Abstract
The dopamine (DA) D3 receptor is posited to be importantly involved in drug reward and addiction, and D3 receptor antagonists have shown extraordinary promise as potential anti-addiction pharmacotherapeutic agents in animal models of drug addiction. SB-277011A is the best characterized D3 receptor antagonist in such models. However, the potential use of SB-277011A in humans is precluded by pharmacokinetic and toxicity problems. We here report a novel D3 receptor antagonist YQA14 that shows similar pharmacological properties as SB-277011A. In vitro receptor binding assays suggest that YQA14 has two binding sites on human cloned D3 receptors with Ki-High (0.68 × 10−4 nM) and Ki-Low (2.11 nM), and displays > 150-fold selectivity for D3 over D2 receptors and > 1000-fold selectivity for D3 over other DA receptors. Systemic administration of YQA14 (6.25–25 mg/kg) or SB-277011A (12.5–25 mg/kg) significantly and dose-dependently reduced intravenous cocaine self-administration under both low fixed-ratio and progressive-ratio reinforcement conditions in rats, while failing to alter oral sucrose self-administration and locomotor activity, suggesting a selective inhibition of drug reward. However, when the drug dose was increased to 50 mg/kg, YQA14 and SB-277011A significantly inhibited basal and cocaine-enhanced locomotion in rats. Finally, both D3 antagonists dose-dependently inhibited intravenous cocaine self-administration in wild-type mice, but not in D3 receptor-knockout mice, suggesting that their action is mediated by D3 receptor blockade. These findings suggest that YQA14 has a similar anti-addiction profile as SB-277011A, and deserves further study and development.
Keywords: Cocaine, D3 receptor, dopamine, SB-277011A, self-administration, YQA14
INTRODUCTION
Drug addiction is characterized by drug-induced reward (the ‘high’) and relapse to drug use after abstinence (Wise 1996, 2005; Shalev, Grimm & Shaham 2002). The mesolimbic dopamine (DA) system, which originates in the midbrain ventral tegmental area (VTA) and projects to the forebrain nucleus accumbens (NAc) and prefrontal cortex (PFC), has been shown to be critically involved in both drug reward and relapse (Wise 1996, 2005; Gardner 2000; Shalev et al. 2002; Pierce & Kumaresan 2006). Thus, brain DA receptors have become important targets in medication development for the treatment of addiction. Five DA receptors (D1–D5) have been identified in the brain (Missale et al. 1998). DA D3 receptors are found to primarily in the mesolimbic DA system, including the VTA, NAc, islands of Calleja, and olfactory tubercle (Sokoloff et al. 1990; Bouthenet et al. 1991; Diaz et al. 1995, 2000). Growing evidence suggests that D3 receptors are critically involved in drug reward and also in motivational and cognitive processes implicated in addiction and relapse to drug-seeking behavior (Sokoloff et al. 1992; Caine & Koob 1993; Heidbreder & Newman 2010). Based on this, it has been proposed that selective DA D3 receptor antagonists may be effective for the treatment of drug addiction (Le Foll, Schwartz & Sokoloff 2000; Heidbreder et al. 2005; Newman, Grundt & Nader 2005; Xi & Gardner 2007).
To date, several compounds with D3 receptor antagonist profiles have been synthesized and tested in experimental animals, including SB-277011A, NGB2904, BP-897, S-33138 and PG-01037 (Yuan et al. 1998; Pilla et al. 1999; Reavill et al. 2000; Grundt et al. 2005; Peng et al. 2009; Higley et al. 2011). Among them, SB-277011A is the most well-characterized D3 receptor antagonist in multiple animal models of drug addiction (Heidbreder et al. 2005). Systemic administration of SB-277011A significantly inhibits reinstatement (relapse) of drug-seeking behavior triggered by cocaine or other addictive drugs, triggered by cocaine- or nicotine-associated environmental cues, and triggered by footshock stress (Xi et al. 2004; Gilbert et al. 2005; Gal & Gyertyan 2006; Cervo et al. 2007). SB-277011A also inhibits certain aspects of addictive drug reward as assessed by conditioned place preference (Vorel et al. 2002; Ashby et al. 2003; Cervo et al. 2005; Pak et al. 2006), intracranial electrical brain-stimulation reward (BSR) (Vorel et al. 2002; Pak et al. 2006; Spiller et al. 2008), drug self-administration under second-order reinforcement (Di Ciano et al. 2003; Di Ciano 2008), and drug self-administration under progressive-ratio (PR) and high fixed-ratio (FR10) reinforcement conditions (Andreoli et al. 2003; Gal & Gyertyan 2003; Xi et al. 2005; Ross et al. 2007). SB-277011A, at doses between 3 mg/kg and 24 mg/kg (i.p.), does not produce aversive or rewarding effects (Vorel et al. 2002; Gal & Gyertyan 2003; Xi et al. 2005; Pak et al. 2006; Ross et al. 2007). All such findings strongly support a potential utility for SB-277011A as an anti-addiction medication. However, further development of SB-277011A has been terminated due to pharmacokinetic and toxicity problems (Macdonald et al. 2003; Heidbreder & Newman 2010). Thus, further development of more selective D3 receptor antagonists with improved bioavailability is called for.
Given that NGB-2904 has higher D3 receptor selectivity than SB-277011A, we used NGB-2904 as a template molecule to develop additional highly selective D3 receptor antagonists. YQA14 is the lead compound in this chemical series. YQA14 has been shown to possess longer half-life than SB-277011A in rodents and also better metabolic stability in humans (Li et al. unpublished). Therefore, in the present study, we first examined the in vitro receptor binding and post-receptor G protein coupling properties of YQA14, and then compared its pharmacological action with SB-277011A in animal models of cocaine addiction. Previous studies have shown that SB-277011A (and other D3 antagonists) does not significantly alter low fixed-ratio (FR1, FR2) cocaine (or nicotine or methamphetamine) self-administration (Andreoli et al. 2003; Gal & Gyertyan 2003; Xi et al. 2005; Higley et al. 2011), although—as noted above—it is highly effective at attenuating drug self-administration under high FR or PR reinforcement conditions. Given that YQA14 appears to have significantly higher affinity and selectivity than SB-277011A for D3 versus D2 and other DA receptors, we used FR2 intravenous cocaine self-administration to determine whether YQA14 is more potent and effective than SB-277011A at inhibiting low FR cocaine self-administration. In addition, we also examined the effects of YQA14 and SB-277011A on oral sucrose self-administration and cocaine-induced hyperactivity in rats. Finally, we, for the first time, used D3 receptor-knockout mice to gather additional data to assess whether the pharmacological action produced by YAQ14 and SB-277011A on cocaine self-administration is mediated by blockade of D3 receptors in vivo.
MATERIALS AND METHODS
Experiment 1: in vitro radioligand binding assays
Cell cultures
The protocols for cell culture were the same as reported previously (Wu et al. 2005). hD3R- and hD4R-pcDNA3.1 (+) were purchased from Missouri S&T cDNA Resource Center (Rolla, MO, USA), and transfected to CHO and HEK293 cells, respectively, according to the manufacturer’s instructions (Gibco™, Grand Island, NY, USA). The HEK293 cell lines expressing hD1, hD2 or hD5 receptors, respectively, were obtained from the Shanghai Institute of Biological Sciences, Chinese Academy of Sciences, Shanghai, China (Yu et al. 2008). The transfected cells were cultured for 4–6 weeks in the presence of 1000 μg/ml G418. A single CHO-hD3R or HEK294-hD4R cell clone was then selected and re-cultured in DMEM/F12 medium supplemented with 2 mM Lglutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, 200 μg/ml G418,10%fetal calf serum(FCS), and5% CO2 at 37°C until 80 to 90% confluence.
