Abstract
Objective
To compare Anti-Mullerian Hormone (AMH) levels in women at high risk for hereditary breast and ovarian cancer compared to healthy, low-risk controls.
Design
Prospective cohort
Setting
Ambulatory
Patient(s)
Reproductive age women with a uterus and both ovaries were analyzed in four groups: BRCA1 carriers, BRCA2 carriers, BRCA negative, and low-risk controls
Intervention(s)
Self-collected dried blood spot (DBS)
Main Outcome Measure(s)
AMH levels
Result(s)
One hundred ninety-five women were included: 55 BRCA1 carriers, 50 BRCA2 carriers, 26 BRCA negative, and 64 low-risk controls. After adjusting for confounders, BRCA2 carriers had AMH levels that were 33% lower than controls (Geometric Mean Ratio(GMR)=0.67, 95% CI 0.47–0.94) and an increased odds of having AMH<1 ng/mL (OR 3.69, 95% CI 1.34–10.19). BRCA1 carriers and BRCA negative women had similar AMH levels to controls. When analysis was restricted to regularly menstruating women younger than 40, BRCA2 carriers continued to demonstrate significantly lower AMH levels and increased likelihood of low AMH. Also, in this restricted group, BRCA negative women demonstrated AMH levels that were 42% lower than controls (GMR=0.58; 95%CI 0.35–0.95). No difference in AMH was observed among BRCA1 carriers.
Conclusion(s)
We observed significantly lower AMH levels among BRCA2 carriers compared to low-risk controls. These results were stable across all models. BRCA negative women also had lower AMH values, but only in models restricted to young, regularly menstruating women. In contrast to previous analyses, BRCA1 carriers had AMH values that were similar to low-risk controls, but this may be due to differences in the population studied.
Keywords: BRCA, Anti-mullerian hormone, ovarian reserve, fertility
Introduction
BRCA1 and 2 mutations are associated with increased risk of breast and ovarian cancer in reproductive age women. Among BRCA carriers, the lifetime risks of breast and ovarian cancer are as high as 65% and 39%, respectively. (1–3) BRCA gene mutations are also common, impacting 2.5% of the Ashkenazi Jewish population and up to one in 300 in the general population.
Several authors have suggested that BRCA carriers may also be at risk for infertility. A small study of breast cancer patients undergoing in vitro fertilization (IVF) for fertility preservation found that BRCA carriers were more likely to have a poor response to ovarian stimulation compared to BRCA negative women. (4) The same group also showed that BRCA1 expression in human oocytes declines with age. (5) Natural menopause may occur at a slightly younger age among BRCA carriers, suggesting early depletion of the follicular pool. (6–8) When considering parity, some studies show lower parity among BRCA carriers (7) while others show no difference (9–12) or higher parity. (13)
BRCA1 and BRCA2 are tumor suppressor genes, acting to ensure the integrity of the genome through repair of DNA double stranded breaks. (14) The hypothesized mechanism of diminished ovarian function is premature depletion of the primordial pool due to impaired repair of double stranded DNA breaks. (5) BRCA also maintains telomeres, and some hypothesize that telomere shortening is associated with ovarian aging and reproductive senescence. (15,16) BRCA1 deficient mice have fewer oocytes in response to ovarian stimulation, smaller litter sizes, (5) and flaws in mitotic spindle formation (17), and BRCA2 deficient mice have increased nuclear abnormalities and depletion of germ cells. (18) Histopathology examination of human ovaries suggests that BRCA mutation carriers undergoing risk-reducing oophorectomy have fewer follicles than women undergoing oophorectomy for other reasons. (19)
If indeed BRCA mutations are associated with diminished ovarian reserve or reduced fertility that would have important implications for BRCA carriers and could help to elucidate genetic mechanisms involved in ovarian aging. Therefore, our objective was to compare AMH levels in women at high risk for hereditary breast and ovarian cancer compared to healthy, low-risk controls. We hypothesized that AMH levels will be significantly lower among BRCA carriers compared to low-risk controls.
Materials and Methods
Participants
The Institutional Review Board approved this prospective cohort study at the University of Pennsylvania. Subjects completed an online questionnaire to determine eligibility. Women ages 18–45 years at high risk for hereditary breast and ovarian cancer who had been tested for a BRCA gene mutation were eligible for inclusion in the exposed groups. Healthy women (18–45 years old) at low risk for hereditary breast and ovarian cancer who had never been tested for BRCA were eligible as controls. Women were deemed low risk if they had less than a 5–10% chance of having an inherited predisposition to breast and ovarian cancer and did not meet criteria for screening based on guidelines from the American Congress of Obstetricians and Gynecologists. (20) Exclusion criteria included prior hysterectomy, unilateral or bilateral oophorectomy, chemotherapy or pelvic radiation, pregnancy or lactation within one month, polycystic ovary syndrome (PCOS), or history of any condition associated with premature ovarian failure (i.e. Turner’s syndrome, Fragile X premutation carrier). Individuals with a diagnosis of cancer for which no systemic treatment was administered were included. Eligible subjects completed a detailed questionnaire, which included medical history, reproductive history, contraceptive use, history of infertility, and prior fertility treatment (Supplemental Figure 1). BRCA carriers were asked to list their specific mutation.
Participants were recruited from February 2014 to February 2016 through national advocacy groups; through the University of Pennsylvania’s Basser Center of BRCA, Women’s Health Clinical Research Center, and Division of Reproductive Endocrinology and Infertility; and through local advertisements. Controls were recruited from the latter three groups. Recruitment of controls was targeted such that age and hormonal contraceptive (HC) use were similar among groups.
Study procedures
Subjects who met inclusion criteria received a collection kit with a link to an instructional video. Participants were asked to collect the dried bloodspot (DBS) on cycle day one through five if possible and to record the first day of their last menstrual period, the date of collection, and recent HC use. Subjects returned the DBS in a prepaid return envelope with a signed release of medical records so that their genetic testing results could be obtained. Bloodspots were stored at −20°.
