Abstract
In response to acute viral infections, activated naïve T cells give rise to effector T cells that clear the pathogen and memory T cells that persist long-term and provide heightened protection. T cell factor 1 (Tcf1) is essential for several of these differentiation processes. Tcf1 is expressed in multiple isoforms, with all isoforms sharing the same HDAC and DNA-binding domains, and the long isoforms containing a unique N-terminal β-catenin-interacting domain. Here we specifically ablated Tcf1 long isoforms in mice, while retaining expression of Tcf1 short isoforms. During CD8+ T cell responses, Tcf1 long isoforms were dispensable for generating cytotoxic CD8+ effector T cells and maintaining memory CD8+ T cell pool size, but contributed to optimal maturation of central memory CD8+ T cells and their optimal secondary expansion in a recall response. On the other hand, Tcf1 long isoforms were required for differentiation of T follicular helper (TFH) cells but not T helper 1 (TH1) effectors elicited by viral infection. Whereas Tcf1 short isoforms adequately supported Bcl6 and ICOS expression in TFH cells, Tcf1 long isoforms remained important for suppressing the expression of Blimp1 and TH1-associated genes and for positively regulating Id3 to restrain germinal center TFH cell differentiation. Furthermore, formation of memory TH1 and memory TFH cells strongly depended on Tcf1 long isoforms. These data reveal that Tcf1 long and short isoforms have distinct yet complementary functions and may represent an evolutionarily conserved means to ensure proper programming of CD8+ and CD4+ T cell responses to viral infection.
In response to a viral infection, naïve T cells that recognize their cognate antigens become activated, expand prolifically, and differentiate into effector T cells equipped with diverse functions. Effector CD8+ T cells acquire cytotoxic functions and eliminate virus-infected cells (1, 2). On the other hand, activated CD4+ T cells predominantly differentiate into two types of effectors, T helper 1 (TH1) cells that secrete interferon-γ (IFN-γ) and enhance the cytotoxicity of effector CD8+ T cells, or T follicular helper (TFH) cells that secret IL-4 and IL-21 and provide essential help to antibody-producing B cells (3–5). Effector T cells are heterogeneous and contain subsets that have different kinetics of contraction following the peak responses, and hence different potential to give rise to memory T cells. Whereas memory CD8+ T cells are more durable than memory CD4+ T cells, both populations contribute to enhanced responses upon re-challenge with the same antigen.
Differentiation of effector T cells and their transition to memory T cells are coordinated by transcriptional regulators (6). In activated CD8+ T cells, T-bet and Blimp1 transcription factors as well as the Id2 cofactor are potently induced, and critically regulate CD8+ effector cell differentiation and acquisition of cytotoxic functions (7–9). On the other hand, Eomes, Bcl6, and Id3 promote the transition and survival of memory CD8+ T cells (9–11). In CD4+ T cells, T-bet and Bcl6 are the lineage-specifying master regulator for TH1 and TFH cells, respectively (3, 4), and induction of Blimp1 and Id2 favors TH1 differentiation at the expense of TFH lineage (12, 13). In contrast to advances in elucidating the transcriptional networks in CD4+ lineage differentiation at the effector phase, little is known about transcriptional regulation involved in memory CD4+ T cell formation and functions.
T cell factor 1 (Tcf1) has been known as a transcription factor acting downstream of the Wnt pathway and can interact with β-catenin coactivator. β-catenin is post-translationally regulated and stabilized by Wnt- or prostaglandin-derived signals. In addition to its essential role for T cell development (14, 15), recent studies have revealed that Tcf1 critically regulates mature T cell responses. Whereas loss of Tcf1 modestly diminished production of effector CD8+ T cells, Tcf1 is essential for maturation, longevity, and secondary expansion of memory CD8+ T cells (16, 17). In activated CD4+ T cells, Tcf1 appears to restrain TH1 differentiation in vitro (18) but is essential for activating the TFH program by acting upstream of Bcl6 (12, 19, 20). Due to differential promoter usage and alternative splicing, multiple Tcf1 isoforms can be detected in T cells (21). All isoforms contain a C-terminal HMG DNA binding domain, which can also interact with Groucho/Transducin-like Enhancer-of-split (TLEs) corepressor proteins, and a newly discovered HDAC domain (22). The Tcf1 long isoforms (p45 and p42) contain an N-terminal β-catenin-binding domain, while the Tcf1 short isoforms (p33 and p30) lack this domain and hence cannot interact with β-catenin. Most of the previous loss-of-function studies ablated all Tcf1 isoforms. The specific requirements for the Tcf1 long isoforms in effector and memory T cell responses have not yet been elucidated.
Here we specifically ablated Tcf1 long isoforms in mouse, and coupled this model with MHC-I- and MHC-II-restricted T cell receptor (TCR) transgenes to dissect the roles of Tcf1 isoforms in regulating mature CD8+ and CD4+ T cell responses. Our data showed that Tcf1 long isoforms were dispensable for generation of cytotoxic CD8+ effector T cells and helper TH1 cells in response to viral infection, while Tcf1 short isoforms were sufficient for maintaining memory CD8+ T cell pool size. On the other hand, Tcf1 long isoforms remained critical for TFH differentiation at the effector phase and for generation of both memory TH1 and memory TFH cells. This study reveals a functional complementation among Tcf1 isoforms in programming mature T cell responses, and further suggests an inter-isoform coordination is necessary to constitute a fully functional gene product.
Materials and Methods
Mice
p45+/GFP (i.e., p45+/−) mice were generated as previously described (23), and cross-bred with P14 or SMARTA TCR transgenes to generate P14 p45−/− or SMARTA p45−/− mice, respectively. Our recent analysis of thymocyte development in the p45-targeted mice showed no detectable differences between p45+/+ and p45+/− animals (23), suggesting that heterozygosity of the p45-targeted allele does not significantly impact T cell biology. As such, we used T cells from p45+/− mice as controls in this study, and the Tcf1-EGFP reporter embedded in the p45+/− allele was utilized to mark Tcf1 expression in CD4+ and CD8+ T cells at different stages of responses to viral infection. C57BL/6J and B6.SJL mice were from the Jackson Laboratory. All mice analyzed were 6–12 weeks of age, and both genders are used without randomization or blinding. All mouse experiments were performed under protocols approved by the Institutional Animal Use and Care Committees of the University of Iowa.
Flow cytometry
Single-cell suspensions were prepared from the spleen, lymph nodes (LNs), or peripheral blood lymphocytes (PBLs), and surface or intracellularly stained as described (19). The fluorochrome-conjugated antibodies were as follows: anti-CD8 (53-6.7), anti-CD4 (RM4-5), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-PD-1 (J43), anti-CD45.1 (A20), anti-CD45.2 (104), anti-ICOS (C398.4A), anti-IFN-γ (XMG1.2), anti-TNFα (MP6-XT22), anti-Eomes (Dan11mag), anti-T-bet (eBio4B10), anti-IL-2 (JES6-5H4), anti-IL-7Rα (eBio17B7) and anti-KLRG1 (2F1) from eBiosciences; anti-Bcl6 (K112-91) from BD Biosciences; anti-human granzyme B (FGB12) and corresponding isotype control from Invitrogen/Life Technologies, anti-SLAM (TC15-12F12.2) from BioLegend. For detection of CXCR5, three-step staining protocol was used with unconjugated anti-CXCR5 (2G8; BD Biosciences) (24). For detection of Bcl6, surface-stained cells were fixed and permeabilized with the Foxp3/Transcription Factor Staining Buffer Set (eBiosciences), followed by incubation with a fluorochrome-conjugated antibody or isotype control. Peptide-stimulated cytokine production and detection by intracellular staining were as described (25). Data were collected on a FACSVerse (BD Biosciences) and were analyzed with FlowJo software (TreeStar).
