Abstract
The association of obesity on survival among patients with colorectal cancer (CRC) has not been well characterized. We investigated the association of pre-diagnostic body mass index (BMI)/waist-hip ratio (WHR) and total/cause-specific mortality in CRC patients. This study included 1,452 patients who participated in two large cohort studies and were diagnosed with CRC during follow-up period. Participants were measured for anthropometrics and interviewed to collect relevant information at baseline, prior to any cancer diagnosis. Data on site-specific cancer incidence and cause-specific mortality were obtained via in-person surveys and annual record linkage with cancer and vital statistics registries. Cox proportional hazard models were used to evaluate the associations of BMI and WHR with survival. A total of 547 participants died during the follow-up period, including 499 who died of CRC. Relative to normal BMI (18.5 to < 25.0 kg/m2), obesity (BMI ≥ 30 kg/m2) was associated with increased mortality resulting from all causes (Hazard Ratio (HR) = 1.5, 95% Confidence Interval (CI): 1.1–2.1) and CRC (HR = 1.5, 95% CI: 1.1–2.1). Elevated risk of death was also found among underweight patients (BMI <18.5 kg/m2), although not all risk estimates were statistically significant. Overweight BMI (25.0 to < 30.0 kg/m2) was not associated with risk of death among CRC patients, nor was WHR. In conclusion, pre-diagnostic BMI was associated with survival among CRC patients following a U-shape pattern; obesity was associated with high mortality after CRC diagnosis. These findings provide support for maintaining healthy weight to improve the survival of CRC patients.
Keywords: body mass index, waist-hip ratio, colorectal cancer, survival, obesity
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world. The five-year survival rate remains low, particularly in developing countries.1–3 With the exception of tumor characteristics, few prognostic factors have been established for CRC. Therefore, it is important to identify additional prognostic factors that may affect survival in CRC patients.
Both general obesity, typically measured by body mass index (BMI), and abdominal obesity, typically measured by waist to hip ratio (WHR), are associated with a high risk of developing CRC.4–6 However, only a few studies have investigated the potential influence of obesity on CRC survival, and their results are inconsistent.7–14 In some studies, an elevated risk of overall mortality was observed in CRC patients with high BMI compared to those with normal BMI.7–9 In other studies, however, high BMI was not associated with or was even associated with a reduced risk of mortality in CRC patients.10–12 Some studies found that BMI was associated with increased mortality only in a specific sex subgroup, or that the association varies with tumor location, molecular subtype or cancer stage.1,7,13,15 Even fewer studies have assessed the association of WHR on CRC mortality, and some of these studies reported that abdominal obesity may better predict all-cause and colorectal cancer mortality than BMI.6,16
Most studies that evaluated the association of obesity with CRC prognosis were conducted in European descendants. The role of obesity on cancer survival has been less well studied among East Asians who, in general, have a lower prevalence of overall obesity but higher prevalence of central obesity compared to those of European descent. It has been reported that Asians may have a higher level of visceral fat than European descendants at the same BMI level and thus are more vulnerable to insulin resistance.17 To date, only four studies have evaluated the possible influence of obesity on CRC survival among Asian populations.18–21 However, these previous Asian studies had small sample sizes, did not adjust for potential confounders, and did not evaluate the association of central obesity with CRC survival.
The aim of the present study is to investigate the influence of pre-diagnosis BMI and WHR on all-cause and CRC-specific mortality using the resources of two large cohort studies, the Shanghai Women’s Health Study (SWHS) and the Shanghai Men’s Health Study (SMHS).
Materials and Methods
Study cohort
The SWHS and the SMHS are two on-going, population-based, prospective cohort studies, conducted in Shanghai, China. Details of the study designs and baseline questionnaires have been published previously.22,23 Briefly, women aged 40–70 years and men aged 40–74 years, who were permanent residents of the study communities, were approached for the study by trained interviewers. At baseline recruitment, anthropometric measurements (including weight, height, and circumferences of waist and hips), information on socio-demographic characteristics, lifestyle factors, and medical history were collected through in-person interviews. BMI was calculated as weight in kilograms divided by the square of height in meters. WHR was calculated as waist circumference divided by hip circumference.
The SWHS recruited 74,942 women from 1997 to 2000 (participation rate 92.7%), and the SMHS recruited 61,480 men from 2002 to 2006 (participation rate 74.0%). These two cohorts have been followed up through a combination of in-person surveys every 2–4 years and annual record linkage with the population-based Shanghai Cancer Registry and the Shanghai Vital Statistics Registry to identify incident cancer cases and cause-specific mortality, with nearly complete follow-up. Cancer cases were verified by home visits and review of medical records and pathological slides. Clinical information was obtained by review of medical charts. By December 31, 2013, 1,627 cohort members had been diagnosed with incident CRC. 14 patients were excluded because of missing information for anthropometric measurements. In order to reduce potential biases due to reverse causality, 161 subjects diagnosed within 2 years of follow-up were excluded. Of the remaining 1,452 eligible patients, 894 were diagnosed with colon cancer and 558 were diagnosed with rectal cancer. There were 573 men and 879 women with CRC in this analysis. On the average, BMI was assessed about 8 years before CRC diagnosis for the patients included in this analysis. The study was approved by the relevant institutional review boards for human research. Written, informed consent was obtained from all participants.