AMH DBS assays were performed by Ansh Labs (Webster, TX) using picoAMH ELISA. The DBS assay for AMH is comparable to serum-based ELISA methods and has been validated in healthy reproductive age women and in cancer survivors. (21–23) The inter-assay and intra-assay variability was 4.7–6.5% and 3.5–7.2%, respectively. The sensitivity was 2 pg/ml, and the range was 3–750 pg/ml. A subgroup of participants also had a serum AMH level performed using ELISA kits (Diagnostic Systems, Gen2, range 0.050–10.0 ng/ml, sensitivity 0.025 ng/ml, inter-assay variability < 8%, intra-assay variability 5%) so that results from the DBS could be validated within this cohort.
We chose to assess ovarian reserve with AMH because it is relatively stable during the menstrual cycle. AMH has been shown to correlate with pregnancy rates and response to ovarian stimulation during IVF (24–26) and is associated with likelihood of pregnancy and time to menopause in a fertile population. (27,28)
Data Analysis
We planned to include 213 subjects. The mean for the AMH DBS reported by McDade et al. was 1.96 ng/mL (standard deviation 1.79 ng/mL) for the entire cohort and 1.29 ng/ml (standard deviation 1.20 ng/mL) for those with infertility.(21) Assuming 2:1 enrollment of BRCA carriers to non-carriers and that BRCA carriers have AMH values similar to the infertility population, the sample size estimate of 213 participants provides 80% power to detect a 0.67 difference in AMH (p=0.05). This difference was thought to be clinically meaningful since it represents the difference in mean AMH observed by McDade and colleagues between healthy and infertile women. We planned to inflate recruitment by 25% to allow for dropout.
After recruitment began, the study design was altered to include a healthy, low-risk control population. Therefore, participants were analyzed in four groups: BRCA1, BRCA2, BRCA negative, and low-risk controls. Women with mutations of undetermined significance and those with deleterious mutations in both BRCA1 and 2 were excluded. Demographic data are presented as means and standard deviations for continuous variables and as percentages for categorical data. Continuous variables were analyzed using Student’s t test or Wilcoxon rank sum test as appropriate, and Pearson’s chi squared and Fisher’s exact test were utilized for categorical data. AMH values were natural log transformed to reduce the impact of large values and analyzed using linear regression. (29)
A multivariable linear regression model was fit to the data controlling for all covariates of interest, including age (linear and quadratic terms were included to account for potential non-linear association between age and log-AMH)(30), HC use, menstrual regularity (yes=1, no=0), African American race, history of cancer, current smoking, menstrual cycle phase of sample collection (early follicular phase=1, other=0), and days from sample collection to assay performance. Backwards elimination was performed, and all covariates with p<0.10 were retained. Final model was adjusted for age, age2, HC use, African American race, and menstrual regularity. Additional linear regression models evaluated each covariate above individually. A subgroup analysis was performed in regularly menstruating women less than 40 years old to limit impact of undiagnosed PCOS and because it has been suggested that the impact of BRCA mutations on fertility occurs only among late reproductive age women. (31) We opted to include regularly-menstruating women taking HC in this subgroup analysis because women with diminished ovarian reserve may use HC for cycle control, and exclusion of HC users could potentially result in exclusion of those with the outcome of interest. Additional subgroup analyses were performed for women without a history of infertility, for women whose BRCA mutation was verified, and for women whose body mass index (BMI) was available. After models were generated, AMH values were transformed from the log scale back to their original scale and presented as geometric means. Geometric mean ratios (GMR) are presented for each model. The geometric mean represents the median of AMH values, while the GMR represents the ratio of the median. (32) We chose to use geometric means as the measure of central tendency because the distribution of AMH values is positively skewed. Compared to the arithmetic mean, the geometric mean is less impacted by outliers and, therefore, provides a more accurate reflection of the data.(33) Geometric mean ratios allow for the groups to be compared to one another. For example, if the GMR for AMH is 0.60, then group A has AMH values that are, on average, 40% lower than group B. Beta coefficients with 95% CIs are included (Supplemental Table 1). Geometric mean AMH values are also presented (Figure 1, Supplemental Table 2). Logistic regression models assessing the likelihood of having a low AMH (defined as AMH <1 ng/mL) are also presented. Pearson’s correlation, rho, was utilized to examine the correlation between AMH values collected from bloodspot and venipuncture. A Bland Altman plot was constructed to show the relationship between the assays and to assess for systematic bias.
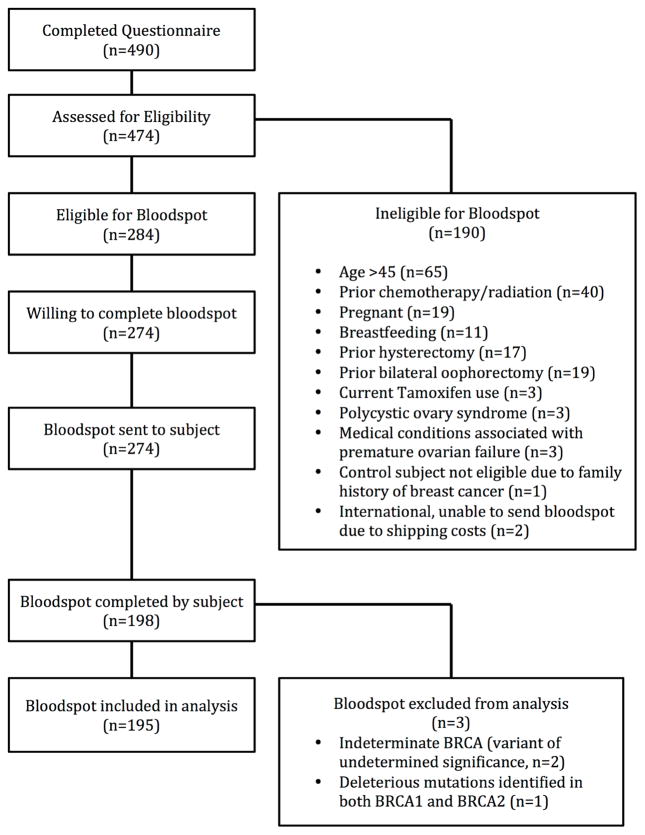
Figure 1.