Adoptive transfer and viral infection
Naïve CD45.2+ P14 CD8+ T cells or SMARTA CD4+ T cells were isolated from the LNs of P14- or SMARTA-transgenic p45+/− or p45−/− mice. For characterization of CD8+ T cell responses, 2 × 104 Vα2+ P14 CD8+ T cells were intravenously (i.v.) injected into CD45.1+ B6.SJL recipient mice and infected intraperitoneally (i.p) with 2 × 105 PFU of lymphocytic choriomeningitis virus, Armstrong strain (LCMV-Arm). For characterization of CD4+ T cell responses, 2 × 105 Vα2+ SMARTA CD4+ T cells were i.v. injected into CD45.1+ hosts and infected similarly. For examining CD4+ T cell responses under a competitive condition, 5,000 p45+/− or p45−/− SMARTA CD4+ T cells were mixed with the same number of WT CD45.1+CD45.2+ SMARTA CD4+ T cells and adoptively transferred. At ≥ 40 dpi, the immune mice were i.v. infected with 2 × 106 PFU of LCMV clone 13 (Cl13) to elicit secondary CD8+ or CD4+ T cell responses.
Immunoblotting
CD44loCD62L+ naïve P14 CD8+ T cells or SMARTA CD4+ T cells were sort-purified from the spleens of P14- or SMARTA-transgenic p45+/− or p45−/− mice. Cell lysates were prepared from the sorted cells, resolved on SDS-PAGE, and immunoblotted with an anti-Tcf1 antibody (C46C7, Cell Signaling Technologies) as previously described (23).
RNA-Seq and quantitative RT-PCR
p45+/− or p45−/− SMARTA CD4+ T cells were adoptively transferred and recipients infected as described above. On 8 dpi, PD-1lo CXCR5+ cells were sort-purified from CD45.2+CD4+ splenocytes. The total RNA was extracted and subjected to RNA-Seq analysis as previously described (19). The RNA-Seq data were deposited at the Gene Expression Omnibus (GEO) under the accession number GSE98347 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse98347). For validation of key gene expression changes, the RNA was reverse-transcribed and analyzed by quantitative PCR as previously described (19). The primers used for Id2 were 5′-catcagcatcctgtccttgc and 5′-gtgttctcctggtgaaatgg, and those for Id3 were 5′-atctcccgatccagacagc and 5′-gagagagggtcccagagtcc.
Chromatin immunoprecipitation (ChIP)
WT SMARTA CD4+ T cells were adoptively transferred and recipients infected as above. On 8 dpi, CXCR5−SLAMhi TH1 and CXCR5+SLAMlo TFH cells were sort-purified from CD45.2+CD4+ splenocytes and cross-linked, and the resulting chromatin fragments were immunoprecipitated with an anti-Tcf1 antibody (C46C7) or normal rabbit IgG, as previously described (19). Enriched Tcf1 binding at the Id3 transcription start site was determined by quantitative PCR.
Statistical analyses
Data from multiple experiments were analyzed with Student’s t-test with two-tailed distribution, assuming equal sample variance when p>0.05 on F-test. When p ≤ 0.05 on F-test comparing the variances, Welch’s correction was applied.
Results
Tcf1 long isoforms are dispensable for CD8+ effector T cell differentiation
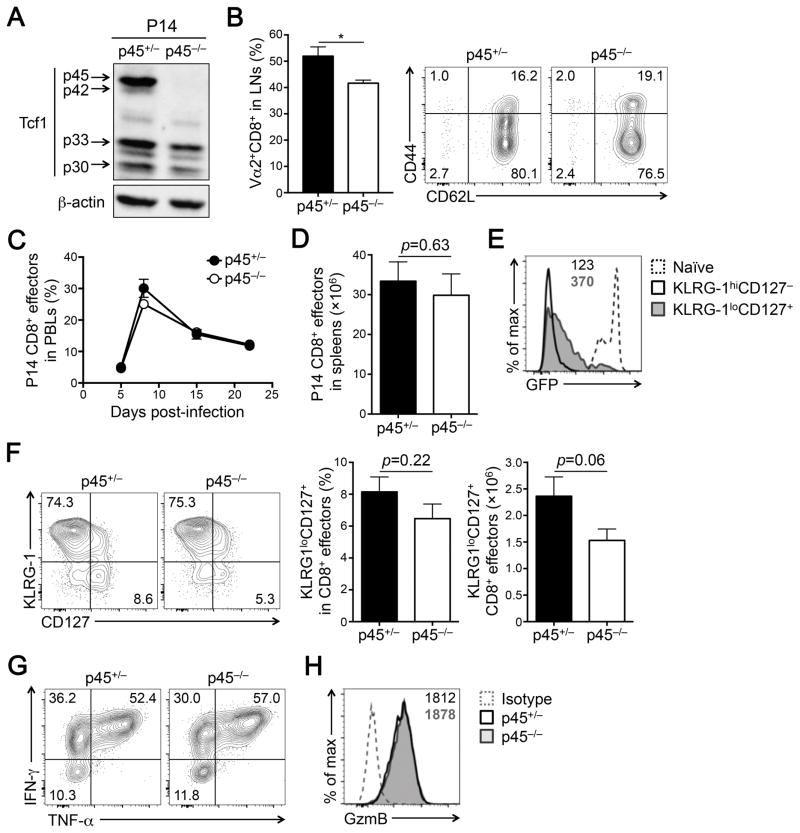
Tcf1 proteins are encoded by the Tcf7 gene, with long isoforms produced from transcripts initiated at exon 1 and short isoforms from transcripts initiated at exon 3. Previously we have generated a Tcf1-EGFP reporter mouse strain, where we inserted an IRES-EGFP cassette into the first intron of Tcf7 gene (26). In front of the cassette, we placed an En2 splice acceptor that forced the splicing of exon 1 to this cassette instead of exon 2. Mice homozygous for the Tcf1-EGFP reporter alleles thus failed to produce Tcf1 long isoforms, p45 and p42; for simplicity, this strain is called p45−/− herein. To assess the importance of Tcf1 long isoforms (p45 and p42) in CD8+ T cell responses to viral infection, we crossed p45−/− mice with the P14 TCR transgene, which encodes a TCR specific for the glycoprotein (GP) 33 epitope of LCMV. As expected, p45−/− P14 CD8+ T cells were deficient for Tcf1 long isoforms but retained the expression of Tcf1 short isoforms, albeit at a reduced amount (Fig. 1A). Loss of Tcf1 long isoforms moderately reduced the frequency of P14 CD8+ T cells in the LNs, but did not result in aberrant activation of peripheral CD8+ T cells, as determined by the CD62Lhi phenotype (Fig. 1B).
Figure 1. Tcf1 long isoforms are dispensable for effector CD8+ T cell responses.