Statistical analysis
The primary endpoints were death from any cause and death from CRC after CRC diagnosis. BMI was categorized according to WHO criteria: underweight, < 18.5 kg/m2; normal weight, 18.5 to < 25.0 kg/m2; overweight, 25.0 to < 30.0 kg/m2; and obese, ≥ 30 kg/m2. WHR was categorized by sex-specific quartiles. The normal weight and lowest WHR were used as reference categories. Follow-up time began on the date of CRC diagnosis and ended on the date of death or December 31, 2013, whichever came first. Differences in socio-demographic characteristics and other risk factors by pre-diagnosis BMI and WHR categories were evaluated using a t-test for continuous variables and a chi-square test for categorical variables. The Kaplan-Meier method was used to estimate survival rates, and the log-rank test was performed to test the differences. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated using Cox proportional hazards models to evaluate the associations of pre-diagnosis BMI and WHR with hazard of death after adjusting for potential confounders. The validity of the proportional hazards assumption was evaluated by creating multiplicative interaction terms between both BMI and WHR and time and comparing Cox models with and without interaction terms using the likelihood ratio test. We identified potential confounders using prior knowledge regarding the association between factors (both lifestyle and clinical) that are potentially related to both BMI and survival, and the 10% change-in-estimate criterion. The following factors were adjusted for in final models: age at diagnosis, sex, income level, cigarette smoking, Charlson comorbidity index, and TNM stage. Adjusting for treatment did not change the results appreciably and thus the final models did not adjust for treatment. We also examined the association for BMI and WHR continuously using restricted cubic spline models with five knots (i.e., 5th, 25th, 50th, 75th, and 95th percentiles) for both all-cause and CRC-specific mortality.24 All analyses were performed using SAS, version 9.4 software (SAS Institute, Inc., Cary, NC), and all tests of statistical significance were set at P < 0.05 for two-sided analyses.
Results
The mean follow-up time was 3.4 years after diagnosis of CRC (median, 2.3 years; range, 2.01 days to 14.1 years). After excluding patients who died in the first two years of follow-up, 547 deaths were documented, of which 499 were due to CRC. The distribution of socio-demographic and other characteristics across BMI and WHR measurements are shown in Table 1. There were no meaningful differences across categories of BMI and WHR for smoke, doing exercise, family history of CRC, subsite of CRC, TNM stage or treatment. Underweight participants were more likely to be regular smokers, and obese participants were more likely to be older, less educated and had lower income. Compared to participants in the lowest category of WHR, participants with a high WHR were more likely to be older, less educated, had lower income, and have severe comorbidity.
Table 1.
Characteristics of Patients Diagnosed with Colorectal Cancer by BMI and WHR in Shanghai Women’s and Shanghai Men’s Cohorts, 1997 to 2013.
| BMI(kg/m2)
|
WHRa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | < 18.5 (n = 40) |
18.5 to <25.0 (n = 796) |
25.0 to < 30.0 (n = 533) |
≥30.0 (n = 83) |
P value | Q1 (n = 338) |
Q2 (n = 350) |
Q3 (n = 342) |
Q4 (n=422) |
P value |
| Age (mean ± SD) | 65.5±9.6 | 66.4±9.3 | 68.5±8.8 | 69.6±7.5 | < 0.001 | 65.2±9.7 | 66.6±9.1 | 67.7±9.0 | 69.3±8.3 | < 0.001 |
| Sex, male (%) | 45.0 | 41.6 | 37.5 | 28.9 | 0.08 | 35.2 | 42.3 | 46.2 | 35.1 | 0.004 |
| Education level (%) | ||||||||||
| < High school | 50.0 | 53.9 | 60.9 | 70.4 | 0.007 | 50.9 | 53.9 | 60.3 | 62.9 | 0.02 |
| High school | 40.0 | 27.0 | 23.8 | 19.8 | 30.7 | 26.4 | 23.5 | 23.1 | ||
| College and above | 10.0 | 19.1 | 15.3 | 9.9 | 18.5 | 19.7 | 16.2 | 14.1 | ||
| Income (%) | ||||||||||
| Low | 12.5 | 14.3 | 17.6 | 24.1 | 0.01 | 15.1 | 15.4 | 12.9 | 19.9 | 0.007 |
| Middle | 77.5 | 76.5 | 77.7 | 71.1 | 75.4 | 74.9 | 82.2 | 74.6 | ||
| High | 10.0 | 9.2 | 4.7 | 4.8 | 9.5 | 9.7 | 5.0 | 5.5 | ||
| Ever regularly smoke cigarettes (%, male only) | 83.3 | 61.6 | 70.0 | 66.7 | 0.09 | 62.2 | 62.8 | 64.6 | 71.6 | 0.31 |
| Ever regularly smoke cigarettes(%, female only) | 9.1 | 1.3 | 2.7 | 5.1 | 0.03 | 2.7 | 1.0 | 2.2 | 2.9 | 0.53 |
| Ever exercise regularly (%) | 37.5 | 44.4 | 43.3 | 38.6 | 0.65 | 43.2 | 46.9 | 42.4 | 41.7 | 0.51 |
| Family history of CRC (%) | 2.5 | 3.9 | 3.2 | 2.4 | 0.82 | 3.3 | 4.9 | 3.5 | 2.6 | 0.40 |
| Charlson comorbidity index (%) | ||||||||||
| ≤1 | 95.0 | 83.9 | 84.1 | 80.7 | 0.23 | 90.5 | 84.9 | 85.4 | 77.3 | < 0.001 |
| ≥2 | 5.0 | 16.1 | 16.0 | 19.3 | 9.5 | 15.1 | 14.6 | 22.8 | ||
| Tumor location (%) | ||||||||||
| Colon | 57.5 | 60.8 | 64.0 | 55.4 | 0.38 | 61.8 | 61.4 | 59.9 | 62.8 | 0.88 |
| Rectum | 42.5 | 39.2 | 36.0 | 44.6 | 38.2 | 38.6 | 40.1 | 37.2 | ||
| TNM stage (%)b | ||||||||||
| I | 14.8 | 22.5 | 22.3 | 20.3 | 0.14 | 23.1 | 21.5 | 20.1 | 23.4 | 0.94 |
| II | 25.9 | 28.1 | 30.5 | 17.2 | 28.4 | 29.4 | 29.9 | 26.1 | ||
| III | 29.6 | 36.4 | 33.1 | 40.6 | 34.1 | 36.6 | 33.9 | 36.0 | ||
| IV | 29.6 | 13.1 | 14.1 | 21.9 | 14.4 | 12.5 | 16.1 | 14.4 | ||
| Treatment (%) | ||||||||||
| Received surgery | 85.0 | 90.0 | 92.1 | 89.2 | 0.32 | 92.0 | 89.1 | 90.6 | 90.5 | 0.65 |
| Received chemotherapy | 60.0 | 71.1 | 70.7 | 66.3 | 0.40 | 73.1 | 69.4 | 69.9 | 69.4 | 0.67 |
| Received radiotherapy | 5.0 | 8.0 | 6.4 | 10.8 | 0.39 | 6.2 | 9.1 | 9.4 | 5.7 | 0.12 |
WHR quartile (female,Q1: WHR < 0.79, Q2:0.79 ≤ WHR ≤ 0.82, Q3:0.82 ≤ WHR ≤ 0.85, Q4:WHR ≥ 0.85; male, Q1:WHR <0.87, Q2:0.87 ≤ WHR ≤ 0.91, Q3:0.91 ≤ WHR ≤ 0.95, Q4:WHR ≥0.95.)