Cell membrane preparations
The protocols for cell membrane preparations were slightly modified from a previous report (Reavill et al. 2000). The transfected cells, as described above, were transferred from 175-cm2 tissue culture flasks to 145-cm2 Petri dishes, and then allowed to continue to culture until 90% confluence. The cultured cells in the Petri dishes were washed first with 5 ml cold phosphate buffer solution, and then dissipated with separation buffer (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 10mM Na2HPO4, 1.0 mM glucose-H2O, 2.0 mM EDTA-Na2. 2H2O, pH 7.4). The separated cells were then harvested and homogenized in lysis buffer (5 mM Tris, 5 m MEDTA-Na2, 5 mM EGTA). The homogenates were centrifuged at 40 000 g at 4°C for 20 minutes. The resulting pellets were re-suspended in 50 mM Tris-HCl, pH 7.4 and centrifuged twice at 40 000 g at 4°C for 20 minutes each. The resulting pellets were re-suspended in reaction buffer (50 mM Tris, 120 mM NaCl, 5 mM KCl, 5 mM EDTA-Na2. 2H2O, 5 mM MgCl2, 1.5 mM CaCl2, pH 7.4) and stored at −70°C. The protein level in each membrane sample was measured using methods reported previously (Bradford 1976).
In vitro radioligand binding assays
The methods for DA receptor binding were slightly modified from those described in a previous report (Shahid et al. 2009). Stored membranes were thawed and suspended in reaction buffer as described above. The receptor binding assay was carried out in reaction buffer (200 μl) containing 25 μg membrane protein and various concentrations of [3H]spiperone (0.075–4.8 nM) were used to construct saturation binding curves to determine receptor binding activity on transfected cells and an optimal concentration of [3H]spiperone to be used in subsequent YQA14 receptor binding assays. The effects of YQA14 or NGB2904 on [3H]spiperone or [3H]SCH23390 binding to cell membrane were determined in the presence of 0.5 nM [3H]spiperone or 1.0 nM [3H]SCH23390 and varying concentrations of YQA14 (10−16–10−5 M) or NGB2904 (10−10–10−5 M). After 1 hour incubation (at 25°C), the reaction was terminated by filtration through Whatman GF/C filters pre-soaked in 0.3% polyethyleneimine for 30 minutes. The filter was then washed five times with 3 ml of cold 50 mM Tris-HCl. Radioactivity on each filter was measured with a liquid scintillation spectrometer (LS6500; Beckman, Brea, CA, USA). Nonspecific binding was determined in the presence of 0.5 nM [3H]spiperone and 10 μM haloperidol or 1 nM [3H]SCH23390 and 10 μM (+)-butaclamol. Specific receptor binding was calculated by subtracting nonspecific binding from total ligand binding. The receptor binding data were analyzed using a non-linear regression model (GraphPad Software, San Diego, CA) (Castelli et al. 2001). IC50 values of YQA14 or NGB2904 were determined using the method described by Cheng & Prusoff (1973). Ki value was calculated using the equation Ki = IC50/(1 + [S]/Kd) ([S] – radioligand substrate concentration) (Cheng & Prusoff 1973). All data are presented as mean values of two to three dependent experimental assays.
[35S]GTPγS-binding assay
Procedures for the [35S]GTPγS binding assay were slightly modified from the methods of Vanhauwe et al. (1999). Briefly, stored membranes were thawed and diluted in reaction buffer (20 mM HEPES, 100 mM NaCl, 3 mM MgCl2, 1 mM EGTA, 0.1 mM dithiothreitol, 1 mM guanosine diphosphate, pH 7.4). Membrane proteins (35 μg) were pre-incubated with unlabeled quinpirole (10−12–10−5 M) in reaction buffer (400 μl, 30°C) containing 3 μM GTP but no [35S]GTPγS for 30 minutes, following which 100 μl 1.0 nM [35S]GTPγS was added. The mixture was incubated for another 30 minutes. Basal [35S]GTPγS binding was measured in the absence of quinpirole. Nonspecific binding was measured in the presence of 0.2 nM [35S]GTPγS and 40 μM unlabeled GTPγS. The intrinsic activity (agonist or antagonist) of YQA14 alone on [35S]GTPγS binding was measured in the absence of quinpirole. The effects of YQA14 on quinpirole-stimulated [35S]GTPγS binding were measured in the presence of varying concentrations of YQA14(10−14–10−5 M), 10 μM quinpirole and 0.2 nM [35S]GTPγS. Reactions were terminated by rapid filtration with Whatman GF/B filters as described above. The filters were then washed with 3 ml of washing buffer (50 mM Tris-HCl, 50 mM NaCl, 5 mM MgCl2-6H2O, pH 7.4, 4°C). The radioactivity on each filter was measured with a scintillation spectrometer (LS6500; Beckman, Brea, CA, USA). GraphPad Prism software was used to calculate EC50 values of quinpirole-stimulated [35S]GTPγS binding and IC50 values of YQA14 on quinpirole-stimulated [35S]GTPγS binding. To obtain a stable maximal [35S]GTPγS binding to CHO-hD3R cell membranes, we used a high concentration of quinpirole (10 μM) as an agonist. However, such concentrations of quinpirole may also affect YQA14 binding to CHO-hD3R cells. Thus, the original IC50 values of YQA14 measured from the functional response curve with the GraphPad Prism software were corrected or normalized to the corrected IC50 (cIC50) according to the following equation: cIC50 = IC50(antagonist)/(1 + [quinpirole]/EC50(quinpirole)) (Vanhauwe et al. 1999).
Experiment 2: intravenous cocaine self-administration in rats
Animals
Male Long–Evans rats (Charles River Laboratories, Raleigh, NC, USA) weighing 250–300 g were used. They were housed individually in a climate-controlled animal colony room on a reversed light/dark cycle with access to food and water ad libitum. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Academy of Sciences and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse.
Surgery
Intravenous (i.v.) catheters were constructed of microrenathane (Braintree Scientific Inc., Braintree, MA, USA). Rats were anesthetized with sodium pentobarbital (65 mg/kg i.p.), and an i.v. catheter was inserted into a jugular vein using standard aseptic surgical procedures. During experimental sessions, the catheter was connected to an infusion pump via tubing encased in a protective metal spring from the head-mounted connector to the top of the experimental chamber. To prevent clogging, catheters were flushed daily with a gentamicin-heparinsaline solution (30 IU/ml heparin; ICN Biochemicals, Cleveland, OH, USA).
Self-administration apparatus
Intravenous cocaine self-administration experiments were conducted in operant response test chambers (32 × 25 × 33 cm) (Med Associates, Saint Albans, VT, USA). Each test chamber had two levers located 6.5 cm above the floor, one active and one inactive. Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no other consequence. A cue-light and a speaker were located 12 cm above the active lever. At the start of each 3-hour test session, the house-light was turned on. When the animal made a lever-pressing response that resulted in cocaine infusion (0.1 ml in 4.6 s), the cue-light (4 W) was illuminated and a cue-sound (tone, 30 Hz, 15 dB) was turned on for the duration of the infusion. Lever presses during the 4.6-second cocaine infusion were counted, but did not lead to further infusions. There was no timeout after the completion of each infusion.
Initial single-dose cocaine self-administration
After 5–7 days of recovery from surgery, each rat was placed into a test chamber and allowed to lever-press for i.v. cocaine (1.0 mg/kg/infusion) infusion on a fixed-ratio 1 (FR1) reinforcement schedule until stable cocaine self-administration was established. The initial cocaine dose of 1 mg/kg per infusion was chosen as our previous experience showed that this dose produces rapid and facile acquisition of cocaine self-administration behavior (Xi et al. 2006). After transition from FR1 reinforcement, subjects were allowed to continue cocaine (0.5 mg/kg per infusion) self-administration under FR2 reinforcement (7–10 days) until the following criteria for stable cocaine-maintained responding were met: less than 10% variability in inter-response interval and less than 10% variability in number of presses on the active lever for at least 3 consecutive days.
Multiple-dose cocaine self-administration
To determine whether the behavioral effects of YQA14 or SB-277011A on cocaine self-administration were dependent on drug (YQA14 or SB-277011A) and cocaine doses, we examined the effects of YQA14 or SB-277011A on cocaine self-administration maintained by a full dose range of cocaine (0.031, 0.0625, 0.125, 0.25, 0.5 and 1.0 mg/kg per infusion) in a single session. The session consisted of five sequential 20-minute components, each preceded by a 20-minute timeout period for changing the cocaine dose. The infusion volumes and durations of each component were identical except that cocaine concentrations for corresponding unit cocaine doses differed. There was a 30-minute extinction period (0 mg/kg cocaine) before each daily cocaine self-administration session. Testing continued until stable cocaine-maintained responding was achieved (i.e. a minimum of 10 mg/kg cocaine infusions per session, with less than 10% variation in total number of cocaine injections for 3 consecutive days, and at least fivefold higher maximal response rates compared with those maintained during extinction). Then, each rat randomly received one of three doses of YQA14 (6.25, 12.5 or 25 mg/kg, i.p.) or vehicle (25% 2-hydroxypropyl-β-cyclodextrin) 20 minutes prior to the test session. Additional rats were used to observe the effects of SB-277011A (12.5 or 25 mg/kg i.p.) on cocaine self-administration using the same procedure as described above. Animals then received an additional 5–7 days of self-administration of cocaine alone until baseline response was re-established prior to testing the next dose of YQA14 or SB-277011A. The order of testing for the various doses of drug or vehicle was counterbalanced.