(A) Detection of Tcf1 isoforms by immunoblotting in naïve CD8+ T cells. CD62LhiCD44loVα2+CD8+ cells were sorted from the LNs of p45+/− or p45−/− P14 transgenic mice and immunoblotted with an anti-Tcf1 or β-actin antibody. (B) Characterization of naïve P14 CD8+ T cells. LNs from uninfected p45+/− or p45−/− P14 transgenic mice were surface-stained to detect the frequency (left) and immuno-phenotype (right) of Vα2+CD8+ T cells (n = 4 from 4 experiments). (C) Kinetics of CD8+ T cell responses in PBLs. CD45.2+ p45+/− or p45−/− P14 CD8+ T cells (2×104 each) were adoptively transferred into CD45.1+ recipients followed by infection with LCMV-Arm. The frequency of CD45.2+ CD8+ effector T cells in CD11ahiCD8dim antigen-experienced CD8+ T cells were monitored in the PBLs on indicated days post-infection (n = 10–20 from 3 experiments). (D) Numbers of P14 CD8+ effector T cells detected in the spleen on 8 dpi (n = 8 from 3 experiments). (E) Detection of Tcf1-EGFP reporter in p45+/− P14 CD8+ effector subsets, and the geometric mean fluorescent intensity (gMFI) of EGFP is marked. Dotted line denotes EGFP expression in naïve CD8+ T cells. (F) Detection of P14 CD8+ effector subsets. CD45.2+ CD8+ effector T cells in the spleen (8 dpi) were analyzed for KLRG1 and CD127 expression, and the percentages of KLRG1hiCD127− and KLRG1loCD127+ subsets are shown in representative contour plots. Cumulative data on the frequency and numbers of the KLRG1loCD127+ subset are summarized in bar graphs (n = 10 from 3 experiments). (G) Cytokine production by P14 CD8+ effector T cells. Splenocytes (8 dpi) were incubated with GP33 peptide for 5 hours, and CD45.2+CD8+ T cells were intracellularly stained to detect IFN-γ and TNF-α. The percentage of each population is shown in representative contour plots from 3 experiments. (H) Granzyme B expression in P14 CD8+ effector T cells detected by intracellular staining. gMFI of Granzyme B is shown in representative histogram. Dotted line, isotype control staining. Data in bar graphs are means ± SEM. *, p < 0.05 by Student’s t-test unless specified otherwise.
To facilitate functional characterization on a per cell basis, we adoptively transferred the same numbers (2×104) of p45+/− or p45−/− P14 CD8+ T cells into congenic recipients followed by infection with LCMV-Arm. As tracked in PBLs, both p45+/− and p45−/− CD45.2+ CD8+ effector T cells showed similar frequency among antigen-experienced CD11ahiCD8int T cells (Fig. 1C). In the spleens, similar numbers of p45+/− and p45−/− CD45.2+CD8+ effector T cells were generated at the peak response, i.e., 8 days post-infection (dpi) (Fig. 1D). By comparison, loss of all Tcf1 isoforms diminishes CD8+ effector T cells by approximately 50% in both PBLs and spleens (16). CD8+ effector T cells are heterogeneous, with KLRG1hiCD127− cells as terminally differentiated effector CD8+ T cells, and the KLRG1loCD127+ subset showing increased potential to give rise to memory CD8+ T cells (6). As observed in p45+/− CD8+ T cells, the Tcf1-EGFP reporter expression is potently diminished in KLRG1hiCD127− cells, but partially retained in the KLRG1loCD127+ subset (Fig. 1E). This is consistent with our previous observations that Tcf1 and Lef1 are required for generation of the KLRG1loCD127+ subset (27). However, the KLRG1loCD127+ subset in p45−/− CD8+ effectors was only modestly reduced in frequency and numbers (Fig. 1F). Both p45+/− and p45−/− CD8+ effectors exhibited similar capacity to produce IFN-γ, TNF-α, and granzyme B (Fig. 1G–1H), and by comparison, these functional aspects are not affected by loss of all Tcf1 isoforms as shown in our previous study (16). Collectively, these data indicate that Tcf1 long isoforms are dispensable for clonal expansion and cytotoxic functions of CD8+ effector T cells.
Tcf1 long isoforms contribute to maturation of central memory CD8+ T cells
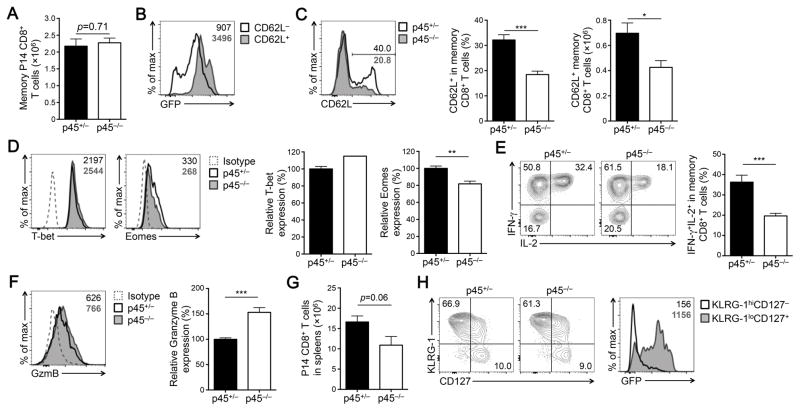
Following the peak response to LCMV infection, p45+/− and p45−/− CD45.2+CD8+ effector T cells contracted similarly (Fig. 1C), and at ≥ 60 dpi, similar numbers of p45+/− and p45−/− memory CD8+ T cells were found in the recipient spleens (Fig. 2A). Memory CD8+ T cells in the secondary lymphoid organs are heterogeneous, with the CD62L− subset as effector memory (TEM) cells that reside in non-lymphoid tissues, and the CD62L+ subset as central memory (TCM) cells that are capable of self-renewal and conferring long-term protection (28). Compared with CD8+ effector T cells, most of the memory CD8+ T cells upregulated Tcf1, and the CD62L+ subset expressed higher levels of Tcf1 as determined by the Tcf1-EGFP reporter activity (Fig. 2B, compare with Fig. 1E). Our previous studies showed that deletion of all Tcf1 isoforms almost completely abrogated generation of CD62L+ TCM cells and caused substantial decrease in Eomes expression (16). In p45−/− memory CD8+ T cell pool, CD62L+ cells were generated, but at diminished frequency and numbers (Fig. 2C). In addition, p45−/− memory CD8+ T cells showed modest reduction in Eomes expression, accompanied by a modest increase in T-bet expression (Fig. 2D). Regarding functional aspects, fewer p45−/− memory CD8+ T cells (in both CD62L− and CD62L+ subsets) were capable of producing IL-2 than p45+/− cells (Fig. 2E), whereas they showed similar production of IFN-γ and TNF-α (data not shown). Additionally, p45−/− memory CD8+ T cells showed modest increase in granzyme B compared with p45+/− cells (Fig. 2F). These observations indicate that loss of Tcf1 long isoforms does not affect the pool size of memory CD8+ T cells but predisposes them to a TEM phenotype.
Figure 2. Tcf1 long isoforms contribute to optimal TCM maturation and secondary expansion.
