Abstract
The Na-K-2Cl cotransporter (NKCC) is a Cl− uptake transporter that is responsible for maintaining a Cl− equilibrium potential positive to the resting potential in neurons. If NKCC is active, GABA and glycine can depolarize neurons. In view of the abundance of GABAergic and glycinergic synapses in retina, we undertook a series of studies using immunocytochemical techniques to determine the distribution of NKCC in retinas of both developing and adult mice. We found NKCC antibody (T4) labeling present in retinas from wild-type mice, but not in NKCC1-deficient mice, suggesting that the NKCC1 subtype is a major Cl− uptake transporter in mouse retina. Strong labeling of NKCC1 was present in horizontal cells and rod-bipolar dendrites in adult mice. Interestingly, we also found that a diffuse labeling pattern was present in photoreceptor terminals. However, NKCC1 was barely detectable in the inner retina of adult mice. Using an antibody against K-Cl cotransporter 2 (KCC2), we found that KCC2, a transporter that extrudes Cl−, was primarily expressed in the inner retina. The expression of NKCC1 in developing mouse retinas was studied from postnatal day (P) 1 to P21, NKCC1 labeling first appeared in the dendrites of horizontal and rod-bipolar cells as early as P7, followed by photoreceptor terminals between P10–P14; with expression gradually increasing concomitantly with the growth of synaptic terminals and dendrites throughout retinal development. In the inner retina, NKCC1 labeling was initially observed in the inner plexiform layer at P1, but labeling diminished after P5. The developmental increase in NKCC expression only occurred in the outer retina. Our results suggest that the distal synapses and synaptogenesis in mouse retinas undergo a unique process with a high intracellular Cl− presence due to NKCC1 expression.
Keywords: NKCC, Mouse retina, Distal retina, Cl− cotransporters, Retinal development
Introduction
The cation Cl− cotransporters, Na-K-2Cl (NKCC) and K-Cl− (KCC), are known to control intracellular Cl− concentrations. The NKCC actively accumulates Cl− in cells, thereby keeping the Cl− equilibrium (ECl) more positive than the resting potential, whereas KCC extrudes Cl− from cells, thus making the ECl more negative than the resting potential. By affecting the ECl in neurons, these cotransporters can determine whether γ-aminobutyric acid (GABA) and glycine depolarize or hyperpolarize neurons (Haas & Forbush, 1998; Sun & Murali, 1999; Kaneko et al., 2004). Usually, NKCC is expressed in immature neurons and GABA and glycine evoke an excitatory effect in the neurons (Cherubini et al., 1991; Russell, 2000). However, as a part of neuronal maturation, NKCC expression declines and is gradually replaced by expression of KCC; consequently, the ECl changes from positive to negative, compared to the resting potential in the neurons. Therefore, GABA and glycine evoke an inhibitory response in adult neurons (Kakazu et al., 1999; Rivera et al., 1999; Ben-Ari, 2002). However, recent studies indicate that GABA can depolarize adult dorsal root ganglions (Sung et al., 2000), spinal neurons (Jang et al., 2001), and olfactory receptor neurons (Reisert et al., 2005) due to the expression of NKCC in these neurons. Thus, NKCC is not only expressed in immature neurons, but also expressed in adult central nervous system neurons.
Previous immunohistochemical study has showed that both NKCC and KCC are present in vertebrate retinas in adult animals (Vu et al., 2000; Vardi et al., 2000, Zhang et al., 2006a, 2006b). Interestingly, the developmental switch of NKCC to KCC seems only present in inner retinal neurons: bipolar terminals, amacrine cells, and ganglion cells (Vu et al., 2000). Electrophysiological studies indicate that GABA and glycine inhibit glutamate transmission by hyperpolarizing the inner retinal neurons. In contrast, an opposite effect has been found in outer retinal neurons where GABA and glycine act to depolarize horizontal cells (HCs) and ON-bipolar cells (BCs) because the ECl is more positive than the resting potentials in these neurons and neuronal regions (Miller & Dacheux, 1983; Blanco et al., 1996; Varela et al., 2005b; Stockton & Slaughter, 1991; Shen, 2005; Duebel et al., 2005). Unlike inner retinal neurons that predominantly express KCC, NKCC are expressed in HCs and ON-bipolar dendrites, whereas KCC are expressed in OFF-bipolar dendrites (Vardi et al., 2000). Consequently, GABA depolarizes HCs and some ON-bipolar dendrites and hyperpolarizes OFF-bipolar dendrites, since ECl is different in these neurons and neuronal regions (Satoh et al., 2001; Varela et al., 2005a). Differential localization of NKCC and KCC would be critical for the synaptic effect of GABA and glycine in retinal neurotransmission. Previous studies concerning NKCC were reported in rabbit, ferret, and primate retinas. However, little is known about NKCC in mouse retinas that are frequently used to study GABAergic and glycinergic synapses in retinal circuitry. NKCC expression during the retinal development is largely unknown.