Enrollment flow diagram
Results
Four hundred and ninety women completed the questionnaire; 284 were eligible to complete the DBS, 274 agreed to complete the DBS, and 198 returned the DBS (Figure 1). There was no significant difference in age, race, smoking status, BRCA group, menstrual regularity, amenorrhea in the past year, parity, or history of infertility among those who returned bloodspots and those who did not (Supplemental Table 3). Three samples were excluded: one subject had a variant of undetermined significance, and two subjects had deleterious mutations in both BRCA1 and BRCA2. Of the 195 subjects included, 55 were BRCA1 positive, 50 were BRCA2 positive, 26 were BRCA negative, and 64 were low-risk controls. A list of individual mutations is included in Supplemental Table 4. The mean age of the cohort was 31.5 years (range 19–45 years). BRCA negative women were, on average, three years older than women in the other groups (p=0.06, Table 1.) There was no difference in race, ethnicity, smoking history, HC use, or menstrual regularity among the groups. Nine subjects reported a history of cancer (4.6%), and all were confirmed to meet inclusion criteria. BRCA negative women were more likely to have a history of cancer compared to other groups (p=0.04).
Table 1.
Demographic data, fertility history, and pregnancy history for BRCA1 carriers, BRCA2 carriers, BRCA negative women, and low-risk controls
| Demographic Data | BRCA1 (n=55) | BRCA2 (n=50) | BRCA - (n=26) | Controls (n=64) |
|---|---|---|---|---|
| Age (years)* | 31.4 (5.5) | 30.9 (6.2) | 34.3 (6.7) | 30.9 (5.6)a |
| Caucasian race, n (%) | 50 (90.9%) | 48 (96.0%) | 24 (92.3%) | 57 (89.1%) |
| African American race, n (%) | 3 (5.5%) | 0 (0%) | 1 (3.8%) | 4 (6.3%) |
| Hispanic ethnicity, n (%) | 2 (3.4%) | 3 (6.0%) | 0 (0%) | 2 (3.1%) |
| Body Mass Index (kg/m2)*ϕ | 25.7 (5.3) | 23.9 (4.1) | 26.9 (7.3) | 24.3 (3.7) |
| Current smoking, n (%) | 2 (3.6%) | 4 (8.0%) | 0 (0%) | 5 (7.8%) |
| History of Smoking, n (%) | 12 (21.8%) | 14 (28.0%) | 6 (23.1%) | 15 (23.4%) |
| History of Cancer, n (%)δ | 3 (5.6%) | 1 (2.0%) | 4 (15.4%) | 1 (1.6%)a |
| Hormonal contraceptive use, n (%) | 16 (29.1%) | 13 (26.0%) | 6 (23.1%) | 20 (31.3%) |
| Progestin-secreting IUD use, n (%) | 10 (18.2%) | 7 (14.0%) | 3 (11.5%) | 7 (10.9%) |
| Regular menses, n (%) | 42 (76.4%) | 38 (76.0%) | 22 (84.6%) | 54 (84.4%) |
| Early follicular phase, n (%) | 38 (69.1%) | 37 (74.0%) | 16 (61.5%) | 49 (76.6%) |
| Graduated college, n (%) | 50 (90.9%) | 42 (84.0%) | 23 (88.5%) | 53 (84.1%) |
| Family history of BRCA mutation, n (%) | 48 (87.3%) | 44 (88.0%) | 16 (61.5%) | 0 (0%)a,b,c |
| Jewish ancestry, n (%) | 18 (32.7%) | 9 (18.0%) | 3 (11.5%) | 5 (7.8%)b |
| Fertility and Pregnancy History | ||||
| Nulligravid, n (%) | 31 (57.4%) | 31 (62.0%) | 11 (42.3%) | 45 (71.4%)a |
| Parous, n (%) | 19 (34.6%) | 14 (28.0%) | 13 (50.0%) | 13 (20.3%)a |
| Age at first birth (years)* | 30.6 (5.1) | 27.1 (5.6) | 28.6 (3.8) | 24.7 (4.7)a,b |
| Tried to conceive, n (%) | 23 (41.8%) | 20 (40.8%) | 13 (50.0%) | 13 (20.3%)a,b,c |
| History of infertility, n (%) | 12 (21.8%) | 12 (24.0%) | 1 (3.8%) | 0 (0%)b,c |
| Any infertility treatment, n (%) | 4 (7.3%) | 7 (14.0%) | 1 (3.8%) | 0 (0%)b,c |
| Prior intrauterine insemination, n (%) | 3 (5.5%) | 2 (4.0%) | 1 (3.8%) | 0 (0%) |
| Prior in vitro fertilization, n (%) | 1 (1.8%) | 2 (4.0%) | 0 (0%) | 0 (0%) |
| Mean, unadjusted AMH (ng/mL)* | 3.26 (2.83) | 2.72 (3.08) | 2.13 (2.31) | 3.20 (2.62)a |
p<0.05 for BRCA negative vs. controls
p<0.05 for BRCA1 vs. control
p<0.05 for BRCA2 vs. control
Presented as mean (standard deviation)
Data available for 80 subjects: 18 BRCA1, 18 BRCA2, 8 BRCA-, and 36 controls
Cancer diagnoses included: Breast cancer (n=4), thyroid cancer (n=2), and skin cancer (n=3).
The majority of subjects were nulligravid. Low-risk controls were less likely to report trying to conceive in the past, were less likely to have children, and were younger at the time of first birth (Table 1). BRCA1 and BRCA2 carriers were more likely to have a history of infertility (defined as 12 consecutive months of unprotected intercourse without pregnancy) and to have received fertility treatment (Table 1). Three subjects, one BRCA1 carrier and two BRCA2 carriers, had undergone IVF; of those, one BRCA2 carrier used preimplantation genetic diagnosis.
Log transformed AMH was negatively associated with age (p<0.001) and quadratic age (p<0.001). After adjustment for age, AMH levels were lower in women reporting HC use (p=0.04) and African American race (p=0.04) and higher in women with irregular menses (p=0.02). Time since collection did not impact the AMH levels (p=0.85), nor did current smoking (p=0.61), body mass index (BMI) (p=0.35), history of cancer (p=0.26), or whether the sample was collected in the early follicular phase (p=0.22).
Mean unadjusted AMH levels were significantly different among the groups (p=0.046, Table 1). Age-adjusted models showed a trend toward lower AMH levels in BRCA2 carriers compared to low-risk controls (GMR 0.72, 95% CI 0.51–1.02, p=0.067, Table 2A). After adjusting for additional confounders, BRCA2 carriers had AMH levels that were 33% lower than controls (GMR 0.67, 95% CI 0.47–0.94, p=0.021, Table 2A).
