Abstract
Candida albicans is the most common fungus in the human intestinal microbiota but not in mice. To make a murine sepsis model more closely resemble human sepsis and to explore the role of intestinal C. albicans, in the absence of candidemia, in bacterial sepsis, live- or heat-killed C. albicans was orally administered to mice at 3h prior to cecal ligation and puncture (CLP). A higher mortality rate of CLP was demonstrated with Candida-administration (live- or heat-killed) prior to CLP. Fecal Candida presented only in experiments with live-Candida administration. Despite the absence of candidemia, serum (1→3)-β-D-glucan (BG) was higher in CLP with Candida-administration than CLP-controls (normal saline administration) at 6h and/or 18h post-CLP. Interestingly, fluconazole attenuated the fecal Candida burden and improved survival in mice with live-Candida administration, but not CLP-control. Microbiota analysis revealed increased Bacteroides spp. and reduced Lactobacillus spp. in feces after Candida administration. Additionally, synergy in the elicitation of cytokine production from bone marrow-derived macrophages, in vitro, was demonstrated by co-exposure to heat-killed E. coli and BG. In conclusion, intestinal abundance of fungi and/or fungal-molecules was associated with increased bacterial sepsis-severity, perhaps through enhanced cytokine elicitation induced by synergistic responses to molecules from gut-derived bacteria and fungi. Conversely, reducing intestinal fungal burdens decreased serum BG and attenuated sepsis in our model.
Introduction
Sepsis is a syndrome of imbalance of host pro- and anti-inflammatory responses to pathogens [1, 2]. Sepsis is a critically important worldwide health-care problem and is the most common cause of death among patients in the intensive care unit [3, 4]. Pathogen associated molecular patterns (PAMPs) derived from gastrointestinal (GI) microorganisms are capable of immune activation and gut-translocation of viable bacteria or bacterial molecules leads to vigorous systemic inflammation [5]. Indeed, gut permeability barrier defects are observed in sepsis [6, 7]. While the significance of gut-translocation of bacterial molecules is appreciated [8], the impact of fungal molecules in bacterial sepsis is unknown.
(1→3)-β-D-glucan (BG) are a major component of the cell wall in most fungi and are released during fungal-growth and the tissue invasion process [9, 10]. BG are bioactive and activate immune responses through several receptors [11, 12]. We have demonstrated that in bacterial sepsis, higher serum BG, from gut-translocation, in the absence of fungemia, is associated with greater sepsis severity [7]. However, the role of intestinal fungi in bacterial sepsis in the absence of fungemia is not well studied. In order to address the role of fungi, we assessed the effect of oral administration of C. albicans in a murine bacterial sepsis model. Because C. albicans is the predominant fungal species in human intestine but not in mouse [13], a murine sepsis model with orally-administered C. albicans might more closely resemble human sepsis. We recently demonstrated that oral administration of C. albicans with mixed-oral antibiotics 5 days prior to cecal ligation and puncture (CLP) enhances the severity of bacterial sepsis in the murine sepsis model [14]. However, oral antibiotics, alone, impact fecal microbiota and sepsis severity in the Candida colonization model [14]. Hence, the influence of C. albicans in bacterial sepsis might be demonstrated more accurately without antibiotic administration.
Accordingly, the importance of intestinal fungi, without fungemia, in bacterial sepsis was investigated using a murine cecal ligation and puncture (CLP) sepsis model with C. albicans administered orally at 3h, but not 5 days, prior to the surgery without oral antibiotics administration.
Materials and methods
Candida albicans preparation
Fluconazole-sensitive Candida albicans ATCC 90028 (Fisher Scientific, Waltham, MA, USA; minimal inhibitory concentration: 0.25–1 μg/ml) was used. C. albicans were cultured over-night on Sabouraud dextrose broth (SDB) (Thermo Scientific, Hampshire, UK) and counted in a hemocytometer (Bright-Line, Denver, CO, USA) before use. Heat-killed C. albicans were prepared by immersion in a water-bath at 60°C for 1h.
Animals and animal models
The US National Institutes of Health (NIH) animal care and use protocol (#85–23, revised 1985) was followed. Male, ICR mice at 8-week-old (National Laboratory Animal Center, Nakhornpathom, Thailand) were used. The animal protocols were approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
Cecal ligation and puncture at 3h after oral-administration of Candida albicans (CLP with immediate Candida administration model)
Live- C. albicans oral administration at 1x106 CFU, with cecal ligation and puncture (CLP) surgery, induced positive fecal fungi without fungemia, within 24h after administration. Oral Candida at higher doses, with CLP, induced positive fungal growth from both feces and blood. Live- or heat-killed C. albicans at 1x106 CFU was administered at 3h prior to cecal ligation and puncture (CLP) surgery to characterize the potential role of (1→3)-β-D-glucan (BG) in bacterial sepsis. CLP procedures were slightly modified from the previously published [7]. Briefly, the cecum was ligated at 10 mm from the cecal tip and punctured twice with a 21-gauge needle. The operation was performed through an abdominal incision under isoflurane anesthesia. Fentanyl at 0.03 mg/kg in 0.5 ml of normal saline solution (NSS) was administered subcutaneously for an analgesia and fluid replacement post-operatively and at 6h. Antibiotic (imipenem/cilastatin) 14 mg/kg in 0.3 ml of NSS was administered subcutaneously at 6h post-surgery. Fluconazole (Sigma-Aldrich, St. Louis, MO, USA) at 10 mg/kg in 0.5 ml of NSS or NSS alone was administered orally, pre-operatively (0.5h), and post-operatively at 3h and 6h to reduce fecal Candida. High dose fluconazole was needed to significantly reduce fecal Candida [15]. In the sham operation, cecum was only identified through the abdominal incision then sutured.
