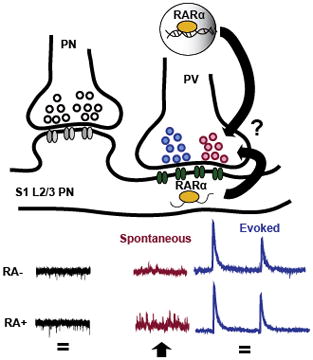
Abstract
Retinoic acid (RA), a developmental morphogen, has emerged in recent studies as a novel synaptic signaling molecule that acts in mature hippocampal neurons to modulate excitatory and inhibitory synaptic transmission in the context of homeostatic synaptic plasticity. However, it is unclear whether RA is capable of modulating neural circuits outside of the hippocampus, and if so, whether the mode of RA’s action at synapses is similar to that within the hippocampal network. Here we explore for the first time RA’s synaptic function outside the hippocampus and uncover a novel function of all-trans retinoic acid at inhibitory synapses. Acute RA treatment increases spontaneous inhibitory synaptic transmission in L2/3 pyramidal neurons of the somatosensory cortex, and this effect requires expression of RA’s receptor RARα both pre- and post-synaptically. Intriguingly, RA does not seem to affect evoked inhibitory transmission assayed with either extracellular stimulation or direct activation of action potentials in presynaptic interneurons of connected pairs of interneuron and pyramidal neurons. Taken together, these results suggest that RA’s action at synapses is not monotonous, but is diverse depending on the type of synaptic connection (excitatory versus inhibitory) and circuit (hippocampal versus cortical). Thus, synaptic signaling of RA may mediate multi-faceted regulation of synaptic plasticity.
Keywords: inhibitory synapses, GABAergic neurons, synaptic RA signaling, homeostatic synaptic plasticity
Graphical Abstract
In addition to its classic roles in brain development, retinoic acid (RA) has recently been shown to regulate excitatory and inhibitory transmission in the adult brain. Here, the authors show that in layer 2/3 (L2/3) of the somatosensory cortex (S1), acute RA induces increases in spontaneous but not action-potential evoked transmission, and that this requires retinoic acid receptor (RARα) both in presynaptic PV-positive interneurons and postsynaptic pyramidal (PN) neurons.
Introduction
The advent of conditional genetics, by allowing us to delete genes at defined time points beyond early development, has revealed that many developmental regulators are repurposed in adults. Retinoic acid (RA), a metabolite of vitamin A, is an early developmental morphogen responsible for early patterning of the brain (Maden, 2007). In recent years, however, studies in mature hippocampal neurons have revealed functionally and mechanistically distinct roles for RA in postnatal neurons. An initial study in mature, dissociated hippocampal neurons indicated that RA mediates homeostatic synaptic strengthening of excitation in response to prolonged activity blockade (Aoto et al., 2008). Chronic (24-hour) pharmacological blockade of synaptic AMPA and NMDA receptor transmission results in decreased postsynaptic calcium levels, which is sensed by the protein phosphatase calcineurin. A reduction in calcineurin activity thus triggers RA synthesis (Aoto et al., 2008; Wang et al., 2011; Arendt et al., 2015a).
In contrast to the canonical paradigm in which RA regulates transcription through genomic actions of retinoic acid receptors (RARs) α, β and γ, RA’s ability to trigger rapid homeostatic plasticity in mature neurons depends instead upon local protein translation in neuronal dendrites (Aoto et al., 2008; Poon and Chen, 2008). In this case, RA binds to a dendritic population of RARα that constitutively represses protein synthesis of a subset of mRNAs. RA binding to RARα relieves this repression to allow de novo synthesis of various proteins including AMPAR GluA1 subunits, which then form new homomeric AMPARs (Aoto et al., 2008; Poon and Chen, 2008; Wang et al., 2011). Insertion of these additional GluA1 AMPARs, via postsynaptic SNARE-mediated exocytosis, manifests as increased amplitude of miniature excitatory postsynaptic currents (mEPSCs) (Aoto et al., 2008; Wang, 2011; Arendt, 2015b). Indeed, highlighting this function of RARα in regulating protein synthesis, a mouse model of Fragile X Syndrome with known defects in protein synthesis, the Fmr1 knockout mouse, completely lacks RA-mediated synaptic scaling (Soden and Chen, 2010).