(A) Numbers of memory P14 CD8+ T cells detected in the spleen at ≥ 60 dpi (n = 5–6 from 2 experiments). (B) Detection of Tcf1-EGFP reporter in CD62L− and CD62L+ memory CD8+ T cell subsets. CD45.2+ memory CD8+ T cells from p45+/− mice were analyzed for EGFP expression, and values in the histogram denote EGFP gMFI. (C) Detection of P14 CD8+ TCM cells. CD45.2+ memory CD8+ T cells in the spleen were analyzed for CD62L expression, and the percentages of CD62L+ TCM cells are shown in representative histograms. Cumulative data on the frequency and numbers of the CD62L+ TCM cells are summarized in bar graphs (n = 5–6 from 2 experiments). (D) T-bet and Eomes expression in memory P14 CD8+ T cells detected by intranuclear staining. gMFI of T-bet or Eomes is shown in representative histograms, and cumulative data are in bar graphs (for T-bet, n = 2–3 from 1 experiment; for Eomes, n = 5–6 from 2 experiments). Dotted line, isotype control staining. (E) Cytokine production by memory P14 CD8+ T cells. Splenocytes were harvested at ≥ 60 dpi and incubated with GP33 peptide, followed by intracellular detection of IFN-γ and IL-2 in CD45.2+CD8+ T cells. The percentage of each population is shown in representative contour plots (n = 8 from 3 experiments), and that of IFN-γ– and IL-2-double producers are summarized in bar graphs. (F) Granzyme B expression in memory P14 CD8+ T cells detected by intracellular staining. gMFI of Granzyme B is shown in representative histogram, and cumulative data are in bar graphs (n = 8 from 3 experiments). Dotted line, isotype control staining. (G) Numbers of secondary CD8+ effector T cells. The immune CD45.1+ recipients were infected with LCMV-Cl13, and five days after infection, the numbers of CD45.2+CD8+ secondary effector T cells were determined in the spleen (n = 5 from 2 experiments). (H) Characterization of P14 CD8+ secondary effector T cells. CD45.2+CD8+ secondary effector T cells in the spleen were surface-stained, and the percentages of KLRG1hiCD127− and KLRG1loCD127+ subsets are shown in representative contour plots (left panels). Both subsets were analyzed for Tcf1-EGFP reporter expression in p45+/− recipients, with EGFP gMFI marked (right panel, n = 5–6 from 2 experiments). Data in bar graphs are means ± SEM. *, p < 0.05; **, p<0.01; ***, p < 0.001 by Student’s t-test unless specified otherwise.
To assess the recall response by memory CD8+ T cells, we re-challenged the immune recipient mice with LCMV-Cl13. As detected on day 5 post-infection, both p45+/− and p45−/− memory CD8+ T cells underwent substantial secondary expansion, although the numbers of p45−/− secondary CD8+ effector T cells in the spleens were modestly decreased (Fig. 2G), which is in contrast to severely impaired secondary expansion in the absence of all Tcf1 isoforms (16). Similar to CD8+ effector T cells in the primary response, the secondary CD8+ effector T cells were heterogeneous, with the KLRG1loCD127+ subset retaining substantially higher Tcf1-EGFP reporter activity than the KLRG1hiCD127− subset (Fig. 2H). Loss of Tcf1 long isoforms did not detectably perturb the subset composition or IFN-γ and TNF-α production in the secondary effectors (Fig. 2H and data not shown). Collectively, these data suggest that Tcf1 long isoforms contribute to optimal maturation of TCM cells and optimal secondary expansion in a recall response. It is also noteworthy that reduced expression of Tcf1 short isoforms in p45−/− CD8+ T cells may also be a contributing factor.
Tcf1 long isoforms are required for TFH differentiation at the effector phase of CD4+ T cell responses
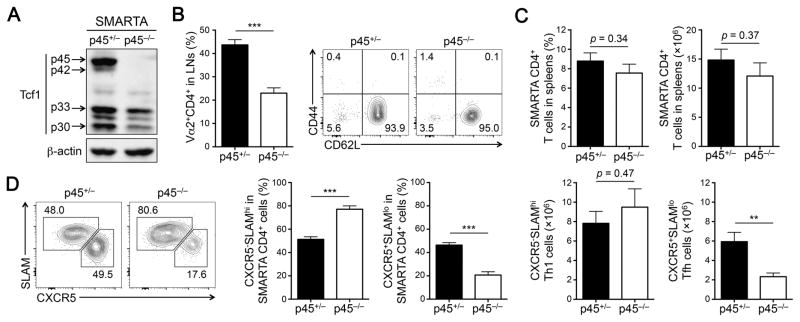
We and others have recently demonstrated that deletion of all Tcf1 isoforms impairs TFH differentiation in response to viral infections (12, 19, 20). To determine the requirements for Tcf1 long isoforms during CD4+ T cell responses, we crossed the p45−/− strain with SMARTA TCR transgene, which encodes a TCR specific for the GP61 epitope of LCMV. By immunoblotting, we validated that p45−/− SMARTA CD4+ T cells were deficient for Tcf1 long isoforms but retained the expression of Tcf1 short isoforms, also at a reduced amount (Fig. 3A). Although the frequency of SMARTA CD4+ T cells in the LNs was diminished in p45−/− mice, the majority of p45−/− SMARTA CD4+ T cells exhibited a CD44loCD62Lhi phenotype, suggesting that loss of Tcf1 long isoforms did not result in aberrant activation of peripheral CD4+ T cells (Fig. 3B).
Figure 3. Tcf1 long isoforms regulate optimal TFH differentiation.
(A) Detection of Tcf1 isoforms by immunoblotting in CD4+ T cells. CD62LhiCD44loVα2+CD4+ cells were sorted from the LNs of p45+/− or p45−/− SMARTA-Tg mice and immunoblotted as in Figure 1A. (B) Characterization of naïve SMARTA CD4+ T cells. LNs were isolated from p45+/− or p45−/− SMARTA-Tg mice and surface-stained to detect the frequency (left) and immunophenotype (right) of Vα2+CD4+ T cells (n = 8 from 8 experiments). (C) Detection of effector SMARTA CD4+ T cells at the peak response. CD45.2+ p45+/− or p45−/− SMARTA CD4+ T cells from LN (2×105 each) were adoptively transferred into CD45.1+ recipients followed by infection with LCMV-Arm. On 8 dpi, CD45.2+CD4+ T cells were detected and enumerated in the spleens of recipient mice (n = 8 from 3 experiments). (D) Detection of TH1 and TFH effector CD4+ T cells. CD45.2+ effector CD4+ T cells (as in C) were analyzed for CXCR5 and SLAM expression. The percentages of CXCR5−SLAMhi TH1 and CXCR5+SLAMlo TFH cells are shown in representative contour plots (left panels). Cumulative data on the frequency and numbers of each subset are summarized in bar graphs (n = 5–8 from 2–3 experiments). Data are means ± SEM. **, p < 0.01; ***, p < 0.001 by Student’s t-test unless specified otherwise.
To investigate the regulatory roles of Tcf1 long isoforms on a per cell basis, we adoptively transferred the same numbers (2×105) of p45+/− or p45−/− SMARTA CD4+ T cells from LNs into congenic recipients followed by infection with LCMV-Arm. At the peak of effector CD4+ T cell responses (i.e., 8 dpi), p45+/− and p45−/− SMARTA CD4+ T cells expanded to similar levels, showing similar frequency and numbers of effector CD4+ T cells in the spleen of recipient mice (Fig. 3C). Viral infection elicits two major forms of effector CD4+ T cells, TH1 and TFH cells, which exhibit CXCR5−SLAMhi and CXCR5+SLAMlo phenotypes, respectively. p45+/− effector CD4+ T cells contained approximately equal amounts of TH1 and TFH cells; in contrast, p45−/− effector CD4+ T cells were strongly skewed toward the TH1 phenotype (Fig. 3D). As a result, p45−/− SMARTA CD4+ T cells generated markedly fewer TFH cells than the p45+/− counterparts (Fig. 3D), similar to TFH differentiation defects observed in the absence of all Tcf1 isoforms (12, 19, 20). On the other hand, both p45−/− and p45+/− SMARTA CD4+ T cells gave rise to similar amounts of effector TH1 cells (Fig. 3D). These data suggest that Tcf1 long isoforms are important in directing effective TFH differentiation, but are dispensable for TH1 responses.