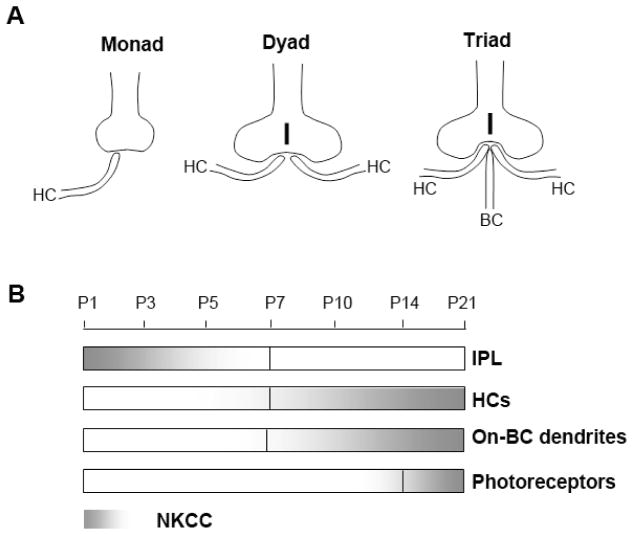
The mouse retina is an excellent model system because the cellular organization and neuronal morphogenesis in the developing retina are well characterized (Rich et al., 1997; Sherry et al., 2003; Sharma et al., 2003). Generally, synaptic formation in rods and cones starts at different time periods during the retinal development. Cones undergo synaptic formation with HCs and BCs at postnatal (P) day five; rods synaptic formation begins three to four days later (Rich et al., 1997; Sherry et al., 2003). Process growth from HCs begins as early as P1 and forms a “monad” contact with a single dendrite touching a cone terminal, by day five. By day seven, an additional HC dendrite is recruited and makes a “dyad” contact to a cone terminal. In comparison, the rod synaptic development occurs later between P7–P9 forming “monad” and “dyad” complexes with HC processes. By P10, a BC dendrite is recruited and makes a “triad” synaptic complex with two HC processes contacting one rod terminal. The distal synaptic complex formation has been summarized in schematic diagrams (Blanks et al., 1974; Sherry et al., 2003). We redrew the diagrams in Fig. 10A. The synaptic formation in the outer retina is complete when the mouse opens its eyes on P14. By day 21, retinal development is complete. This time table served as a reference for the present study of the temporal expression of NKCC using mouse retinal development.
Fig. 10.
(A) Schematics of the outer retinal morphogenesis in forming “monad,” “dyad,” and “triad” complexes in mouse outer retinal development (redrawn from Blanks et al., 1974; Sherry et al., 2003). Cones begin “monad” formation at P4–P5, “dyad” formation at P6, and “triad” formation at P7–P8. Rods form “monads” at P8, “dyads” at P9, and “triads” at P10. (B) Scale showing the temporal labeling of NKCC1 during outer retinal development. (HCs: horizontal cells, BCs: bipolar cells; IPL: inner plexiform layer.)
We investigated the localization of NKCC in both developing and adult mouse retinas using a monoclonal antibody against NKCC subtypes NKCC1 and NKCC2. Our study shows that NKCC1 is the predominant subtype in the adult mouse retina and is expressed in the outer synaptic layer, not only in the processes of HCs and rod-BCs, but also in photoreceptor terminals. Both NKCC1 and KCC2 were expressed on ON-bipolar dendrites. In addition, expression of NKCC1 in the developing retina paralleled the formation of “monad,” “dyad,” and “triad” complexes in photoreceptor terminals.