Participants with unknown TNM-stage were excluded.
Abbreviation: SD, standard deviation.
Adjusted HRs for all-cause and CRC-specific mortality by selected demographic and clinical characteristics are shown in Table 2. In addition to cancer stage, age at diagnosis, sex, and income were associated with both all-cause and CRC-specific mortality. In Kaplan-Meier curves showing survival rates according to pre-diagnosis BMI, underweight and obese participants had higher CRC-specific (log-rank p < 0.001) and all-cause mortality (log-rank p < 0.001) (Figure 1).
Table 2.
Association of All-Cause and CRC-specific Mortality with Selected Demographic and Clinical Characteristics in Chinese Patients with Colorectal Cancer, Shanghai, 1997–2013.
| All-cause mortality
|
CRC-specific mortality
|
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Number of cases | Number of Events | 5-year survival (%) | HRa (95%CI) | Number of Events | 5-year survival (%) | HRa (95%CI) |
| Age | |||||||
| <60 | 343 | 105 | 67.1 | 1.00(ref) | 100 | 67.7 | 1.00(ref) |
| 60–70 | 464 | 162 | 60.7 | 1.37(1.07–1.76) | 147 | 63.6 | 1.32(1.02–1.71) |
| 70–75 | 312 | 136 | 53.3 | 1.81(1.39–2.36) | 122 | 57.2 | 1.71(1.30–2.25) |
| >=75 | 333 | 144 | 43.3 | 2.17(1.66–2.84) | 130 | 47.5 | 2.02(1.53–2.67) |
| P for trend | <0.0001 | <0.0001 | |||||
| Sex | |||||||
| Female | 879 | 346 | 58.7 | 1.00(ref) | 318 | 61.1 | 1.00(ref) |
| Male | 573 | 201 | 54.4 | 1.70(1.32–2.19) | 181 | 58.0 | 1.65(1.26–2.15) |
| Education levelb | |||||||
| <High school | 826 | 333 | 54.8 | 1.00(ref) | 305 | 57.7 | 1.00(ref) |
| High school | 371 | 128 | 60.3 | 0.92(0.74–1.14) | 117 | 62.7 | 0.90(0.72–1.13) |
| College and above | 244 | 83 | 60.8 | 0.81(0.62–1.06) | 75 | 63.8 | 0.80(0.61–1.05) |
| P for trend | 0.12 | 0.10 | |||||
| Income | |||||||
| Low | 233 | 106 | 48.3 | 1.00(ref) | 97 | 52.0 | 1.00(ref) |
| Middle | 1113 | 408 | 58.2 | 0.73(0.58–0.92) | 371 | 61.0 | 0.74(0.58–0.93) |
| High | 106 | 33 | 65.8 | 0.65(0.43–1.00) | 31 | 67.5 | 0.69(0.44–1.06) |
| P for trend | 0.01 | 0.02 | |||||
| Cigarette smoking (males only) | |||||||
| Never | 198 | 75 | 50.3 | 1.00(ref) | 65 | 56.2 | 1.00(ref) |
| Former | 91 | 37 | 45.8 | 0.83(0.55–1.25) | 33 | 51.2 | 0.86(0.56–1.34) |
| Current | 284 | 89 | 60.2 | 0.80(0.57–1.12) | 83 | 61.6 | 0.84(0.59–1.19) |
| P for trend | 0.20 | 0.33 | |||||
| Tumor location | |||||||
| Colon | 894 | 348 | 55.0 | 1.00(ref) | 319 | 57.9 | 1.00(ref) |
| Rectum | 558 | 199 | 60.7 | 0.90(0.75–1.07) | 180 | 63.4 | 0.88(0.73–1.06) |
| TNM stageb | |||||||
| I | 251 | 29 | 87.5 | 1.00(ref) | 21 | 90.7 | 1.00(ref) |
| II | 322 | 80 | 73.5 | 2.32(1.51–3.56) | 67 | 76.3 | 2.65(1.62–4.34) |
| III | 400 | 177 | 51.3 | 5.28(3.56–7.83) | 165 | 53.2 | 6.73(4.27–10.62) |
| IV | 163 | 141 | 6.2 | 23.91(15.85–36.06) | 136 | 6.8 | 30.01(18.76–48.00) |
| P for trend | <0.0001 | <0.0001 | |||||
Adjusted by age at diagnosis, sex, education, income, smoke, tumor location and TNM stage.
Some counts do not add to totals because of missing data (Education missing = 11, TNM stage missing=316).
Figure 1.