Table 2A.
Linear regression models for Anti-Mullerian Hormone for BRCA1 carriers, BRCA2 carriers, and BRCA Negative women compared to low-risk controls. Data modeled as natural log of AMH and presented as geometric mean ratio (GMR) with 95% confidence intervals (CI).
| Model | BRCA1 | BRCA2 | BRCA - | |||
|---|---|---|---|---|---|---|
| GMR (95% CI) | P value | GMR (95% CI) | P value | GMR (95% CI) | P value | |
| Age-adjusted (n=195)1 | 1.03 (0.73–1.44) | 0.873 | 0.72 (0.51–1.02) | 0.067 | 0.86 (0.55–1.33) | 0.490 |
| Fully-adjusted (n=195)2 | 0.99 (0.71–1.38) | 0.966 | 0.67 (0.47–0.94) | 0.021 | 0.84 (0.55–1.28) | 0.414 |
| Fully-adjusted, mutation verified (n=171)2 | 0.93 (0.65–1.35) | 0.719 | 0.59 (0.41–0.86) | 0.006 | 0.84 (0.54–1.30) | 0.429 |
| Fully-adjusted, excluding subjects with history of infertility (n=170) 2 | 0.98 (0.68–1.41) | 0.893 | 0.66 (0.45–0.98) | 0.038 | 0.81 (0.52–1.26) | 0.346 |
| Fully-adjusted, including only subjects with BMI available (n=80)3 | 0.86 (0.49–1.52) | 0.607 | 0.51 (0.28–0.91) | 0.023 | 0.58 (0.27–1.26) | 0.165 |
| Fully-adjusted, restricted to women < 40 years old with regular menses (n=135)4 | 1.00 (0.70–1.44) | 0.999 | 0.67 (0.45–0.98) | 0.037 | 0.58 (0.35–0.95) | 0.031 |
| Fully-adjusted, restricted to women < 40 years old with regular menses, mutation verified (n=122)4 | 0.93 (0.63–1.38) | 0.733 | 0.59 (0.39–0.89) | 0.012 | 0.58 (0.35–0.96) | 0.036 |
| Fully-adjusted, restricted to women < 40 years old with regular menses, mutation verified and BMI available (n=58)5 | 0.91 (0.49–1.68) | 0.747 | 0.33 (0.17–0.63) | 0.001 | 0.44 (0.21–0.94) | 0.035 |
Adjusted for age and age2
Adjusted for age, age2, hormonal contraceptive use, African American race, and regular menses.
Adjusted for age, age2, hormonal contraceptive use, African American race, regular menses, and body mass index.
Adjusted for age, age2, hormonal contraceptive use, and African American race.
Adjusted for age, age2, hormonal contraceptive use, African American race, and body mass index.
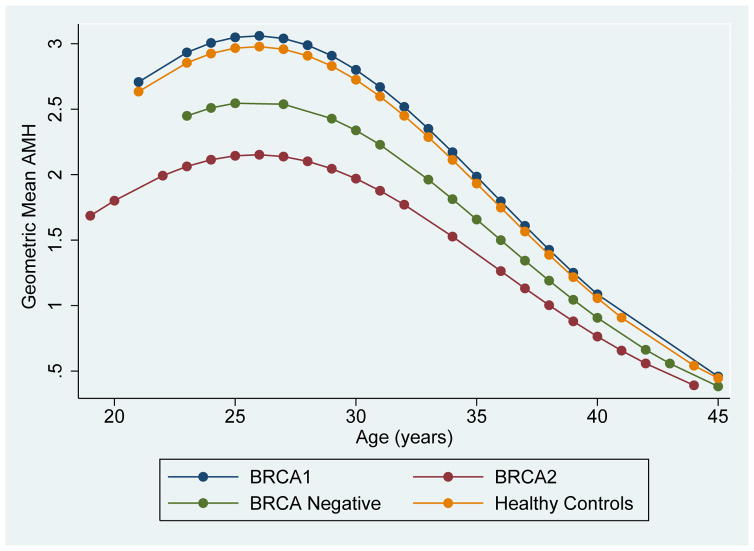
Predicted geometric mean AMH levels adjusted for age were calculated and are presented in Figure 2. In all groups, AMH was noted to increase until age 26 and then decline, which is consistent with AMH trends observed in healthy, low-risk populations. (34) To demonstrate differences in AMH levels between groups at varying ages, geometric mean AMH levels were calculated at five-year intervals (Supplemental Table 4).
Figure 2.
Predicted geometric mean Anti-Mullerian Hormone (AMH) levels among BRCA1 carriers, BRCA2 carriers, BRCA negative women, and low-risk controls. Adjusted for age (linear and quadratic terms).
Several subgroup analyses were performed to assess confounding and confirm the robustness of the findings. First, when the analysis was restricted to women with verified mutation status, results for BRCA2 carriers were even more significant (GMR 0.59, 95% CI 0.41–0.86, p=0.006, Table 2A). Similar results were obtained when subjects with a history of infertility were excluded (GMR 0.66, 95% CI 0.45–0.98, p=0.038), when BMI was included in the model (GMR 0.51 (0.28–0.91, p=0.023), and when Bonferroni correction was used to control for multiple comparisons (data not shown). When the analysis was restricted to women less than 40 years of age with regular menses, AMH levels continued to be 33% lower in BRCA2 carriers compared to low-risk controls (GMR 0.67, 95% CI 0.45–0.98, p=0.037). Interestingly, in this subgroup, BRCA negative women also had significantly lower AMH levels compared to controls (GMR 0.58, 95% CI 0.35–0.95, p=0.031). BRCA1 carriers had similar AMH levels to low-risk controls in all models.
In order to further explore these associations, AMH was evaluated as a dichotomous outcome with a low AMH defined as a value in the lowest quartile corresponding to an AMH of less than 1 ng/ml. After backwards selection, linear age, HC use and regular menses remained significant and were included in the model. When the entire cohort was examined, BRCA2 carriers were more likely to have a low AMH (OR 3.69, 95% 1.34–10.19, p=0.012, Table 2B) while BRCA1 and BRCA negative women did not. Similar results were observed in restricted models.
Table 2B.