Mouse sample analysis
Blood collection was performed at the indicated time-points by tail vein nicking and at sacrifice with cardiac puncture under isoflurane anesthesia. For blood bacterial quantitative analysis, blood (25 μl) was spread directly onto blood agar plates (Oxoid, Hampshire, UK), incubated at 37°C and bacterial colonies were enumerated at 24-48h. Serum was separated by centrifugation and kept at -80°C until analysis. The QuantiChrom Creatinine Assay kit (DICT-500; Bioassay, Hayward, CA, USA) and EnzyChrom Alanine Transaminase assay (EALT-100, BioAssay) were used for serum creatinine (Scr) and alanine transaminase (ALT) measurement, respectively. Serum cytokines (TNF-α, IL-6 and IL-10) were measured with ELISA (ReproTech, NJ, USA). BG was analyzed with Fungitell® (Associates of Cape Cod, Inc., East Falmouth, MA). BG values at <7.8 and >523.4 pg/ml (beyond the lower and upper range of the standard curve) were recorded as 0 and 523 pg/ml, respectively. All assays were performed according to the manufacturer’s protocol. In addition, at 18h post-CLP, internal organs; liver, spleen, and mesenteric lymph nodes were weighed, homogenized and plated onto blood agar plates and processed as previously mentioned.
Culture of fungi
Individual mice were placed in an empty cage prior to CLP for fecal collection (0h time-point of CLP). At 6h and 18h post-CLP, mice were sacrificed and feces from descending colon and/or rectum were collected. Feces and phosphate buffer solution (PBS) in a ratio of 1μg/ 1μl were well-mixed before plating directly onto Sabouraud dextrose agar (SDA) with 0.1% chloramphenicol (Thermo Scientific). The plates were incubated at 35°C, for 72h, before fungal colony enumeration. In addition, at 18h post-CLP, internal organs; liver, spleen, and mesenteric lymph nodes were weighed, homogenized and plated onto SDA with 0.1% chloramphenicol and processed as previously mentioned.
Fecal microbiome
Microbiota analysis, as previously reported [16], was used to explore the alteration of fecal microbiota in each manipulation. Briefly, feces from individual mice (0.25g) were processed for metagenomic DNA extractions. 3 independent extractions were performed per sample. Total nucleic acid was extracted by power DNA Isolation Kit (MoBio, Carlsbad, CA, USA). Agarose gel electrophoresis and nanodrop spectrophotometry were used to assess metagenomic DNA quality. Universal prokaryotic 515F (forward; (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (reverse; 5′-GGACTACHVGGGTWTCTAAT-3′), with appended 5′ Illumina adapter and 3′ Golay barcode sequences, were used for 16S rRNA gene V4 library construction [16]. Each 25-μl PCR reaction comprised 1× EmeraldAmp® GT PCR Master Mix (TaKaRa), 0.2 μM of each primer, and 75 ng of the metagenomic DNA. Independently triplicate polymerase chain reactions (PCRs) were performed and pooled to prevent stochastic PCR bias. The 16S rDNA amplicons of 381 basepairs (bp) were purified from agarose gel, using the GenepHlow™ Gel Extraction Kit (Geneaid Biotech Ltd., New Taipei City, Taiwan), and quantified by Picogreen (Invitrogen, Eugene, Oregon, USA). Each sample (240 ng) was pooled for Miseq300 platform sequencing (Illumina, San Diego, CA, USA), by the sequencing primers and index sequence described [17].
Mothur’s standard quality screening operating procedures for MiSeq platform was used for the quality screening of the raw sequences [18] then aligned and assigned taxon (operational taxonomic unit, OTU) based on a default parameter [18]. Samples were normalized to an equal sampling depth (N = 118121 reads per sample) followed Mothur’s computations method [18].
Bone marrow derived macrophage preparation
A previous published method of preparing bone marrow (BM) derived-macrophages was used [19]. In brief, femur BM cells were incubated for 7 days in supplemented Dulbecco's Modified Eagle Medium (DMEM) with 20% conditioned medium of the L929 cell line, as a source of macrophage-colony stimulating factor (M-CSF), in a humidified 5% CO2 incubator at 37°C. Cells were harvested with cold PBS. Anti-F4/80 and anti-CD11c antibody staining (BioLegend, San Diego, CA, USA) by flow cytometry were used for characterization of the macrophage phenotype.
Induction of macrophage cytokine production
The preparation of heat-killed E. coli (Escherichia coli ATCC 25922; ATCC, Manassas, VA, USA) was used as representative of bacterial molecules. Bacteria (3.3x107 cells/ ml) were killed by heating at 65°C for 15 minutes followed by sonication with a High Intensity Ultrasonic Processor (VC/VCX 130, 500,750) at 25 percent amplitude until a clear solution was obtained. The lysate was centrifuged at 10,000 rpm for 5 minutes, and the supernatant was decanted and filtered with 0.22 μm filters (Millex®, Tullagreen, Carrigtwohill, Ireland). For a representative BG, CM-Pachyman, (Megazyme, Bray, Ireland) was used. [20]. Pachyman at different doses, with or without E. coli preparations, were incubated with macrophages (1x105 cells/well) in culture plates. The total volume was adjusted by PBS addition to 200 μl/well. Supernatant was collected at specific time-points and cytokines were measured by ELISA assay (ReproTech).