Materials and methods
Animals and tissue preparation
Wild-type C57/B1 mice (Jackson Laboratories, Bar Harbor, ME) were housed in a room with a 12:12 light/dark circle and handled according to the guidelines of the National Institute of Health, and were approved by Florida Atlantic University’s Institute of Animal Care and Use Committee (IACUC). Both, 2-month-old adult mice and pups from postnatal days were used: P1 (within 24 h after birth), P3, P5, P7, P10, P14, and P21. Young mice (P1–P10) were sacrificed by decapitation. Older mice were anesthetized with an injection of sodium pentobarbital (100 mg/kg) followed by decapitation. Following decapitation, eyes were enucleated and fixed in a 0.1 M phosphate buffer saline (PBS) with 4% paraformaldehyde (pH 7.4) for 10 min. Following fixation, the cornea, lens, and vitreous humor were carefully removed. Eyecups were fixed for another 20 min at room temperature, washed in PBS and crytoprotected in 15% sucrose for 30 min, followed by a 1-h rinse in 20% sucrose and stored overnight in 30% sucrose at 4°C. On the following day, eyecups were embedded in OCT (Ted Pella, Redding, CA), and sectioned vertically at 12 μm with a cryostat. Frozen sections were collected on silicone-coated slides, air-dried, and stored at −80°C for immunocytochemical experiments.
Immunocytochemistry
Single-labeling
Sections were rinsed in PBS three times and transferred to blocking solution consisting of 5% normal goat serum, 1% BSA, 0.3% Triton X-100, 0.3% Tween in PBS, and blocked for 20 min. Next, sections were incubated in PBS containing primary antibodies and 3% normal goat serum overnight at 4°C. The sections were rinsed three times with PBS for 10 min each, then incubated in PBS with a secondary antibody, either FITC conjugated goat anti-mouse or Cy3-conjugated goat anti-rabbit antibody, in the dark for 40 min. After secondary antibody treatment, sections were rinsed and mounted using Vectorshield (Vector Laboratories, Burlingame, CA).
Double-labeling
Retinal sections were incubated in PBS with primary antibody and 10% goat serum for 2 h at room temperature. The dilutions of primary antibodies were the same as noted above. The sections were rinsed and incubated in PBS with two secondary antibodies: FITC-conjugated goat anti-mouse and Cy3-conjugated goat anti-rabbit IgG for 40 min at room temperature in the dark.
Negative control
The control experiment for the T4 antibody specificity was done in NKCC1-deficient mouse retinas. No detectable labeling was observed when other control experiments were performed by omitting the primary antibody or replacing the primary antibody with normal IgG. In double-labeling controls, one primary antibody was applied, followed by the application of an inappropriate secondary antibody. No cross-reactivity was detected.
Detecting and image processing
Immunofluorescence-labeled sections were viewed using 60X oil objective on a Bio-Rad Radiance 2100 confocal laser scanning microscope (Nikon Eclipse TE300) equipped with an Ar 488 laser and a Green He-Ne laser. For double-labeled slides, confocal laser images were sequentially collected for Cy3 and FITC labeling to prevent spectral bleed-through. Antibody labeling in retinal sections was detected in single optical scanning procedures. Adobe Photoshop 7.0 program was used to merge images, as well as to adjust brightness and contrast levels when necessary.
Antibodies
Primary antibodies
Nine primary antibodies were used in this study (see Table 1). One of the principle primary antibodies used in this study was mouse monoclonal anti-NKCC (T4, supernatant), purchased from DSHB (Developmental Studies Hybridoma Bank at the University of Iowa, Iowa City, IA). The T4 antibody binds the carboxyterminus portion (MET-902 to SER-1212) of human NKCC1 protein, and also recognizes isoforms of NKCC1 and NKCC2. For the T4 antibody in immunolabeling in retinal sections, we followed the procedure suggested by the original producer of the T4 antibody, Dr. Christian Lytle (Division of Biomedical Science, University of California). Since the T4 antibody can only recognize a denatured NKCC protein, we treated the retinal frozen sections in 1% SDS to denature the NKCC protein before performing a general immunolabeling procedure. In negative control experiments, we did not treat the retinal sections in SDS solution and no specific labeling was observed (data not shown). All other primary antibodies used in this study are listed in Table 1, including the sources, dilutions used, and references.
Table 1.