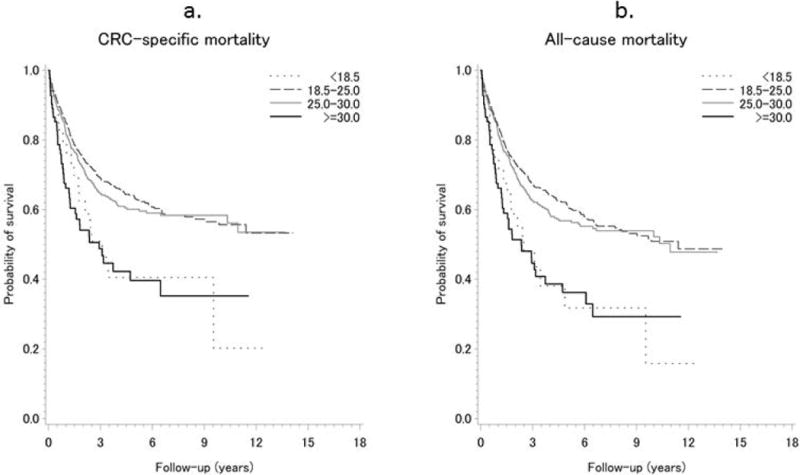
Kaplan-Meier Estimates of Survival Functions for CRC-specific (A) and All-cause (B) Mortality across BMI Categories Among Patients with Colorectal Cancer in Shanghai Women’s and Shanghai Men’s Cohorts, 1997–2013.
Dotted line: BMI < 18.5 kg/m2; Dashed line: BMI 18.5–25.0 kg/m2; Gray line: BMI 25.0–30.0 kg/m2; Black line: BMI ≥ 30.0 kg/m2.
P value: log-rank P < 0.001 for both CRC-specific and all-cause mortality.
No violation of the validity of the proportional hazards assumption was observed. Multivariable-adjusted HRs for all-cause and CRC-specific mortality by pre-diagnosis BMI categories are shown in Table 3. In men and women combined, pre-diagnosis obese BMI compared with normal BMI was associated with 54% higher risk of all-cause death and 51% higher risk of CRC-specific death. Underweight was also associated with an elevated mortality, although the HRs were not statistically significant likely due to a small sample size. No statistically significant increases in hazard of death were found in the overweight group. When BMI was considered as a continuous variable by using restricted cubic splines, a nadir of the U-shaped association of BMI with all-cause mortality was observed at BMI of 20–25 kg/m2 (P for non-linearity = 0.0001; Figure 2). A similar pattern was observed for BMI and CRC-specific mortality (Supplemental Figure 2).
Table 3.
All-Cause and CRC-specific Mortality Among Patients with Colorectal Cancer by BMI in Shanghai Women’s and Shanghai Men’s Cohorts, 1997–2013.
| Subgroup | BMI(kg/m2)
|
||||
|---|---|---|---|---|---|
| <18.5 | 18.5 to <25.0 | 25.0 to <30.0 | ≥30.0 | P for linear trenda | |
| All-causes mortality | |||||
| Both sexes | |||||
| No. | 22/40 | 281/796 | 198/533 | 46/83 | |
| HR (95%CI)b | 1.47(0.95–2.29) | 1.00(ref) | 1.04(0.87–1.25) | 1.54(1.12–2.12) | 0.22 |
| Women | |||||
| No. | 14/22 | 167/465 | 129/333 | 36/59 | |
| HR (95%CI)b | 1.37(0.78–2.39) | 1.00(ref) | 1.08(0.86–1.37) | 1.68(1.16–2.44) | 0.09 |
| Men | |||||
| No. | 8/18 | 114/331 | 69/200 | 10/24 | |
| HR (95%CI)b | 1.99(0.95–4.18) | 1.00(ref) | 1.02(0.75–1.38) | 1.45(0.74–2.84) | 0.94 |
| Colon cancer | |||||
| No. | 11/23 | 179/484 | 134/341 | 24/46 | |
| HR (95%CI)b | 1.25(0.67–2.31) | 1.00(ref) | 0.99(0.79–1.24) | 1.47(0.95–2.27) | 0.69 |
| Rectal cancer | |||||
| No. | 11/17 | 102/312 | 64/192 | 22/37 | |
| HR (95%CI)b | 2.01(1.05–3.84) | 1.00(ref) | 1.15(0.84–1.58) | 1.60(0.99–2.60) | 0.28 |
| CRC-specific mortality | |||||
| Both sexes | |||||
| No. | 19/40 | 257/796 | 181/533 | 42/83 | |
| HR (95%CI)b | 1.34(0.84–2.16) | 1.00(ref) | 1.04(0.86–1.26) | 1.51(1.08–2.11) | 0.22 |
| Women | |||||
| No. | 12/22 | 154/465 | 119/333 | 33/59 | |
| HR (95%CI)b | 1.23(0.68–2.25) | 1.00(ref) | 1.08(0.85–1.37) | 1.63(1.11–2.41) | 0.14 |
| Men | |||||
| No. | 7/18 | 103/331 | 62/200 | 9/24 | |
| HR (95%CI)b | 1.86(0.85–4.10) | 1.00(ref) | 1.00(0.73–1.38) | 1.42(0.70–2.87) | 0.87 |
| Colon cancer | |||||
| No. | 11/23 | 165/484 | 121/341 | 22/46 | |
| HR (95%CI)b | 1.30(0.70–2.42) | 1.00(ref) | 0.96(0.76–1.22) | 1.42(0.90–2.23) | 0.80 |
| Rectal cancer | |||||
| No. | 8/17 | 92/312 | 60/192 | 20/37 | |
| HR (95%CI)b | 1.62(0.77–3.42) | 1.00(ref) | 1.18(0.85–1.64) | 1.64(0.98–2.72) | 0.19 |
No.: Number of events/patients at risk.
Patients with BMI<18.5 kg/m2 were excluded in the trend test.
Adjusted by age at diagnosis, sex, income level, smoking status, comorbidity and TNM stage.
P interaction with sex: All-causes mortality: 0.78; CRC-specific mortality: 0.75.