Statistical analysis
Data was presented as mean ±standard error (SE) and the differences between groups were examined for statistical significance by one-way analysis of variance (ANOVA) followed by Tukey’s analysis or the Mann-Whitney U test for the comparisons of multiple or 2 groups, respectively. Survival analysis was performed by log-rank test. The repeated measures analysis of variance (ANOVA) with Bonferroni post hoc analysis was used for the analysis of the time-course experiments. All statistical analyses were performed with SPSS 11.5 software (SPSS, IL, USA). In addition, the area under the curve (AUC) of cytokine response was calculated with Graphpad Prism4.0 software and compared between the mouse strains with one-way ANOVA followed by Tukey’s analysis (SPSS). A P value < 0.05 was considered to be statistically significant.
Results
Oral administration of live- or heat-killed- Candida albicans before cecal ligation and puncture increased serum (1→3)-β-D-glucan and worsened sepsis severity
Without Candida spp. administration, fecal fungi were undetectable, with, or without, CLP (data not shown), as observed previously [13, 21, 22]. Oral administration of live Candida at 104, 106 and 1010 CFU/mouse alone, without CLP, did not cause candidemia. However, a low level of candidemia (70±34 CFU/ml; data from 8 mice) was demonstrated at 18h post-CLP with Candida gavage at 1010 CFU/mouse. Subsequently, live- or heat-killed- Candida at 106 CFU, an optimal Candida dose which did not induce candidemia, was selected for the further studies.
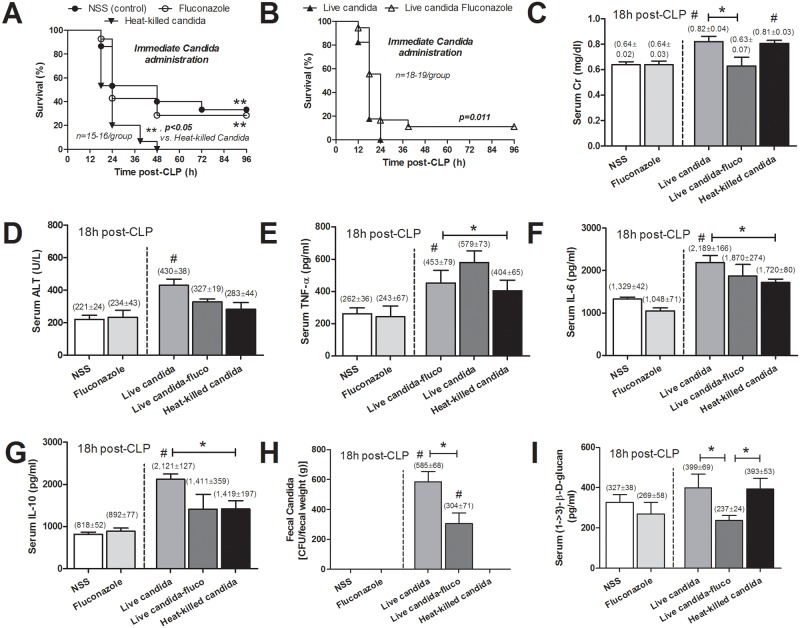
Live- or heat-killed C. albicans was orally administered at 3h before CLP to evaluate the effect of live- fungi or fungal molecules upon bacterial sepsis. The administration of either live- or heat-killed C. albicans increased sepsis mortality rate to 100% mortality within 48h after surgery in both models; compared with 67% in CLP with NSS control (CLP-control) (Fig 1A and 1B). In addition, enhanced sepsis severity was also demonstrated by Scr (kidney injury), ALT (liver injury) and cytokines (TNF-α, IL-6 and IL-10) at 18h post-CLP (Fig 1C–1G). All of these parameters, in CLP with live-Candida administration (CLP-live-Candida), were higher than CLP-control. In addition, fecal Candida was detectable only in CLP after live Candida administration and fecal Candida burdens were higher in CLP-live-Candida without fluconazole (Fig 1H). On the other hand, serum BG was higher in CLP-live-Candida with fluconazole compare to CLP with live- or heat-killed Candida (Fig 1I). This implied the enhanced serum BG after an oral BG administration.
Fig 1.
Survival analysis of mice with cecal ligation and puncture (CLP) with normal saline (NSS), NSS with fluconazole and heat-killed Candida oral administration (A) and with live-Candida with and without fluconazole (B) at the time of operation is shown. Organ injury, as demonstrated by serum creatinine (Scr) for kidney injury (C), alanine transaminase (ALT) for liver injury (D), serum cytokines (TNF-α, IL-6 and IL-10) (E-G), fecal Candida burdens (H) and serum (1→3)-β-D-glucan (BG) (I) was analyzed.(n = 6-7/group); #, p < 0.05 vs. NSS; *, p < 0.05
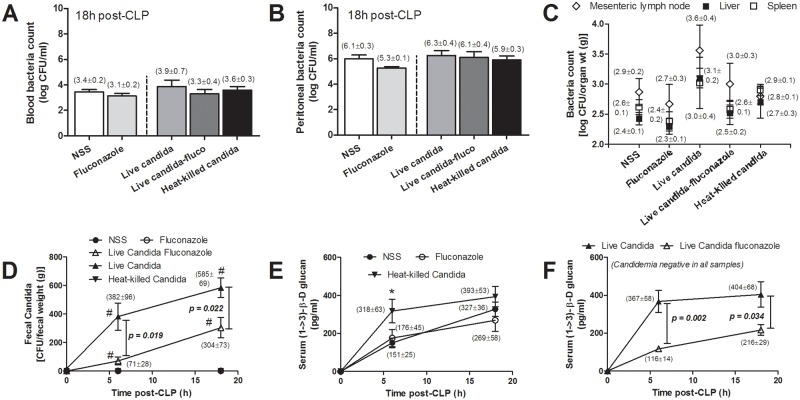
Moreover, Scr but not serum BG, ALT and cytokines in CLP with heat killed-Candida was higher than CLP-control (Fig 1C–1G). Serum cytokines, but not Scr and ALT, in CLP-live-Candida were higher than CLP with heat-killed-Candida. On the other hand, quantitative analysis of blood bacterial count, peritoneal bacterial count and bacterial count from the internal organs at 18h post-CLP were positive for mixed bacterial colonies but the levels were not different among groups (Fig 2A–2C).