Antibodies
| Antibody | Host | Source | Lot No. | Dilution | References |
|---|---|---|---|---|---|
| NKCC1 (T4) | Mouse | DSHB | 1:90 | Yan et al., 2003 | |
| PSD 95 | Rabbit | Abcam | 132364 | 1:500 | Jellali et al., 2002 |
| SV2 | Mouse | DSHB | 1:10 | Wang et al., 2003 | |
| PKC | Rabbit | Upstate | 06870 | 1:800 | Wässle et al., 1991 |
| PKC | Mouse | Sigma | 065K4877 | 1:200 | Wässle et al., 1991 |
| KCC2 | Rabbit | Upstate | 24369 | 1:600 | Vu et al., 2000 |
| Calbindin | Mouse | Sigma | 016K4786 | 1:2000 | Sharma et al., 2003 |
| Calbindin | Rabbit | Sigma | 035K4765 | 1:1000 | Beltran et al., 2005 |
| HCN4 | Rabbit | F. Müller | 1:2 | Müller et al., 2003 |
Secondary antibodies
FITC-conjugated goat anti-mouse and Cy3-conjugated goat anti-rabbit antibodies were purchased from the Jackson Immunoresearch (West Grove, PA). The working concentrations used were 1:400 and 1:600, respectively.
Western blotting
Membrane proteins were extracted from mouse tissues using a general procedure. Four to eight retinas or brain tissues were homogenized in 1 ml of lysis buffer solution (mM): 250 sucrose, 10 Tris, 10 HEPES, and 1 EDTA, pH =7.2, added a protease inhibitor “cocktail” (Roche). The homogenates were centrifuged at 2500 rpm for 10 min in 4°C. The supernatants were collected and centrifuged further at 30000 rpm for 2 h at 4°C. The final pellets were then suspended in lysis buffer solution. Protein concentration was determined using a BCA™ Protein Assay Kit (Lot No. GH98667, Pierce, Rockford, IL). Membrane proteins were resolved by SDS-PAGE using a 4–12% Bis-Tris gel. Proteins were transferred to a Hybond™-ECL™ Nitrocellulose membrane (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). The membranes were blocked in a block solution (7% non-fat dry milk in PBST, pH =7.4) for 1 h at room temperature, then incubated in a block solution with anti-NKCC1 (1:50) for 2 h at room temperature. After washing in a block solution, the membranes were incubated in a secondary antibody solution (horseradish peroxidase-conjugated goat anti-mouse IgG; 1:2000) for 2 h at room temperature. Next, the membranes were washed in PBST solution. Positive staining bands were detected on the membrane using a chemiluminescence (ECL) assay (GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
Results
Detection of NKCC1 in adult mouse retinas
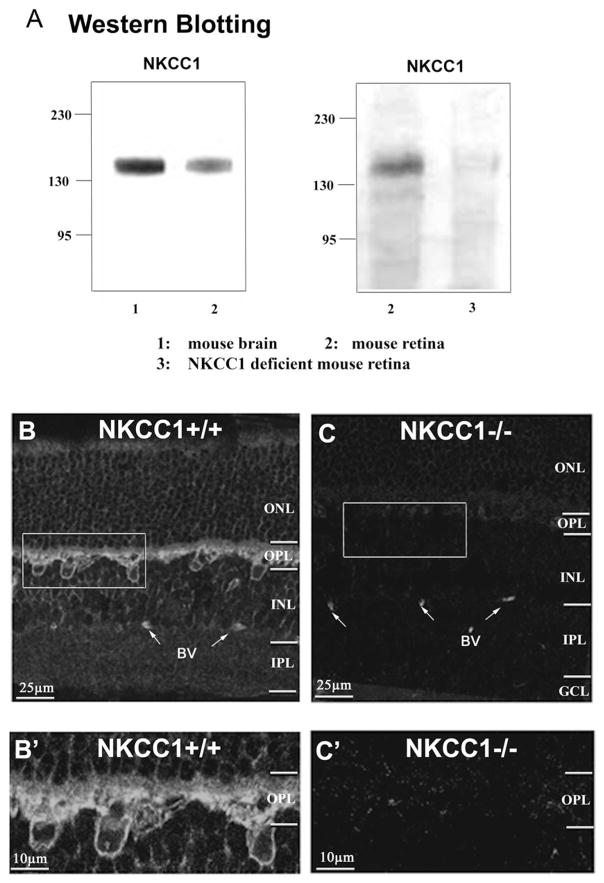
The T4 antibody is known to bind both NKCC1 and NKCC2 (Yan et al., 2003). The specificity of the antibody for NKCC1 has been extensively characterized in rat cortical neurons (Sun & Murali, 1999; Yan et al., 2003) and hippocampal neurons (Marty et al., 2002). To characterize the specificity of the T4 antibody in mouse retinas, both wild-type and NKCC1-deficient mouse retinas (gifts from Dr. Kingwai Yau’s Lab) were used in Western blotting assays. The NKCC1-deficient mice were created by inserting the reporter gene lac-Z to the exon 9 of NKCC1 to interrupt the regular gene expression (Delpire et al., 1999). The lack of NKCC1 protein in the deficient mice was confirmed in auditory and olfactory tissue (Delpire et al., 1999; Reisert et al., 2005). Our Western blots showed that the antibody recognized a single band at 155 ±8 kDa in the homogenates of wild-type mouse retina and brain (Fig. 1A, left panel). This is the predicted molecular mass for NKCC1 in the mammalian brain (Sun & Murali, 1999; Yan