Abstract
Intracochlear ATP is an important mediator in regulating hearing function. ATP can activate ionotropic purinergic (P2x) and metabotropic purinergic (P2y) receptors to influence cell functions. In this paper, we report that ATP can activate P2x receptors directly to modify outer hair cell (OHC) electromotility, which is an active cochlear amplifier determining hearing sensitivity and frequency selectivity in mammals. We found that ATP, but not UTP, a P2y receptor agonist, reduced the OHC electromotility-associated nonlinear capacitance (NLC) and shifted its voltage dependence to the right (depolarizing) direction. Blockage of the activation of P2x receptors by pyridox-alphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), suramin, and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) could block the ATP effect. This modification also required extracellular Ca++ participation. Removal of extracellular Ca++ abolished the ATP effect. However, chelation of intracellular Ca++ concentration by a fast calcium-chelating reagent 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA, 10 mM) did not affect the effect of ATP on NLC. The effect is also independent of K+ ions. Substitution of Cs+ for intracellular or extracellular K+ did not affect the ATP effect. Our findings indicate that ATP activates P2x receptors instead of P2y receptors to modify OHC electromotility. Extracellular Ca++ is required for this modification.
Keywords: ATP, P2x receptor, Outer hair cell electromotility, Calcium, Prestin
Introduction
ATP, acting as a major extracellular signaling molecule, can influence many physiological functions including hearing. An early experiment demonstrated that the application of ATP to the perilymph in the guinea pig cochlea affected auditory nerve activity [1]. Intracochlear perfusion of ATP also reduced the cochlear microphonics (CM) and the compound action potential [2–5]. It has been reported that ATP can evoke inward currents and raise the intracellular Ca++ concentration in cochlear outer and inner hair cells, thereby modifying sound transduction and neurotransmission [6–11]. ATP can also reduce the cubic component (2f1 − f2) in distortion product otoacoustic emissions (DPOAEs) [2, 12], which is generated by active cochlear mechanics.
Outer hair cell (OHC) electromotility [13] is an active cochlear amplifier in mammals that boosts vibration of the basilar membrane to increase auditory sensitivity and frequency selectivity [14, 15]. Recently, we have demonstrated that gap junction hemichannels in the cochlear supporting cells can release ATP to mediate OHC electromotility [16]. However, the mechanism underlying the effect of ATP on OHC electromotility remains unclear. ATP can activate purinergic (P2) receptors to influence cell functions [17, 18]. P2 receptors have two subgroups: ATP-gated ionotropic (P2x) receptors and G protein-coupled metabotropic (P2y) receptors. P2x receptors contain intrinsic pores that switch conformation from closed to open while binding ATP, allowing ions to flow and changing the membrane potential and local ion concentrations. In contrast, P2y receptors couple to intracellular second messenger systems through heteromeric G proteins. Each subgroup has several subtypes [17, 18]. OHCs have both P2x and P2y expressions [10, 16, 19–24]. In this study, the P2 receptor activity and ionic dependence underlying the effect of ATP on OHC electromotility were studied. The nonlinear capacitance (NLC) as an electrical index of OHC electromotility was measured by patch clamp recording. We found that ATP activated P2x receptors rather than P2y receptors directly to modify OHC electromotility-associated NLC. We also show that the extracellular Ca++ is required for this modification.
A preliminary report of this work has been presented in abstract form [25].
Materials and methods
Outer hair cell preparation
The OHCs were freshly isolated from adult guinea pigs (250–400 g) as previously described [10, 15, 26–27]. Briefly, the temporal bones were removed after decapitation. The isolated otic capsule was dissected in a normal extracellular solution (NES) (130 NaCl, 5.37 KCl, 1.47 MgCl2, 2 CaCl2, and 10 HEPES in mM; 300 mOsm and pH 7.2) and the organ of Corti was exposed. After the removal of the stria vascularis and spiral ligament, the sensory epithelium (organ of Corti) was picked away with a sharpened needle and further dissociated by trypsin (1 mg/ml) for 5–10 min. The dissociated cells were transferred to a recording dish for recording. All experimental procedures were performed at room temperature (23°C) and conducted in accordance with the policies of University of Kentucky’s Animal Care and Use Committee.
Patch clamp recording and nonlinear capacitance measurement
The cells were continuously perfused with the NES (0.5 ml/min) and the classical whole-cell recording was performed. A patch pipette was filled with an intracellular solution (140 KCl, 10 ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 2 MgCl2, 10 HEPES in mM; 300 mOsm and pH 7.2) and had an initial resistance of 2.5–3.5 MΩ in the bath solution. The patch pipette was patched at the basal nuclear pole of the OHC under a whole-cell configuration using Axopatch 200B (Axon, CA, USA). The recording was performed by jClamp (SciSoft, New Haven, CT, USA). The OHC electromotility-associated NLC was measured with a two-sinusoidal wave voltage stimulus in jClamp [27, 28]. This voltage stimulus was composed of a ramp command (−150 to +150 mV) summed with two-sinusoidal commands (f1=390.6 Hz, f2= 781.3 Hz, 25 mV peak to peak). The signal was filtered by a four-pole low-pass Bessel filter with a cut-off frequency of 10 kHz and digitized utilizing a Digidata 1322A (Axon, CA, USA). The capacitance was calculated by admittance analysis of the current response [30]. The peak of NLC and the voltage corresponding to the peak capacitance (Vpk) were also continuously recorded by a phase-tracking technique (sampling rate=4/s, tracking step=0.25 mV) [27, 28].