In concert with excitatory synaptic transmission changes, further work in dissociated hippocampal cultures demonstrated that RA decreases amplitudes of miniature inhibitory synaptic currents (mIPSC) in response to chronic blockade of excitatory synaptic activity, via similar protein translation and non-nuclear RARα-dependent mechanisms. In this case, RA appears to trigger GABAAR endocytosis, likely by allowing the translation of proteins promoting GABAAR endocytosis (Sarti et al., 2013). It seems that RA is capable of orchestrating shifts in synaptic excitation-inhibition balance, which in turn may shift the threshold of neuronal spiking as well as Hebbian plasticity. RA’s ability to mediate disinhibition and excitatory upscaling have both been validated in hippocampal slice cultures, which preserve more of the intact circuitry than do dissociated cultures; the disinhibitory effect of RA has been further shown in acute slices from young (P10) hippocampus (Sarti et al., 2013; Arendt et al., 2015a,b).
Although studies on RA so far have mostly pointed towards increasing network activity in response to activity blockade, it is possible that RA might participate in different types of plasticity depending upon context. While the mechanisms and scope of RA-mediated plasticity are becoming increasingly clear using dissociated and cultured slice hippocampal preparations, what is the role of RA in more intact circuits and in different brain regions, and at more advanced stages of development? Beginning towards the goal of understanding RA in a greater variety of brain regions and circuits, we tested the effect of acute RA application in an acute slice preparation of somatosensory cortex. We found that in contrast to previous findings in cultured hippocampal preparations, RA induced increases in spontaneous inhibitory transmission, with no effect on evoked transmission at two different, identified inhibitory neuronal subtypes.
Materials and Methods
Mouse Husbandry and Genotyping
All animals were housed according to Stanford University APLAC guidelines. Unless otherwise stated, RA incubation experiments were performed in “wildtype” RARα homozygous floxed mice (RARαfl/fl), as previously described (Chapellier et al., 2002, Sarti et al., 2012). For KO experiments, RARαfl/fl mice were crossed to CAMKII-Cre, RARαfl/fl double heterozygotes (CAMKII-Cre/+; RARαfl/+) or PV-Cre, RARα flox double heterozygotes (PV-Cre/+; RARαfl/+) (Tsien et al., 1999, Hippenmeyer et al., 2005). Litters were genotyped for flox by PCR using the following previously described primers (Sarti et al., 2012): Fwd 5′-GTGTGTGTGTGTATTCGCGTGC-3′, Rev 5′-ACAAAGCAAGGCTTGTAGATGC-3′, annealing at decreasing temperatures in the range from 62°C to 56°C to increase product specificity. They were also genotyped for Cre using primers Fwd 5′-GCCTGCATTACCGGTCGATGCAACGA-3′, Rev 5′-GTGGCAGATGGCGCGGCAACACCATT-3′, annealing at 60°C.
Acute slice electrophysiology
Mice age P21 to P25 were anesthetized with isoflurane, and the brains quickly removed into ice-cold high sucrose solution (HSS) containing the following (in mM): 75 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 25 glucose, 75 sucrose, 2 MgCl2, and 0.5 CaCl2. Across-row slices, which allow for accurate identification of whisker barrels, were prepared by placing the brain on a metal platform chilled over ice and making a 45°-to-midline cut to remove the caudal end of one hemisphere, as described by Finnerty et al., 1999 and Allen et al., 2000. From the remaining rostral end, slices of 400 μm in thickness were cut at 45° from midline in HSS using a vibratome (Leica, VT1200). After cutting, slices were immediately moved to 32–34°C artificial CSF (ACSF) containing the following (in mM): 125 NaCl, 27.5 NaHCO3, 2.6 KCl, 11.5 glucose, 2 CaCl2, 1.3 MgSO4, and 1.0 NaH2PO4. ACSF and HSS are balanced with 5% CO2 and 95% O2. Slices were allowed to recover at 32–34°C for 30 min, after which the slices were moved to the room temperature. RA (2 μM) or DMSO (“mock”) was added to the incubating ACSF at room temperature; for each experiment, adjacent slices from the same animal were included in each treatment group. To compensate for the loss of RA resulting from oxidation by bubbling with 5% CO2/95% O2, two additional supplements of RA (2 μM) were added 45 min and 90 min after the first treatment. Electrophysiology recordings were done between 2 and 4 h after the first RA treatment. Whole-cell voltage-clamp recordings were made using borosilicate glass pipettes with tip resistance 2–4 MΩ. Evoked IPSCs were recorded at the excitatory reversal potential of 0 mV, with an internal solution containing the following (in mM): 122.5 Cs-Gluconate, 6.3 CsCl, 10 HEPES, 10 EGTA, 4 MgATP, 20 Na-phosphocreatine, 0.3 NaGTP, pH 7.3. In some experiments, 10 μM CNQX, and 50 μM D-APV were also included during IPSC recordings; in other experiments, no synaptic blockers were included and EPSCs were additionally recorded at a holding potential of −70 mV. mIPSCs and mEPSCs were recorded with the same solutions and respective holding potentials, but in the presence of 1 μM TTX. Electrical stimulation was achieved using a bipolar stimulating electrode or homemade electrode made from formvar-insulated nichrome wire of 0.002-inches in diameter (AM Systems).