et al., 2003; Marty et al., 2002). However, antibody labeling was absent in the NKCC1-deficient mouse retina (Fig. 1A, right panel). Since the T4 antibody didn’t recognized protein bands in NKCC1-deficient mouse retina, we concluded that it is a reliable antibody that specifically labels NKCC1 in the wild-type mouse retina. Possibly, there is no T4-sensitive NKCC2 in mouse retina, although there may be a variant of NKCC2 that is T4-insensitive, as shown recently for rabbit starburst amacrine cells (Gavrikov et al., 2006).
Fig. 1.
Immunoblotting and immunocytolabeling for NKCC1 in wide-type and NKCC1-deficient mouse retinas. (A) Western Blot assays to detect NKCC1 proteins in homogenates of (1) mouse brain tissues, (2) wild-type mouse retinas, and (3) NKCC1-deficient mouse retinas. The monoclonal antibody T4 recognizes a single protein band at MW 155 ±8 kDa (the predicted MW for NKCC1) in wide-type mouse brain and retinas, but not in a NKCC1-deficient mouse retina homogenate. (B) A confocal single optical section shows that the T4 antibody labels NKCC1 in the outer retina of wild-type mouse. (B′) Enlarged inset from (B) shows a detailed pattern of NKCC1 labeling in the outer retina. Two sub-layers are differentiated in the OPL: a faint, diffuse layer in the pre-synaptic area, and a strong, clear staining layer in the postsynaptic area. (C and C′) The labeling is completely eliminated in NKCC1-deficient mouse retina, shown in both a vertical section and an enlarged inset in the OPL. (BV: blood vessel.)
NKCC1 was present predominantly in the distal retinas of adult mice
The spatial distribution of NKCC1 was studied in vertical sections of the mouse retina. Retinal sections were treated in a 1% SDS solution prior to the general procedure for NKCC1 immunolabeling (see Materials and methods). Confocal imaging indicates that the T4 strongly labeled the outer plexiform layer (OPL, Fig. 1B). The enlarged inset in Fig. 1B shows that a faint diffuse band was observed in the upper layer of the OPL, while a stronger band was present in the lower region of the OPL, as well as in the distal inner nuclear layer (INL) where the somas of HCs and BCs are located. However, no significant NKCC1 labeling was observed either in the inner plexiform layer (IPL) or in the ganglion cell layer (GCL, see Fig. 1B).
The same experimental protocol was applied to the NKCC1-deficient mouse retinas. T4 immunoreactivity was absent in an NKCC1-deficient mouse retinal section (Figs. 1C, and 1C′). Thus, T4 is a reliable antibody for labeling NKCC1 in wild-type mouse retinas.
NKCC1 in photoreceptor terminals in adult mouse retinas
Double-labeling for NKCC1 and the postsynaptic density protein PSD95 confirmed that photoreceptor terminals were faintly labeled by NKCC1 antibody. These two primary antibodies were obtained from different hosts: rabbits and mice. PSD95 is known to label rod spherules and cone pedicles in mouse retinas (Jellali et al., 2002). Figs. 2A1 and 2A2 show confocal single optical sections of NKCC1 and PSD95 labeling in the outer retina from a section double-labeled with two antibodies. The superimposed figure shows that a faint, diffuse staining band of NKCC1 was present in the upper region of the OPL, overlapped by PSD-95 in photoreceptor terminals (Fig. 2A3). Higher magnified insets of Fig. 2A show colocalizations of two antibodies in photoreceptor terminals.