P interaction with cancer location: All-causes mortality: 0.72; CRC-specific mortality: 0.70.
Figure 2.
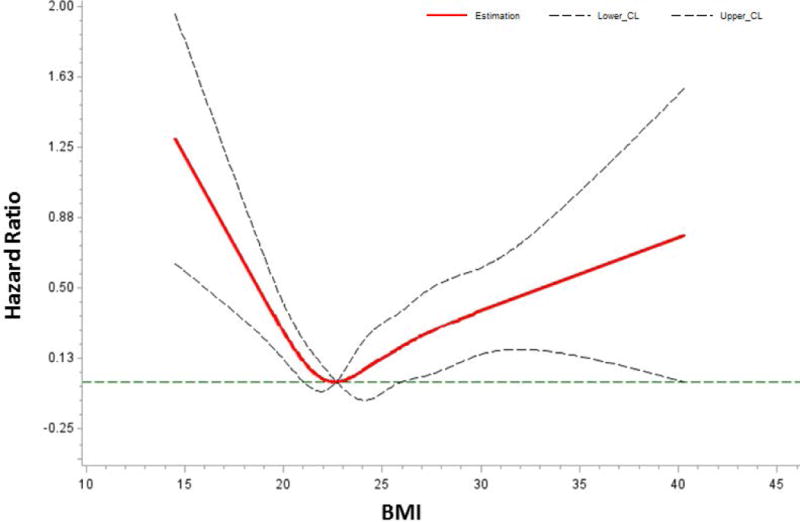
Dose-response Using Restricted Cubic Spline Model for the Association between Pre-diagnosis BMI and All-cause Mortality in Shanghai Men’s and Shanghai Women’s Cohorts, 1997–2013. Adjusted by Age at Diagnosis, Sex, Income Level, Smoking Status, Comorbidity and TNM Stage.
P for non-linearity = 0.0001.
When stratified by sex and subsite of CRC, the association between pre-diagnosis obese BMI and mortality was more pronounced for women (all-cause: HR = 1.68, 95% CI: 1.16–2.44; CRC-specific: HR = 1.63, 95% CI: 1.11–2.41) than men, and the HR for the association of underweight BMI with all-cause mortality was significant only among patients diagnosed with rectal cancer (all-cause: HR = 2.01, 95%CI: 1.05–3.84). However, neither test for interaction by sex or CRC subsite was statistically significant (P ≥0.70) (Table 3).
We also examined the association of pre-diagnosis obese BMI relative to normal BMI with CRC-specific mortality across the strata of other potential predictors of survival, including cancer stage, physical activity, income and smoking status (Figure 3). Due to a small number of events among female smokers (n=6), analyses among female smokers were not conducted. There was no evidence for significant effect measure modification by any of these variables (all P for heterogeneity > 0.05). Additionally, we conducted survival analyses stratified by diagnosis within 8 years of follow-up and diagnosis after 8 years; however the results were very similar in these two strata (all P heterogeneity > 0.05; Figure 3).
Figure 3.

Adjusted Hazard Ratios for CRC-specific Mortality between Obese BMI and Normal BMI Stratified by Stage, Physical Activity, Income, Smoke and Different Intervals between Measurement and Diagnosis.
HRs: Adjusted by age at diagnosis, sex, income level, smoking status, comorbidity and TNM stage.
NE = Not estimated due to small number of events.
*HRs and 95% CI for obese BMI compared to Normal BMI.
No significant association was found between pre-diagnosis WHR and all-cause (highest relative to lowest WHR quartile: HR =0.94, 95%CI: 0.81–1.10) or CRC-specific mortality (highest relative to lowest WHR quartile: HR = 0.94, 95% CI: 0.80–1.10) in multivariable-Cox regression analyses (Table 4) or the Kaplan-Meier survival analysis (all-cause: log-rank p = 0.78; CRC-specific: log-rank p = 0.71; Supplementary Figure S1). All P values for interaction by sex and CRC subsite were 0.18 or higher (Table 4). Analyses using continuous variables also revealed no significant association of WHR with total and CRC-specific mortality (Supplemental Figures 3 and 4). In stratified analyses, no evidence was found for potential effect modification on the association of WHR and CRC survival outcomes (all P heterogeneity > 0.05; Supplementary Figure S5).
Table 4.
All-Cause and CRC-specific Mortality Among Patients with Colorectal Cancer by WHR in Shanghai Women’s and Shanghai Men’s Cohorts, 1997–2013.