For paired recordings, presynaptic cells were recorded in current clamp with an internal solution containing the following (in mM): 100 K-Gluconate, 50mM KCl, 10 HEPES, 4 MgATP, 10 Na-phosphocreatine, 1 NaGTP, pH 7.2. For fast-spiking to pyramidal cell pairs, postsynaptic cells were recorded in voltage clamp at −70mV, using the same internal as presynaptic cells. For somatostatin-positive interneuron pairs, postsynaptic cells were recorded in voltage clamp at −70mV, using a Cs-based internal as follows (in mM): 100 Cs-Methanesulfonate, 50mM CsCl, 10 HEPES, 4 MgATP, 10 Na-phosphocreatine, 1 NaGTP, pH 7.2. No additional drugs were present in the bath. Two presynaptic action potentials (AP) were evoked in the presynaptic cell by two 2-ms current injections at a 50ms interval, and connected postsynaptic cells identified by detection of a postsynaptic unitary IPSC (uIPSC) above noise, at short latency after the AP. 50–100 sweeps were collected for each cell, at 0.1Hz. Success rate was calculated as the fraction of sweeps in which a first uIPSC was detected above threshold. uIPSC amplitudes were calculated as the mean amplitude of the first uIPSC, including sweeps in which no event was detected (“failures”). Paired-pulse ratio (PPR) was calculated as the ratio of the two uIPSCs (IPSC2/IPSC1).
All electrophysiological recordings were performed with Multiclamp 700A/B amplifiers. Junction potentials were compensated and recordings Bessel filtered at 2kHz (for mEPSCs and mIPSCs) or 10kHz (evoked IPSCs). Data was analyzed using Clampfit (Axon Laboratories) and Mini Analysis Program (Synaptosoft).
Statistical Analysis
Statistical analysis was performed using SigmaPlot Version 11.0 (Systat Software). For paired-pulse ratio experiments, p values represent results of a two-way ANOVA. For all other experiments, p values represent results of a student’s t-test.
Drugs and Chemicals
All-trans RA was purchased from Sigma. TTX was purchased from Tocris or Abcam. CNQX from Tocris, and D-APV from Abcam.
Results
Acute Retinoic acid treatment increases mIPSC frequency
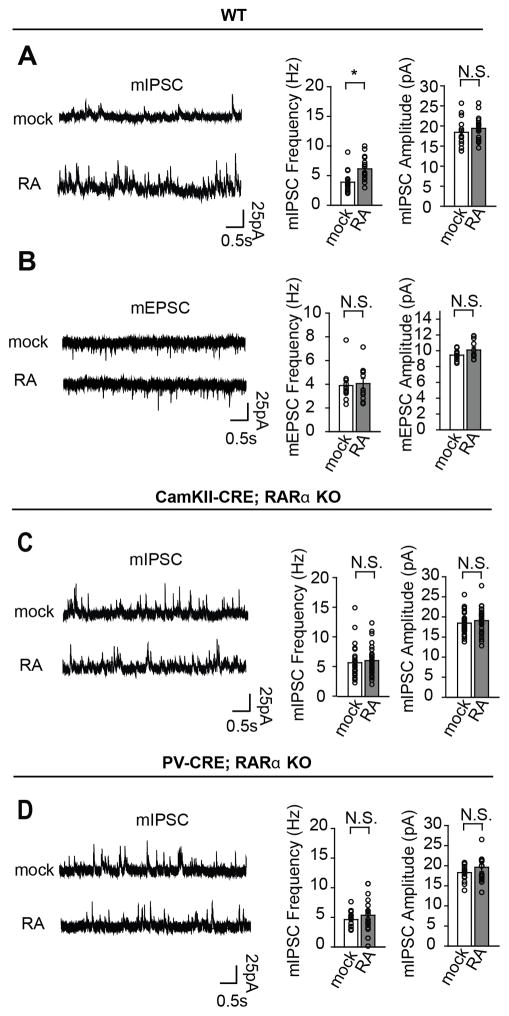
In cultured hippocampal systems, RA is known to rapidly regulate synaptic strength, reflected in changes of both spontaneous and evoked miniature synaptic transmission at excitatory and inhibitory synapses (Aoto et al., 2008; Sarti et al., 2013; Arendt et al., 2015a). To begin to test whether RA would have effects on mature synapses of other brain regions and under conditions that more closely mimic endogenous circuitry, we began by testing the effect of acute (two hour) RA application on layer 2/3 pyramidal cells in an acute slice preparation of somatosensory cortex (S1). We found that RA induced an increase in mIPSC frequency in these cells (mock, 3.89 ± 0.477 Hz; RA, 6.13 ± 0.466 Hz) (Fig. 1A). Interestingly, in contrast to our previous findings that mIPSC amplitude was decreased by RA treatment in cultured hippocampal cells and acute hippocampal slices from young animals (postnatal day 10) (Sarti et al., 2013), we found that with treatment of RA in these more mature cortical slices, mIPSC amplitude remained unchanged (mock, 18.4 ± 0.857pA; RA, 19.389 ± 0.699pA) (Fig. 1A). This suggests a difference in RA’s effect on synapses either as a consequence of maturation (postnatal day 21) or brain region. Note that mEPSCs were also completely unchanged by RA treatment (frequency: mock, 3.88 ± 0.391Hz; RA, 4.06 ± 0.443Hz; amplitude: mock, 9.45 ± 0.183pA; RA, 10.10 ± 0.368pA), demonstrating again that RA’s effects diverge depending on age and brain region (Fig. 1B).