Fig. 2.
Colocalization of NKCC1 with photoreceptor terminal markers in adult mouse retinas. (A1 and A2) Single-labeling patterns for NKCC1 (green) and PSD95 (red, a photoreceptor terminal marker) in the outer retina of a vertical section double-labeled for both antibodies. (A3) The merged image indicates that a faint, diffuse staining of NKCC1 is colocalized with PSD95 in photoreceptor terminals. The colocalizations can be clearly viewed in higher amplified insets of (A1), (A2), and (A3), focusing at photoreceptor terminals. (B1 and B2) Single-channel optical sections of KCC2 (red) and SV2 (green) in the outer retina. The superimposed image shows that KCC2 staining does not overlap with SV2 in photoreceptor terminals. (ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer; BV: blood vessel.)
We also examined KCC2 immunoreactivity in photoreceptor terminals. KCC2 has been identified in retinas and the KCC2 antibody used in this study was previously used to study rat retinas (Vardi et al., 2000; Vu et al., 2000). Since the KCC2 antibody is from rabbit, an anti-mouse monoclonal antibody to synaptic vesicle protein SV2 was used to mark photoreceptor terminals to avoid antibody cross-reaction with the same host. SV2 is an integral membrane protein expressed in both synaptic and ribbon synaptic vesicles (Balkema & Rizkalla, 1996), and is a common marker for photoreceptor terminals, BCs and amacrine cells (Rich et al., 1997; Wang et al., 2003). Confocal imaging shows KCC2 and SV2 immunoreactivity in the outer retina (Figs. 2B1–2B3). KCC2 labeling was present in the OPL and INL, but failed to colocalize with SV2 in photoreceptor terminals; instead, the labeling was present in the post-synaptic sites of photoreceptor terminals and the INL. The absence of KCC2 in the photoreceptor terminals is similar to previous results in rat and primate retinas (Vu et al., 2000;Vardi et al., 2000), but not ferret retinas in which KCC2 is detected in photoreceptors (Zhang et al., 2006a, 2006b).
Our immunolabeling was robust in all retinal sections from 11 mice from age 30 days to 2 months, suggesting that NKCC, rather than KCC, is the predominant cation-chloride cotransporter in photoreceptor terminals.
Distribution of NKCC1 on HCs
To verify that HCs are NKCC1-positive neurons, retinal sections were double-labeled with antibodies for NKCC1 (mouse host) and calbindin (rabbit host). Calbindin is a calcium-binding protein found in mouse retinal HCs. A calbindin antibody serves as a marker for HCs in mouse retinas (Sharma et al., 2003). Both monoclonal and polyclonal calbindin antibodies have been used to label HCs for mammalian retinas (Sharma et al., 2003; Beltran et al., 2005). Our double-labeling results indicated that calbindin-positive HCs were strongly immunoreactive to NKCC1 and the colocalization was substantial on both somas and processes (Fig. 3A). Neither NKCC1 nor calbindin labeled the inner retina. On the other hand, double labeling for calbindin (mouse host) and KCC2 (rabbit host) indicated that KCC2 strongly labeled the IPL, INL, and the OPL, but barely colocalized with the calbindin-positive HCs (Fig. 3B), consistent with previous results in the rabbit (Vardi et al., 2000).
Fig. 3.
Localizations of NKCC1 and KCC2 in adult mouse retinal HCs. HCs were labeled with calbindin. (A) Double-labeling for calbindin and NKCC1 shows NKCC1 binding colocalized with calbindin in the processes and somatic membranes of HCs. (B) KCC2 immunolabeling does not overlap with calbindin-positive HCs. (A1–A3 and B1–B3) Higher magnification views of the insets in (A) and (B) in single-channel optical sections and superimpositions.
NKCC1 and KCC2 in the dendrites of rod-BCs and Off-cone BCs
Interestingly, the dendritic regions of the rod-driven ON-BCs were immunoreactive to both NKCC1 and KCC2. This was identified by double-labeling for protein kinase C (PKC) with either NKCC1 or KCC2. The PKC antibody is commonly used to label rod-connected ON-BCs in mammalian retinas (Wässle et al., 1991). PKC binding illustrated the entire connection of rod-BCs with long axons ending in the sublamina-b of the IPL (the ON-BC synaptic region). Double-labeling for PKC and NKCC1 showed punctate yellow spots (the overlap areas) were only present in the dendritic regions of rod-BCs in the OPL (Fig. 4A), which could be clearly viewed at a higher magnification by superimposing single-optical channels (Figs. 4B1–4B3).