| Subgroup | WHRa
|
|||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Per 0.1 increase | P for linear trend | |
| All-cause mortality | ||||||
| Both sexes | ||||||
| No. | 125/338 | 128/350 | 128/342 | 166/422 | ||
| HR (95%CI)b | 1.00(ref) | 1.04(0.81–1.33) | 1.00(0.78–1.28) | 0.95(0.75–1.20) | 0.94(0.81–1.10) | 0.44 |
| Women | ||||||
| No. | 83/219 | 75/202 | 71/184 | 117/274 | ||
| HR (95%CI)b | 1.00(ref) | 0.95(0.69–1.31) | 0.93(0.67–1.28) | 1.05(0.78–1.42) | 1.11(0.90–1.36) | 0.33 |
| Men | ||||||
| No. | 42/119 | 53/148 | 57/158 | 49/148 | ||
| HR (95%CI)b | 1.00(ref) | 1.35(0.89–2.03) | 1.19(0.79–1.78) | 0.85(0.56–1.29) | 0.78(0.62–0.98) | 0.03 |
| Colon cancer | ||||||
| No. | 79/209 | 79/215 | 87/205 | 103/265 | ||
| HR (95%CI)b | 1.00(ref) | 1.17(0.85–1.60) | 1.15(0.85–1.57) | 0.99(0.73–1.34) | 0.94(0.78–1.14) | 0.54 |
| Rectal cancer | ||||||
| No. | 46/129 | 49/135 | 41/137 | 63/157 | ||
| HR (95%CI)b | 1.00(ref) | 0.91 (0.61–1.37) | 0.75(0.49–1.15) | 0.85(0.57–1.26) | 0.89(0.68–1.17) | 0.40 |
| CRC-specific mortality | ||||||
| Both | ||||||
| No. | 112/338 | 118/350 | 119/342 | 150/422 | ||
| HR (95%CI)b | 1.00(ref) | 1.07(0.83–1.39) | 1.04(0.80–1.35) | 0.96(0.75–1.24) | 0.94(0.80–1.10) | 0.43 |
| Women | ||||||
| No. | 73/219 | 72/202 | 68/184 | 105/274 | ||
| HR (95%CI)b | 1.00(ref) | 1.04(0.75–1.45) | 1.00(0.72–1.40) | 1.08(0.79–1.47) | 1.09(0.88–1.35) | 0.44 |
| Men | ||||||
| No. | 39/119 | 46/148 | 51/158 | 45/148 | ||
| HR (95%CI)b | 1.00(ref) | 1.23(0.80–1.89) | 1.14(0.75–1.74) | 0.82(0.53–1.27) | 0.77(0.61–0.99) | 0.04 |
| Colon cancer | ||||||
| No. | 71/209 | 72/215 | 82/205 | 94/265 | ||
| HR (95%CI)b | 1.00(ref) | 1.21 (0.86–1.68) | 1.22 (0.88–1.68) | 1.01 (0.73–1.39) | 0.94(0.78–1.15) | 0.57 |
| Rectal cancer | ||||||
| No. | 41/129 | 46/135 | 37/137 | 56/157 | ||
| HR (95%CI)b | 1.00(ref) | 0.96(0.63–1.47) | 0.76(0.49–1.20) | 0.85 (0.56–1.29) | 0.87(0.66–1.16) | 0.35 |
No.: Number of events/patients at risk.
WHR quartile (female patients,Q1: WHR < 0.79, Q2:0.79 ≤ WHR ≤ 0.82, Q3:0.82 ≤ WHR ≤ 0.85, Q4:WHR ≥ 0.85; male patients, Q1:WHR < 0.87, Q2:0.87 ≤ WHR ≤ 0.91, Q3:0.91 ≤ WHR ≤ 0.95, Q4:WHR ≥ 0.95.)
Adjusted by age at diagnosis, sex, income level, smoking status, comorbidity and TNM stage.
P interaction with sex: All-causes mortality: 0.18; CRC-specific mortality: 0.47.
P interaction with cancer location: All-causes mortality: 0.46; CRC-specific mortality: 0.47.
Discussion
In these two prospective cohort studies, pre-diagnostic BMI was associated with survival among CRC patients following a U-shape pattern; both underweight and obesity determined prior to cancer diagnosis was associated with increased all-cause and CRC-specific mortality compared with normal BMI, whereas no significant association was observed for overweight BMI (25 – 29.9 kg/m2). No significant association was observed between WHR and mortality outcomes in this study.
Only a few studies investigated the association between pre-diagnosis BMI and CRC survival, and the results were not consistent, but generally, high BMI is associated with poor survival results.7,9,11,16,25 Two recent papers showed differences in association between BMI and CRC survival. Kocarnik et al. found that overweight (BMI 25.0–29.9) was associated with increased mortality among patients with Stage I disease, and decreased mortality among those with Stages II–IV disease13. Kroenke et al. observed that BMI measure at the time of cancer diagnosis was associated with CRC mortality in a nonlinear fashion, but compared with the BMI group of 18.5 to <23.0, a reduced mortality was found for each of higher BMI groups (23.0 to <25.0, 25.0 to <28.0 and 28.0 to <30.0)14. Because BMI was measured at the time of cancer diagnosis, a potential bias due to reverse causation is a concern. In general, similar to our results, most previous European studies indicate a U-shaped pattern of association for the relation between BMI and mortality.
Only two studies to date have evaluated the association between pre-diagnosis WHR and the survival of patients with CRC, and both of them were conducted in European descendants. Fedirko et al.6 used quintile of WHR cut off points to analyze the association between pre-diagnosis WHR and survival after colorectal cancer diagnosis, while Prizment et al.16 adopted tertile of WHR as cut off points. Unlike the present study, their results showed that higher WHR was associated with increased risk of death.6,16 However, in the stratified analysis by sex of Fedirko’s results, the significant association was observed only in men with highest WHR category (WHR>1.01), whereas no significant association was observed for any WHR groups in women and for other categories in men. Compared to European descendants, Asians tend to have lower WHR. It is possible that the increased hazard of death may only be observed in individuals with a very high WHR, which could be difficult to detect Chinese living in Shanghai. We observed an association of poor survival outcome with high BMI but not WHR in this study. The reasons for a different association with BMI and WHR are unclear. BMI is a measure of general obesity while WHR measures primarily central obesity. Perhaps survival outcomes may be more closely related to general obesity than central obesity in Chinese CRC patients. Additional research is needed to understand the mechanisms of the associations observed in this study.
To our knowledge, this is the first cohort study to examine the relationship between pre-diagnosis BMI/WHR and prognosis in Asian populations with CRC. Four studies have been conducted in Asian colorectal patients to evaluate BMI measured at cancer diagnosis in relation to outcome. Results from these studies were conflicting, showing no association19,20, a borderline decreased disease-free survival21 or even an improved overall survival18 associated with overweight. All these previous studies had small sample sizes, limiting their ability to rigorously evaluate the association of BMI and mortality among CRC patients. The previous studies also used different BMI categories in the analysis, making a direct comparison of their results with ours difficult. Furthermore, these previous studies assessed BMI at diagnosis rather than pre-diagnostic BMI. Because of possible weight loss during the cancer development and progression in some patients, reverse causation could affect the validity of the study results.