Cocaine self-administration under PR reinforcement
After stable cocaine self-administration under FR2 reinforcement was established, subjects were switched to cocaine self-administration (0.5 mg/kg per injection) under PR reinforcement, during which the work requirement (lever presses) needed to receive a single i.v. cocaine infusion was progressively raised within each test session (see details in Richardson & Roberts 1996) according to the following PR series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492 and 603 until the break-point was reached. The break-point was defined as the maximal workload (i.e. number of lever presses) completed for the last cocaine infusion prior to a 1-hour period during which no infusions were obtained by the animal. Animals were allowed to continue daily sessions of cocaine self-administration under PR reinforcement conditions until day-to-day variability in break-point fell within 1–2 ratio increments for 3 consecutive days. Once a stable break-point was established, subjects were assigned to seven subgroups. Then, each group randomly received vehicle (25% 2-hydroxypropyl-β-cyclodextrin), one of three doses YQA14 (1.00, 6.25 or 12.5 mg/kg, i.p.), or one of three doses of SB-277011A (6, 12 or 25 mg/kg, i.p.) 20 minutes prior to the test session.
Experiment 3. Oral sucrose self-administration in rats
The procedures for oral sucrose self-administration testing were identical to the procedures used for cocaine self-administration, except for the following minor differences: (1) no surgery was carried out in this experiment; and (2) active lever presses led to delivery of 0.1 ml of 5% sucrose solution into a liquid food tray on the operant chamber wall. The effects of the same doses of YQA14 or SB-277011A on oral sucrose self-administration were evaluated in this group of rats.
Experiment 4. Locomotion behavior in rats
Before receiving any drug, rats were placed in a locomotor detection chamber (Accuscan, Columbus, OH, USA) for habituation for 1 hour per day for 3 days. Rats were then divided into four groups. Two groups of rats were used to determine whether YAQ14 or SB-277011A alone altered locomotion, whereas two other groups of rats were used to determine whether pre-treatment with YQA14 or SB-277011A altered cocaine-enhanced locomotion. On test days, each rat randomly received vehicle or one of two doses of YQA14 (12.5 or 25 mg/kg, i.p.) or SB-277011A (12.5 or 25 mg/kg, i.p.). Twenty minutes later, the first two groups of rats were placed in the locomotor detection chambers for 2 hours, whereas the other two pre-treatment groups of rats received 10 mg/kg cocaine immediately before they were placed into the locomotion detection chambers. After each test, animals received 2–3 additional days of habituation training (1 hour per day) in the same test chambers before the next dose was tested. The order of testing for various doses of YQA14, SB-277011A or vehicle was counterbalanced. Total distance counts were used to evaluate the effect of YQA14 and SB-277011A on basal and cocaine-enhanced locomotion.
Experiment 5: Intravenous cocaine self-administration in mice
Animals
Male wild-type (WT) and DA D3 receptor knockout (D3−/−) mice with C57BL/6J genetic backgrounds were bred at the National Institute on Drug Abuse from three D3+/− breeding pairs purchased from the Jackson Laboratory. All the mice were genotyped in our own laboratory according to the mouse tail D3R-DNA-PCR protocol used by Charles River Laboratories (Wilmington, MA, USA). WT and D3−/− mice were matched for age (8–14 weeks) and weight (25–35 grams). They were housed individually in a climate-controlled animal colony room on a reversed light–dark cycle (lights on at 1900 h, lights off at 700 h) with free access to food and water. The animals were maintained in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Academy of Sciences, and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the US National Institutes of Health.
Surgery
Mice were prepared for experimentation by surgical catheterization of the right external jugular vein. Catheterization was performed under 4% chloral hydrate (10 μl/g) using aseptic surgical technique. A 6.0-cm length of MicroRenathane tubing (ID 0.012″, OD 0.025″) (Braintree Scientific Inc., Braintree, MA, USA) was inserted 1.2 cm into the right jugular vein, and the distal end of the tubing was anchored to a 24-gauge steel cannula (Plastics One, Roanoke, VA, USA) that was bent at a 100° angle and mounted to the skull with cyanoacrylate glue and dental acrylic. A 2.5-cm extension of flexible tubing was connected to the distal end of the cannula. During cocaine self-administration sessions, the flexible tubing extension was connected to a perfusion pump (Razel Scientific Instruments, Stamford, CT, USA) via a PE50 tubing connector. To keep the implanted catheters patent, they were flushed daily with a 0.05 ml saline solution containing 20 IU/ml heparin and 0.33 mg/ml gentamycin. To avoid cocaine overdose, each animal was limited to a maximum of 30 cocaine infusions per 3-hour session.
Apparatus
Intravenous cocaine self-administration experiments were conducted in operant response test chambers (Model ENV-307A, Medical Associates, Georgia, VT, USA). Each test chamber had two levers located 2.5 cm above the floor (one active and one inactive). Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no consequence. A cue light and a speaker were located 5 cm above the active lever. A house chamber light was turned on at the start of each 3-hour test session. When the animal performed a lever-press that resulted in a drug infusion, it was exposed to two drug-paired environmental cues: a cue-light and a cue-sound (tone) that lasted for the duration of the infusion. Scheduling of experimental events and data collection were accomplished using Medical Associates software.
Cocaine self-administration under FR1 reinforcement
After recovery from surgery, each mouse was placed into a test chamber and allowed to lever-press for i.v. cocaine (1 mg/kg per infusion) delivered in 0.015 ml over 4.2 seconds on an FR1 reinforcement schedule. For the first 3–5 days, all animals received five free cocaine infusions within a 2- to 5-minute time interval at the beginning of each self-administration session to prime the animal for drug-seeking and drug-taking behavior. These five free drug infusions were subtracted from the total number of drug infusions in data analysis. During the 4.2-second injection period, additional responses on the active lever were recorded but did not lead to additional infusions. Each session lasted 3 hours. After 1 week of cocaine self-administration, the cocaine dose was switched from 1 to 0.5 mg/kg per infusion for an additional 1–2 weeks of cocaine self-administration until stable day-to-day self-administration was established, which was defined as ≥ 20 cocaine infusions per session with a steady self-administration pattern for at least 3 consecutive days. Then, each mouse randomly received vehicle (25% 2-hydroxypropyl-β-cyclodextrin solution) or one of two doses of YQA14 (25 or 50 mg/kg, i.p.) or SB-277011A (50 or 100 mg/kg, i.p.) at 20 minutes prior to testing. We chose two to four times higher drug doses in mice than those used in rats based on our pilot preliminary data about the minimal effective doses and the fact that drug metabolism is in general faster in smaller animals (such as mice) than in larger ones (such as rats) (Zhao & Ishizaki 1997; Bun et al. 1999). Thus, higher drug doses are required to produce effective pharmacological effects in mice than in rats. Animals then received an additional 5–7 days of cocaine self-administration until baseline response was re-established prior to testing the next dose of the drug.
Cocaine self-administration under PR reinforcement
The procedure for PR cocaine self-administration was identical to that used in rats (described above). In brief, mice were initially trained under FR1 reinforcement as outlined above. After stable cocaine self-administration was established, animals were switched from FR1 to PR reinforcement, under which the work requirement (lever presses) to receive a cocaine infusion was progressively raised within each test session (Roberts 1989; Roberts, Loh & Vickers 1989; Rodefer & Carroll 1996). Once a stable break-point was established, subjects randomly received vehicle (25% 2-hydroxypropyl-β-cyclodextrin) or one of two doses of YQA14 (50 or 75 mg/kg, i.p.) or SB-277011A (50 or 100 mg/kg, i.p.) 20 minutes prior to PR cocaine self-administration testing.