Fig. 4.
NKCC1 and KCC2 in the somatodendritic regions of rod-BCs in the adult mouse retina. Rod-BCs were labeled with PKC in retinal vertical sections. (A) Double-labeling for NKCC1 and PKC shows colocalization in rod-BC dendrites. (B1, B2) Enlarged single-optical sections for NKCC1 and PKC staining in the somas and dendrites of rod-BCs. (B3) The superimposition shows colocalization in the dendrites of rod-BCs. (C) A superimposed image of double-labeling for KCC2 and PKC in a retinal vertical section. The KCC2 and PKC binding is colocalized in some rod-BC dendrites, somas, axons (thick arrows) and axon terminals. Some rod-BC axons are PKC-labeled (arrow) and some are KCC2-labeled (arrowhead). (C1 and C2) The enlarged inset of (C), KCC2, and PKC in single-channel optical sections. (C3) The co-localization is identified in the superimposed image.
The double-labeling for KCC2 and PKC indicates that the KCC2 staining significantly overlapped with the PKC-positive somas, axons and axon terminals of the rod-BCs, all of which were negative for NKCC1 labeling (Fig. 4C). Interestingly, KCC2 staining was also present in the PKC-positive dendrites, shown in higher magnification views of the inset in Fig. 4C (Figs. 4C1, 4C2, and 4C3). The merged images of two single optical sections revealed the colocalization of KCC2 in the somatodendritic regions of rod-BCs. The colocalization of KCC2 and PKC in the dendrites of rod-BCs is quite interesting since previous studies indicated that only NKCC1 was found on ON-bipolar dendrites of primate retinas (Vardi et al., 2000). We repeated the double-labeling experiments in five other retinal sections from five mice and the results were identical.
Furthermore, colocalization of NKCC1 and KCC2 in OFF-bipolar dendrites was also examined by double-labeling for either Cl− cotransporters or an antibody to HCN4. HCN4, a hyperpolarization and cyclic nucleotide-gated channel, has been found in type 3a OFF-BCs in rat and mouse retinas (Müller et al., 2003; Feigenspan et al., 2004; Mataruga et al., 2007). According to recent studies (Mataruga et al., 2007), Type 3a OFF-BCs are in contact with not only cone terminals, but also rod terminals. Double-labeling for NKCC1 and HCN4 showed that HCN4 stained dendrites, somas and axon terminals that stratified in the sublamina-b, the OFF-sublamina of the IPL (Fig. 5), which are similar to the type 3, OFF-cone BCs described in previous studies (Müller et al., 2003). Both HCN4 and NKCC1 stained the OPL. However, HCN4-positive dendrites were missing NKCC1, as shown in the enlarged inset of the OPL (Figs. 5A1–5A3). The results indicated that the dendrites of OFF-cone BCs might be NKCC1-negative. Contrarily, KCC2 was highly colocalized with HCN4 in the OPL. The dashed staining pattern of HCN4 overlapped with KCC2 staining in a double-labeled retinal section (Fig. 5B). The colocalization of HCN4 and KCC2 was not only present in the axon terminals of the OFF-cone BCs in the IPL, but also in the dendrites of the outer retina viewed at a higher magnification by superimposing single-optical channels (Figs. 5B1, 5B2, and 5B3).
Fig. 5.
Localization of NKCC1 and KCC2 on OFF-cone bipolar dendrites in the adult mouse retina. The OFF-cone dendrites were labeled with HCN4. (A) Double-labeling for NKCC1 and HCN4 in a vertical section. At a higher magnification, NKCC1 and HCN4 do not colocalize in Off-cone dendrites of the outer retina in a single optical section (A1–A3). In contrast, KCC2 immunolabeling is clearly colocalized with HCN4 in the dendrites of OFF-cone BCs [shown in (B), and (B1–B3)].
