google Scholar]
- 77.Elliott JM, Elliott JA. The effect of the rate of temperature increase on the critical thermal maximum for parr of Atlantic salmon and brown trout. J Fish Biol. 1995;47: 917–919. doi: 10.1111/j.1095-8649.1995.tb06014.x [Google Scholar]
- 78.Morley SA, Bates AE, Lamare M, Richard J, Nguyen KD, Brown J, et al. Rates of warming and the global sensitivity of shallow water marine invertebrates to elevated temperature. J Mar Biol Assoc U K. 2016;96: 159–165. doi: 10.1017/S0025315414000307 [Google Scholar]
- 79.Moyano M, Illing B, Peschutter P, Huebert KB, Peck MA. Thermal impacts on the growth, development and ontogeny of critical swimming speed in Atlantic herring larvae. Comp Biochem Physiol A Mol Integr Physiol. 2016;197: 23–34. doi: 10.1016/j.cbpa.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 80.Rasband WS. ImageJ [Internet]. U. S. National Institutes of Health, Bethesda, Maryland, USA; 2014. Available: http://imagej.nih.gov/ij/
- 81.Pinheiro J, Bates D, DebRoy S, Sarkar D, R-Development-Core-Team. Nlme: Linear and Nonlinear Mixed Effects Models. 2013.
- 82.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010;1: 3–14. doi: 10.1111/j.2041-210X.2009.00001.x [Google Scholar]
- 83.Hufnagl M, Peck MA. Physiological individual-based modelling of larval Atlantic herring (Clupea harengus) foraging and growth: insights on climate-driven life-history scheduling. ICES J Mar Sci. 2011;68: 1170–1188. doi: 10.1093/icesjms/fsr078 [Google Scholar]
- 84.R Core Development Team. R: a language and environment for statistical computing, 3.2.1 [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2013. Available: http://www.r-project.org/
- 85.Leis JM, Caselle JE, Bradbury IR, Kristiansen T, Llopiz JK, Miller MJ, et al. Does fish larval dispersal differ between high and low latitudes? Proc R Soc B Biol Sci. 2013;280: 20130327–20130327. doi: 10.1098/rspb.2013.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oeberst R, Dickey-Collas M, Nash RDM. Mean daily growth of herring larvae in relation to temperature over a range of 5–20°C, based on weekly repeated cruises in the Greifswalder Bodden. ICES J Mar Sci. 2009;66: 1696–1701. doi: 10.1093/icesjms/fsp193 [Google Scholar]
- 87.Fey DP. Differences in temperature conditions and somatic growth rate of larval and early juvenile spring-spawned herring from the Vistula Lagoon, Baltic Sea manifested in the otolith to fish size relationship. J Fish Biol. 2001;58: 1257–1273. doi: 10.1111/j.1095-8649.2001.tb02284.x [Google Scholar]
- 88.Claireaux G, Théron M, Prineau M, Dussauze M, Merlin F- X, Le Floch S. Effects of oil exposure and dispersant use upon environmental adaptation performance and fitness in the European sea bass, Dicentrarchus labrax. Aquat Toxicol. 2013;130–131: 160–170. doi: 10.1016/j.aquatox.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 89.Sanchez-Vazquez FJ, Muñoz-Cueto JA, editors. Biology of European seabass. Boca Ratón, FL: CRC Press; 2015. [Google Scholar]
- 90.Thompson BM, Harrop RT. The distribution and abundance of bass (Dicentrarchus labrax) eggs and larvae in the English Channel and southern North Sea. J Mar Biol Assoc UK. 1987;67: 263–274. [Google Scholar]
- 91.Vinagre C, Madeira D, Narciso L, Cabral H, Diniz M. Impact of climate change on coastal versus estuarine nursery areas: cellular and whole-animal indicators in juvenile seabass Dicentrarchus labrax. Mar Ecol Prog Ser. 2012;464: 237–243. doi: 10.3354/meps09885 [Google Scholar]
- 92.Madeira D, Narciso L, Cabral HN, Vinagre C. Thermal tolerance and potential impacts of climate change on coastal and estuarine organisms. J Sea Res. 2012;70: 32–41. doi: 10.1016/j.seares.2012.03.002 [Google Scholar]
- 93.Peck LS, Clark MS, Morley SA, Massey A, Rossetti H. Animal temperature limits and ecological relevance: effects of size, activity and rates of change. Funct Ecol. 2009;23: 248–256. doi: 10.1111/j.1365-2435.2008.01537.x [Google Scholar]
