Abstract
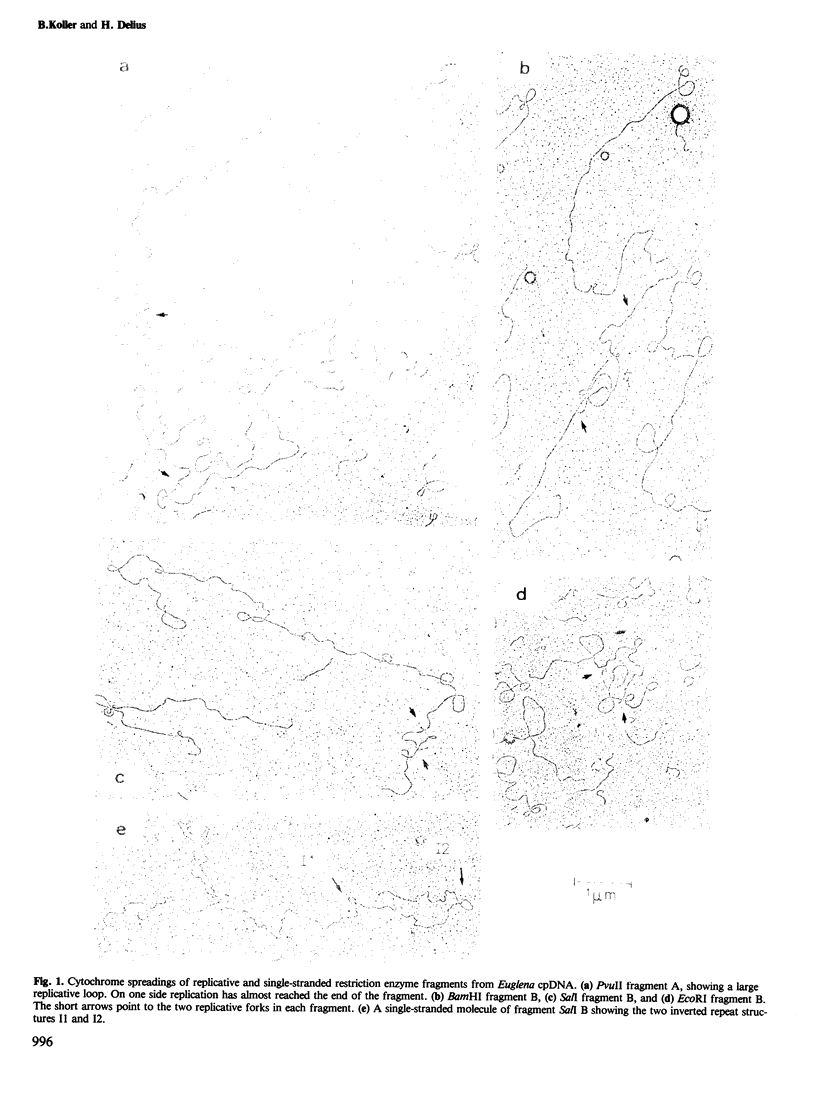
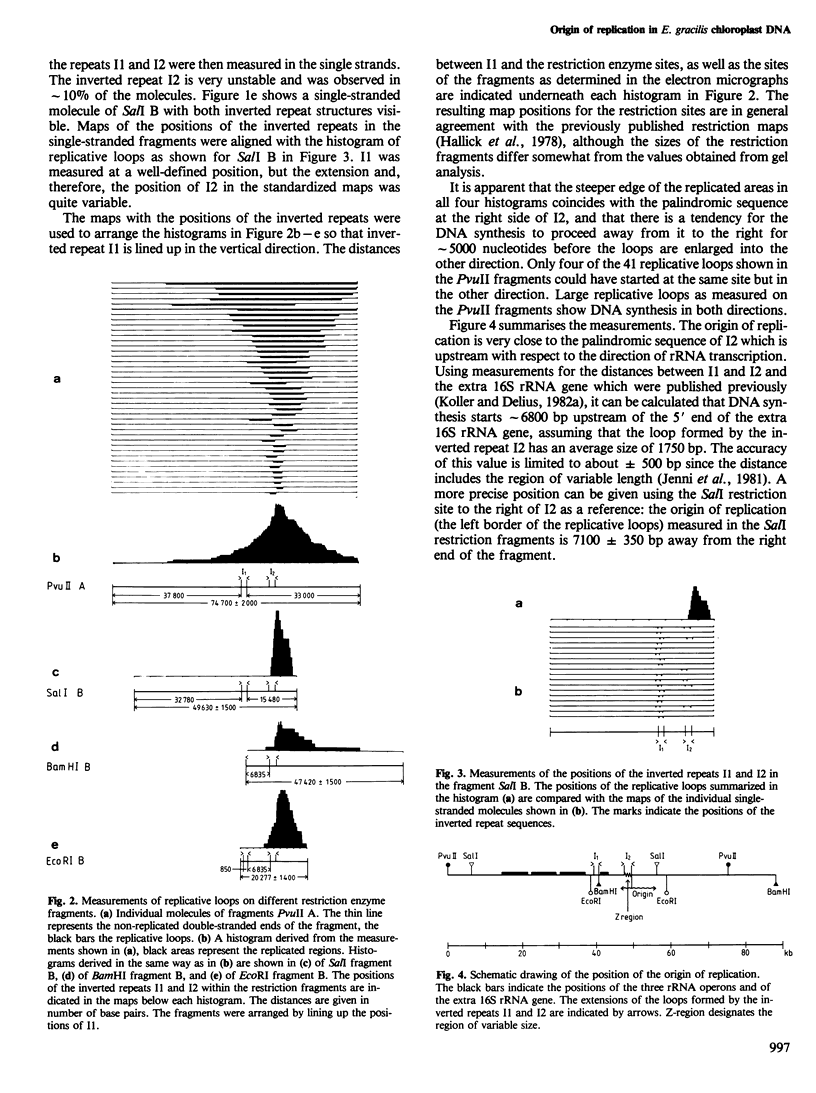
Chloroplast DNA (cpDNA), containing ˜10% replicative molecules, was isolated 2 h after onset of the dark period from cultures of Euglena gracilis strain Z. The DNA was digested with the restriction enzymes PvuII, SalI, BamHI, or EcoRI. Fragments that contained intact replicative loops were measured to determine the position of replicated sequences in relation to the restriction enzyme sites. It was found that replication starts at a unique position near one of the palindromic sequences I2 (Koller and Delius, 1982a) which is located upstream (with respect to the direction of rRNA transcription) of the AT-rich region of variable size (Jenni et al., 1981; Schlunegger et al., in preparation). In the majority of cases DNA synthesis proceeds unidirectionally away from this region for ˜5000 nucleotides before it starts in the other direction (in the same sense as the rRNA transcription) through the Z-region and the second palindromic sequence.
Keywords: chloroplast DNA, origin of replication, Z-region, inverted repeat, Euglena gracilis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fauron C. M., Wolstenholme D. R. Structural heterogeneity of mitochondrial DNA molecules within the genus Drosophila. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3623–3627. doi: 10.1073/pnas.73.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Wolstenholme D. R. Origin and direction of replication in mitochondrial DNA molecules from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3886–3890. doi: 10.1073/pnas.75.