Data processing
Data analysis was performed with jClamp and MATLAB. The voltage-dependent NLC was fitted to the first derivative of a two-state Boltzmann function:
| (1) |
where Qmax is the maximum charge transferred, Vpk is the potential that corresponds to the peak of NLC and also has an equal charge distribution, z is the number of elementary charge (e), k is Boltzmann’s constant, T is the absolute temperature, and Clin is the cell membrane capacitance. Curve fitting and figure plotting was performed with SigmaPlot software. Membrane potential (Vm) was corrected for pipette series resistance (Rs).
Chemicals and chemical perfusion
All chemicals were purchased from Sigma Chemical Company (St. Louis, USA). ATP and chemicals were locally delivered by a Y-tube perfusion system [15, 16], which was controlled by either programming or manually. The perfusion delay was less than 1 s. The bath perfusion was stopped as the local Y-tube perfusion was performed.
Results
The effect of ATP on OHC electromotility
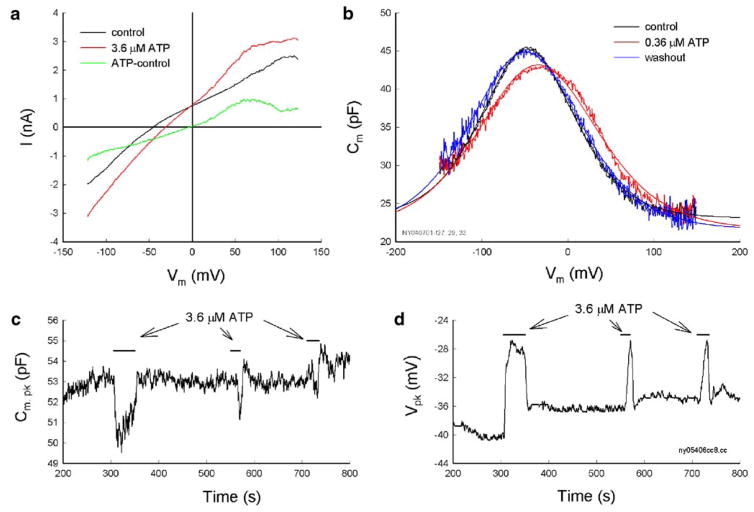
The OHC electromotility-associated NLC was recorded in OHCs varying in length from 40 to 80 μm. The range of the zero-current potential (Vz) was from −93 to −22 mV; the mean was −52±13 mV (SD, n=122). The recorded Vpk of the NLC varied from −98 to −10 mV; the mean was −37± 16 mV. Application of ATP-evoked inward current and depolarized cells (Fig. 1a). The Vz shifted to −37.5±9 mV (n=10) for application of 3.6 μM ATP. Application of ATP also shifted the voltage dependence of NLC to the depolarizing (right) direction and reduced the peak capacitance (Fig. 1b–d). The effect was reversible. After washout, the NLC was returned back to the control level (Fig. 1b). The ATP effect could also reoccur as ATP was repeatedly applied (Fig. 1c and d). The Vpk of NLC was shifted by almost the same value (Fig. 1d). The reduction in NLC, however, appeared to be slightly reduced (Fig. 1c). The reversible and repeatable ATP responses could be observed in all tested ATP concentrations from 3.6 nM to 36 μM. The EC50 value of the ATP effect on NLC was 79.7 nM (Fig. 3), close to the nanomolar concentration of ATP measured in the cochlea [29].
Fig. 1.
The ATP-evoked OHC electric response and the effect on OHC electromotility-associated NLC. a Current–voltage relations for the ATP application. A voltage ramp (−150 to +150 mV) was applied from a holding potential of −40 mV and corrected for pipette series resistance (Rs=8.7 MΩ). The zero-current potential had a shift from −45.3 to −31.2 mV for the ATP application. The subtracted reversal potential for the ATP response was −3.5 mV. b ATP reduces NLC and shifts its voltage dependence to the depolarization direction. Smooth lines represent curve fitting by the Boltzmann function. The parameters of fitting are Qmax=3.26, 4.03, and 4.03 pC; z=0.70, 0.55, and 0.60; Vpk= −46.9,−34.9, and −49.5 mV; and Clin=23.1, 21.5, and 21.6 pF for control, 0.36 μM ATP perfusion, and washout, respectively. c and d The ATP effect is reversible and repeatable. The peak of NLC (Cm, pk) and the voltage of the peak capacitance were continuously recorded by the phase-tracking technique. The horizontal bars represent the perfusion of ATP
Fig. 3.