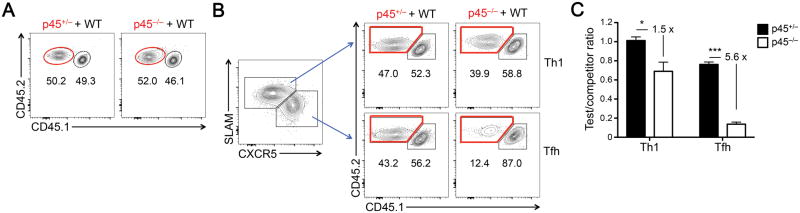
In the experiments above, we transferred a relatively large number of SMARTA CD4+ T cells so as to obtain sufficient cells for RNA-Seq and memory phase analysis (see below). To exclude a secondary impact derived from a higher precursor frequency and demonstrate an intrinsic requirement for Tcf1 long isoforms, we transferred a small number of p45+/− or p45−/− SMARTA CD4+ T cells in a competitive setting. Specifically, we mixed CD45.2+ p45+/− or p45−/− SMARTA CD4+ T cells (as test cells) with the same number of CD45.1+CD45.2+ wild-type (WT) SMARTA CD4+ T cells (as competitors) (Fig. 4A). We then adoptively transferred a total of 10,000 SMARTA CD4+ T cells (5,000 each of test cells and competitors) into CD45.1+ recipients, followed by LCMV-Arm infection. On 8 dpi, within the TH1 compartment, the p45+/−:WT ratio was largely maintained at 1:1, and the p45−/−:WT ratio was modestly decreased (Fig. 4B–4C). Within the TFH cells, however, the p45−/−:WT ratio was reduced by >5 fold compared with the p45+/−:WT ratio (Fig. 4B–4C). Thus, analysis under the competitive setting at a lower precursor frequency further corroborated a specific, intrinsic requirement for Tcf1 long isoforms in TFH differentiation.
Figure 4. CD4+ T cells lacking Tcf1 long isoforms are less competitive in TFH differentiation.
(A) Relative abundance of CD45.2+ test cells (p45+/− or p45−/−) and CD45.1+CD45.2+ competitors (WT CD4+ T cells) in the cell mixture used in competitive transfer experiments. (B) Detection of relative contribution of test and competitor SMARTA CD4+ T cells to TH1 and TFH responses. CXCR5−SLAMhi TH1 cells and CXCR5+SLAMlo TFH cells were detected in the recipients on 8 dpi (left). Within each subset, the percentages of test and competitor cells were determined and shown in representative contour plots. (C) Cumulative data of test/competitor ratio within TH1 or TFH subsets at the effector phase (8 dpi). Data are from 2 experiments (n = 5–6). Data are means ± SEM. *, p < 0.05; ***, p < 0.001 by Student’s t-test.
Tcf1 long isoforms regulate a unique subset of genes in the Tfh program
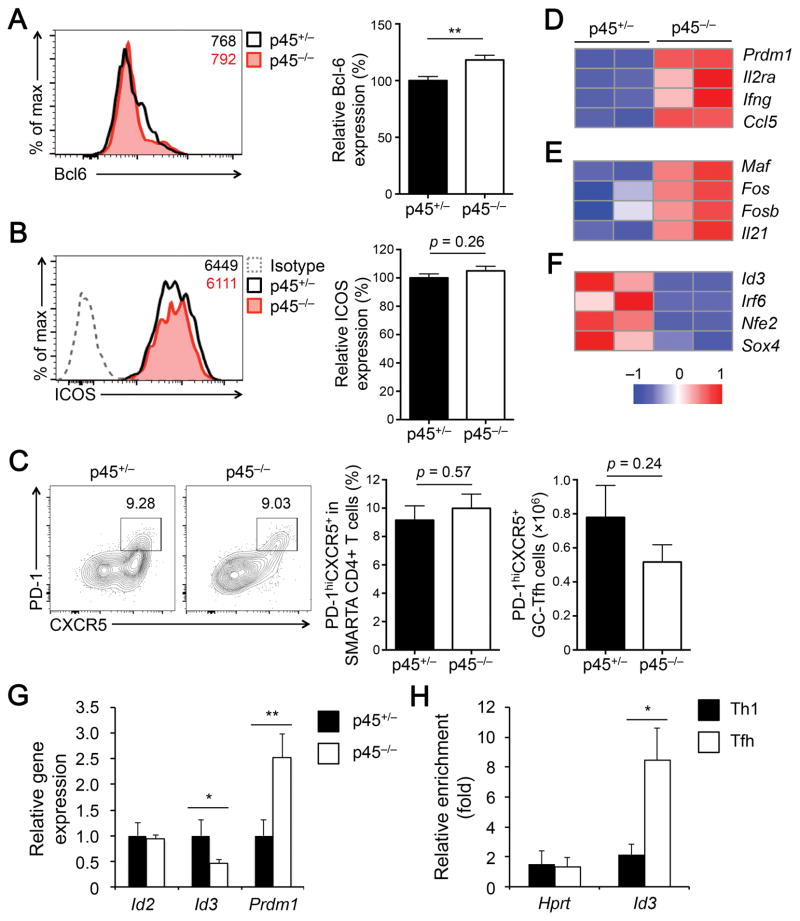
We previously showed that Tcf1 critically regulates activation of the TFH program in primed CD4+ T cells, and deletion of all Tcf1 isoforms diminishes the expression of Bcl6 and ICOS (19). However, p45+/− and p45−/− TFH cells showed similar Bcl6 and ICOS expression (Fig. 5A–5B), suggesting that Tcf1 short isoforms are sufficient for supporting Bcl6 and ICOS induction, in spite of reduced protein expression. TFH differentiation is known as a step-wise process (4), with TFH lineage-committed cells undergoing further maturation and becoming germinal center (GC)-TFH cells, as marked by high PD-1 expression. Although there was an overall reduction of p45−/− TFH cells, PD-1hiCXCR5+ GC-TFH cells were detected at similar frequency and numbers in p45+/− and p45−/− effector SMARTA CD4+ T cells (Fig. 5C). Thus, loss of Tcf1 long isoforms showed stronger impact on non-GC-TFH cells, causing subset composition changes within the TFH population. By comparison, both non-GC TFH and GC TFH cells were diminished in the absence of all T cell isoforms (19). Therefore, Tcf1 short isoforms appears to be adequate for promoting GC-TFH maturation.
Figure 5. Tcf1 long isoforms are essential for regulating a subset of TFH transcriptional program.