Fig 2.
Blood bacterial count (A), peritoneal bacterial count (B) and bacterial count from internal organs (C) are shown. (n = 7/ group). The time-course of fecal Candida burdens (D) and serum (1→3)-β-D-glucan (BG) in different treatment groups (E, F) is described. [n (at 0h) = 10-12/group and n (at 6h and 18h) = 5-6/group]. #, p < 0.05 vs. 0h in each group; *, p < 0.05 vs. NSS
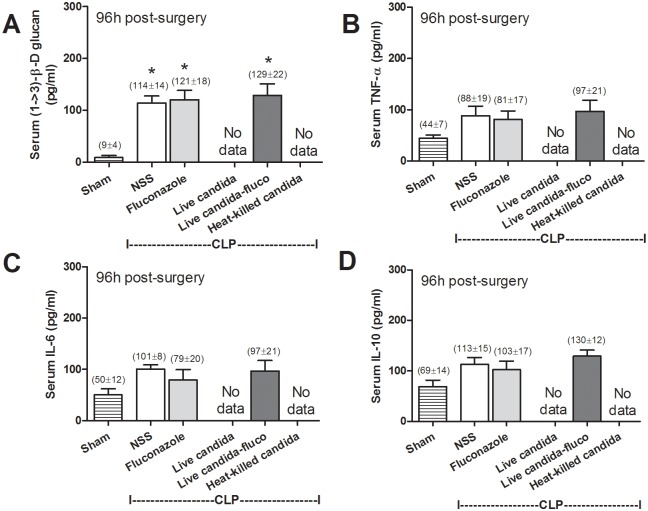
Oral live-Candida administration converted fecal Candida culture from negative to positive in a time dependent manner after CLP despite the absence of candidemia (Fig 2D). In comparison with CLP-control, serum BG was higher in CLP with heat-killed Candida administration at 6h but not at 18h after CLP (Fig 2E). And serum BG was higher in CLP-live-Candida at 6h and 18h in comparison with CLP-control (Fig 2F). At 6h, serum BG of CLP-control, heat-killed- and live- Candida were 150±25, 318±63 and 367±58 pg/ml, respectively; p<0.05 by one-way ANOVA. Of note, fungal culture was negative in blood, peritoneal fluid and the internal organs (data not shown). Interestingly, fluconazole reduced intestinal Candida abundance (Fig 2D) and attenuated sepsis severity in CLP-live-Candida (Fig 1B). Survival in CLP with live-Candida, CLP-control and CLP-fluconazole were 2/18 (11%), 5/15 (33%) and 4/14 (29%), respectively (Fig 1A). In parallel, fluconazole in CLP-live-Candida attenuated fecal Candida, serum BG, mortality rate and Scr, but not ALT and serum cytokines (Fig 2D–2F, Fig 1). In contrast, fluconazole was non-effective for sepsis attenuation in CLP-control group (Figs 1 and 2). It is interesting to note that both serum BG and serum cytokine levels were lower in the survivors at 96h post-CLP in comparison with 18h-post CLP. However, while the serum cytokines were normalized, serum BG level was still higher than sham control (Fig 3).
Fig 3. Serum (1→3)-β-D-glucan (BG) (A) and serum cytokines (TNF-α, IL-6 and IL-10) (B-D) in the mice at 96h post-sham surgery (n = 5) and mice survived at 96h post-CLP administered with normal saline (NSS; n = 5), fluconazole (n = 4) and live-Candida with fluconazole (n = 2) are shown (survival rate of these groups were demonstrated in Fig 1A and 1B).
*, p < 0.05 vs. sham; No data, non survivor in this group.
Oral administration of either live- or heat-killed-Candida albicans before cecal ligation and puncture increased several intestinal anaerobic bacteria but not aerobic bacteria
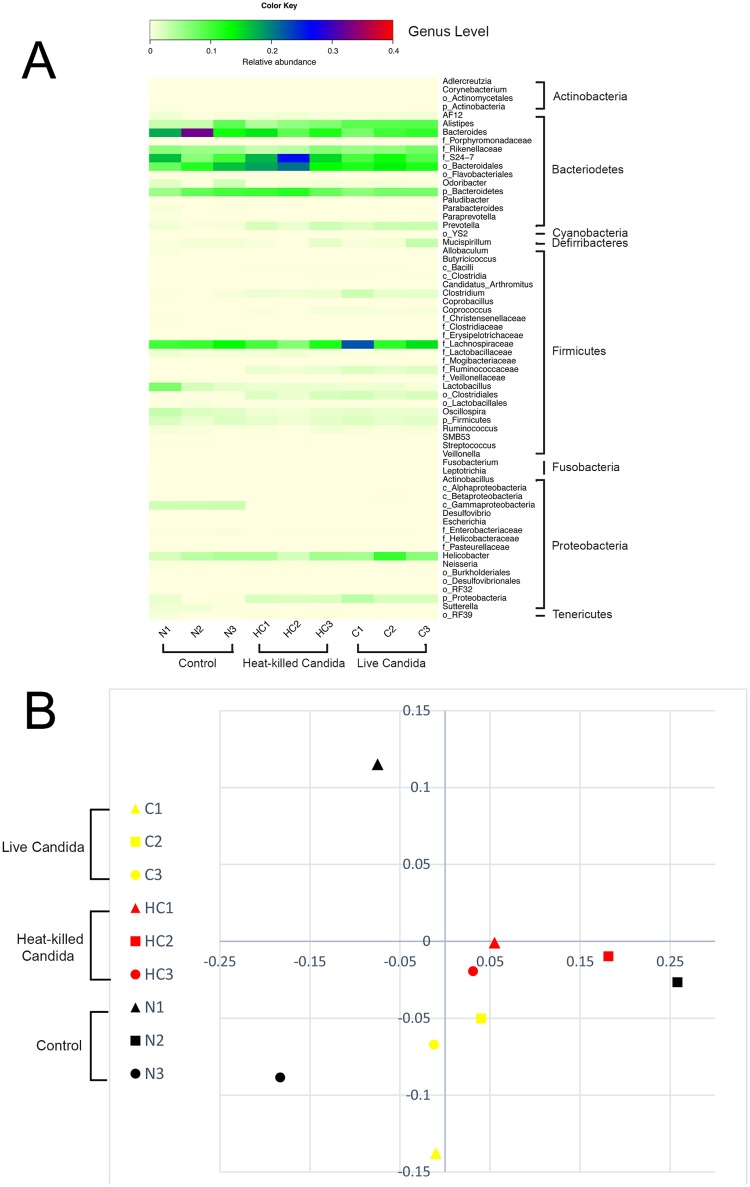