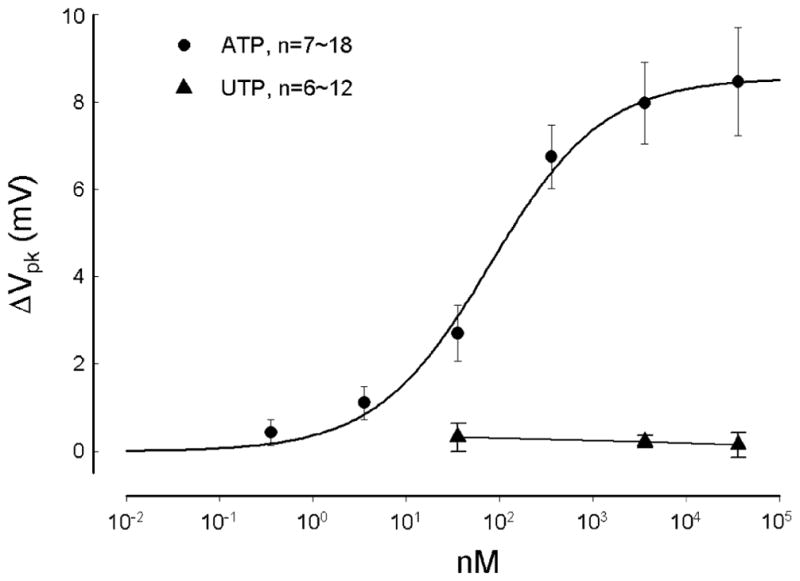
Dose curves of the effects of ATP and UTP on the Vpk of NLC. The smooth line represents data fitting to a Hill’s function ΔVpk=a× Cn/(Kn+Cn) where n=0.71 (Hill coefficient) and K=79.7 nM (EC50) for ATP
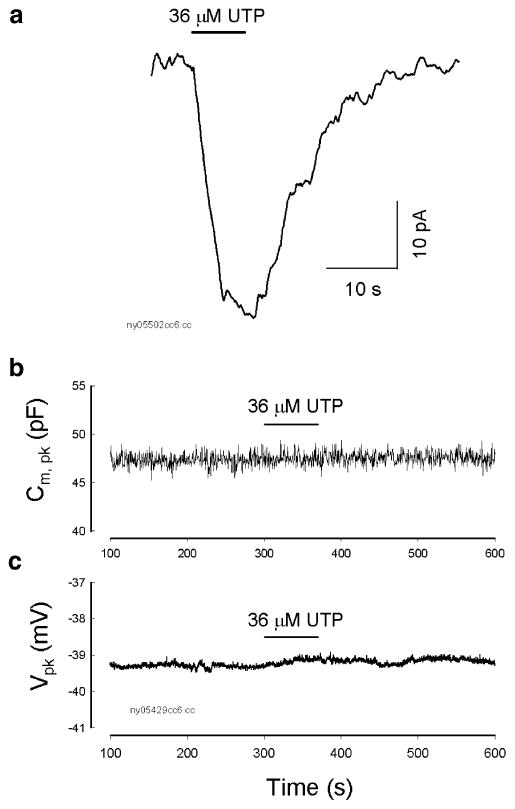
Ineffectiveness of UTP on OHC electromotility
Unlike ATP that can activate both P2x and P2y receptors, UTP is a P2y receptor agonist [17, 18]. Extracellular perfusion of UTP could also evoke an inward current in some OHCs (n=6/18, Fig. 2a). However, UTP never shifted or reduced NLC. Figure 2b and c shows that the NLC and Vpk were not changed by the perfusion of UTP. Dose curve also shows that UTP did not affect NLC even though high concentrations of UTP were used (Fig. 3). Thus, the activation of P2y receptors does not directly influence OHC electromotility.
Fig. 2.
Ineffectiveness of a P2y receptor agonist UTP on OHC electromotility-associated NLC. a A UTP-evoked inward current in an OHC. The cell was held at −40 mV. The horizontal bar indicates the perfusion of 36 μM UTP. b and c UTP did not affect OHC electromotility. The NLC and Vpk were continuously recorded. The horizontal bars represent the perfusion of 36 μM UTP. Perfusion of UPT did not reduce NLC and shift its Vpk
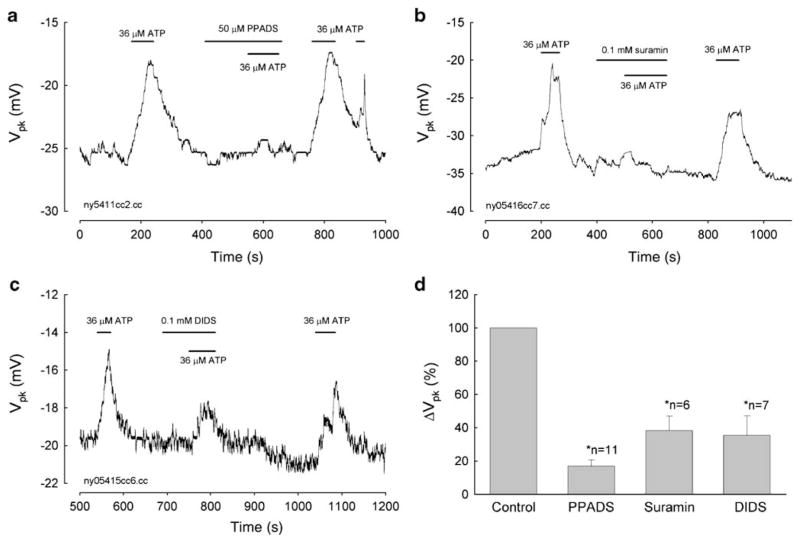
Blocking the effect of ATP on OHC electromotility by P2x receptor antagonists
Figure 4a–c shows the reduction in the ATP effect by blocking the activation of P2x receptors. After preapplication of pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS, 50 μM), suramin (0.1 mM), and 4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid (DIDS, 0.1 mM), ATP-induced Vpk shift in NLC was significantly reduced to 16.93±3.67%, 38.28±8.74%, and 35.37±11.79%, respectively (Fig. 4d). However, the antagonist itself had no effect on NLC (see the response to the beginning of the middle bar for antagonist perfusion in Fig. 4a–c). The blockage was reversible. After washout of antagonists, the ATP effect was restored. This further indicates the effect of ATP on OHC electromotility through the activation of P2x receptors.
Fig. 4.