- 94.Mauduit F, Domenici P, Farrell AP, Lacroix C, Le Floch S, Lemaire P, et al. Assessing chronic fish health: An application to a case of an acute exposure to chemically treated crude oil. Aquat Toxicol. 2016;178: 197–208. doi: 10.1016/j.aquatox.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 95.Ribeiro PL, Camacho A, Navas CA. Considerations for assessing maximum critical temperatures in small ectothermic animals: insights from leaf-cutting ants. Dornhaus A, editor. PLoS ONE. 2012;7: e32083 doi: 10.1371/journal.pone.0032083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mora C, Maya MF. Effect of the rate of temperature increase of the dynamic method on the heat tolerance of fishes. J Therm Biol. 2006;31: 337–341. doi: 10.1016/j.jtherbio.2006.01.005 [Google Scholar]
- 97.Pörtner H. Integrating climate-related stressor effects on marine organisms: unifying principles linking molecule to ecosystem-level changes. Mar Ecol Prog Ser. 2012;470: 273–290. doi: 10.3354/meps10123 [Google Scholar]
- 98.Roze T, Christen F, Amerand A, Claireaux G. Trade-off between thermal sensitivity, hypoxia tolerance and growth in fish. J Therm Biol. 2013;38: 98–106. doi: 10.1016/j.jtherbio.2012.12.001 [Google Scholar]
- 99.Meyer S, Caldarone EM, Chícharo MA, Clemmesen C, Faria AM, Faulk C, et al. On the edge of death: Rates of decline and lower thresholds of biochemical condition in food-deprived fish larvae and juveniles. J Mar Syst. 2012;93: 11–24. doi: 10.1016/j.jmarsys.2011.09.010 [Google Scholar]
- 100.Angilletta MJ. Thermal adaptation: A theoretical and empirical synthesis. Oxford: Oxford University Press; 2009. [Google Scholar]
- 101.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci. 2008;105: 6668–6672. doi: 10.1073/pnas.0709472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sandblom E, Clark TD, Gräns A, Ekström A, Brijs J, Sundström LF, et al. Physiological constraints to climate warming in fish follow principles of plastic floors and concrete ceilings. Nat Commun. 2016;7: 11447 doi: 10.1038/ncomms11447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brett JR. The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can. 1964;21: 1183–1226. doi: 10.1139/f64-103 [Google Scholar]
- 104.Fischer K, Kölzow N, Höltje H, Karl I. Assay conditions in laboratory experiments: is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia. 2011;166: 23–33. doi: 10.1007/s00442-011-1917-0 [DOI] [PubMed] [Google Scholar]
- 105.Kingsolver JG, MacLean HJ, Goddin SB, Augustine KE. Plasticity of upper thermal limits to acute and chronic temperature variation in Manduca sexta larvae. J Exp Biol. 2016;219: 1290–1294. doi: 10.1242/jeb.138321 [DOI] [PubMed] [Google Scholar]
- 106.Scott GR, Johnston IA. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc Natl Acad Sci. 2012;109: 14247–14252. doi: 10.1073/pnas.1205012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Donelson JM, Munday PL, McCormick MI, Pitcher CR. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat Clim Change. 2012;2: 30–32. doi: 10.1038/nclimate1323 [Google Scholar]
- 108.Salinas S, Munch SB. Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol Lett. 2012;15: 159–163. doi: 10.1111/j.1461-0248.2011.01721.x [DOI] [PubMed] [Google Scholar]
- 109.Gunderson AR, Stillman JH. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc R Soc B Biol Sci. 2015;282: 20150401–20150401. doi: 10.1098/rspb.2015.0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pörtner HO. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol. 2010;213: 881–893. doi: 10.1242/jeb.037523 [DOI] [PubMed] [Google Scholar]
- 111.Jørgensen C, Peck MA, Antognarelli F, Azzurro E, Burrows MT, Cheung WWL, et al. Conservation physiology of marine fishes: advancing the predictive capacity of models. Biol Lett. 2012;8: 900–3. doi: 10.1098/rsbl.2012.0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The y-axis represents the residuals from the Generalized Linear Model (GLM) presented in Fig 1. No significant effect of body size on CTmax was observed (see text). Symbols are shape-coded by acclimation temperature (circles, 7°C, triangles, 13°C).
(TIF)
(DOCX)
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.t5453.