Logistic regression models for odds of low Anti-Mullerian Hormone (<1.0 ng/mL) for BRCA1 carriers, BRCA2 carriers, and BRCA negative women compared to low-risk controls. Data presented as Odds Ratio (OR) with 95% CI.
| Model | BRCA1 | BRCA2 | BRCA - | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age-adjusted (n=195)1 | 1.32 (0.48–3.61) | 0.593 | 3.26 (1.22–3.61) | 0.018 | 1.15 (0.34–3.85) | 0.826 |
| Fully-adjusted (n=195)2 | 1.46 (0.53–4.06) | 0.476 | 3.69 (1.34–10.19) | 0.012 | 1.11 (0.32–3.83) | 0.869 |
| Fully-adjusted, mutation verified (n=171)2 | 1.86 (0.64–5.46) | 0.256 | 5.28 (1.77–15.78) | 0.003 | 1.11 (0.32–3.89) | 0.864 |
| Fully-adjusted, excluding subjects with history of infertility (n=170) 2 | 1.23 (0.41–3.63) | 0.714 | 3.08 (1.01–9.36) | 0.048 | 1.21 (0.36–4.08) | 0.759 |
| Fully-adjusted, including only subjects with BMI available (n=80)3 | 1.75 (0.32–9.71) | 0.520 | 6.06 (1.11–33.1) | 0.038 | 0.33 (0.02–6.32) | 0.460 |
| Fully-adjusted, restricted to women < 40 years old with regular menses (n=135)4 | 1.59 (0.50–5.09) | 0.436 | 2.94 (0.91–9.49) | 0.072 | 1.30 (0.27–6.21) | 0.741 |
| Fully-adjusted, restricted to women < 40 years old with regular menses, mutation verified (n=122)4 | 2.12 (0.64–7.05) | 0.733 | 4.74 (1.37–16.44) | 0.014 | 1.28 (0.26–6.16) | 0.759 |
| Fully-adjusted, restricted to women < 40 years old with regular menses, mutation verified and BMI available (n=58)5 | 1.15 (0.14–9.42) | 0.894 | 11.40 (1.55–83.8) | 0.017 | 1.23 (0.08–18.42) | 0.879 |
Adjusted for age only
Adjusted for age, hormonal contraceptive use, and regular menses.
Adjusted for age, hormonal contraceptive use, regular menses and body mass index.
Adjusted for age and hormonal contraceptive use.
Adjusted for age, hormonal contraceptive use, and body mass index
Both DBS and serum AMH values were obtained in 21 subjects. When serum and bloodspot log AMH values were compared, the results were highly correlated (rho=0.79, p<0.0001). A Bland Altman plot, which demonstrates the difference in log AMH measured with DBS and serum relative to the average of the two measurements, showed no evidence of systematic error (Supplemental Figure 2).
Discussion
We observed significantly lower AMH levels in BRCA2 mutation carriers compared to low-risk controls. Geometric mean ratios for BRCA2 carriers ranged from 0.59 to 0.72 across models, suggesting that geometric mean AMH values were 28–41% lower compared to controls. BRCA2 carriers were also three to five times more likely to have low AMH compared to controls. Furthermore, low AMH levels were observed among BRCA2 carriers throughout the reproductive lifespan, a finding that challenges the current hypothesis that the reproductive impact of BRCA2 mutations only occurs among women of advanced reproductive age. (31) Interestingly, we also observed lower AMH values in BRCA negative women, but only when the cohort was restricted to young, regularly menstruating women. We did not observe differences in AMH among BRCA1 carriers compared to controls.
Several authors have demonstrated decreased AMH levels among BRCA1 carriers. A study comparing unadjusted AMH levels in 15 BRCA1 carriers, 9 BRCA2 carriers, and 60 non-carriers demonstrated lower AMH values in BRCA1 carriers compared to non-carriers (1.12 vs. 2.23 ng/ml, p<0.001). There was a trend toward lower AMH values in BRCA2 carriers (1.39 vs. 2.23 ng/ml) but the difference was not significant (p=0.127). (5) Additionally, a study examining AMH values among 62 BRCA1 carriers, 27 BRCA2 carriers, and 54 controls found significantly lower AMH levels in BRCA1 carriers but not in BRCA2 carriers after adjusting for age and BMI. (35) Another study examining 124 BRCA1 carriers and controls observed that BRCA1 carriers have increased odds of low AMH but only among women older than 35. (36)
One of the largest studies to date examined 693 women ages 25 to 45 years in Australia and New Zealand who were enrolled in a prospective cohort study. (37) Similar to our findings, increased age was associated with declining AMH, and HC users had 28% lower AMH levels than non-users. The authors observed that AMH values were 25% lower in BRCA1 carriers compared to BRCA1 non-carriers. The findings were robust after controlling for HC use and other confounders. They observed no difference in AMH between BRCA2 carriers and non-carriers.
While these studies suggest diminished ovarian reserve in BRCA1 and not in BRCA2 carriers, others have reached different conclusions. Michaelson and colleagues examined AMH in 41 healthy BRCA carriers (26 BRCA1, 12 BRCA2, 3 BRCA1and2) and found no difference in AMH levels when compared to age-based AMH normograms. (38) In addition, Valentini et al. examined chemotherapy-induced amenorrhea in 1,506 BRCA1 carriers and 433 BRCA2 carriers. Compared to BRCA1 carriers, BRCA2 carriers were more likely to have chemotherapy-induced amenorrhea (47% vs. 33%, p<0.001). The difference remained significant after adjusting for age at diagnosis and when excluding women treated with Tamoxifen. (39) Finally, a large study examining age of natural menopause in BRCA carriers showed that menopause occurred slightly earlier in BRCA carriers compared to controls. When BRCA1 and BRCA2 carriers were analyzed separately, a statistically significant difference remained for BRCA2 carriers but not for BRCA1 carriers.(6)
There are several possible explanations as to why we observed lower AMH values in BRCA2 carriers while others have not. With one exception, we included more BRCA2 carriers than previous studies, yielding more statistical power to detect a difference. In addition, we controlled for several important confounders, including age, HC use, race, and menstrual irregularity. HC use and African American race have been shown to be associated with lower AMH levels, (30,40–42), and menstrual irregularity is associated with higher AMH levels, especially in the setting of PCOS. (43–46) In the current study, history of PCOS was specifically evaluated, as it was part of the exclusion criteria. Furthermore, we assessed menstrual cycle regularity and performed a subgroup analysis in regularly menstruating women to reduce the impact that participants with undiagnosed PCOS would have on the results.