Blockage of the ATP effect on NLC by P2x receptor antagonists. a–c Applications of PPADS, suramin, and DIDS eliminate the effect of ATP on OHC electromotility-associated NLC. The block is reversible. The horizontal bars represent ATP and blocker applications. d Elimination of the ATP effect on the Vpk of NLC by P2 receptor antagonists. The Vpk shift for the application of 36 μM ATP under the treatments of P2 receptor antagonists was normalized to the response at the pretreatment in the same cell and then averaged. Error bars represent SE. Asterisks indicate that there is a significant difference in statistical analysis (P<0.001, paired t test)
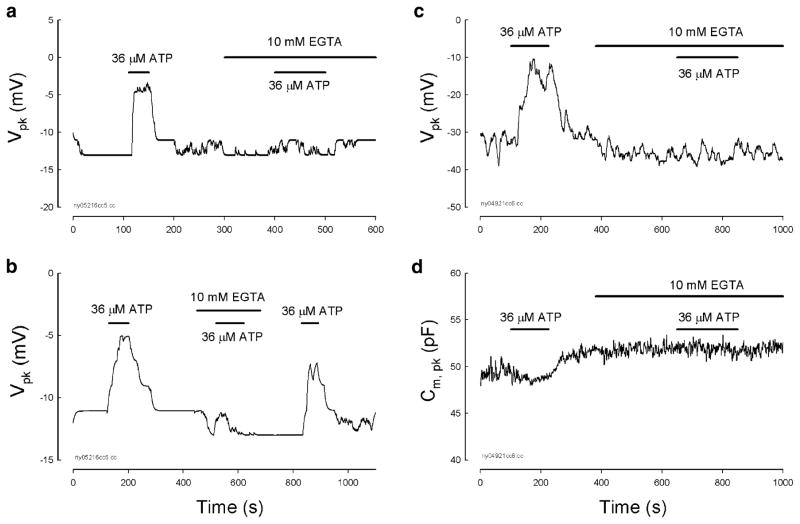
Ca++ dependence of the ATP effect on OHC electromotility
One important characteristic of P2x receptors is its permeability to K+ and Ca++ cationic ions [17, 18]. We found that extracellular perfusion of 10 mM EGTA Ca++-free extracellular solution (130 NaCl, 5.37 KCl, 1.47 MgCl2, 10 EGTA, and 10 HEPES in mM; 300 mOsm and pH 7.2) to remove extracellular Ca++ abolished the ATP-evoked changes in NLC (Fig. 5). This inhibition was reversible. After reperfusion of the NES to restore the extracellular Ca++ concentration (2 mM), the ATP response was also restored (Fig. 5b). However, removal of the extracellular Ca++ alone did not affect NLC (see the response to the beginning of the middle bar for EGTA perfusion in Fig. 5a–d). On the other hand, intracellular Ca++ chelated by a fast Ca++ chelator of 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA, 10 mM) did not affect the ATP-evoked changes in NLC (Figs. 5c–d and 6). As the patch pipette was filled with an intracellular solution buffered with 10 mM BAPTA, the application of 36 μM ATP could still evoke a positive Vpk shift (Figs. 5c–d and 6b–c). Perfusion of 10 mM EGTA Ca++-free extracellular solution to remove extracellular Ca++ also abolished the ATP effect on NLC (Fig. 5c–d).
Fig. 5.
Extracellular Ca++ is required for ATP-modifying OHC electromotility-associated NLC. a and b The patch pipette was filled with normal intracellular solution and the bath was perfused with NES. Removal of extracellular Ca++ ions by application of 10 mM EGTA Ca++-free extracellular solution eliminated the ATP effect. The elimination is reversible. Reperfusion of NES restored the ATP effect (b). c and d Ineffectiveness of intracellular Ca++ on the ATP effect. The patch pipette was filled by the intracellular solution with 10 mM BAPTA to chelate intracellular Ca++ concentration; intracellular K+ was also replaced with Cs+ (140 CsCl, 10 BAPTA, 2 MgCl2, and 10 HEPES in mM). The bath was perfused with NES. The effect of ATP on NLC remained. Extracellular perfusion of 10 mM EGTA also abolished the ATP effect
Fig. 6.