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann P., Doly J., Hussein Y., Nicolas P., Nigon V., Bernardi G. The chloroplast genome of bleached mutants of Euglena gracilis. Biochim Biophys Acta. 1981 May 29;653(3):412–415. doi: 10.1016/0005-2787(81)90197-0. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Replication of circular DNA in eukaryotic cells. Annu Rev Biochem. 1974;43(0):695–719. doi: 10.1146/annurev.bi.43.070174.003403. [DOI] [PubMed] [Google Scholar]
- Koller B., Delius H. Electron microscopic analysis of the extra 16 SrRNA gene and its neighbourhood in chloroplast DNA from Euglena gracilis strain Z. FEBS Lett. 1982 Mar 8;139(1):86–92. doi: 10.1016/0014-5793(82)80493-6. [DOI] [PubMed] [Google Scholar]
- Kolodner R. D., Tewari K. K. Chloroplast DNA from higher plants replicates by both the Cairns and the rolling circle mechanism. Nature. 1975 Aug 28;256(5520):708–711. doi: 10.1038/256708a0. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Presence of displacement loops in the covalently closed circular chloroplast deoxyribonucleic acid from higher plants. J Biol Chem. 1975 Nov 25;250(22):8840–8847. [PubMed] [Google Scholar]
- Kolter R., Helinski D. R. Regulation of initiation of DNA replication. Annu Rev Genet. 1979;13:355–391. doi: 10.1146/annurev.ge.13.120179.002035. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Richards O. C. Synthesis and turnover of Euglena gracilis nuclear and chlorplast deoxyribonucleic acid. Biochemistry. 1972 May 23;11(11):2036–2043. doi: 10.1021/bi00761a007. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]