Figure 1.
Acute RA treatment increases frequency of miniature inhibitory postsynaptic currents (mIPSCs) in an RARα-dependent manner. A: Example traces (left) and quantification (right) of mIPSCs recorded from layer 2/3 pyramidal cells of primary somatosensory cortex (S1) (mock n=15 cells and RA n=18 cells, 6 animals). B: Miniature excitatory postsynaptic potentials (mEPSCs) recorded from layer 2/3 pyramidal neurons in S1 (mock n=12 cells, RA n=11, 4 animals). C: mIPSCs recorded in CamKII-Cre; RARα conditional KO mouse (mock n=27 cells, RA n=30 cells, 5 animals). D: mIPSCs recorded in PV-Cre; RARα conditional KO mouse (mock n=17 cells and RA n=17 cells, 4 animals). Individual circles represent single cells, bars are mean ± S.E.M. (*p<0.01, N.S p>0.1).
Expression of RARα is required for mIPSC increase by RA
RA-mediated homeostatic synaptic plasticity is thought to be exclusively mediated by one of three RA receptors, namely, retinoic acid receptor alpha (RARα), which regulates postsynaptic translation of de novo GluA1 AMPA receptor subunits (Aoto et al. 2008). To assess whether the effect of RA on mIPSC frequency is also RARα-mediated, we removed RARα from excitatory pyramidal neurons using a conditional knock-out approach in which we crossed a CAMKII-Cre line with mice bearing flox sites flanking exon three of the RARα gene (Tsien et al., 1999; Sarti et al., 2012). Acute slices from these knockout mice (CAMKII-Cre; RARαfl/fl) or their heterozygote littermates (CAMKII-Cre; RARαfl/+) were then incubated in RA and assayed for mIPSCs. We found that the RARα KO (CAMKII-Cre; RARαfl/fl) neurons exhibited an elevated baseline mIPSC frequency, which failed to increase further after RA treatment (frequency: KO mock, 5.6 ± 0.535 Hz; KO RA, 6.05 ± 0.449 Hz; amplitude: KO mock, 18.45 ± 0.533 pA; KO RA, 19.08 ± 0.573 pA) (Fig. 1C). Presumably, this is due to a postsynaptic requirement for RARα because the CAMKII-Cre driver is thought to be specific to excitatory neurons. In order to assess the potential action of RARα in presynaptic, parvalbumin (PV)-positive inhibitory neurons, we also crossed our RARα conditional line with a PV-Cre driver line and treated acute slices from these KOs with RA. Comparing the effect of RA in knockout (PV-Cre;RARαfl/fl) versus heterozygote littermates (PV-Cre;RARαfl/+), we found that this too blocked RA-induced increases in mIPSCs frequency, but without significantly elevating the baseline frequency (frequency: KO mock, 4.6 ± 0.334 Hz; KO RA, 5.37 ± 0.719 Hz; amplitude: KO mock, 18.28 ± 0.471 pA; KO RA, 19.619 ± 1.119 pA) (Fig. 1D). To date, all studies on the postnatal function of RA have demonstrated that RARα mediates RA’s effect on excitatory and inhibitory transmission through a postsynaptic locus. Thus, these results surprisingly suggest that in contrast to previous studies, RA’s effect on spontaneous transmission requires actions of RARα at both presynaptic and postsynaptic locations.