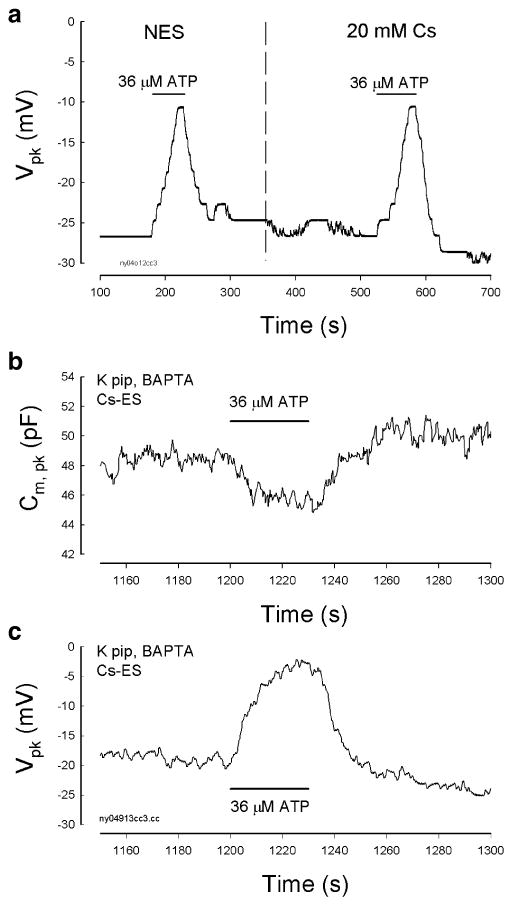
Independence of the ATP effect on K+ and Na+. a The effect of ATP is independent of K+ ions. The dashed vertical line indicates that an OHC was alternatively perfused with NES and 20 mM Cs+ extracellular solution (130 NaCl, 20 CsCl, 2 CaCl2, 1.47 MgCl2, and 10 HEPES in mM) to replace extracellular K+. The patch pipette was also filled with 140 mM Cs+ to replace K+ (Cs pipette: 140 CsCl, 10 EGTA, 2 MgCl2, and 10 HEPES in mM). b and c The effect of ATP on OHC electromotility-associated NLC is independent of extracellular Na+. The patch pipette was filled with a BAPTA-buffered intracellular solution. The OHC was perfused with a Cs-based extracellular solution (Cs-ES), which Na+ and K+ were replaced with 140 mM Cs+ (140 CsCl, 2 CaCl2, 1.47 MgCl2, and 10 HEPES in mM)
The effect of ATP on OHC electromotility is independent of K+ and Na+ ions
OHC electromotility is independent of K+ and Na+. Intracellular and extracellular K+ and Na+ were also not required for the ATP modification on OHC electromotility (Figs. 5, 6, and 7). When the intracellular K+ was replaced with Cs+, the zero-current membrane potential became −17.9±7.3 mV (n=23). However, the effect of ATP on NLC were not affected (Figs. 5c–d and 6a). Moreover, ATP-evoked response still existed as the extracellular K+ was replaced with Cs+ (Fig. 6a). Because the P2x receptors are permeable to Na+ cations as well [18], we used Cs+ to replace extracellular Na+ (Fig. 6b–c); the effect of ATP on NLC remained and had no changes.
Fig. 7.
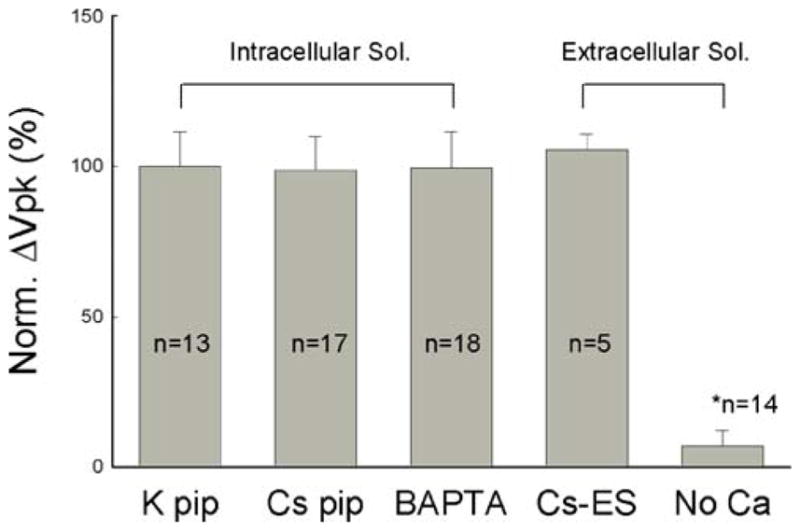
Ionic effect on ATP-modifying OHC electromotility-associated NLC. Vpk shifts for the application of 36 μM ATP under different ionic conditions were normalized to the Vpk shift under the normal condition (K pipette). Cs pip Cs+ to replace K+ in the intracellular solution, BAPTA BAPTA-buffered pipette, Cs-ES the Cs-based extracellular solution in which Na+ and K+ were replaced with Cs+, No Ca Ca++-free extracellular solution that contains 0 mM Ca++ and 10 mM EGTA. Error bars represent SE. The asterisk indicates a statistically significant difference (P<0.001, ANOVA)
The ionic dependence of the ATP effect on OHC electromotility was summarized in Fig. 7. The effect of ATP on OHC electromotility did not have changes when the intracellular K+ was substituted by Cs+ or the intracellular Ca++ concentration was chelated by BAPTA. The ATP effect was also not changed as the extracellular Na+ and K+ substituted by Cs+. However, the removal of extracellular Ca++ abolished the effect of ATP on NLC. The Vpk shift reduced to 6.96±5.06% (Fig. 7).
The ATP effect cannot be abolished by the inactivation of calcium channels. Figure 8 shows that as the OHC was depolarized at +40 mV, the application of ATP could still reduce the peak of NLC and shift the NLC curve to the positive direction (Fig. 8). The Cm, pk reduced from 49.0 to 47.9 pF and the Vpk shifted from −45.8 to −30.9 mV for the ATP application. Positive holding potential could not block the effect of ATP on NLC (n=10).
Fig. 8.