Because gram negative bacteria are well-known causative agents of severe bacterial sepsis, it is possible that oral Candida administration might enhance gram negative bacteria in gut. Accordingly, we characterized the fecal gut bacterial microbiota (Fig 4) to evaluate the effect of live or dead Candida administration. Surprisingly, fecal anaerobic bacteria in the genera Bacteroidales, Lachonospiraceae, Clostridiales and Helicobacter, but not gram negative bacteria, were increased after live- or heat-killed fungi administration. In parallel, bacteria in the genus of Lactobacillus (Fermicutes group) were slightly decreased compared with control mice. Bacteroides fragilis is the most common anaerobic bacteria isolated from intra-abdominal sepsis either in patients [23] or mice [24] and Lactobacilli help in controlling gut-pathogenic bacteria [25]. Because of the slow progressive clinical characteristics of anaerobic bacterial infection [26], the alteration of the distribution and prevalence of the gut microbiota components did not explain the rapid progressive sepsis in CLP with Candida administration.
Fig 4. Gut microbiota analysis from individual mouse feces.
Control (N1-3), heat-killed Candida administration (HC1-3) and live-Candida (C1-3) by relative abundance of bacterial diversity at genus level (A) are shown. Abbreviation “p”, “c” and “o” means unclassified family sequences in phylum, class and order, respectively, and Genera that contain ≤ 0.01% relative abundance were removed. A representative of gut microbiota structures demonstrated by non-metric multidimensional scaling (NMDS) are shown (B).
Purified (1→3)-β-D-glucan (Pachyman) with heat-killed E. coli preparation, synergistically increased macrophage cytokine production
Due to the importance of macrophages in sepsis-pathogenesis [27], the immune responses of bone marrow-derived macrophages were tested in vitro. Purified (1→3)-β-D-glucan (Pachyman) and heat-killed E. coli preparation have previously been as representative of Pathogen Associated Molecular Patterns of fungi (19) and bacteria, respectively. These were utilized to assess macrophage responses to them individually and together.
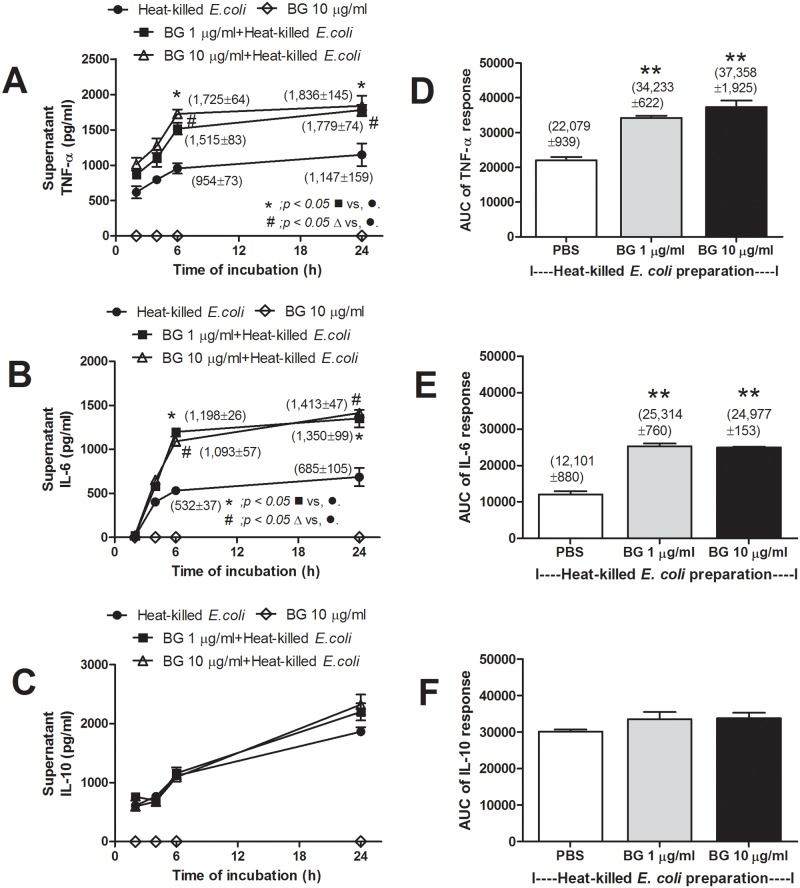
BG alone, either at 1 or 10 μg/ml could not activate cytokine production from macrophages (Fig 5A–5C; only BG at 10 μg/ml was shown). However, incubation of macrophages with BG plus heat-killed E. coli resulted in higher levels of TNF-α and IL-6, but not IL-10, and as early as 6h after incubation (Fig 5A–5C). The area under the curve (AUC) of cytokine responses from the data in time-points supported the synergistic effect of BG and E. coli in TNF-α and IL-6 elicitation but not IL-10 (Fig 5D–5F). However, the elicitation synergy was not BG-dose dependent, above 1 μg/ml.