Presynaptic release probability of synaptic inhibition is unchanged by RA
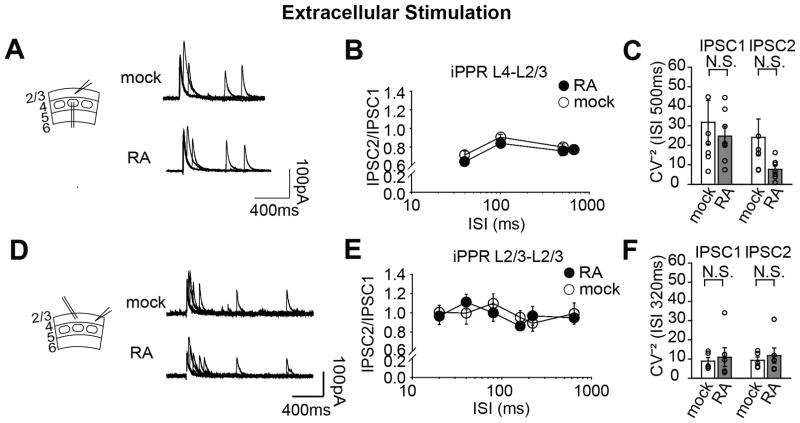
The changes in mIPSC frequency but not amplitude suggested to us that, as opposed to a change in postsynaptic receptor quantity such as that which occurs during RA-mediated mEPSC scaling in hippocampus, here in S1, there might be a change in presynaptic efficacy. To directly test this, we measured the paired-pulse ratio of evoked IPSCs, which is a standard assay for probing changes in release probability. By extracellularly stimulating layer 4 of the same barrel column as the recorded cell in layer 2/3, we tested whether the paired-pulse ratio at layer 4-to-layer 2/3 synapses was altered by RA application. Surprisingly, we found that it was unchanged across all interpulse intervals (Fig. 2A). Although layer 4 is thought to be the main input to layer 2/3, horizontal inputs across layer 2/3 are also present (Adesnik and Scanziani, 2010). To test whether this input might account for the change in mIPSC frequency, we stimulated layer 2/3 of the column neighboring to the recorded cell. However, paired-pulsed ratios at this input also remained unchanged (Fig. 2B). Taken together, it seems unlikely that RA alters presynaptic release probability at inhibitory terminals.
Figure 2.
RA does not change inhibitory paired-pulse ratios (PPR) evoked by extracellular stimulation. A: Recording configuration and sample traces (left) of PPR at layer 4-layer 2/3 synapses, with or without RA treatment. Stimulation electrode was placed in layer 4 barrel and pyramidal cells recorded from the same column. Quantification (right) of PPR at layer 4-layer 2/3 synapses, across varying time intervals (mock n=7 cells and RA n=7 cells, 3 animals; p=0.06, Two-way ANOVA). B: Recording configuration and sample traces (left) of PPR at layer 2/3-layer 2/3 synapses, with or without RA treatment. Stimulation electrode was placed in layer 2/3 in column adjacent to recorded cell. Quantification (right) of PPR at layer 2/3-layer 2/3 synapses (mock n=5 and RA n=6, 3 animals; p=0.785, Two-way ANOVA). Large circles represent mean ± S.E.M. C: Coefficient of variation (CV-2) calculated for each layer 4-layer 2/3 IPSC for the 500ms interval D: CV-2 calculated for each layer 2/3-layer 2/3 IPSC for the 320ms interval. Individual circles represent single cells, bars are mean ± S.E.M. (N.S p>0.1).
Another factor that could result in reduced presynaptic efficacy is number of presynaptic boutons or active release sites. To begin to address this, we calculated the coefficient of variation (CV-2), whose inverse should be proportional to N, the number of active release sites. We found, however, that this was also unchanged (Figs. 2C and 2D).