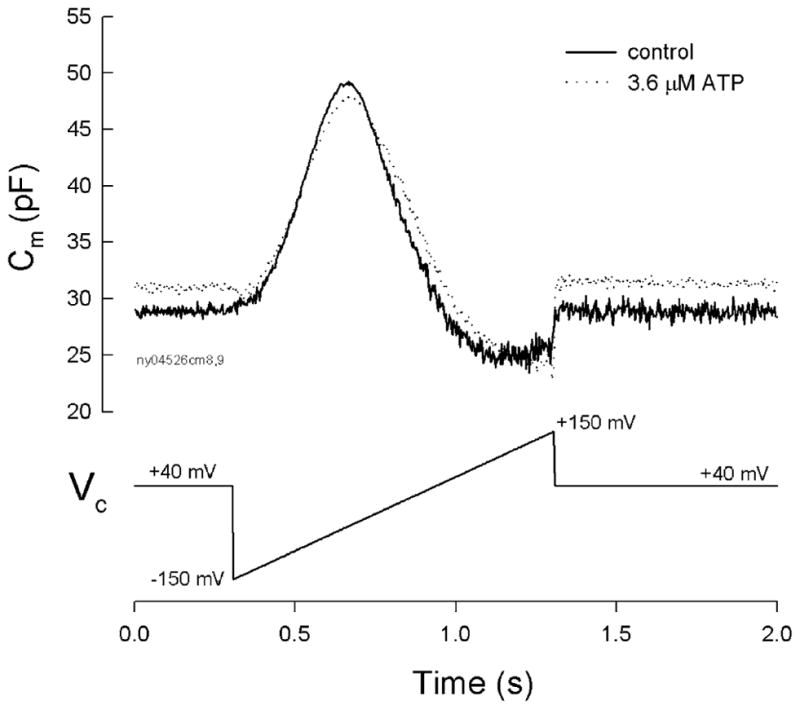
Ineffectiveness of positive holding potential on the ATP influence. The ATP effect on NLC was measured at the holding potential of +40 mV. Solid and dotted lines represent the membrane capacitance measured at control and 3.6 μM ATP application for 10 min, respectively. ATP reduced the peak of NLC and shifted the NLC curve to the positive direction. Note that there is a small (approximately 2 pF) increase rather than decrease in capacitance at the holding potential of +40 mV due to the right shift of the NLC curve in the presence of ATP. A trace at the bottom of the figure shows the voltage command
Discussion
In this study, we found that ATP, but not UTP, a P2y receptor agonist, reduced OHC electromotility-associated NLC and shifted the NLC curve in a positive direction (Figs. 1, 2, and 3). Blockage of the activation of P2x receptors could eliminate the effect of ATP on NLC (Fig. 4). We also demonstrated that extracellular Ca++ was required for this modification; the removal of extracellular Ca++ abolished the ATP effect (Figs. 5 and 7). However, the ATP effect still existed as the OHC intracellular Ca++ was chelated by BAPTA (Figs. 5, 6, and 7).
ATP modifies OHC electromotility through the activation of P2x receptors
ATP can activate both P2x and P2y receptors to influence cell functions. OHCs have predominant expressions of P2x2 and P2x7 receptors [10, 16, 21–23]; they also have P2y2 and P2y4 expressions [23, 24, 30] but have no P2y1 and P2y12 expressions [30]. It has been proposed that P2y receptors may be involved in setting the operating point of OHC transduction [31]. In this experiment, we found that a P2y receptor agonist UTP may be able to evoke an inward current (Fig. 2a) but did not alter OHC electromotility-associated NLC (Figs. 2b and c, 3). Moreover, blockage of P2x receptor activation could eliminate the ATP effect on NLC (Fig. 4). A previous experiment demonstrated that UTP had no effect on EP and CM [4]. Taken together, these results indicate that the activation of P2x receptors rather than P2y receptors is mainly responsible for this purinergic regulation on OHC electromotility.
Extracellular Ca++ ions are required for ATP regulation on OHC electromotility
In the present study, we found that extracellular Ca++ is required for ATP modulating OHC electromotility (Figs. 5 and 7), indicating that extracellular Ca++ plays a key role in the effect of ATP on OHC electromotility. It has been hypothesized that Ca++ modulates OHC electromotility via two pathways: modifying cytoskeletal stiffness [32] and shifting the voltage sensitivity of OHC electromotility [33]. The effect of ATP on OHC electromotility may follow the same concept, activating the P2x receptors allowing Ca++ influxing to modify OHC electromotility.
P2x receptors can permeate to Ca++ and K+ cationic ions to induce an inward cationic current after activation by ATP [17, 18]. This has been demonstrated in different cochlear cells, including hair cells [34]. There are multiple expressions of P2x receptors in OHCs [10, 16, 22, 23, 30]. P2x2 is a predominant isoform expressed at the stereocilia and the cuticular plate [10, 16, 22, 23]. It has been further hypothesized that the application of ATP may also result in the diffusion of a G protein to a specialized inositol 1,4,5-trisphosphate (IP3) receptor-gated Ca++ store (Hensen’s body) present beneath the cuticular plate to raise intracellular Ca++ and affect the mechanoelectrical conductance, particularly the adaptation rate [35]. In this experiment, the chelation of intracellular Ca++ by a fast Ca++ chelator BAPTA did not alter the effect of ATP on OHC electromotility (Figs. 5, 6, and 7). We also found that depolarization to suppress Ca++ entry via calcium channels did not eliminate the ATP effect (Fig. 8). These findings indicate that this long-distance mechanism is unlikely to play an important role in the modification of ATP on OHC electromotility.
However, our data cannot rule out the possibility of the Ca++ local effect. It has been reported that P2x7 receptors are predominantly expressed at the OHC basolateral wall [16, 30]. Also, there is a dense distribution of IP3 receptors along the OHC lateral wall just beneath the plasma membrane [33, 35]. It has been proposed that the application of acetylcholine (Ach) can induce the release of Ca++ from intracellular stores located near the lateral plasma membrane to influence OHC function [33, 36, 37]. Currently, the subcellular mechanism underlying the regulation of OHC electromotility is little known. Our preliminary data shows that the cytoskeleton in the extracisternal space between the plasma membrane and the subsurface cisternae at the OHC lateral wall plays an important role in the OHC electromotility modification, including the modification of ATP on OHC electromotility [38]. The complete mechanism underlying the effect of ATP on OHC electromotility may be complex and requires further studies to elucidate it.