When participants were stratified according to tumor location, we found that the BMI-mortality association did not differ between colon and rectal cancer. While, in a similar cohort study of CRC with predominantly European-descent participants, pre-diagnosis obese BMI was associated with higher risk of all-cause mortality among participants with rectal cancer but not colon cancer.9 However, in another study, both underweight and obese patients were at increased risk of colon cancer death, no association was observed for those with rectal cancer.7 Although it is still unclear whether obesity exerts different impacts on the prognosis of colon cancer and rectal cancer, it has been suggested that obesity adds to the technical challenge of pelvic operations in both open and laparoscopic radical proctectomy.26 Some studies have reported that obesity was associated with shorter disease-free survival, retrieval of fewer lymph nodes, longer operative times, lower rates of sphincter preservation, higher rates of postoperative complications, and higher likelihood of local recurrence in patients with rectal cancer.21,27,28
Except for increasing the risk of suboptimal treatment and complications, the underlying mechanisms by which obesity affects CRC prognosis are largely unclear. It has been shown that obesity is associated with insulin resistance and higher levels of circulating insulin, and insulin could enhance the bioactivity of IGF-I, which can promote cell proliferation and protect cancer cells from apoptosis.29 Other pathophysiological and biological mechanisms have also been suggested to impact cancer mortality, including adipokines, obesity-related inflammatory markers, nuclear factor kappa beta system, and oxidative stresses.30 Furthermore, some studies have demonstrated that the adverse effect of obesity on patient survival was modified by specific gene expression.31,32 Some studies have also found that underweight BMI was associated with increased mortality compared with normal BMI, an association possibly reflecting underlying comorbidities that increased mortality.7,8 In addition, underweight participants were more likely to develop chemotherapy-related toxicities and complications33, compromising the effect of cancer treatment.
The strengths of our study include a large sample size, a standardized assessment of anthropometrics, ability to adjust for a wide range of potential confounding variables, and the nearly complete follow-up on outcomes. Limitations to our study include a lack of data on post-diagnosis BMI/WHR information, treatment related complications, and detailed cancer treatment data. Because men and women were recruited in somewhat different time periods, potential period effects should be considered when comparing the results between men and women. Although the pre-diagnosis BMI used in this study was measured at different times before diagnosis, results from our survival analyses stratified by different intervals between measurement and diagnosis were very similar.
In conclusion, our cohort study found that pre-diagnostic BMI was associated with survival among CRC patients following a U-shape pattern; obesity determined prior to cancer diagnosis was independently associated with poor prognosis for CRC patients, whereas no statistically significant associations were observed for WHR. These findings indicate the importance of maintaining a healthy weight which may help to potentially improve prognosis following a colorectal cancer diagnosis.
Supplementary Material
Brief description of the novelty and impact of the work.
Previous studies showed an inconsistent association of general obesity and survival in patients with colorectal cancer (CRC), and this association has been understudied among Asian populations. Central obesity, as often measured using waist-to-hip ratio, has even less adequately studied in relation to survival outcome of CRC patients. In our study, pre-diagnostic BMI was associated with survival among CRC patients following a U-shape pattern, while no statistically significant associations were observed for WHR.
Acknowledgments
This work was supported by grants from the United States National Institutes of Health (R37 CA070867, UM1 CA182910, and UM1 CA173640).
Abbreviations
- CRC
colorectal cancer
- BMI
body mass index
- WHR
waist-hip ratio
- SWHS
the Shanghai Women’s Health Study
- SMHS
the Shanghai Men’s Health Study
- WHO
World Health Organization
- HR
Hazard ratio
- 95% CI
95% confidence interval
References
- 1.Vrieling A, Kampman E. The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: a review of the literature. Am J Clin Nutr. 2010;92:471–90. doi: 10.3945/ajcn.2010.29005. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet Lond Engl. 2014;383:1490–502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X-S, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet Lond Engl. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Institute for Cancer Research, World Cancer Research Fund. Food, nutrition, physical activity and the prevention of cancer: a global perspective: a project of World Cancer Research Fund International. Washington, D.C: American Institute for Cancer Research; 2007. p. 517. [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet Lond Engl. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Fedirko V, Romieu I, Aleksandrova K, Pischon T, Trichopoulos D, Peeters PH, Romaguera-Bosch D, Bueno-de-Mesquita HB, Dahm CC, Overvad K, Chirlaque M-D, Johansen C, et al. Pre-diagnostic anthropometry and survival after colorectal cancer diagnosis in Western European populations: Anthropometry and colorectal cancer survival. Int J Cancer. 2014;135:1949–60. doi: 10.1002/ijc.28841. [DOI] [PubMed] [Google Scholar]
- 7.Doria-Rose VP, Newcomb PA, Morimoto LM, Hampton JM, Trentham-Dietz A. Body Mass Index and the Risk of Death Following the Diagnosis of Colorectal Cancer in Postmenopausal Women (United States) Cancer Causes Control. 2006;17:63–70. doi: 10.1007/s10552-005-0360-0. [DOI] [PubMed] [Google Scholar]