Evoked IPSCs at two distinct inhibitory synapse types is unchanged by RA
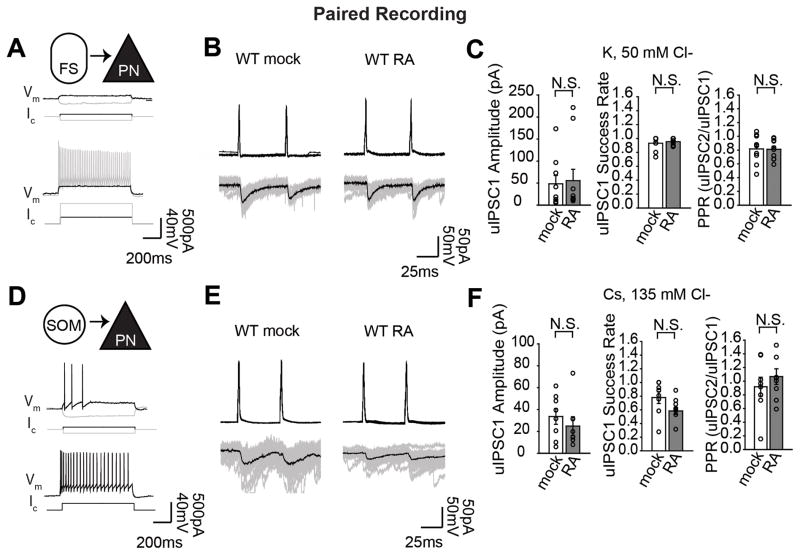
The lack of a change in either release probability or N, together with our initial finding that spontaneous miniature transmission was increased by RA treatment left us with the possibility that evoked, action potential-driven and spontaneous, action potential-independent transmission are differentially regulated by RA. To further explore this, we decided to examine the evoked response of cell type-specific inhibitory input using paired recordings from synaptically-coupled pairs of interneuron and pyramidal (PN) neurons. We were able to record from two types of identified presynaptic, GABAergic interneuron cell types, fast-spiking (FS) cells and somatostatin-positive (SOM) low-threshold spiking cells. The identity of the presynaptic neuron was verified using current injection under current clamp mode (Fig. 3A and 3D), or in the case of the somatostatin cell type, with the aid of a genetic GFP-labeled line (Oliva et al., 2000). After establishing the whole-cell recordings of the pair, synaptic connection was confirmed by presence of a postsynaptic unitary IPSC (uIPSC) at short latency after an action potential evoked in the presynaptic cell (Fig. 3B and 3E) (see Methods). Consistent with the idea that RA affects spontaneous but not evoked transmission, neither FS-PN pairs nor SOM-PN pairs exhibited changes in uIPSC amplitude, calculated as the average amplitude over 50 trials, including failures (FS-PN: mock, 48.4 ± 17.0 pA; RA, 55.5 ± 25.8 pA; SOM-PN: mock, 30.2 ± 9.2 pA, RA 16.20 ± 4.0 pA), success rate (FS-PN: mock, 0.933 ± 0.033; RA, 0.955 ± 0.150; SOM-PN: mock 0.779 ± 0.087, RA 0.583 ± 0.051), or paired-pulse ratio (FS-PN: mock, 0.816 ± 0.07; RA 0.811 ± 0.142, SOM-PN: mock, 0.930 ± 0.189, RA 1.1012 ± 0.151) in response to RA application (Fig. 3C and 3F).
Figure 3.
RA does not change evoked transmission at fast-spiking interneuron or somatostatin-positive interneuron-onto-pyramidal cell synapses. A: Paired recordings were performed between fast-spiking (FS) cells and pyramidal (PN) cells in layer 2/3; shown are current-clamp recordings characterizing firing patterns of FS cells. Ic: current injected, Vm: voltage responses. Black or gray Ic trace corresponds to black or gray Vm trace, respectively. B: Sample traces of presynaptic action potentials (APs) evoked by current injection (top traces) and postsynaptic uIPSCs recorded at −70 mV (bottom traces, gray represents individual trials, black is average) from connected FS-PN pairs. C: Average amplitude (including failures), success rate, and paired-pulse ratio of uIPSCs measured from PV-PN pairs (mock n=9 cells and RA n=10 cells, 4 animals). D: Paired recordings between somatostatin-positive (SOM) cells and PN cells in layer 2/3; shown are current-clamp recordings characterizing firing patterns of SOM cells. E: Sample traces of presynaptic APs and uIPSCs from connected SOM-PN pairs. F: Average amplitude (including failures), success rate, and paired-pulse ratio of uIPSCs measured from SOM-PN connected pairs (mock n=8 cells and RA n=7 cells, 5 animals). Individual circles represent individual pairs, bars represent mean ± S.E.M. (N.S p>0.05)
Discussion
Our main finding in this study is that RA differentially upregulates spontaneous synaptic inhibition onto layer 2/3 pyramidal neurons in the somatosensory cortex without affecting evoked inhibition. This effect is very different from those previously reported in hippocampus (Sarti et al., 2013). In cultured hippocampal neurons and young acute hippocampal slices, RA selectively decreases eIPSC amplitude as well as mIPSC amplitude without affecting mIPSC frequency. In the present study, RA increased mIPSCs in frequency but not amplitude, and this effect is not reflected in the evoked response. At least two factors may contribute to such differences. First, the current work was performed in an acute slice preparation of P21 animals, whereas previous work was performed in either acute slice preparations from P10 animals, or in cultured slices and cultured dissociated neurons prepared from young animals (P7 and P0, respectively) and recorded at 7–14 DIV. In older slices with relatively intact local circuitry, the effect of RA on a particular synapse may be complicated by altered network activity due to RA’s simultaneous effects on other, surrounding synapses within the network. Additionally, there may be differences in the molecular machinery downstream of RA’s action in neurons across development. Either case or the combination of the two could lead to the observed differences in RA’s effects on synaptic inhibition. Indeed, both activity blockade and RA treatments have been shown to increase mEPSC frequency in dissociated neurons at 21 DIV but not 14 DIV, supporting the idea that these effects can be developmentally regulated (Jakawich et al., 2010, Wang et al., 2011).