Possible mechanisms of ATP regulation in hearing function
OHC electromotility is an active cochlear amplifier that determines the hearing sensitivity in mammals [14]. Extracellular perfusion of ATP shifted OHC electromotility to the depolarizing direction and reduced NLC (Figs. 1, 3, 4, and 5). These changes can reduce the gain of the cochlear amplifier to decrease hearing sensitivity. It has been found that intracochlear perfusion of ATP could reduce both the endocochlear potential (EP) and CM [3]. The intracochlear perfusion of ATP also decreased DPOAEs [2, 3]. These proposed changes resulted from shifting the operating point of the cochlear amplifier to reduce the amplifier gain [31]. In the present study, we found that ATP shifted the NLC to the positive (depolarizing) direction (Figs. 1, 3, 4, and 5). This provides strong evidence that ATP can directly shift the operating point of active cochlear amplifier.
We have reported that gap junction hemichannels in the cochlear supporting cells can release ATP [16, 39]. ATP shifted the NLC curve in the depolarization direction (Figs. 1, 3, 4, 5, 6, and 8), thereby possibly decreasing hearing sensitivity. We also found that the hemichannel-mediated ATP release is increased as mechanical stimulation (sound stimulation) increased [16]. It has been reported that acoustic overstimulation can increase Ca++ concentration in the OHC and cause dynamic contractions of the organ of Corti in vivo [40]. This indicates that this purinergic hearing control can play an important role in the regulation of hearing sensitivity.
Acknowledgments
This work was supported by NIDCD DC 05989. We thank P.G. Wilson for the technical support.
Contributor Information
Ning Yu, Department of Surgery–Otolaryngology, University of Kentucky Medical Center, 800 Rose Street, Lexington, KY 40536-0293, USA. Department of Otorhinolaryngology, Institute of Otolaryngology, Chinese PLA General Hospital, Beijing 100853, People’s Republic of China.
Hong-Bo Zhao, Department of Surgery–Otolaryngology, University of Kentucky Medical Center, 800 Rose Street, Lexington, KY 40536-0293, USA.
References
- 1.Bobbin RP, Thompson MH. Effects of putative transmitters on afferent cochlear transmission. Ann Otol Rhinol Larygnol. 1978;87:185–190. doi: 10.1177/000348947808700207. [DOI] [PubMed] [Google Scholar]
- 2.Kujawa SG, Erostegui C, Fallon M, Crist J, Bobbin RP. Effects of adenosine 5′-triphosphate and related agonists on cochlear function. Hear Res. 1994;76:87–100. doi: 10.1016/0378-5955(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 3.Munoz DJ, Thorne PR, Housley GD, Billett TE, Battersby JM. Extracellular adenosine 5′-triphosphate (ATP) in the endolymphatic compartment influences cochlear function. Hear Res. 1995;90:106–118. doi: 10.1016/0378-5955(95)00152-3. [DOI] [PubMed] [Google Scholar]
- 4.Munoz DJ, Thorne PR, Housley GD. P2X receptor-mediated changes in cochlear potentials arising from exogenous adenosine 5′-triphosphate in endolymph. Hear Res. 1999;138:56–64. doi: 10.1016/s0378-5955(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 5.Sueta T, Paki B, Everett AW, Robertson D. Purinergic receptors in auditory neurotransmission. Hear Res. 2003;183:97–108. doi: 10.1016/s0378-5955(03)00221-1. [DOI] [PubMed] [Google Scholar]
- 6.Ashmore JF, Ohmori H. Control of intracellular calcium by ATP in isolated outer hair cells of the guinea-pig cochlea. J Physiol. 1990;428:109–131. doi: 10.1113/jphysiol.1990.sp018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa T, Akaike N, Kimitsuki T, Komune S, Arima T. ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol. 1990;63:1068–1074. doi: 10.1152/jn.1990.63.5.1068. [DOI] [PubMed] [Google Scholar]
- 8.Dulon D, Mollard P, Aran JM. Extracellular ATP elevates cytosolic Ca2+ in cochlear inner hair cells. NeuroReport. 1991;2:69–72. doi: 10.1097/00001756-199102000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Housley GD, Greenwood D, Ashmore JF. Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc R Soc Lond B. 1992;249:265–273. doi: 10.1098/rspb.1992.0113. [DOI] [PubMed] [Google Scholar]
- 10.Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugasawa M, Erostegui C, Blanchet C, Dulon D. ATP activates non-selective cation channels and calcium release in inner hair cells of the guinea-pig cochlea. J Physiol. 1996;491:707–718. doi: 10.1113/jphysiol.1996.sp021251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skellett RA, Chen C, Fallon M, Nenov AP, Bobbin RP. Pharmacological evidence that endogenous ATP modulates cochlear mechanics. Hear Res. 1997;111:42–54. doi: 10.1016/s0378-5955(97)00093-2. [DOI] [PubMed] [Google Scholar]
- 13.Brownell WE, Bader CR, Bertrand D, Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- 14.Dallos P. The active cochlea. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao HB, Santos-Sacchi J. Auditory collusion and a coupled couple of outer hair cells. Nature. 1999;399:359–362. doi: 10.1038/20686. [DOI] [PubMed] [Google Scholar]
- 16.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PubMed] [Google Scholar]