(A–B) Detection of Bcl6 (A) and ICOS (B) in p45+/− and p45−/− CXCR5+SLAMlo TFH cells (8 dpi). gMFI of each protein is shown in representative histograms, and cumulative data on the relative expression between p45+/− and p45−/− TFH cells are in bar graphs (n = 11 from 4 experiments). (C) Detection of PD-1hiCXCR5+ GC-TFH cells (8 dpi). The percentages of GC-TFH cells among CD45.2+ SMARTA CD4+ T cells are shown in representative contour plots, and cumulative data in bar graph are from 2 experiment (n = 6). (D–F) Heatmaps of select differentially expressed genes. p45+/− and p45−/− PD-1loCXCR5+ non-GC TFH cells were sorted from splenic CD45.2+ SMARTA CD4+ T cells in the recipients on 8 dpi. Two biological replicates were collected for each genotype and analyzed with RNA-Seq. (G) Detection of select gene expression in non-GC TFH cells by quantitative RT-PCR. The cells were sorted using the same strategy as above. For each gene of interest, its expression in p45+/− cells was set as 1, and its relative expression in p45−/− cells was calculated accordingly. Data are from two independent experiments (n = 3–4). (H) Detection of enriched Tcf1 binding. WT TH1 and TFH cells were sorted and subjected to ChIP analysis. The relative enrichment at the control Hprt locus and Id3 transcription start site by an anti-Tcf1 antibody was determined by quantitative PCR. Data are from two independent experiments with each sample measured in duplicates or triplicates. Data in bar graphs are means ± SEM. *, p < 0.05; **, p < 0.01 by Student’s t-test unless specified otherwise.
To search for downstream genes that account for defective p45−/− TFH differentiation, we specifically sorted p45+/− and p45−/− PD-1lo CXCR5+, non-GC-TFH cells on 8 dpi and performed RNA-Seq analysis. By a setting of ≥ 2 fold expression changes and false discovery rate (FDR) q value <0.05, we found 89 downregulated and 119 upregulated genes in p45−/− TFH cells compared with p45+/− counterparts (Supplemental Table 1). Transcriptomic changes have been profiled in TFH cells lacking all Tcf1 isoforms or both Tcf1 and Lef1 proteins (19, 20). Whereas differences in specific cell subsets analyzed and profiling platforms utilized among these studies prevented a meaningful and fair comparison for specific genes affected, the number of differentially expressed genes was substantially lower in p45−/− TFH cells than that found in Tcf1/Lef1-deficient TFH cells, suggesting a relatively limited impact of deficiency in Tcf1 long isoforms on TFH transcriptome. Functional annotation using the DAVID Bioinformatics Resources revealed a partial activation of TH1 program in p45−/− TFH cells, including upregulation of Prdm1, Il2ra, and Ifng (encoding Blimp1 transcription factor, CD25, and IFN-γ, respectively) (Fig. 5D). Increased expression of Prdm1 was validated by quantitative RT-PCR (Fig. 5G). Blimp1 is known to promote activated CD4+ T cells to a TH1 fate at the expense of TFH differentiation (29), and Tcf1 and Bcl6 co-occupy an intron 3 regulatory region in the Prdm1 gene locus (19, 30). Our data suggest that Tcf1 long isoforms are critical in repressing the TH1 potential in TFH cells.
Among the upregulated genes included Maf, Fos and Fosb (Fig. 5E). Maf is necessary for promoting TFH differentiation and inducing IL-21 (31, 32). AP-1 factors including Fos and FosB may contribute to recruiting Bcl6 to its target genes in TFH cells (30). Among the downregulated genes, a prominent subset was involved in transcriptional regulation, including Irf6, Nfe2, Sox4, and Id3 (Fig. 5F). Importantly, the first three genes were detected at low abundance in TFH cells (< 5 FPKMs); in contrast, Id3 was highly abundant in TFH cells (132 PFKMs on average). As validated in Fig. 5G, Id3 expression was diminished in p45−/− TFH cells, whereas Id2 was not detectably altered. By chromatin immunoprecipitation, we further found that Tcf1 exhibited enriched binding to the transcription start site of Id3 gene in TFH cells but not TH1 cells (Fig. 5H), establishing Id3 as a direct target gene of Tcf1 in TFH cells. It has been recently shown that Id3 deficiency results in evident accumulation of GC-TFH cells (13). These data collectively suggest that another important function of Tcf1 long isoforms appears to be restraining GC-TFH differentiation by positive regulation of Id3, maybe aided by suppression of Maf and AP-1 factors.
Tcf1 long isoforms are essential for formation of memory TH1 and TFH cells
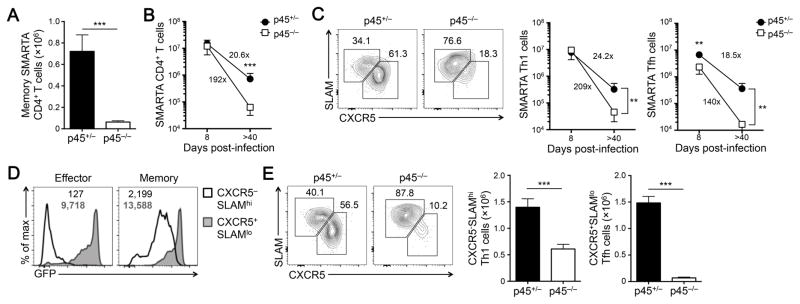
We continued to track the SMARTA CD4+ T cell responses at the memory phase, i.e., > 40 dpi, and found significantly fewer p45−/− memory CD4+ T cells than the p45+/− counterparts (Fig. 6A). Compared with CD4+ effector T cells, the number of p45+/− memory CD4+ T cells was a result of approximately 20-fold contraction; in contrast, the number of p45−/− memory CD4+ T cells represented an almost 200-fold contraction of corresponding CD4+ effector T cells (Fig. 6B). Phenotypic analysis using SLAM and CXCR5 expression further revealed that p45−/− memory CD4+ T cells were strongly skewed toward TH1, with very few SLAMloCXCR5+ memory TFH cells detected (Fig. 6C). In spite of the lineage bias in the absence of Tcf1 long isoforms, p45−/− memory TH1 and TFH cells both showed more potent contraction than their the p45+/− counterparts (Fig 6C). As detected by the Tcf1-GFP reporter in p45+/− CD4+ T cells, although Tcf1 was downregulated in TH1 effectors, it was upregulated in memory TH1 cells, whereas Tcf1-GFP reporter was detected at high levels in both effector and memory TFH cells (Fig. 6D). We next assessed the recall response by memory CD4+ T cells, by re-challenging the immune recipient mice with LCMV-Cl13. As detected on day 5 post-infection, p45−/− secondary CD4+ effectors were greatly diminished in numbers and remained strongly skewed toward TH1 cells, containing very few TFH cells (Fig. 6E). These data suggest that Tcf1 long isoforms are essential for generation of memory TH1 as well as memory TFH cells and ensuing secondary responses. It should be noted that reduced expression of Tcf1 short isoforms in p45−/− CD4+ T cells may also have contributed to defects in memory CD4+ T cells.
Figure 6. Tcf1 long isoforms are critical for formation of memory TH1 and memory TFH cells.