Secondly, and maybe more importantly, the current study was performed in the somatosensory cortex, whereas the experiments described above were performed in acute slices or cultures from the hippocampus. It is conceivable that RARα activates a different set of signaling programs in somatosensory cortex neurons versus in hippocampal neurons. Both cortical and hippocampal neurons are capable of expressing homeostatic synaptic plasticity, however, the mechanism may differ (He K et al., 2011). Although unlikely given the acute two-hour RA treatment, the RA-dependent increase in mIPSC frequency might be a result of a transcription-dependent instead of translational mechanism. Moreover, different brain regions may achieve homeostatic plasticity using distinct alterations in local microcircuitry. For example, while multiple groups have reported visual deprivation-induced homeostatic scaling of mEPSCs in visual cortex (Desai et al., 2002; Goel et al., 2007; Keck et al., 2013), evidence for a similar cell-wide type of excitatory synaptic scaling in somatosensory cortex by whisker deprivation has yet to be discovered (Li et al., 2014; Wen et al., 2013). It thus stands to reason that RA may show different, perhaps even non-homeostatic or Hebbian effects in somatosensory cortex versus visual cortex or hippocampus; a complete account of how RA differentially regulates excitatory and inhibitory synaptic transmission remains to be pursued.
Our experiments testing how pyramidal-cell versus fast spiking-cell specific knockout of RARα can eliminate RA’s acute effect on spontaneous transmission suggest that RA may be working partially through previously characterized postsynaptic mechanisms (Aoto et al., 2008), but additionally affects spontaneous transmission through presynaptic effects on GABAergic interneurons. For example, RA may simultaneously affect GABAergic interneuron firing and neurotransmitter release through a more classic transcriptional regulation mechanism, while concurrently activating postsynaptic translation. The fact that excitatory postsynaptic neuron-specific RARα KO appears to block RA-induced increases in inhibition through an “occlusion” effect—e.g. by increasing baseline mIPSC frequency in the KO—seems consistent with the notion of constitutive repression of translation by RARα (Poon and Chen, 2008). However, it is also possible that synthesis of a retrogradely acting molecule, which increases presynaptic spontaneous release, is constitutively repressed by RARα, and that this repression is relieved by RA binding to RARα.
It is puzzling that in the presence of a clear effect on mIPSCs, RA did not affect evoked synaptic transmission onto pyramidal neurons from at least two identified, major inhibitory cell types of the brain: fast-spiking (FS), PV-positive cells are thought to comprise approximately 40% of interneurons in the somatosensory cortex, and somatostatin-positive another 30% (Markram, 2004; Fishell and Rudy, 2011). We cannot formally rule out the possibility that changes at a third class of inhibitory interneuron accounts for the observed mIPSC responses to RA. For example, in somatosensory cortex, a distinct 5-HT3AR-expression population (~30% of interneurons, largely separated from PV and SOM-expressing cells) exists, and may include irregular spiking VIP, NPY and CCK-expressing interneurons (Rudy et al., 2011; Lee et al. 2010). If a third group were responsible, however, it is puzzling that PV-specific RARα KO should completely block RA’s effect; since FS neurons may be further subdivided, for example, into PV-expressing large and small basket cells as well as chandelier cells, it may be that RA selectively affects a subpopulation that our methods (electrophysiological identification of FS neurons) did not resolve, or that a non-fast spiking, PV+ population (such as the multipolar cell reported by Blatow et al. (2003)) accounts for the phenotype. Alternatively, mounting evidence suggests that spontaneous and evoked synaptic transmission may be differentially regulated and may involve different synaptic vesicle pools (Kavalali, 2015). It is thus conceivable that, at least at a subset of synapses, RA triggers signaling events that preferentially affect spontaneous vesicle release with minor influence on evoked responses.
Recent experiments have shown that deprivation paradigms in whisker barrel cortex can induce increased or decreased synaptic inhibition (House et al., 2010; Shao et al., 2013; Li et al., 2014; Gainey et al., 2016), in addition to synapse-specific changes in excitation (Wen et al., Greenhill et al., 2015). Just as the circuit mechanisms of cortical plasticity are diverse and complex, RA’s action at synapse can very well be multifarious depending on brain subregion, cortical layer and animal age. It will be of interest to explore the possibility that RA may underlie some but not all such changes and to probe what downstream effectors of RA and their expression patterns account for the functional differences observed in different contexts.
Acknowledgments
The work was supported by NIH grants MH086403 (L.C.) and MH091193 (L.C.),.
Footnotes
Conflict of Interest
The authors do not have any conflict of interest to declare.