- 8.Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O’Connell MJ, Wolmark N. Body Mass Index and Outcomes in Patients Who Receive Adjuvant Chemotherapy for Colon Cancer. JNCI J Natl Cancer Inst. 2006;98:1647–54. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 9.Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of Body Mass Index on Survival After Colorectal Cancer Diagnosis: The Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 10.Ballian N, Yamane B, Leverson G, Harms B, Heise CP, Foley EF, Kennedy GD. Body Mass Index does not Affect Postoperative Morbidity and Oncologic Outcomes of Total Mesorectal Excision for Rectal Adenocarcinoma. Ann Surg Oncol. 2010;17:1606–13. doi: 10.1245/s10434-010-0908-4. [DOI] [PubMed] [Google Scholar]
- 11.Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, Lane DS, Wactawski-Wende J, Hou L, Jackson RD, Kampman E, Newcomb PA. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012;23:1939–48. doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simkens LHJ, Koopman M, Mol L, Veldhuis GJ, Ten Bokkel Huinink D, Muller EW, Derleyn VA, Teerenstra S, Punt CJA. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur J Cancer. 2011;47:2560–7. doi: 10.1016/j.ejca.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Kocarnik JM, Chan AT, Slattery ML, Potter JD, Meyerhardt J, Phipps A, Nan H, Harrison T, Rohan TE, Qi L, Hou L, Caan B, et al. Relationship of prediagnostic body mass index with survival after colorectal cancer: Stage-specific associations. Int J Cancer. 2016;139:1065–72. doi: 10.1002/ijc.30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, Xiao J, Caan BJ. Analysis of Body Mass Index and Mortality in Patients With Colorectal Cancer Using Causal Diagrams. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogino S, Nosho K, Baba Y, Kure S, Shima K, Irahara N, Toyoda S, Chen L, Kirkner GJ, Wolpin BM, Chan AT, Giovannucci EL, et al. A Cohort Study of STMN1 Expression in Colorectal Cancer: Body Mass Index and Prognosis. Am J Gastroenterol. 2009;104:2047–56. doi: 10.1038/ajg.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prizment AE, Flood A, Anderson KE, Folsom AR. Survival of Women with Colon Cancer in Relation to Precancer Anthropometric Characteristics: the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:2229–37. doi: 10.1158/1055-9965.EPI-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma RCW, Chan JCN. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States: Diabetes in East Asians. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min YW, Kim S-A, Lee JH, Kim JY, Chang DK, Rhee P-L, Kim JJ, Rhee JC, Kim Y-H. Overweight is Associated with a Favorable Survival in Patients with Colorectal Cancer: A Prospective Cohort Study in an Asian Population. Ann Surg Oncol. 2012;19:3460–4. doi: 10.1245/s10434-012-2436-x. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto N, Fujii S, Sato T, Oshima T, Rino Y, Kunisaki C, Masuda M, Imada T. Impact of body mass index and visceral adiposity on outcomes in colorectal cancer: Obesity and outcome in colorectal cancer. Asia Pac J Clin Oncol. 2012;8:337–45. doi: 10.1111/j.1743-7563.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu D, Li Q, Yang Z, Hu X, Qian W, Du Y, Liu B. Association of body mass index and smoking on outcome of Chinese patients with colorectal cancer. World J Surg Oncol. 2013;11:271. doi: 10.1186/1477-7819-11-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon H-G, Ju Y-T, Jeong C-Y, Jung E-J, Lee Y-J, Hong S-C, Ha W-S, Park S-T, Choi S-K. Visceral Obesity May Affect Oncologic Outcome in Patients with Colorectal Cancer. Ann Surg Oncol. 2008;15:1918–22. doi: 10.1245/s10434-008-9891-4. [DOI] [PubMed] [Google Scholar]
- 22.Zheng W. The Shanghai Women’s Health Study: Rationale, Study Design, and Baseline Characteristics. Am J Epidemiol. 2005;162:1123–31. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 23.Shu X-O, Li H, Yang G, Gao J, Cai H, Takata Y, Zheng W, Xiang Y-B. Cohort Profile: The Shanghai Men’s Health Study. Int J Epidemiol. 2015;44:810–8. doi: 10.1093/ije/dyv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Li W, Johnson J, Heymsfield SB, Cefalu WT, Ryan DH, Hu G. Body mass index and the risk of all-cause mortality among patients with type 2 diabetes mellitus. Circulation. 2014;130:2143–51. doi: 10.1161/CIRCULATIONAHA.114.009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkin E, O’Reilly DA, Sherlock DJ, Manoharan P, Renehan AG. Excess adiposity and survival in patients with colorectal cancer: a systematic review. Obes Rev Off J Int Assoc Study Obes. 2014;15:434–51. doi: 10.1111/obr.12140. [DOI] [PubMed] [Google Scholar]
- 26.Denost Q, Quintane L, Buscail E, Martenot M, Laurent C, Rullier E. Short- and long-term impact of body mass index on laparoscopic rectal cancer surgery: Body mass index and laparoscopic rectal cancer surgery. Colorectal Dis. 2013;15:463–9. doi: 10.1111/codi.12026. [DOI] [PubMed] [Google Scholar]
- 27.Görög D, Nagy P, Péter A, Perner F. Influence of obesity on lymph node recovery from rectal resection specimens. Pathol Oncol Res POR. 2003;9:180–3. doi: 10.1007/BF03033734. [DOI] [PubMed] [Google Scholar]
- 28.Ishii Y, Hasegawa H, Nishibori H, Watanabe M, Kitajima M. Impact of visceral obesity on surgical outcome after laparoscopic surgery for rectal cancer. Br J Surg. 2005;92:1261–2. doi: 10.1002/bjs.5069. [DOI] [PubMed] [Google Scholar]
- 29.Hardwick JCH, Van Den Brink GR, Offerhaus GJ, Van Deventer SJH, Peppelenbosch MP. Leptin Is a Growth Factor for Colonic Epithelial Cells. Gastroenterology. 2001;121:79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- 30.Renehan AG, Roberts DL, Dive C. Obesity and cancer: Pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 31.Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, Giovannucci EL, Loda M, Fuchs CS. Cohort Study of Fatty Acid Synthase Expression and Patient Survival in Colon Cancer. J Clin Oncol. 2008;26:5713–20. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morikawa T, Kuchiba A, Liao X, Imamura Y, Yamauchi M, Qian ZR, Nishihara R, Sato K, Meyerhardt JA, Fuchs CS, Ogino S. Tumor TP53 expression status, body mass index and prognosis in colorectal cancer. Int J Cancer. 2012;131:1169–78. doi: 10.1002/ijc.26495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB, Macdonald JS, Fuchs CS. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–95. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