We did not observe lower AMH levels in BRCA1 carriers. On the contrary, BRCA1 carriers had AMH levels that were similar to low-risk controls. This difference may be due to our heterogeneous population. Use of self-collected DBS allowed us to recruit women nationwide, which resulted in a diverse population. Only 13 BRCA1 carriers and 5 BRCA2 carriers in our study had an Ashkenazi Jewish mutation compared to 73% and 62%, respectively, of BRCA1 and BRCA2 carriers studied by Wang and colleagues. (35) Similarly, all subjects included by Phillips et al. were recruited from Australia and New Zealand, thus representing a different population. (37) Additionally, the current study included healthy, untested women at low risk for hereditary breast and ovarian cancer as the control group while most other investigators have utilized a control group composed of BRCA negative women. BRCA negative women are, necessarily, at high risk for hereditary breast and ovarian cancer due to personal or family history of cancer. It is possible that BRCA negative women are also at risk for diminished ovarian reserve through BRCA-independent mechanisms. If BRCA negative women are also at risk for diminished ovarian reserve, then that could help to explain varying results across studies.
In addition, it has been hypothesized that women most severely affected by BRCA mutations will have early-onset cancer and will not be eligible for inclusion in studies assessing ovarian reserve. (31) If one assumes that the BRCA mutations with the most oncogenic potential also cause the most damage to the ovarian follicle, then it is possible that exclusion of women at the highest risk for early onset cancer also resulted in exclusion of those women with the highest risk of diminished ovarian reserve. This could impact BRCA1 carriers preferentially since the average age of cancer diagnosis is earlier than BRCA2 carriers, and BRCA1 carriers may undergo prophylactic oophorectomy earlier in life. When we examined participants who were not eligible for the study, there was no difference in the percentage of BRCA1 carriers and BRCA2 carriers who were excluded due to prior bilateral oophorectomy (29% vs. 26%, p=0.54) or due to prior chemotherapy (11% vs. 9%, p=0.61). In addition, among those excluded for bilateral oophorectomy or chemotherapy, there was no difference in age of last menstrual period between BRCA1 and BRCA 2 carriers (41 vs. 42 years, p=0.56). Therefore, there does not appear to be preferential exclusion of BRCA1 carriers in our cohort. Nonetheless, it is possible that young BRCA1 carriers with early onset disease or early oophorectomy were less likely to enroll, limiting our ability to detect low AMH in that group. While this hypothesis could explain why we did not observe low AMH in BRCA1 carriers, it does not explain why we observed low AMH in BRCA2 carriers and among BRCA negative women in some models.
A recent large-scale genome wide analysis suggests an alternate hypothesis. Day et al. examined genes associated with age of natural menopause and showed associations with DNA damage response genes. Early age at natural menopause was highly correlated with four common BRCA1 variants; however, all four are considered “not clinically important” for hereditary breast and ovarian cancer risk. (47) These findings could suggest a potential for misclassification bias since most studies include only women with deleterious mutations in the affected group. Additionally, the authors identified 15 signals involving various DNA repair mechanisms. At least one of these genes, RAD51, is also important in the BRCA2 pathway. (48) It is possible that the lower AMH levels that we observed in BRCA2 carriers occurred due to variants in other DNA repair genes associated with the BRCA2 pathway. While there is mounting evidence that DNA repair genes are involved in regulation of the follicular pool, further studies are needed to more precisely identify the mechanisms and candidate genes.
Interestingly, we observed lower AMH levels in BRCA negative women in some models, which was unexpected. A possible explanation for this finding can be extrapolated from the breast cancer literature. Some authors have demonstrated that women who come from BRCA1/2 positive families who themselves test negative for a BRCA gene mutation develop breast cancer at a higher rate than expected.(49–51) It has been hypothesized that there are inherited genetic modifiers in these families that increase the risk of breast cancer even in the absence of a deleterious BRCA mutation. It stands to reason that the same could be true for ovarian reserve. BRCA negative women may be more likely to inherit genetic modifiers that predispose to early depletion of the follicular pool even in the absence of a deleterious BRCA gene mutation. It is important to note that while some studies have shown increased risk of breast cancer in BRCA negative women, others have not.(52,53) It is possible that these well-designed studies found different conclusions because of differences in the patient populations studied and the inability to control for unknown genetic and environmental factors. Similar to oncogenesis, the regulation of the follicular pool likely involves a complex interplay between multiple genetic pathways and environmental factors.
This study has several strengths. First, we used a validated, self-collected, dried bloodspot to assess AMH levels. This novel approach allowed us to recruit women from a geographically diverse population. We rigorously evaluated potential confounders and included important confounders in our models. Multiple subgroup analyses were performed, and results for BRCA1 and BRCA2 carriers were consistent across models. In addition, mutation status was confirmed for most participants, and restricting the analysis to those with confirmed mutation status strengthened the findings observed in BRCA2 carriers. Furthermore, we excluded women with PCOS, and we limited the impact of undiagnosed PCOS by controlling for menstrual cycle regularity and performing a subgroup analysis of regularly menstruating women.
The weaknesses of this study include small sample size. Dropout between questionnaire and bloodspot was higher than expected, so we did not reach our target sample size. It is possible that we did not detect a difference in AMH among BRCA1 carriers due to small sample size. However, BRCA1 carriers and controls had similar AMH levels, and a trend toward lower levels among BRCA1 carriers was not observed. In addition, our results may have been influenced by selection bias. It is possible that women with low ovarian reserve or infertility were more likely to participate. However, the number of BRCA carriers with infertility was low, and subgroup analysis excluding women with infertility revealed similar findings. Additionally, BMI was only available for 41% of subjects, limiting our ability to assess BMI as a potential confounder. Nonetheless, when comparing BMI for those with data available, there was no significant difference between groups. Similar to results reported in other cohorts (46), linear regression analysis revealed no association between BMI and AMH in this cohort. Furthermore, restricting the analysis to those with BMI available and including BMI in the model had no impact on the results (Table 2, Supplemental Table 1). Finally, to limit confounding, we did not include women who had been treated with chemotherapy, which resulted in exclusion of most women with breast cancer. Similarly, women choosing to undergo risk-reducing salpingo-oophorectomy at an early age were also excluded. Therefore, the results may not be generalizable to those with severe mutations or those at the highest risk for early onset breast cancer.