(A) Detection of memory SMARTA CD4+ T cells. Adoptive transfer and infection were performed as in Figure 3. On >40 dpi, CD45.2+CD4+ T cells were detected and enumerated in the spleens of recipient mice (n = 7–8 from 3 experiments). (B) Population fold changes due to contraction. p45+/− and p45−/− SMARTA CD4+ T cells were enumerated at the peak (8 dpi) and memory (> 40 dpi) phases in response to viral infection, and fold reduction between the two phases is calculated (n = 8 from 3 experiments). (C) Detection of memory TH1 and TFH T cells. CD45.2+CD4+ T cells were analyzed for CXCR5 and SLAM expression. The percentages of CXCR5−SLAMhi memory TH1 and CXCR5+SLAMlo memory TFH cells are shown in representative contour plots (left panels). Cumulative data on the numbers of TH1 and TFH cells at both effector and memory phases are summarized in line graphs (right panels), and the fold reduction during contraction of each cell type is calculated (n = 8 from 3 experiments). (D) Detection of Tcf1-EGFP reporter in CXCR5−SLAMhi TH1 and CXCR5+SLAMlo TFH cells. p45+/− TH1 and TFH cells at the effector (8 dpi, left) and memory (>40 dpi, right) phases were analyzed for EGFP expression, and values in the histograms denote EGFP gMFI. (E) Detection of secondary TH1 and TFH effector responses. The immune CD45.1+ recipients were infected with LCMV-Cl13 and five days later, CD45.2+ secondary TH1 and TFH effector T cells were detected in the spleen. The frequency of each subset is shown in representative contour plots, and the cumulative data on cell numbers are summarized in bar graphs (n =6–8 from 3 experiments). Data are means ± SEM. For all paired comparison, **, p <0.01; ***, p<0.001 by Student’s t-test.
DISCUSSION
Tcf1 is known for its versatile functions in regulating T cell development and mature CD8+ and CD4+ T cell responses to infections (15, 33). Most of the previous studies involved deletion of all Tcf1 isoforms. Until recently, Tcf1 short isoforms were generally considered to function as dominant negatives for the full-length molecule, due to their inability to interact with the co-activator β-catenin. Using a new mouse model where generation of transcripts for Tcf1 long isoforms was abrogated, we achieved specific retention of Tcf1 short isoforms only. We have recently showed that Tcf1 short isoforms are sufficient to support developing thymocytes to traverse through maturation stages without causing obvious developmental blocks, whereas Tcf1 long isoforms remain necessary for optimal thymic survival (23). Coupled with MHC-I- or MHC-II-restricted TCR transgenes, we employed the Tcf1 long isoform-targeted mice to reveal isoform-specific requirements for CD8+ and CD4+ T cell differentiation in response to viral infection.
At the peak of CD8+ T cell response to LCMV, the terminally differentiated KLRG1hiCD127− CD8+ effector T cells exhibit strong downregulation of all Tcf1 isoforms. It is therefore not surprising that loss of Tcf1 long isoforms did not show a detectable impact on the numbers or functions of CD8+ effector T cells. Recent studies examined early events of CD8+ T cell activation in vivo and found that Tcf1 expression is retained at high levels in all daughter cells during the first 3–4 divisions. In the ensuing divisions, due to asymmetrical signaling via the PI3K pathway, most daughter cells obliterate Tcf1 expression and become differentiated CD8+ effector T cells, while some daughter cells maintain higher Tcf1 expression, acquire ‘self-renewal’ capacity, and give rise to memory CD8+ T cells (34, 35). In this scenario, retention of Tcf1 short isoforms only appears to be adequate to maintain the self-renewing Tcf1hi population in spite of diminished amount of protein expression because the memory CD8+ T cell pool was similar between p45+/− and p45−/− genotypes. It is currently unknown if the Tcf1hi population at the effector phase is responsible for generation of both TEM and TCM cells. Loss of all Tcf1 isoforms almost completely abrogated TCM maturation (16); in contrast, p45−/− TCM cells were indeed generated, albeit at a modestly lower frequency. These data suggest that Tcf1 long isoforms remain necessary to promote optimal TCM maturation.
Similar to the terminally differentiated CD8+ effector T cells, CXCR5−SLAMhi TH1 cells at the effector phase of CD4+ T cell responses potently downregulated Tcf1 expression, and loss of Tcf1 long isoforms was rather inconsequential for generation of TH1 effectors. Unlike memory CD8+ T cells which were unaffected in numbers by loss of Tcf1 long isoforms, p45−/− memory TH1 cells were greatly diminished. It has also been shown that after 4–5 divisions during early CD4+ T cell activation, Tcf1 expression is specifically retained in a subset of activated CD4+ T cells due to asymmetrical signaling (36). The Tcf1hi CD4+ cells seem to possess ‘self-renewal’ capacity as well, although whether they are direct precursors of memory CD4+ T cells remains to be conclusively demonstrated. Nonetheless, formation of memory TH1 cells is strongly dependent on Tcf1 long isoforms.
Another major CD4+ T cell response to viral infection is differentiation to TFH lineage, and deletion of all Tcf1 isoforms severely impairs generation of TFH and GC-TFH cells due to insufficient induction of Bcl6 and ICOS (12, 19, 20). This study shows that loss Tcf1 long isoforms greatly diminished the total number of TFH cells but only modestly affected GC-TFH cells. Transcriptomic analysis, coupled with phenotypic characterization, revealed that ablating Tcf1 long isoforms had a rather limited impact on the gene expression profile of TFH cells compared with deleting all Tcf1 isoforms, similar to what we have observed in early thymocytes (23). Apparently, Tcf1 short isoforms, even at a reduced amount of protein, appeared to be sufficient to support induction of Bcl6 and ICOS in TFH cells. Differentiation of non-GC TFH to GC TFH is a controlled process, and a recent study identified Id3 as a restraining factor (13). Our data demonstrated that Tcf1 directly binds to the Id3 gene locus and Tcf1 short isoforms alone are not sufficient to support optimal Id3 expression in TFH cells. Paradoxically, p45−/− TFH cells upregulated Maf, another key TFH regulator, and AP-1 factors which potentially cooperate with Bcl6. These gene expression changes may all indirectly contribute to promoting GC-TFH differentiation, in addition to direct activation of Bcl6 transcription by Tcf1 itself. p45−/− TFH cells also exhibited inappropriate activation of TH1-associated genes, including Prdm1, Il2ra and Ifng, indicating another unique requirement for Tcf1 long isoforms in protecting the TFH lineage integrity (37).
Recent studies have provided strong evidence that TH1 and TFH cells are largely committed to respective lineages at both effector and memory phases (38, 39). Loss of Tcf1 long isoforms not only impaired TFH differentiation at the effector phase of CD4+ T cell responses, but also caused a stronger contraction, resulting in even fewer memory TFH cells. It has been suggested that stronger TCR stimulation during CD4+ effector differentiation is associated with predisposition to form memory CD4+ T cells (40). An increased strength of TCR signaling, resulting from extended dwell time of TCR with peptide-MHC II complex, is considered to favor TFH differentiation (41). Since both memory CD4+ T cells and TFH differentiation were affected by loss of Tcf1 long isoforms, an interesting possibility is that Tcf1 may contribute to regulation of TCR signaling outcome, albeit not affecting TCR avidity or dwell time per se. In light of our recent finding that Tcf1 has intrinsic HDAC activity (22), Tcf1 may have global impact on the epigenome of T cells and hence affect their response to external stimuli. This scenario merits further investigation.
Collectively, our study identified differential requirements for Tcf1 long and short isoforms in mature CD8+ and CD4+ T cell responses. It is of interest to note the expression levels of Tcf1 short isoforms in p45−/− cells were modestly reduced compared with those in control cells. In spite of this, the Tcf1 short isoforms were adequate in supporting the memory CD8+ T cell pool and induction of Bcl6 and ICOS in TFH cells, indicating a positive regulatory role rather than being merely dominant negatives. On the other hand, Tcf1 long isoforms, plus appropriate amount of short isoforms, remained important for supporting TFH differentiation at the effector phase and memory TH1 and TFH formation. It remains unknown if the defects associated with p45−/− cells are solely ascribed to lack of interaction with β-catenin. Nevertheless, our study supports a notion that generation of multiple isoforms from one gene may represent an evolutionarily conserved, fail-safe mechanism to ensure preservation of key biological functions.