Fig 5.
Time-course of cytokines in supernatant of macrophages after incubation with heat-killed E. coli preparation (see Method) with or without purified (1→3)-β-D-glucan (Pachyman) (BG) (at 1 or 10 μg/ml) or BG 10 μg/ml alone was shown (independent experiments were done in triplicate) (A-C). Area under the curve of cytokine responses of graph A-C are demonstrated (D-F). **, p < 0.05 vs. PBS+heat-killed E. coli.
Discussion
A positive impact of human commensal intestinal fungi upon murine bacterial sepsis, in the absence of fungemia, was demonstrated. Indeed, oral-administration of C. albicans (live- and heat-killed) prior to CLP increased serum BG and enhanced sepsis severity. Interestingly, fluconazole reduced intestinal fungi and attenuated sepsis severity. Gut-translocation of BG, and likely other PAMPs, from commensal fungi appeared to enhance sepsis severity, possibly through synergistic stimulation of pro-inflammatory cytokine production by macrophages.
Commensal organisms are necessary for the host immune maturation and gut barrier function [28] but they are also capable of inflammatory response activation [29]. As such, excess probiotic administration activates inflammatory responses and causes inflammatory diarrhea [30, 31]. Because overgrowth of C. albicans, the predominant gut commensal fungi of human, is observed with antibiotic administration [32, 33], significant Candida colonization is presumed in patients with sepsis. Interestingly, the “Candida colonization index” is an indicator for candidiasis prediction in patients with sepsis but not the index for bacterial sepsis severity [34, 35]. However, the potential exacerbation of bacterial sepsis due to intestinal Candida has not been previously explored. Accordingly, Candida oral-administration in a dose that was adequate for the induction of fecal Candida, without the production of candidemia, was selected to evaluate its effects in a bacterial sepsis model.
Although CLP with oral administration of C. albicans at highly elevated dose resulted in candidemia, C. albicans dosing at 106 CFU induced positive fecal fungi culture without candidemia and was selected for use in our experiments. Live- or heat-killed- Candida administered prior to CLP produced more elevated serum BG and more severe sepsis. Sepsis alone induced elevated serum BG and Candida-administration, with sepsis, showed further increases in the BG level. Additionally, CLP with heat-killed Candida induced higher serum BG than CLP alone. Further, fungal recovery from the mesenteric lymph node, an indicator of gut-translocation, was negative. These data imply the importance of gut-translocation of fungal molecules but, perhaps, not viable cells. Moreover, fluconazole reduced fecal fungal burdens, serum BG and attenuated sepsis severity in CLP with live-Candida administration but not CLP-alone. This supports the potential importance of intestinal fungi as a source of serum BG in bacterial sepsis.
Another factor that could be responsible for the more severe sepsis after an oral fungi administration is the alteration in gut microbiota. As such, microbiota analysis showed the increase in Bacteroidales, Lachonospiraceae, Clostridiales and Helicobacter but decreased Lactobacillus group after fungi administration. Although bacteremia from these bacteria is not frequently found or presents with limited severity [23], Bacteroidales bacteremia is commonly demonstrated in patients, with relatively high virulence, such as with Bacteroides fragillis infection [36]. The Bacteroidales are also the predominant component of the bacterial microbiota in human intestine. It is also generally observed that E. coli is the most active cause of infection within 24h after intra-abdominal injury. In addition, anaerobic bacteria induce a chronic stage of infection [26]. Hence, Bacteroides infection could not explain the rapid progression of sepsis (most of the mice died within 24h) of CLP with Candida administration. On the other hand, Lactobacillus spp., which are helpful components of the intestinal microbiota for the attenuation of gut leakage [25], were decreased after fungi administration. We recently demonstrated the attenuation of gut leakage with Lactobacilli in a C. difficile-induced sepsis model [37]. Thus, decreased Lactobacilli might enhance gut-leakage in our model resulting in the translocation of gut BG.
Although the increased sepsis severity in our models could be responsible from fungal factors (fecal Candida burdens and serum BG) and/or bacterial factor (gut microbiome alteration), the data from the CLP-Candida model that i) the oral administration of live- or heat killed- Candida increased sepsis severity and ii) the reduction of fecal Candida with fluconazole, without additional antibacterial drugs, attenuated sepsis severity implied a more prominent influence of fungal factors over bacterial factor in bacterial sepsis severity in our models. It is also interesting to note that the impact of intestinal Candida on bacterial sepsis severity was also more predominant than the effect from fecal-microbiome alteration in the 5 days Candida colonization model, a model with the influence of mixed-oral antibiotics [14].