References
- Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464(7292):1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Arendt KL, Zhang Z, Ganesan S, Hintze M, Shin MM, Tang Y, Cho A, Graef IA, Chen L. Calcineurin mediates homeostatic synaptic plasticity by regulating retinoic acid synthesis. Proc Natl Acad Sci U S A. 2015;112:E5744–E5752. doi: 10.1073/pnas.1510239112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Zhang Y, Jurado S, Malenka RC, Südhof TC, Chen L. Retinoic Acid and LTP Recruit Postsynaptic AMPA Receptors Using Distinct SNARE-Dependent Mechanisms. Neuron. 2015;86:442–456. doi: 10.1016/j.neuron.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A Novel Network of Multipolar Bursting Interneurons Generates Theta Frequency Oscillations in Neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- Chapellier B1, Mark M, Bastien J, Dierich A, LeMeur M, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor beta (RARbeta) gene. Genesis. 2002;32:91–94. doi: 10.1002/gene.10073. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Roberts LS, Connors BW. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature. 1999;400:367–371. doi: 10.1038/22553. [DOI] [PubMed] [Google Scholar]
- Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainey MA, Wolfe R, Pourzia O, Feldman DE. Whisker Deprivation Drives Two Phases of Inhibitory Synapse Weakening in Layer 4 of Rat Somatosensory Cortex. PLoS ONE. 2016;11:e0148227. doi: 10.1371/journal.pone.0148227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Lee HK. The Persistence of Experience-Induced Homeostatic Synaptic Plasticity through Adulthood in Superficial Layers of Mouse Visual Cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill SD, Ranson A, Fox K. Hebbian and Homeostatic Plasticity Mechanisms in Regular Spiking and Intrinsic Bursting Cells of Cortical Layer 5. Neuron. 2015;88:539–552. doi: 10.1016/j.neuron.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House DRC, Elstrott J, Koh E, Chung J, Feldman DE. Parallel Regulation of Feedforward Inhibition and Excitation During Whisker Map Plasticity. Neuron. 2011;72:819–831. doi: 10.1016/j.neuron.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, Carruthers CJ, Sutton MA. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143–1158. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Goel A, Ciarkowski CE, Song L, Lee HK. Brain area specific regulation of synaptic AMPA receptors by phosphorylation. Commun Integr Biol. 2011;4:569–572. doi: 10.4161/cib.4.5.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2015;16:5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- Keck T, Keller GB, Jacobsen RI, Eysel UT, Bonhoeffer T, Hübener M. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron. 2013;80:327–334. doi: 10.1016/j.neuron.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The Largest Group of Superficial Neocortical GABAergic Interneurons Expresses Ionotropic Serotonin Receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Gainey MA, Goldbeck JE, Feldman DE. Rapid homeostasis by disinhibition during whisker map plasticity. Proc Natl Acad Sci U S A. 2014;111:1616–1621. doi: 10.1073/pnas.1312455111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neuro. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Maghsoodi B, Poon MM, Nam CI, Aoto J, Ting P, Chen L. Retinoic acid regulates RARαlpha-mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proc Natl Acad Sci U S A. 2008;105:16015–16020. doi: 10.1073/pnas.0804801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon MM, Chen L. Retinoic acid-gated sequence-specific translational control by RARalpha. Proc Natl Acad Sci U S A. 2008;105:20303–20308. doi: 10.1073/pnas.0807740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three Groups of Interneurons Account for Nearly 100% of Neocortical GABAergic Neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti F, Zhang Z, Schroeder J, Chen L. Rapid Suppression of Inhibitory Synaptic Transmission by Retinoic Acid. J Neurosci. 2013;33(28):11440–11450. doi: 10.1523/JNEUROSCI.1710-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti F, Schroeder J, Aoto J, Chen L. Conditional RARα knockout mice reveal acute requirement for retinoic acid and RARα in homeostatic plasticity. Front Mol Neurosci. 2012;15:16. doi: 10.3389/fnmol.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Yu R, Isett Brian R, Miyashita4 Toshio, Chung5 Jason, Pourzia Olivia, Gasperini Robert J, Feldman Daniel E. Plasticity of Recurrent L2/3 Inhibition and Gamma Oscillations by Whisker Experience. Neuron. 2013;80:210–222. doi: 10.1016/j.neuron.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang Z, Hintze M, Chen L. Decrease in calcium concentration triggers neuronal retinoic acid synthesis during homeostatic synaptic plasticity. J Neurosci. 2011;31:17764–17771. doi: 10.1523/JNEUROSCI.3964-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JA, DeBlois MC, Barth AL. Initiation, Labile, and Stabilization Phases of Experience-dependent plasticity at neocortical synapses. J Neurosci. 2013;33:8483–8493. doi: 10.1523/JNEUROSCI.3575-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]