Drugs
DMEM/F12 medium, geneticin (G418) and lipofectamine were purchased from Invitrogen Corporation (Gibco™, Grand Island, NY, USA). Fetal bovine serum was purchased from HyClone-Pierce (HyClone®, South Logan, UT, USA). [3H]spiperone (105 Ci/mmol), [3H]SCH23390 (73.1 Ci/mmol) and [35S]guanosine 5′-[γ-thio]-triphosphate ([35S]GTPγS) (1250 Ci/mmol) were purchased from PerkinElmer Life Sciences (NEN, Boston, MA, USA). Other drugs used in experiment 1 were obtained from Sigma (St. Louis, MO, USA). Cocaine HCl (Sigma Chemical Co., Saint Louis, MO, USA) was dissolved in physiological saline. YQA14 was synthesized at the Beijing Institute of Pharmacology and Toxicology. SB-277011A (Trans-N-[4-[2-(6-cyano 1, 2, 3, 4-tetrahydroisoquinolin-2-yl) ethyl] cyclohexyl]-4-quinolinecarboxamide) was synthesized at MegaPharma Kft., Budapest, Hungary. YQA14 or SB-277011Awas dissolved in vehicle, i.e. 25% 2-hydroxypropyl-β-cyclodextrin (Sigma).
Data analyses
All data are presented as means (±SEM). Radioligand and GTPγS binding data were analyzed using a one- or two site non-linear regression model with Prism software. One- or two-way analysis of variance (ANOVA) was used to analyze the data reflecting the effects of YQA14 or SB-277011A on cocaine or sucrose self-administration or on basal or cocaine-enhanced locomotion in rats and mice. Individual group comparisons were performed with the Turkey method.
RESULTS
In vitro radioligand binding properties of YQA14 and NGB2904 on DA receptors
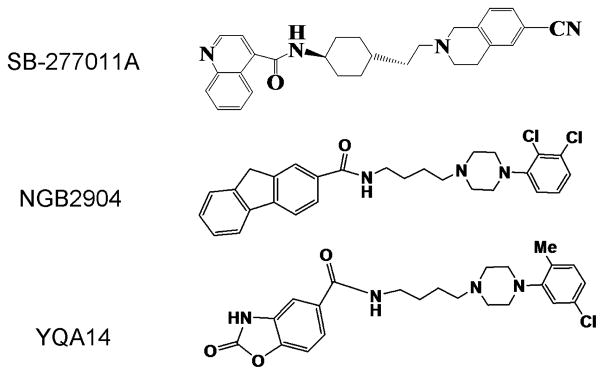
Figure 1 shows the chemical structures of YQA14, NGB-2904 and SB-277011A. Table 1 shows the Ki values of YQA14 on D1–D5 receptors expressed on CHO or HEK293 cells, demonstrating that YQA14 has the highest binding affinity for the D3 receptor over other DA receptors, and has two specific binding sites on D3 receptors with Ki-High (0.68 × 10−4 nM) and Ki-Low (2.11 nM). In contrast, NGB2904 has only one high-affinity binding site on D3 receptors with Ki value of 4.36 nM. Given that the Ki values of YQA14 (335.3 nM) and NGB2904 (502.3 nM) on D2 receptors are significantly higher than those on D3 receptors, this suggests that YQA14 may have ~5 000 000-fold and 150-fold higher selectivity, respectively, for D3 over D2 receptors at each binding site. Table 2 shows the Ki values of SB-277011A, NGB2904 and YQA14, demonstrating that YQA14 has similar or higher potency and selectivity than SB-277011A or NGB2904 for D3 over D2 receptors.
Figure 1.
Chemical structures of SB-277011A, NGB2904 and YQA14
Table 1.
In vitro receptor binding properties of YQA14 on each DA receptor subtype.
| Receptor | Cell line | Radioligand (nM) | Ki (nM) (n) | Selectivity (D*/D3) |
|---|---|---|---|---|
| D1 | HEK293 | [3H]SCH23390 | >105 (2) | >105 |
| D5 | HEK293 | [3H]SCH23390 | >105 (2) | >105 |
| D2 | HEK293 | [3H]spiperone | 335.3 (3) | ~5 × 105 150 |
| D3 | CHO | [3H]spiperone | 0.68 × 10−4; 2.11 (5) | – |
| D4 | HEK293 | [3H]spiperone | >105 (2) | >105 |
D*: D1, D2, D4, or D5.
Table 2.
Comparisons of receptor binding affinity (Ki, nM) and selectivity of SB-277011A, NGB 2904 and YQA14 on cloned D3 receptors.
| Compound | Ki (nM) (at D2R) | Ki (nM) (at D3R) | Selectivity (D2/D3 Ratio) | Reference |
|---|---|---|---|---|
| SB-277011A | 2820 (rodent) | 10.7 (rodent) | 263 | Reavill et al. (2000) |
| 1050 (human) | 11.2 (human) | 93 | Reavill et al. (2000) | |
| NGB 2904 | 217 ± 12 (human) | 1.4 ± 0.6 (human) | 155 | Yuan et al. (1998) |
| 911 ± 190 (primate) | 1.1 ± 0.2 (primate) | 830 | Robarge et al. (2001) | |
| 112 ± 22 (human) | 2.0 3 ±.4 (human) | 56 | Grundt et al. (2005) | |
| 502.3 ± 15.4 (human) | 4.36 ± 1.08 (human) | 115 | Present study | |
| YQA14 | 335.3 ± 111.6 (human) | Ki-High: (0.68 ± 0.39) × 10−4 | > 4 000 000 | Present study |
| Ki-Low: 2.11 ± 0.66 (human) | 158 |
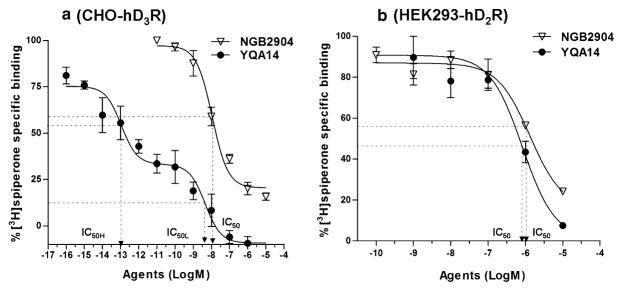
Figure 2a illustrates the inhibitory effects of YQA14 and NGB2904 on [3H]spiperone binding on CHO-hD3R cells. YQA14 had high affinity for cloned human D3 receptors showing two binding sites (IC50-High = ~0.1 pM; IC50-Low = ~5 nM). NGB2904 also had high affinity for D3 receptors, with a single binding site (IC50 = ~10 nM). Figure 2b illustrates the inhibitory effects of YQA14 or NGB2904 on [3H]spiperone binding to HEK293-hD2 cells, demonstrating much lower affinities of YQA14 or NGB2904 for the human D2 receptor, and a single binding site (IC50 = ~1 μM).
Figure 2.
In vitro receptor binding properties of YQA14 and NGB2904 for cloned human D2 and D3 receptors expressed on HEK293 or CHO cells. (a) YQA14 or NGB2904 dose-dependently inhibits 0.5 nM [3H]spiperone binding at CHO-hD3R cells. YQA14 displays two high-affinity binding sites (IC50-High, IC50-Low), whereas NGB2904 has only one binding site at CHO-hD3R cells. (b) YQA14 and NGB2904, at 100-fold higher concentrations (see IC50), also dose-dependently inhibits [3H]spiperone binding at HEK293-hD2R cells (IC50H–IC50-High; IC50L–IC50-Low)
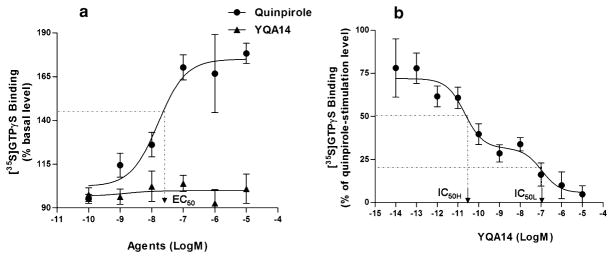
YQA14 inhibits quinpirole-stimulated [35S]GTPγS binding at CHO-hD3 cells
Figure 3a illustrates the dose–effect relationship of quinpirole-activated [35S]GTPγS binding on CHO-hD3R cells (EC50 = 23.7 ± 1.6 nM, Ymax = 187.7 ± 14.4%, n = 3), whereas YQA14 alone had no effect on [35S]GTPγS binding to CHO-hD3R. Figure 3b illustrates the dose-dependent effect of YQA14 on 10 μM quinpirole-stimulated [35S]GTPγS binding, also demonstrating two specific binding sites of YQA14 on CHO-hD3R cells with raw IC50-High (~10 pM) and raw IC50-Low (~0.1 μM). As described above, a very high concentration of quinpirole (10 μM)was used to stimulate [35S]GTPγS binding, which may have affected YQA14 binding to D3 receptors. Therefore, the raw IC50 values were normalized to cIC50. The cIC50 values at both binding sites were cIC50-High (0.61 ± 0.51 pM) and cIC50-Low (0.42 ± 0.18 nM), which are close to the IC50 values (0.1 pM and 5 nM) or Ki values (0.068 pM and 2.11 nM) as measured in the present receptor binding assays above.