Supplementary Material
Acknowledgments
This study is supported by grants from the NIH (AI112579 and AI115149 to HHX, AI042767 to JTH, AI119160 to HHX and VPB, AI114543 and GM113961 to VPB, AI121080 to HHX and WP, AI113806 to WP) and the US Department of Veteran Affairs (I01 BX002903 to HHX). JAG is a recipient of the University of Iowa Presidential Graduate Research Fellowship, T32 Pre-doctoral Training Grant in Immunology (AI007485), and the Ballard and Seashore Dissertation Fellowship. The flow cytometry core facility at the University of Iowa is supported by the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center, and also by grants from the NCI (P30CA086862) and the National Center for Research Resources of the NIH (S10 OD016199). The authors declare no conflict of interests.
We thank the University of Iowa Flow Cytometry Core facility (J. Fishbaugh, H. Vignes, M. Shey, and G. Rasmussen) for cell sorting, and Igor Antoshechkin (California Institute of Technology) for RNA-Seq.
Abbreviations
- Tcf1
T cell factor 1
- TFH
follicular helper T cells
- TH1
helper 1 T cells
- LCMV
lymphocytic choriomeningitis virus
References
- 1.Harty JT, V, Badovinac P. Shaping and reshaping CD8+ T-cell memory. Nature reviews Immunology. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 2.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nature immunology. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nature reviews Immunology. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature reviews Immunology. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin A, Masson F, Liao Y, Preston S, Guan T, Gloury R, Olshansky M, Lin JX, Li P, Speed TP, Smyth GK, Ernst M, Leonard WJ, Pellegrini M, Kaech SM, Nutt SL, Shi W, Belz GT, Kallies A. A molecular threshold for effector CD8(+) T cell differentiation controlled by transcription factors Blimp-1 and T-bet. Nature immunology. 2016;17:422–432. doi: 10.1038/ni.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D’Cruz LM, Watowich SS, Murre C, Goldrath AW. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nature immunology. 2011;12:1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu T, Shin HM, Moseman EA, Ji Y, Huang B, Harly C, Sen JM, Berg LJ, Gattinoni L, McGavern DB, Schwartzberg PL. TCF1 Is Required for the T Follicular Helper Cell Response to Viral Infection. Cell reports. 2015 doi: 10.1016/j.celrep.2015.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw LA, Belanger S, Omilusik KD, Cho S, Scott-Browne JP, Nance JP, Goulding J, Lasorella A, Lu LF, Crotty S, Goldrath AW. Id2 reinforces TH1 differentiation and inhibits E2A to repress TFH differentiation. Nature immunology. 2016;17:834–843. doi: 10.1038/ni.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur J Immunol. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue HH, Zhao DM. Regulation of mature T cell responses by the Wnt signaling pathway. Annals of the New York Academy of Sciences. 2012;1247:16–33. doi: 10.1111/j.1749-6632.2011.06302.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, Zhu Z, Sen JM. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nature immunology. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, Love PE, Peng W, Xue HH, Crotty S. LEF-1 and TCF-1 orchestrate TFH differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nature immunology. 2015;16:980–990. doi: 10.1038/ni.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Cao Y, Xie Z, Huang Q, Bai Q, Yang X, He R, Hao Y, Wang H, Zhao T, Fan Z, Qin A, Ye J, Zhou X, Ye L, Wu Y. The transcription factor TCF-1 initiates the differentiation of TFH cells during acute viral infection. Nature immunology. 2015;16:991–999. doi: 10.1038/ni.3229. [DOI] [PubMed] [Google Scholar]
- 21.Steinke FC, Yu S, Zhou X, He B, Yang W, Zhou B, Kawamoto H, Zhu J, Tan K, Xue HH. TCF-1 and LEF-1 act upstream of Th-POK to promote the CD4(+) T cell fate and interact with Runx3 to silence Cd4 in CD8(+) T cells. Nature immunology. 2014;15:646–656. doi: 10.1038/ni.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing S, Li F, Zeng Z, Zhao Y, Yu S, Shan Q, Li Y, Phillips FC, Maina PK, Qi HH, Liu C, Zhu J, Pope RM, Musselman CA, Zeng C, Peng W, Xue HH. Tcf1 and Lef1 transcription factors establish CD8(+) T cell identity through intrinsic HDAC activity. Nature immunology. 2016;17:695–703. doi: 10.1038/ni.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Xing S, Shan Q, Gullicksrud JA, Bair TB, Du Y, Liu C, Xue HH. Cutting Edge: beta-Catenin-Interacting Tcf1 Isoforms Are Essential for Thymocyte Survival but Dispensable for Thymic Maturation Transitions. J Immunol. 2017;198:3404–3409. doi: 10.4049/jimmunol.1602139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, Love PE, Peng W, Xue HH, Crotty S. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol. 2015;16:980–990. doi: 10.1038/ni.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue HH. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Q, Li F, Harly C, Xing S, Ye L, Xia X, Wang H, Wang X, Yu S, Zhou X, Cam M, Xue HH, Bhandoola A. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nature immunology. 2015;16:1044–1050. doi: 10.1038/ni.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Xue HH. Cutting edge: generation of memory precursors and functional memory CD8+ T cells depends on T cell factor-1 and lymphoid enhancer-binding factor-1. J Immunol. 2012;189:2722–2726. doi: 10.4049/jimmunol.1201150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefrancois L, Marzo AL. The descent of memory T-cell subsets. Nature reviews Immunology. 2006;6:618–623. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- 29.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, Crotty S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. 2015;212:539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinke FC, Xue HH. From inception to output, Tcf1 and Lef1 safeguard development of T cells and innate immune cells. Immunol Res. 2014;59:45–55. doi: 10.1007/s12026-014-8545-9. [DOI] [PubMed] [Google Scholar]
- 34.Lin WW, Nish SA, Yen B, Chen YH, Adams WC, Kratchmarov R, Rothman NJ, Bhandoola A, Xue HH, Reiner SL. CD8+ T Lymphocyte Self-Renewal during Effector Cell Determination. Cell reports. 2016;17:1773–1782. doi: 10.1016/j.celrep.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin WH, Adams WC, Nish SA, Chen YH, Yen B, Rothman NJ, Kratchmarov R, Okada T, Klein U, Reiner SL. Asymmetric PI3K Signaling Driving Developmental and Regenerative Cell Fate Bifurcation. Cell reports. 2015;13:2203–2218. doi: 10.1016/j.celrep.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nish SA, Zens KD, Kratchmarov R, Lin WW, Adams WC, Chen YH, Yen B, Rothman NJ, Bhandoola A, Xue HH, Farber DL, Reiner SL. CD4+ T cell effector commitment coupled to self-renewal by asymmetric cell divisions. J Exp Med. 2017;214:39–47. doi: 10.1084/jem.20161046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gullicksrud JA, Shan Q, Xue HH. Front Biol. 2017 doi: 10.1007/s11515-017-1445-3. [DOI] [Google Scholar]
- 38.Tubo NJ, Fife BT, Pagan AJ, Kotov DI, Goldberg MF, Jenkins MK. Most microbe-specific naive CD4(+) T cells produce memory cells during infection. Science. 2016;351:511–514. doi: 10.1126/science.aad0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.