Because serum BG in normal mice is usually absent or very low (< 30 pg/ml), it is interesting to note that serum BG elevation is demonstrated after CLP without Candida administration (Fig 2E). Without Candida introduction, measurement of BG in gut contents was already at the highest value of the assay (> 523 pg/ml). Thus the intestinal contents contribution of BG is due to either foodstuffs or un-culturable fungi. However, Candida administration increases gut BG as indirectly demonstrated by the higher serum BG in Candida-CLP compared to CLP alone. Taken together, Candida administration possibly increases pathogenic anaerobes, decreases beneficial bacteria, and enhances gut translocation of BG (from foodstuffs and/ or gut-fungi). These Candida-driven factors may be responsible for the exacerbation of sepsis. In the late phase of sepsis, serum BG reduced in survivor mice at 96h post-CLP but not reach the level of the sham control, in contrast to serum cytokines that already normalized at 96h post-CLP. The compatibility between serum BG and serum cytokines in the early phase of sepsis (18h post-CLP) and the discordance of these parameters in the late phase of sepsis (96h post-CLP) implies the different influence of serum BG in the different phase of sepsis. More studies in this topic are needed. The incubation of BG with heat-killed E. coli preparations enhanced pro-inflammatory cytokine production from macrophages in comparison with E. coli preparation alone. Although there was no direct in vivo data from our models, the in vitro macrophage characteristic implied the alteration of macrophage functions through dual-stimulation by both bacterial and fungal molecules [20, 38, 39]. All in all, the potential importance of fecal Candida to bacterial sepsis, which has been overlooked, was demonstrated. Our data also supported the potential usefulness of serum BG for determining propensity to, or severity of, sepsis.
Translationally, an evaluation of fungal abundance in patient stool and the evaluation of serum BG might be useful in the characterization of sepsis severity. However, stool collection in critical care conditions is labor intensive and potentiates contamination. Hence, the rapid estimation of intestinal contents translocation using a surrogate marker such as serum BG may be appropriate in clinical practice. Because serum BG correlated with intestinal fungal-abundance in sepsis (the present data), associated with impaired gut-permeability barrier [7], and correlated with bacterial sepsis severity [14], we propose the detection of serum BG, but not fecal fungi, as an additional biomarker for sepsis severity. Whether serial characterization of serum BG titers can be useful in the management of septic patients will require significant additional clinical study.
In conclusion, increased intestinal burdens of fungal material, live or dead, was shown to cause increased sepsis severity, in a murine CLP model. This was associated, at least in part, with translocation of fungal material from the gut lumen, enhancing cytokine production and inflammation.
Supporting information
(PDF)
Acknowledgments
AL worked under Center of Excellence in Immunology and Immune-mediated Diseases, Department of Microbiology, Faculty of Medicine.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by Ratchadapiseksompotch Fund (RA60/035), Faculty of Medicine, Grant for Development of New Faculty Staff Chulalongkorn University and Thailand Research fund (RSA60). WP was supported by Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (PHD/0316/2552). BG assays were partly supported by Associates of Cape Cod, Inc. The Associates of Cape Cod, Inc., provided support in the form of salaries for authors [MF], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74. doi: 10.1038/nri3552 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srisawat N, Kellum JA. Acute kidney injury: definition, epidemiology, and outcome. Curr Opin Crit Care. 2011;17(6):548–55. doi: 10.1097/MCC.0b013e32834cd349 . [DOI] [PubMed] [Google Scholar]
- 4.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. doi: 10.4161/viru.27372 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Leeuwen PA, Boermeester MA, Houdijk AP, Ferwerda CC, Cuesta MA, Meyer S, et al. Clinical significance of translocation. Gut. 1994;35(1 Suppl):S28–34. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai CW, Sun TL, Lo W, Tang ZH, Wu S, Chang YJ, et al. Shedding-induced gap formation contributes to gut barrier dysfunction in endotoxemia. J Trauma Acute Care Surg. 2013;74(1):203–13. doi: 10.1097/TA.0b013e3182788083 . [DOI] [PubMed] [Google Scholar]
- 7.Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, Jutivorakool K, Somparn P, Issara-Amphorn J, et al. Gastrointestinal Leakage Detected by Serum (1—>3)-beta-D-Glucan in Mouse Models and a Pilot Study in Patients with Sepsis. Shock. 2016. Epub 2016/05/14. doi: 10.1097/SHK.0000000000000645 . [DOI] [PubMed] [Google Scholar]
- 8.Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. 2012;10(6):350–6. doi: 10.1016/j.surge.2012.03.003 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruhnke M, Bohme A, Buchheidt D, Cornely O, Donhuijsen K, Einsele H, et al. Diagnosis of invasive fungal infections in hematology and oncology—guidelines from the Infectious Diseases Working Party in Haematology and Oncology of the German Society for Haematology and Oncology (AGIHO). Ann Oncol. 2012;23(4):823–33. doi: 10.1093/annonc/mdr407 . [DOI] [PubMed] [Google Scholar]
- 10.Hsu JL, Ruoss SJ, Bower ND, Lin M, Holodniy M, Stevens DA. Diagnosing invasive fungal disease in critically ill patients. Crit Rev Microbiol. 2011;37(4):277–312. doi: 10.3109/1040841X.2011.581223 . [DOI] [PubMed] [Google Scholar]
- 11.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, et al. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169(7):3876–82. . [DOI] [PubMed] [Google Scholar]
- 12.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178(5):3107–15. . [DOI] [PubMed] [Google Scholar]