Figure 3.
Functional activity assays of hD3 receptors expressed on CHO cells. (a) Quinpirole dose-dependently increases [35S]GTPγS binding to CHO-hD3R cells. (b) YQA14 dose-dependently inhibits 10 μM quinpirole-stimulated [35S]GTPγS binding at CHO-hD3R cells membranes. YQA14 displays two functional binding sites on CHOhD3R cells in this functional [35S]GTPγS binding assay (IC50H–IC50-High; IC50L–IC50-Low)
Effects of YQA14 and SB-277011A on cocaine self-administration in rats
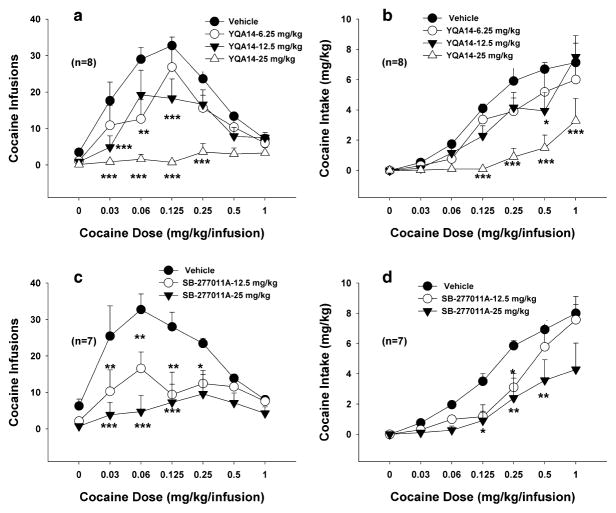
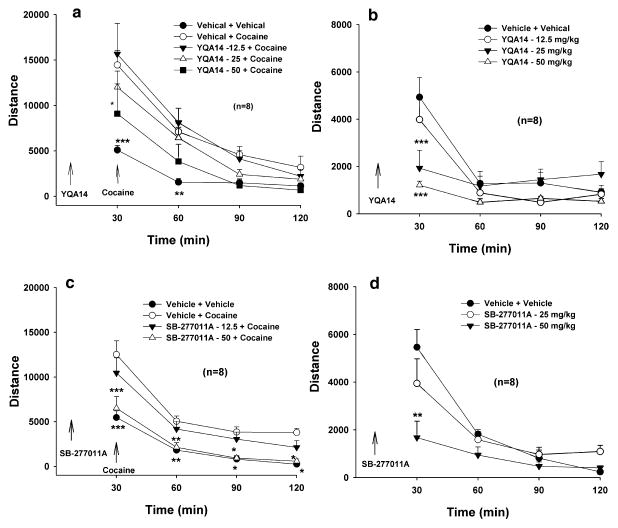
Figure 4 illustrates that YQA14 (12.5 or 25 mg/kg, i.p.) significantly and dose-dependently inhibited cocaine self-administration and shifted the cocaine dose–response self-administration curve downward (Fig. 4a) and shifted cocaine intake dose–response curve downward and to the right (Fig. 4b). Two-way ANOVA for repeated measures over cocaine dose revealed a significant treatment (vehicle versus YQA14) main effect (Fig. 4a, F3,21 = 19.221, P < 0.001; Fig. 4b, F3,21 = 11.16, P < 0.001), significant cocaine dose main effect (Fig. 4a, F6,42 = 19.180, P < 0.001; Fig. 4b, F6,42 = 54.41, P < 0.001) and a significant treatment × dose interaction (Fig. 4a, F18,126 = 5.12, P < 0.001; Fig. 4b, F18,126 = 2.82, P < 0.001). Individual group comparisons revealed a statistically significant reduction in cocaine self-administration maintained by lower doses (0.03–0.25 mg/kg per infusion) of cocaine after 6.25 mg/kg (Fig. 4a, q = 4.065, P < 0.05; Fig. 4b, q = 2.58, P = NS), 12.5 mg/kg (Fig. 4a, q = 4.836, P = 0.013; Fig. 4b, q = 2.72, P = NS) or 25 mg/kg (Fig. 4a, q = 10.64, P < 0.001; Fig. 4b, q = 7.97, P < 0.001) YQA14, when compared to the vehicle control group. Figure 4c and d illustrate that the same doses of SB-277011A also produced a dose-dependent inhibition of cocaine self-administration. Two-way ANOVA for repeated measures over cocaine dose revealed a significant treatment (vehicle versus SB-277011A) main effect (Fig. 4c, F2,12 = 14.798, P < 0.001; Fig. 4d, F2,12 = 7.00, P < 0.01), significant cocaine dose main effect (Fig. 4c, F6,36 = 5.653, P < 0.001; Fig. 4d, F6,36 = 31.51, P < 0.001) and a significant treatment × dose interaction (Fig. 4c, F12,72 = 2.455, P = 0.01; Fig. 4d, F12,72 = 2.12, P < 0.05). Individual group comparisons revealed a statistically significant reduction in cocaine self-administration maintained by lower doses (0.03–0.25 mg/kg per infusion) of cocaine after 12.5 mg/kg (Fig. 4c, q = 5.098, P < 0.01) or 25 mg/kg (Fig. 4c, q = 7.539, P < 0.001; Fig. 4d, q = 5.29, P < 0.01) SB-277011A, when compared to the vehicle control group.
Figure 4.
Effects of YQA14 and SB-277011A on intravenous cocaine self-administration in rats. Systemic administration of YQA14 (a) or SB-277011A (c) dose-dependently shifts the cocaine dose–response self-administration curve downward. Systemic administration of YQA14 (b) or SB-277011A (d) dose-dependently shifted cocaine intake dose–response curve downward and to the right. * P < 0.05, ** P < 0.01, *** P < 0.001, compared to vehicle control group at each cocaine dose
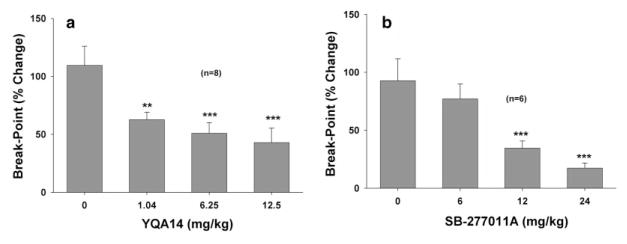
To determine whether the YQA14-induced reduction in cocaine self-administration was due to a reduction in cocaine’s rewarding efficacy, we further observed the effects of YQA14 on i.v. cocaine self-administration under PR reinforcement. Figure 5 shows that systemic administration of YQA14 (Panel a, F3,28 = 11.035, P < 0.001) or SB-277011A (Panel b, F3,20 = 8.187, P < 0.001) significantly and dose-dependently lowered break-point for cocaine self-administration (shown as % change). Individual group comparisons revealed a statistically significant reduction in break-point levels after 1.04 mg/kg (q = 4.527, P < 0.05), 6.25 mg/kg (q = 5.649, P < 0.01) or 12.5 mg/kg YQA14 (q = 7.897, P< 0.001), and after 12 mg/kg (q = 4.706, P = 0.016) or 24 mg/kg (q = 6.11, P = 0.001) of SB-277011A, when compared to the vehicle control group.
Figure 5.