It is important to note that while we observed significantly lower AMH values in BRCA2 carriers, the differences observed between groups are small. It is unclear if differences in AMH will correlate with lower fecundity or impaired response to ovarian stimulation during IVF.
In conclusion, in this cohort, BRCA2 carriers had significantly lower AMH levels compared to healthy, low-risk women and had increased odds of having a low AMH. BRCA negative women were also noted to have lower AMH levels in some, but not all, models. BRCA1 carriers had AMH levels that were similar to low-risk women. These findings may suggest that the BRCA2 DNA repair pathway is involved in regulation of the follicular pool. Further studies are needed to validate these findings and elucidate the underlying cellular mechanism.
Supplementary Material
Acknowledgments
Funding support: Supported by the NIH T32 Reproductive Epidemiology Training Grant (HD 007440) (LJ), ASRM Fertility Preservation Grant, Basser Center for BRCA, and Penn Presbyterian Harrison Fund
Footnotes
Financial disclosures
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77:2318–24. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2318::AID-CNCR21>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28:240–4. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5:172ra21. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finch A, Valentini A, Greenblatt E, Lynch HT, Ghadirian P, Armel S, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril. 2013;99:1724–8. doi: 10.1016/j.fertnstert.2013.01.109. [DOI] [PubMed] [Google Scholar]
- 7.Lin WT, Beattie M, Chen L, Oktay K, Crawford SL, Gold EB, et al. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer. 2013;119:1652–9. doi: 10.1002/cncr.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rzepka-Gorska I, Tarnowski B, Chudecka-Głaz A, Gorski B, Zielińska D, Tołoczko-Grabarek A. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat. 2006;100:59–63. doi: 10.1007/s10549-006-9220-1. [DOI] [PubMed] [Google Scholar]
- 9.Pal T, Keefe D, Sun P, Narod SA Hereditary Breast Cancer Clinical Study Group. Fertility in women with BRCA mutations: a case-control study. Fertil Steril. 2010;93:1805–8. doi: 10.1016/j.fertnstert.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 10.Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, Weideman PC, et al. Do BRCA1 and BRCA2 mutation carriers have earlier natural menopause than their noncarrier relatives? Results from the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. J Clin Oncol. 2013;31:3920–5. doi: 10.1200/JCO.2013.49.3007. [DOI] [PubMed] [Google Scholar]
- 11.Friedman E, Kotsopoulos J, Lubinski J, Lynch HT, Ghadirian P, Neuhausen SL, et al. Spontaneous and therapeutic abortions and the risk of breast cancer among BRCA mutation carriers. Breast Cancer Res. 2006;8:R15. doi: 10.1186/bcr1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moslehi R, Singh R, Lessner L, Friedman JM. Impact of BRCA mutations on female fertility and offspring sex ratio. Am J Hum Biol. 2010;22:201–5. doi: 10.1002/ajhb.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith KR, Hanson HA, Mineau GP, Buys SS. Effects of BRCA1 and BRCA2 mutations on female fertility. Proc Biol Sci. 2012;279:1389–95. doi: 10.1098/rspb.2011.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–82. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 15.McPherson JP, Hande MP, Poonepalli A, Lemmers B, Zablocki E, Migon E, et al. A role for Brca1 in chromosome end maintenance. Hum Mol Genet. 2006;15:831–8. doi: 10.1093/hmg/ddl002. [DOI] [PubMed] [Google Scholar]
- 16.Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol. 2006;18:280–5. doi: 10.1097/01.gco.0000193019.05686.49. [DOI] [PubMed] [Google Scholar]
- 17.Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharan SK, Pyle A, Coppola V, Babus J, Swaminathan S, Benedict J, et al. BRCA2 deficiency in mice leads to meiotic impairment and infertility. Development. 2004;131:131–42. doi: 10.1242/dev.00888. [DOI] [PubMed] [Google Scholar]
- 19.Pavone ME, Hirshfeld-Cytron J, Tingen C, Thomas C, Thomas J, Lowe MP, et al. Human ovarian tissue cortex surrounding benign and malignant lesions. Reprod Sci. 2014;21:582–9. doi: 10.1177/1933719113506498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists, ACOG Committee on Practice Bulletins--Gynecology, ACOG Committee on Genetics, Society of Gynecologic Oncologists. ACOG Practice Bulletin No. 103: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2009;113:957–66. doi: 10.1097/AOG.0b013e3181a106d4. [DOI] [PubMed] [Google Scholar]
- 21.McDade TW, Woodruff TK, Huang Y-Y, Funk WE, Prewitt M, Kondapalli L, et al. Quantification of anti-Mullerian hormone (AMH) in dried blood spots: validation of a minimally invasive method for assessing ovarian reserve. Hum Reprod. 2012;27:2503–8. doi: 10.1093/humrep/des194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts SC, Seav SM, McDade TW, Dominick SA, Gorman JR, Whitcomb BW, et al. Self-collected dried blood spots as a tool for measuring ovarian reserve in young female cancer survivors. Hum Reprod. 2016;31:1570–8. doi: 10.1093/humrep/dew114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su HI, Sammel MD, Homer MV, Bui K, Haunschild C, Stanczyk FZ. Comparability of antimullerian hormone levels among commercially available immunoassays. Fertil Steril. 2014;101:1766–72. doi: 10.1016/j.fertnstert.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468–71. doi: 10.1016/s0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 25.van Rooij IA, Broekmans FJ, te Velde ER, Fauser BJ, Bancsi LF, de Jong FH, et al. Serum anti-mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–71. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 26.Kwee J, Schats R, McDonnell J, Themmen A, de Jong FH, Lambalk C. Evaluation of anti-Mullerian hormone as a test for the prediction of ovarian reserve. Fertil Steril. 2008;90:737–43. doi: 10.1016/j.fertnstert.2007.07.1293. [DOI] [PubMed] [Google Scholar]