- 13.Koh AY. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot Cell. 2013;12(11):1416–22. doi: 10.1128/EC.00196-13 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panpetch W, Somboonna N, Bulan DE, Issara-Amphorn J, Worasilchai N, Finkelman M, et al. Gastrointestinal Colonization of Candida Albicans Increases Serum (1—>3)-beta-D-Glucan, without Candidemia, and Worsens Cecal Ligation and Puncture Sepsis in Murine Model. Shock. 2017. doi: 10.1097/SHK.0000000000000896 . [DOI] [PubMed] [Google Scholar]
- 15.Kinsman OS, Pitblado K. Candida albicans gastrointestinal colonization and invasion in the mouse: effect of antibacterial dosing, antifungal therapy and immunosuppression. Mycoses. 1989;32(12):664–74. . [DOI] [PubMed] [Google Scholar]
- 16.Somboonna N, Wilantho A, Jankaew K, Assawamakin A, Sangsrakru D, Tangphatsornruang S, et al. Microbial ecology of Thailand tsunami and non-tsunami affected terrestrials. PLoS One. 2014;9(4):e94236 doi: 10.1371/journal.pone.0094236 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–4. doi: 10.1038/ismej.2012.8 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–41. doi: 10.1128/AEM.01541-09 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boonyatecha N, Sangphech N, Wongchana W, Kueanjinda P, Palaga T. Involvement of Notch signaling pathway in regulating IL-12 expression via c-Rel in activated macrophages. Mol Immunol. 2012;51(3–4):255–62. doi: 10.1016/j.molimm.2012.03.017 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kikkert R, Bulder I, de Groot ER, Aarden LA, Finkelman MA. Potentiation of Toll-like receptor-induced cytokine production by (1—>3)-beta-D-glucans: implications for the monocyte activation test. J Endotoxin Res. 2007;13(3):140–9. doi: 10.1177/0968051907080024 . [DOI] [PubMed] [Google Scholar]
- 21.Samonis G, Anastassiadou H, Dassiou M, Tselentis Y, Bodey GP. Effects of broad-spectrum antibiotics on colonization of gastrointestinal tracts of mice by Candida albicans. Antimicrob Agents Chemother. 1994;38(3):602–3. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi N, Sonoyama K, Kikuchi H, Nagura T, Aritsuka T, Kawabata J. Gastric colonization of Candida albicans differs in mice fed commercial and purified diets. J Nutr. 2005;135(1):109–15. . [DOI] [PubMed] [Google Scholar]
- 23.Goldstein EJ. Anaerobic bacteremia. Clin Infect Dis. 1996;23 Suppl 1:S97–101. . [DOI] [PubMed] [Google Scholar]
- 24.Hyde SR, Stith RD, McCallum RE. Mortality and bacteriology of sepsis following cecal ligation and puncture in aged mice. Infect Immun. 1990;58(3):619–24. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid G. The scientific basis for probiotic strains of Lactobacillus. Appl Environ Microbiol. 1999;65(9):3763–6. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20(4):593–621. doi: 10.1128/CMR.00008-07 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavaillon JM, Adib-Conquy M. Monocytes/macrophages and sepsis. Crit Care Med. 2005;33(12 Suppl):S506–9. . [DOI] [PubMed] [Google Scholar]
- 28.Jang HR, Gandolfo MT, Ko GJ, Satpute S, Racusen L, Rabb H. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297(5):F1457–65. doi: 10.1152/ajprenal.90769.2008 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen K, Li G, Bui T, Liu F, Li Y, Kocher J, et al. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine. 2012;30(6):1198–207. doi: 10.1016/j.vaccine.2011.11.107 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XQ, Zhu YH, Zhang HF, Yue Y, Cai ZX, Lu QP, et al. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: intestinal microbiota and immune imbalances. PLoS One. 2012;7(7):e40666 doi: 10.1371/journal.pone.0040666 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Li N, Caicedo R, Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J Nutr. 2005;135(7):1752–6. . [DOI] [PubMed] [Google Scholar]
- 32.Samonis G, Gikas A, Toloudis P, Maraki S, Vrentzos G, Tselentis Y, et al. Prospective study of the impact of broad-spectrum antibiotics on the yeast flora of the human gut. Eur J Clin Microbiol Infect Dis. 1994;13(8):665–7. . [DOI] [PubMed] [Google Scholar]
- 33.Samonis G, Gikas A, Anaissie EJ, Vrenzos G, Maraki S, Tselentis Y, et al. Prospective evaluation of effects of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans. Antimicrob Agents Chemother. 1993;37(1):51–3. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eggimann P, Pittet D. Candida colonization index and subsequent infection in critically ill surgical patients: 20 years later. Intensive Care Med. 2014;40(10):1429–48. doi: 10.1007/s00134-014-3355-z ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vardakas KZ, Michalopoulos A, Kiriakidou KG, Siampli EP, Samonis G, Falagas ME. Candidaemia: incidence, risk factors, characteristics and outcomes in immunocompetent critically ill patients. Clin Microbiol Infect. 2009;15(3):289–92. doi: 10.1111/j.1469-0691.2008.02653.x . [DOI] [PubMed] [Google Scholar]
- 36.Aldridge KE, Ashcraft D, O'Brien M, Sanders CV. Bacteremia due to Bacteroides fragilis group: distribution of species, beta-lactamase production, and antimicrobial susceptibility patterns. Antimicrob Agents Chemother. 2003;47(1):148–53. doi: 10.1128/AAC.47.1.148-153.2003 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leelahavanichkul A, Panpetch W, Worasilchai N, Somparn P, Chancharoenthana W, Nilgate S, et al. Evaluation of gastrointestinal leakage using serum (1—>3)-beta-D-glucan in a Clostridium difficile murine model. FEMS Microbiol Lett. 2016;363(18). doi: 10.1093/femsle/fnw204 . [DOI] [PubMed] [Google Scholar]
- 38.Hoffman OA, Olson EJ, Limper AH. Fungal beta-glucans modulate macrophage release of tumor necrosis factor-alpha in response to bacterial lipopolysaccharide. Immunol Lett. 1993;37(1):19–25. . [DOI] [PubMed] [Google Scholar]
- 39.Seong SK, Kim HW. Potentiation of Innate Immunity by beta-Glucans. Mycobiology. 2010;38(2):144–8. doi: 10.4489/MYCO.2010.38.2.144 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.