Effect of YQA14 and SB-277011A on cocaine self-administration under PR reinforcement in rats. Systemic administration of YQA14 (a) or SB- 277011A (b) dose-dependently lowered PR break-point for cocaine self-administration (shown as % change) on. **P < 0.01, ***P < 0.001, compared to vehicle pre-treatment group
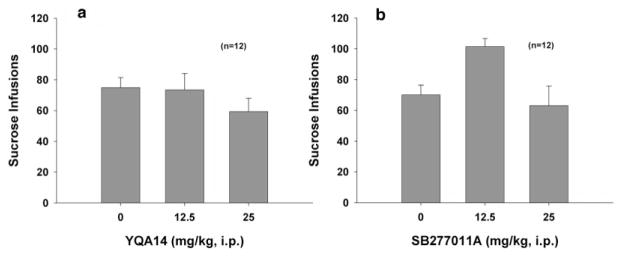
Effects of YQA14 and SB-277011A on oral sucrose self-administration in rats
Figure 6 illustrates that neither YQA14 (Panel a, F2,20 = 2.635, P = NS) nor SB-277011A (Panel b, F2,14 = 4.952, P = NS), at the same doses that inhibit cocaine self-administration, altered oral sucrose self-administration.
Figure 6.
Effects of YQA14 and SB-277011A on oral sucrose self-administration in rats. Systemic administration of YQA14 (a) or SB-277011A (b) has no effect on oral sucrose self-administration behavior under FR2 reinforcement
Effects of YQA14 and SB-277011A on basal and cocaine-augmented locomotion in rats
To determine whether the effects of D3 receptor antagonists on cocaine self-administration generalize to other effects of cocaine, we observed the effect of YQA14 or SB-277011A on basal or cocaine-enhanced locomotion. Figure 7 shows that both YQA14 and SB-277011A, at the same doses (12.5–25 mg/kg) that significantly inhibited cocaine self-administration, did not significantly alter basal or cocaine-enhanced locomotion. However, when the dose was increased to 50 mg/kg, YQA14 or SB-277011A produced a significant inhibition of basal and cocaine-enhanced locomotion. Two-way ANOVA for repeated measurements over time revealed a statistically significant YQA14 treatment main effect (Panel a, F4,28 = 7.866 P < 0.001; Panel b, F3,21 = 3.627 P < 0.001), and SB-277011A treatment main effect (Panel c, F3,21 = 13.362 P < 0.001; Panel d, F2,14 = 4.208 P < 0.05). Individual group comparisons revealed a statistically significant reduction in cocaine-augmented locomotion after 50 mg/kg YQA14 (Panel a, q = 4.347, P < 0.05) or 50 mg/kg SB-277011A (Panel c, q = 6.870, P < 0.001), and in basal levels of locomotion after 50 mg/kg YQA14 (Panel b, q = 4.62, P < 0.05) or 50 mg/kg SB-277011A (Panel d, q = 3.81, P < 0.05).
Figure 7.
Effect of YQA14 and SB-277011A on basal and cocaine-enhanced locomotion in rats. Neither YQA14 (a, b) nor SB-277011A (c, d), at the same doses (12.5 or 25 mg/kg) that inhibit cocaine self-administration, has any effect on either basal (b, d) or 10 mg/kg cocaine-enhanced (a, c) locomotion. However, when the dose was increased to 50 mg/kg, both YQA14 and SB-277011A produced a significant reduction in basal and cocaine-enhanced locomotion. *P < 0.05, **P < 0.01, ***P < 0.001, compared to vehicle treatment group at each time point
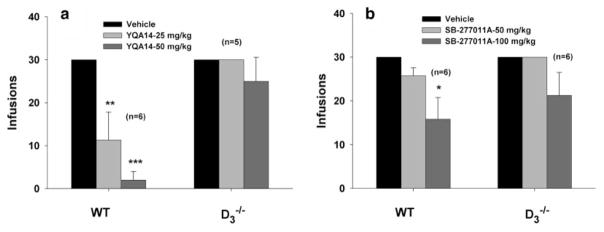
Effects of YQA14 and SB-277011A on cocaine self-administration in WT and D3−/− mice
To further determine whether the observed pharmacological effects of YQA14 and SB-277011A on cocaine self-administration are mediated by blockade of brain DA D3 receptors, we investigated and compared the effects of YQA14 (25 or 50 mg/kg, i.p.) and SB-277011A (50 or 100 mg/kg, i.p.) on cocaine self-administration in WT and D3−/− mice. Figure 8a illustrates that systemic (i.p.) administration of YQA14 produced a significant and dose-dependent reduction in cocaine self-administration under FR1 reinforcement conditions in WT (F2,10 = 19.421, P < 0.001), but not in D3−/− mice (F2,8 = 2.279, P = NS). Figure 8b illustrates that systemic administration of SB-277011A also dose-dependently inhibited cocaine self-administration under FR1 reinforcement in WT mice (F2, 10 = 4.443, P < 0.05), but not in D3−/− mice (F2, 10 = 3.497, P = NS).
Figure 8.
Effects of YQA14 and SB-277011A on i.v. cocaine self-administration under FR1 reinforcement in mice. Systemic administration YQA14 (25 or 50 mg/kg) or SB-277011A (50 or 100 mg/kg) dose-dependently inhibited cocaine self-administration only in WT, but not in D3−/− mice. *P < 0.05, **P < 0.01, ***P < 0.001, compared to vehicle control groups
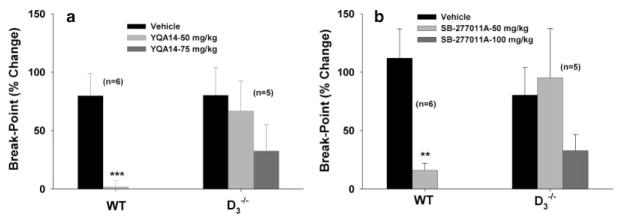
Finally, to determine whether YQA14 or SB-277011A also inhibits mouse cocaine self-administration under PR reinforcement conditions, we observed the effects of YQA14 (50 or 75 mg/kg i.p.) and SB-277011A (50 or 100 mg/kg i.p.) on PR break-point levels for cocaine self-administration in both strains of mice. Figure 9a shows that YQA14 (50 mg/kg) almost completely inhibited cocaine self-administration in WT, but had no effect in D3−/−, mice. However, when the dose was increased to 75 mg/kg, YQA14 appeared to produce an inhibitory tendency in D3−/− mice, although this tendency toward a reduction in break-point was not statistically significant (F2,8 = 2.451, P = NS). Figure 9b shows similar findings, with SB-277011A (50 mg/kg) also almost completely inhibiting PR cocaine self-administration in WT, but not in D3−/− mice. However, at 100 mg/kg, SB-277011A appeared to produce a tendency toward a lower breakpoint level for cocaine self-administration in D3−/− mice, although this tendency was not statistically significant (F2,8 = 1.526, P = NS).
Figure 9.
Effects of YQA14 and SB-277011A on cocaine self-administration under PR reinforcement in mice. Both YQA14 and SB-277011A, at 50 mg/kg, almost completely inhibited PR cocaine self-administration in WT mice, but not in D3−/− mice. Higher doses of YQA14 (75 mg/kg) or SB-277011A (100 mg/kg) appeared to show a tendency toward inhibition of PR cocaine self-administration in D3−/− mice, although this tendency was not statistically significant. **P < 0.01, ***P < 0.001, compared to vehicle control groups
DISCUSSION
In the present study, we report the profile of a novel D3 receptor antagonist (YQA14), which appears to be similarly potent and selective as SB-277011A or NGB2904 for D3 over D2 receptors (see Table 2). Strikingly, this new compound, like endogenous DA itself, has two binding sites on D3 receptors with Ki values of 0.68 × 10−4 and 2.11 nM, respectively. It also shows a longer t1/2 than SB-277011A in rodents and better stability when incubated with human hepatic microsomal enzyme (Li et al. unpublished), suggesting that the novel compound YQA14 has even better pharmacokinetic properties than SB-277011A, the most well-characterized selective D3 receptor antagonist to date in animal models of addiction (Heidbreder et al. 2005).