- 27.Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, et al. Antimullerian Hormone as a Predictor of Natural Fecundability in Women Aged 30–42 Years. Obstet Gynecol. 2011;117:798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-Mullerian Hormone as a Predictor of Time to Menopause in Late Reproductive Age Women. J Clin Endocrinol Metab. 2012;97:1673–80. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su HI, Flatt SW, Natarajan L, DeMichele A, Steiner AZ. Impact of breast cancer on anti-mullerian hormone levels in young women. Breast Cancer Res Treat. 2013;137:571–7. doi: 10.1007/s10549-012-2361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleil ME, Gregorich SE, Adler NE, Sternfeld B, Rosen MP, Cedars MI. Race/ethnic disparities in reproductive age: an examination of ovarian reserve estimates across four race/ethnic groups of healthy, regularly cycling women. Fertil Steril. 2014;101:199–207. doi: 10.1016/j.fertnstert.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oktay K, Turan V, Titus S, Stobezki R, Liu L. BRCA Mutations, DNA Repair Deficiency, and Ovarian Aging. Biol Reprod. 2015;93:67–7. doi: 10.1095/biolreprod.115.132290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quan H, Zhang J. Estimate of standard deviation for a log-transformed variable using arithmetic means and standard deviations. Stat Med. 2003;22:2723–36. doi: 10.1002/sim.1525. [DOI] [PubMed] [Google Scholar]
- 33.Olivier J, Johnson WD, Marshall GD. The logarithmic transformation and the geometric mean in reporting experimental IgE results: what are they and when and why to use them? Ann Allergy Asthma Immunol. 2008;100:333–7. doi: 10.1016/S1081-1206(10)60595-9. [DOI] [PubMed] [Google Scholar]
- 34.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS ONE. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang ET, Pisarksa MD, Bresee C, Chen YD, Alexander C, Karlan B. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril. 2014;102:1723–8. doi: 10.1016/j.fertnstert.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giordano S, Garrett-Mayer E, Mittal N, Smith K, Shulman L, Passaglia C, et al. Association of BRCA1 Mutations with Impaired Ovarian Reserve: Connection Between Infertility and Breast/Ovarian Cancer Risk. J Adolesc Young Adult Oncol. 2016;5:337–43. doi: 10.1089/jayao.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips K-A, Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, et al. Anti-Mullerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations. Hum Reprod. 2016;31:1126–32. doi: 10.1093/humrep/dew044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaelson-Cohen R, Mor P, Srebnik N, Beller U, Levy-Lahad E, Eldar-Geva T. BRCA mutation carriers do not have compromised ovarian reserve. Int J Gynecol Cancer. 2014;24:233–7. doi: 10.1097/IGC.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 39.Valentini A, Finch A, Lubinski J, Byrski T, Ghadirian P, Kim-Sing C, et al. Chemotherapy-induced amenorrhea in patients with breast cancer with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2013;31:3914–9. doi: 10.1200/JCO.2012.47.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson LNC, Sammel MD, Dillon KE, Lechtenberg L, Schanne A, Gracia CR. Antimullerian hormone and antral follicle count are lower in female cancer survivors and healthy women taking hormonal contraception. Fertil Steril. 2014;102:774–81. doi: 10.1016/j.fertnstert.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bentzen JG, Forman JL, Pinborg A, Lidegaard O, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25:612–9. doi: 10.1016/j.rbmo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Birch Petersen K, Hvidman HW, Forman JL, Pinborg A, Larsen EC, Macklon KT, et al. Ovarian reserve assessment in users of oral contraception seeking fertility advice on their reproductive lifespan. Hum Reprod. 2015;30:2364–75. doi: 10.1093/humrep/dev197. [DOI] [PubMed] [Google Scholar]
- 43.Kristensen SL, Ramlau-Hansen CH, Andersen CY, Ernst E, Olsen SF, Bonde JP, et al. The association between circulating levels of antimullerian hormone and follicle number, androgens, and menstrual cycle characteristics in young women. Fertil Steril. 2012;97:779–85. doi: 10.1016/j.fertnstert.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–9. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 45.Casadei L, Madrigale A, Puca F, Manicuti C, Emidi E, Piccione E, et al. The role of serum anti-Mullerian hormone (AMH) in the hormonal diagnosis of polycystic ovary syndrome. Gynecol Endocrinol. 2013;29:545–50. doi: 10.3109/09513590.2013.777415. [DOI] [PubMed] [Google Scholar]
- 46.Dolleman M, Verschuren WM, Eijkemans MJ, Dolle ME, Jansen EH, Broekmans FJ, et al. Reproductive and lifestyle determinants of anti-Mullerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106–15. doi: 10.1210/jc.2012-3995. [DOI] [PubMed] [Google Scholar]
- 47.Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47:1294–303. doi: 10.1038/ng.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holloman WK. Unraveling the mechanism of BRCA2 in homologous recombination. Nat Struct Mol Biol. 2011;18:748–54. doi: 10.1038/nsmb.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evans DGR, Ingham SL, Buchan I, Woodward ER, Byers H, Howell A, et al. Increased rate of phenocopies in all age groups in BRCA1/BRCA2 mutation kindred, but increased prospective breast cancer risk is confined to BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2013;22:2269–76. doi: 10.1158/1055-9965.EPI-13-0316-T. [DOI] [PubMed] [Google Scholar]
- 50.Smith A, Moran A, Boyd MC, Bulman M, Shenton A, Smith L, et al. Phenocopies in BRCA1 and BRCA2 families: evidence for modifier genes and implications for screening. J Med Genet. 2007;44:10–5. doi: 10.1136/jmg.2006.043091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gronwald J, Cybulski C, Lubinski J, Narod SA. Phenocopies in breast cancer 1 (BRCA1) families: implications for genetic counselling. J Med Genet. 2007;44:e76. doi: 10.1136/jmg.2006.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Domchek SM, Gaudet MM, Stopfer JE, Fleischaut MH, Powers J, Kauff N, et al. Breast cancer risks in individuals testing negative for a known family mutation in BRCA1 or BRCA2. Breast Cancer Res Treat. 2010;119:409–14. doi: 10.1007/s10549-009-0611-y. [DOI] [PubMed] [Google Scholar]
- 53.Kurian AW, Gong GD, John EM, Johnston DA, Felberg A, West DW, et al. Breast cancer risk for noncarriers of family-specific BRCA1 and BRCA2 mutations: findings from the Breast Cancer Family Registry. J Clin Oncol. 2011;29:4505–9. doi: 10.1200/JCO.2010.34.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.