- 18.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 19.Raybould NP, Housley GD. Variation in expression of the outer hair cell P2X receptor conductance along the guinea-pig cochlea. J Physiol. 1997;498:717–727. doi: 10.1113/jphysiol.1997.sp021896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandle U, Zenner HP, Ruppersberg JP. Gene expression of P2x7-receptors in the developing inner ear of the rat. Neurosci Lett. 1999;273:105–108. doi: 10.1016/s0304-3940(99)00648-5. [DOI] [PubMed] [Google Scholar]
- 21.Jarlebark L, Housley GD, Raybould NP, Vlajkovic S, Thorne PR. ATP-gated ion channels assembled from P2X2 receptor subunits in the mouse cochlea. NeuroReport. 2002;13:1979–1984. doi: 10.1097/00001756-200210280-00030. [DOI] [PubMed] [Google Scholar]
- 22.Jarlebark LE, Housley GD, Thorne PR. Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X2 receptor subunits in adult and developing rat cochlea. J Comp Neurol. 2000;421:289–301. doi: 10.1002/(sici)1096-9861(20000605)421:3<289::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Szucs A, Szappanos H, Toth A, Farkas Z, Panyi G, Csernoch L, Sziklai I. Differential expression of purinergic receptor subtypes in the outer hair cells of the guinea pig. Hear Res. 2004;196:2–7. doi: 10.1016/j.heares.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Parker MS, Nkeiruka NO, Bobbin RP. Localization of the P2Y4 receptor in the guinea pig organ of Corti. J Am Acad Audiol. 2003;14:286–295. [PubMed] [Google Scholar]
- 25.Yu N, Zhao HB. Extracellular ATP mediates outer hair cell electromotility. Proceedings of the 28th Association for Research in Otolaryngology Annual Meeting; New Orleans, LA. 19–24 February; 2005. Available at http://www.aro.org. [Google Scholar]
- 26.Yu N, Zhu ML, Zhao HB. Prestin is expressed on the whole outer hair cell basolateral surface. Brain Res. 2006;1095:51–58. doi: 10.1016/j.brainres.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos-Sacchi J, Zhao HB. Excitation of fluorescent dyes inactivates the outer hair cell integral membrane motor protein prestin and betrays its lateral mobility. Pflugers Arch. 2003;446:617–622. doi: 10.1007/s00424-003-1053-8. [DOI] [PubMed] [Google Scholar]
- 28.Santos-Sacchi J, Kakehata S, Takahashi S. Effects of membrane potential on the voltage dependence of motility-related charge in outer hair cells of the guinea-pig. J Physiol. 1998;510:225–235. doi: 10.1111/j.1469-7793.1998.225bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz DJ, Thorne PR, Housley GD, Billett TE. Adenosine 5′-triphosphate (ATP) concentrations in the endolymph and perilymph of the guinea-pig cochlea. Hear Res. 1995;90:119–125. doi: 10.1016/0378-5955(95)00153-5. [DOI] [PubMed] [Google Scholar]
- 30.Ji N, Zhao HB. Expressions of ATP-gated purinergic (P2) receptors in the cochlear outer hair cells. Proceedings of the 28th Association for Research in Otolaryngology Annual Meeting; New Orleans, LA. 19–24 February; 2005. Available at http://www.aro.org. [Google Scholar]
- 31.Bobbin RP, Salt AN. ATP-gamma-S shifts the operating point of outer hair cell transduction towards scala tympani. Hear Res. 2005;205:35–43. doi: 10.1016/j.heares.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Dallos P, He DZZ, Sziklai I, Metha S, Evans BN. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci. 1997;17:2212–2226. doi: 10.1523/JNEUROSCI.17-06-02212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frolenkov GI, Mammano F, Belyantseva IA, Coling D, Kachar B. Two distinct Ca2+-dependent signaling pathways regulate the motor output of cochlear outer hair cells. J Neurosci. 2000;20:5940–5948. doi: 10.1523/JNEUROSCI.20-16-05940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Housley GD, Marcotti W, Navaratnam D, Yamoah EN. Hair cells—beyond the transducer. J Membr Biol. 2006;209:89–118. doi: 10.1007/s00232-005-0835-7. [DOI] [PubMed] [Google Scholar]
- 35.Mammano F, Frolenkov GI, Lagostena L, Belyantseva IA, Kurc M, Dodane V, Colavita A, Kachar B. ATP-induced Ca2+ release in cochlear outer hair cells: localization of an inositol triphosphate-gated Ca2+ store to the base of the sensory hair bundle. J Neurosci. 1999;19:6918–6929. doi: 10.1523/JNEUROSCI.19-16-06918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanchet C, Erostegui C, Sugasawa M, Dulon D. Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J Neurosci. 1996;16:2574–2584. doi: 10.1523/JNEUROSCI.16-08-02574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans MG. Acetylcholine activates two currents in guinea-pig outer hair cells. J Physiol. 1996;491:563–578. doi: 10.1113/jphysiol.1996.sp021240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu N, Zhao HB. Cytoskeleton mediates outer hair cell electromotility and memory function. Proceedings of the 31st Association for Research in Otolaryngology Annual Meeting; Phoenix, AZ. 16–21 February; 2008. Available at http://www.aro.org. [Google Scholar]
- 39.Zhao HB. Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signaling and metabolic communications. Eur J Neurosci. 2005;21:1859–1868. doi: 10.1111/j.1460-9568.2005.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fridberger A, Flock A, Ulfendahl M, Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc Natl Acad Sci U S A. 1998;95:7127–7132. doi: 10.1073/pnas.95.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]