In the present study, we evaluated and compared the pharmacological actions of YQA14 against those of SB-277011A in animal models of drug addiction. Drug self-administration and reinstatement of extinguished drug-seeking behavior are the most commonly used animal models to investigate drug reward and relapse (Gardner 2000; Shalev et al. 2002). Previous studies have shown that systemic administration of the D3 receptor antagonists SB-277011A, NGB-2904 and others significantly and dose-dependently inhibit reinstatement of drug-seeking behavior triggered by addictive drugs (including cocaine, methamphetamine, nicotine and alcohol), drug-associated cues, or footshock stress (Xi et al. 2004; Gilbert et al. 2005; Gal & Gyertyan 2006; Cervo et al. 2007), but fail to inhibit i.v. self-administration of most of these drugs (excepting alcohol) under low FR reinforcement conditions (Di Ciano et al. 2003; Xi et al. 2005, 2006), suggesting a possible limitation of D3 receptor antagonists in medication development for the treatment of drug addiction.
Given that YQA14 appears to be a more potent and selective D3 receptor antagonist than SB-277011A (Table 2), and given the significant efficacy shown by SB-277011A across a wide range of preclinical animal models of addiction (Heidbreder et al. 2005), we hypothesized that YQA14 might be more effective than SB-277011A in attenuating cocaine self-administration. To address this issue, we used the multiple-dose cocaine self-administration paradigm, which has hitherto not been used to evaluate D3 receptor antagonist effects on cocaine’s actions. In this paradigm, animals display a characteristic inverted U-shaped dose-response self-administration curve (Panlilio, Thorndike & Schindler 2006; Xi et al. 2010), which allows a more complete assessment of cocaine’s dose–response effects and the effects of test drugs (e.g. YQA14 or SB-277011A) on cocaine self-administration. In this study, we found that both YQA14 and SB-277011A significantly shifted the cocaine dose–response curve downward and to the right (see the cocaine dose–response intake curves), suggesting antagonism by YQA14 or SB-277011A of cocaine’s acute rewarding effect. This is congruent with SB-277011A’s acute attenuation of cocaine-enhanced electrical brain-stimulation reward (Vorel et al. 2002). By careful examination of drug interaction effects seen with different doses of cocaine and YQA14 or SB-277011A, we found that both D3 receptor antagonists significantly and dose-dependently inhibited intravenous cocaine self-administration maintained by lower doses (0.03–0.25 mg/kg per infusion), but not by higher doses (0.5 or 1.0 mg/kg/infusion) of cocaine (Fig. 3), suggesting that the attenuation of cocaine’s effects produced by D3 receptor antagonists is surmountable by increased doses of cocaine. This finding is consistent with previous reports that SB-277011A or NGB-2904 significantly inhibit cocaine-induced conditioned place preference (Vorel et al. 2002), cocaine- or nicotine-enhanced brain-stimulation reward (Pak et al. 2006; Xi et al. 2006) and cocaine or nicotine self-administration under high fixed-ratio (FR10) or PR reinforcement schedules (Xi et al. 2005, 2006; Ross et al. 2007; Xi & Gardner 2007) in which the cumulative cocaine doses are much lower than those in cocaine self-administration under low fixed-ratio (FR1, FR2) reinforcement (Andreoli et al. 2003; Gal & Gyertyan 2003; Xi et al. 2005, 2006), wherein selective D3 receptor antagonism has little or no effect.
To assess whether YQA14-induced attenuation of FR cocaine self-administration is mediated by attenuation of cocaine’s rewarding efficacy, we also observed and compared the effects of YQA14 and SB-277011A on cocaine self-administration under PR reinforcement. The PR paradigm is considered to measure the rewarding efficacy of addictive drugs (Roberts 1989; Rodefer & Carroll 1996; Arnold & Roberts 1997). Given that the PR breakpoint level is largely cocaine dose-dependent, it has been thought to be an index of reward strength. The present study shows that both YQA14 and SB-277011A significantly and dose-dependently lowered break-point for cocaine self-administration under PR reinforcement, suggesting a reduction in cocaine reward after D3 receptor blockade. This is congruent with the previous findings that several D3 receptor antagonists (SB-277011A, NGB2904, S33138, PG-01037) significantly inhibit PR cocaine self-administration as reflected by lowed PR break-points (Di Ciano et al. 2003; Xi et al. 2005, 2006; Peng et al. 2009; Higley et al. 2011).
We believe that the inhibition of cocaine self-administration described above is unlikely due to YQA14-induced locomotor impairment, because neither YQA14 nor SB-277011A, at doses that inhibit cocaine self-administration, significantly altered oral sucrose (nondrug reward) self-administration or locomotor activity. These findings also suggest that blockade of D3 receptors selectively inhibit cocaine, but not natural food reward, consistent with our previous reports (Vorel et al. 2002; Xi et al. 2006). However, with increased doses, both D3 antagonists significantly inhibited basal and cocaine-enhanced locomotion, suggesting that the antagonism by YQA14 and SB-277011A of cocaine self-administration can generalize (at high enough doses) to other effects of cocaine in rats.
Finally, to determine whether this inhibition of cocaine’s rewarding and psychomotor-stimulating effects is mediated by blockade of brain D3 receptors in vivo, we investigated the effects of YQA14 and SB-277011A on cocaine self-administration in WT mice and D3−/− mice. We found that both YQA14 (25 or 50 mg/kg, i.p.) and SB-277011A (50 or 100 mg/kg, i.p.) significantly and dose-dependently inhibited cocaine self-administration under FR1 reinforcement in WT mice, but not in D3−/− mice. In addition, both compounds, at 50 mg/kg, almost completely inhibited cocaine self-administration under PR reinforcement in WT mice, but not in D3−/− mice. However, at higher doses, YQA14 (75 mg/kg) or SB-277011A (100 mg/kg) showed a tendency to inhibit PR cocaine self-administration in D3−/− mice, although this was not statistically significant. We suggest that this tendency, if confirmed to be statistically significant by follow-up studies, may reflect a non-D3 (most likely D2) receptor-mediated effect at very high doses. Further research is required to study D3 versus D2 receptor occupancy in vivo after administration of different doses of YQA14. The present data suggest that the inhibition of cocaine self-administration by lower doses of YQA14 or SB-277011A in both rats and mice is mediated by blockade of brain D3 receptors in vivo.
By comparing the effective drug dose and the amplitude of pharmacological action produced by both D3 receptor antagonists, we found that YQA14 appears to be as effective as SB-277011A in antagonizing FR cocaine self-administration (Fig. 4) and cocaine-enhanced locomotion (Fig. 7) in rats, whereas more effective than SB-277011A in inhibiting PR cocaine self-administration in rats (Fig. 5) and cocaine self-administration under both FR and PR reinforcement in mice (Figs 8 and 9). We note that these relatively small differences in pharmacological action in vivo do not agree with the tremendous differences in their respective receptor binding properties in vitro. As shown on Tables 1 and 2, YQA14 has two binding sites with high affinities (Ki-High = 0.68 × 10−4 nM; Ki-Low = 2.11 nM) for human D3 receptors. The affinities of YQA14 for both binding sites are over 100 000- (Ki-High) and 5-fold (Ki-Low) higher, respectively, than that of SB-277011A (Ki = 11.2 nM) for human D3 receptors, suggesting that the effective in vivo drug dose of YQA14 should be much lower than that of SB-277011A. The reasons underlying the conflicting findings observed in vivo and in vitro are unclear. We conjecture that they may relate to the following factors. First, D3 receptors display significant species differences in D3 receptor gene and receptor expression between human, rat and mouse (Fu et al. 1995; Griffon et al. 1996; Smits et al. 2004). In the present study, we only investigated the receptor binding and intracellular signal-coupling properties of YQA14 in cloned human D3 receptors, not in cloned rodent D3 receptors (which are experiments yet to be undertaken). Thus, it is unclear whether two binding sites also exist on rodent D3 receptors. Second, it was recently reported that D3 receptors may form D1–D3 or D2–D3 receptor heteromers (Marcellino et al. 2008; Maggio & Millan 2010). Thus, it seems not unlikely that receptor heteromerization may block YQA14 binding to one of the binding sites. Finally, differential pharmacokinetic profiles of both D3 receptor antagonists may also contribute to the conflicting findings as stated above. Clearly, more study is needed to address this issue.
In conclusion, the present study demonstrates that YQA14 is a novel selective D3 receptor antagonist. It displays similar or higher potency and selectivity than SB-277011A for D3 over D2 and other DA receptors in receptor binding and intracellular signal-coupling assays in vitro, and similar or more potent pharmacological action than SB-277011A in antagonizing cocaine’s action in vivo. Given that YQA14, at doses that inhibit cocaine self-administration, fails to inhibit locomotion and sucrose self-administration, it is suggested that YQA14 may produce fewer unwanted side effects (such as sedation and natural reward suppression) if used for treating cocaine addiction at the human level. Taken together, the present experiments support the conclusion that YQA14 deserves further research as a potential medication for the treatment of cocaine or psychostimulant addiction.
Acknowledgments
This work was supported by the US National Institute on Drug Abuse Intramural Research Program (for the in vivo behavioral studies) and the National Basic Research Program of China (No. 2009CB522008) and the National Key Technology Research and Development Program of China (No. 2007BAI07B02) (for the in vitro binding assays). We thank Dr Xue-Chu Zhen for providing us with DA D1-, D2- and D5-transfected cells we used in these experiments.
Footnotes
Authors Contribution
R.S. conducted both the in vitro binding assays and in vivo behavioural tests, analyzed data and wrote the preliminary draft of the manuscript. R.F.Y. synthesized YQA14. N.W. participated in vitro binding assays. R.B.S. and J.L. initiated this research project and supervised the in vitro binding assays. X.Q.P. and X.L. participated in vivo behavioral tests. Z.X.X. and E.L.G. supervised in vivo behavioral tests and revised the manuscripts.
References
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bun H, Disdier B, Aubert C, Catalin J. Interspecies variability and drug interactions of clozapine metabolism by microsomes. Fundam Clin Pharmacol. 1999;13:577–581. doi: 10.1111/j.1472-8206.1999.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Mocci I, Sanna AM, Gessa GL, Pani L. S amisulpride binds with high affinity to cloned dopamine D(3) and D(2) receptors. Eur J Pharmacol. 2001;432:143–147. doi: 10.1016/s0014-2999(01)01484-4. [DOI] [PubMed] [Google Scholar]
- Cervo L, Burbassi S, Colovic M, Caccia S. Selective antagonist at D3 receptors, but not non-selective partial agonists, influences the expression of cocaine-induced conditioned place preference in free-feeding rats. Pharmacol Biochem Behav. 2005;82:727–734. doi: 10.1016/j.pbb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychopharmacol. 2007;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Prusoff WH. Mouse ascites sarcoma 180 thymidylate kinase. General properties, kinetic analysis, and inhibition studies. Biochemistry. 1973;12:2612–2619. doi: 10.1021/bi00738a010. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci. 2008;122:129–139. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Diaz J, Levesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC, Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–745. doi: 10.1016/0306-4522(94)00527-c. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Skryabin BV, Brosius J, Robakis NK. Molecular cloning and characterization of the mouse dopamine D3 receptor gene: an additional intron and an mRNA variant. DNA Cell Biol. 1995;14:485–492. doi: 10.1089/dna.1995.14.485. [DOI] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cueinduced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffon N, Crocq MA, Pilon C, Martres MP, Mayerova A, Uyanik G, Burgert E, Duval F, Macher JP, Javoy-Agid F, Tamminga CA, Schwartz JC, Sokoloff P. Dopamine D3 receptor gene: organization, transcript variants, and polymorphism associated with schizophrenia. Am J Med Genet. 1996;67:63–70. doi: 10.1002/(SICI)1096-8628(19960216)67:1<63::AID-AJMG11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZZ, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacol. 2011;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Schwartz JC, Sokoloff P. Dopamine D3 receptor agents as potential new medications for drug addiction. Eur Psychiatry. 2000;15:140–146. doi: 10.1016/s0924-9338(00)00219-4. [DOI] [PubMed] [Google Scholar]
- Macdonald GJ, Branch CL, Hadley MS, Johnson CN, Nash DJ, Smith AB, Stemp G, Thewlis KM, Vong AK, Austin NE, Jeffrey P, Winborn KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Watson JM, Wood M, Parker SG, Ashby CR., Jr Design and synthesis of trans-3-(2-(4-((3-(3-(5-methyl-1,2,4-oxadiazolyl))-phenyl)carboxamido)cyclohexyl)ethyl)-7-methylsulfonyl-2,3,4,5-tetrahydro-1 H-3-benzazepine (SB-414796): a potent and selective dopamine D3 receptor antagonist. J Med Chem. 2003;46:4952–4964. doi: 10.1021/jm030817d. [DOI] [PubMed] [Google Scholar]
- Maggio R, Millan MJ. Dopamine D2-D3 receptor heteromers: pharmacological properties and therapeutic significance. Curr Opin Pharmacol. 2010;10:100–107. doi: 10.1016/j.coph.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem. 2008;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Jr, Heidbreder CA, Pilla M, Gilbert J, Xi ZX, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. Cocaine self-administration under variable-dose schedules in squirrel monkeys. Pharmacol Biochem Behav. 2006;84:235–243. doi: 10.1016/j.pbb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Peng XQ, Ashby CR, Jr, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaer E, Munoz C, Gardner EL, Xi ZX. The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharmacology. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DN, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AK, Hagan JJ. Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robarge MJ, Husbands SM, Kieltyka A, Brodbeck R, Thurkauf A, Newman AH. Design and synthesis of [(2,3-dichlorophenyl)piperazin-1-yl]alkylfluorenylcarboxamides as novel ligands selective for the dopamine D3 receptor subtype. J Med Chem. 2001;44:3175–3186. doi: 10.1021/jm010146o. [DOI] [PubMed] [Google Scholar]
- Roberts DC. Breaking points on a progressive ratio schedule reinforced by intravenous apomorphine increase daily following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1989;32:43–47. doi: 10.1016/0091-3057(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Carroll ME. Progressive ratio and behavioral economic evaluation of the reinforcing efficacy of orally delivered phencyclidine and ethanol in monkeys: effects of feeding conditions. Psychopharmacology (Berl) 1996;128:265–273. doi: 10.1007/s002130050134. [DOI] [PubMed] [Google Scholar]
- Ross JT, Corrigall WA, Heidbreder CA, LeSage MG. Effects of the selective dopamine D3 receptor antagonist SB-277011A on the reinforcing effects of nicotine as measured by a progressive-ratio schedule in rats. Eur J Pharmacol. 2007;559:173–179. doi: 10.1016/j.ejphar.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Shahid M, Walker GB, Zorn SH, Wong EH. Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009;23:65–73. doi: 10.1177/0269881107082944. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Smits BM, D’Souza UM, Berezikov E, Cuppen E, Sluyter F. Identifying polymorphisms in the Rattus norvegicus D3 dopamine receptor gene and regulatory region. Genes Brain Behav. 2004;3:138–148. doi: 10.1111/j.1601-183x.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Martres MP, Giros B, Bouthenet ML, Schwartz JC. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol. 1992;43:659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Peng XQ, Newman AH, Ashby CR, Jr, Heidbreder C, Gaal J, Gardner EL. The selective dopamine D3 receptor antagonists SB-277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology (Berl) 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhauwe JF, Fraeyman N, Francken BJ, Luyten WH, Leysen JE. Comparison of the ligand binding and signaling properties of human dopamine D(2) and D(3) receptors in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1999;290:908–916. [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Su RB, Xu B, Lu XQ, Liu Y, Zheng JQ, Piletz JE, Li J, Qin BY. IRAS, a candidate for I1-imidazoline receptor, mediates inhibitory effect of agmatine on cellular morphine dependence. Biochem Pharmacol. 2005;70:1079–1087. doi: 10.1016/j.bcp.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berl) 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 2010;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang JR, Sun PH, Guo Y, Zhang ZJ, Jin GZ, Zhen X. Neuroprotective effects of atypical D1 receptor agonist SKF83959 are mediated via D1 receptor-dependent inhibition of glycogen synthase kinase-3 beta and a receptor-independent anti-oxidative action. J Neurochem. 2008;104:946–956. doi: 10.1111/j.1471-4159.2007.05062.x. [DOI] [PubMed] [Google Scholar]
- Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JW, Thurkauf A. NGB 2904 and NGB 2849: two highly selective dopamine D3 receptor antagonists. Bioorg Med Chem Lett. 1998;8:2715–2718. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]
- Zhao XJ, Ishizaki T. The In vitro hepatic metabolism of quinine in mice, rats and dogs: comparison with human liver microsomes. J Pharmacol Exp Ther. 1997;283:1168–1176. [PubMed] [Google Scholar]