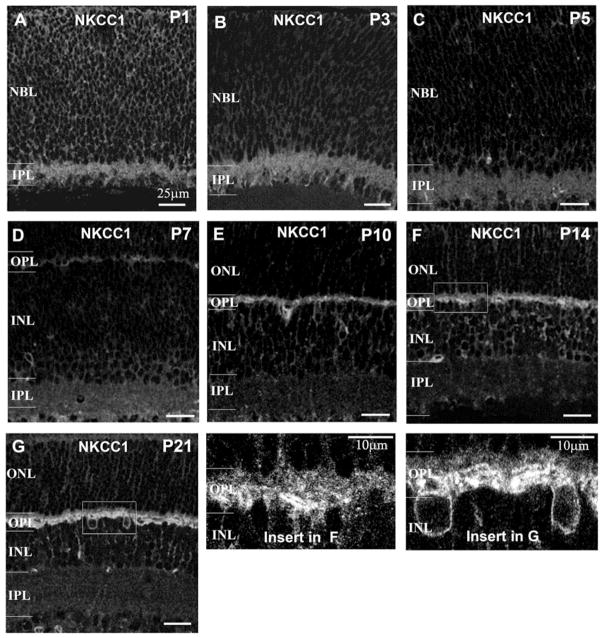
Localization of NKCC1 in developing mouse retinas
The temporal and spatial distribution of NKCC1 in mouse retinal development was sequentially examined from P1 to P21. At P1, a bright, punctate staining band for NKCC1 was observed in the IPL (Fig. 6A). Also, radial processes were faintly-stained in the neuroblastic layer (NBL) at P1–P3, indicating that a certain amount of NKCC1 was expressed in the IPL and neuroblastic cells at a very early postnatal age (Figs. 6A and 6B). The intensity of the staining declined significantly in the IPL of a P5 retina (Fig. 6C). When the HC’s processes invaginate into cones at age P7 (Blanks et al., 1974; Rich et al., 1997; Sherry et al., 2003), a band of weak staining appeared in the OPL (Fig. 6D). However, labeling in the IPL attenuated at P7 (Fig. 6E). NKCC1 staining significantly increased in the OPL at P10 when ribbon synapses first appear in rod terminals, as well as the HC’s processes making “triad” contact with rod terminals (Sherry et al., 2003). By P14, when the mouse eye opens, photoreceptor synaptogenesis is complete and NKCC1 reactivity in the OPL can be differentiated between a faintly labeled band and intensively labeled band at the upper and lower portions of the OPL (Fig. 6F). However, no labeling was observed in the inner retina after P10 (Fig. 6E). By P21, the NKCC1 staining pattern was similar to that observed in adult retinas (Figs. 6G and 1B) indicated by a faint band and strong band in the upper and lower portion of the OPL, respectively. Similar results were obtained in three additional sets of P1 to P21 mouse retinas.
Fig. 6.
Spatial and temporal expression of NKCC1 in mouse retinal development. NKCC1 was localized in developing mouse retina from postnatal day 1 to day 21 (P1 to P21) in single optical sections. (A, B, C and D) From P1 to P7, NKCC1 staining is present in the IPL, but gradually declines during maturation. At P7, NKCC1 labeling first appears in the outer retina. The intensity of the labeling increases dramatically by P10 (E) but NKCC1 labeling is absent in the IPL after P7. (F and G) The outer retinal synaptogenesis is completed when the mouse’s eyes open at P14. From P14 to P21, the intensity of labeling in the outer retinas remains the same, but the pattern becomes more evident (as seen in enlarged insets of (F) and (G)). Scale bar in (A) to (G) represents 25 μm. (NBL: neuroblast layer.)
NKCC1 in photoreceptor synaptic development
PSD95 was used to label photoreceptor terminals in the developing retinas. The photoreceptor terminals were lightly labeled by PSD95 at P7; however, labeling did not overlap with NKCC1 (Figs. 7A and 7A′) suggesting that NKCC1 is present in the post-synaptic region of the OPL. In P10 to P14 retinas, NKCC1 labeling was present in the outer retina and a faint labeling band appeared on the outer layer of the OPL at P14. PSD95 labeling lightly overlapped with NKCC1 staining at P10 and the overlap is noticeable in photoreceptor terminals at P14 (Figs. 7B, 7B′, 7C, and 7C′). By P21, substantial colocalization of NKCC1 and PSD95 was present in photoreceptor terminals and the staining pattern was similar to that observed in the adult retina (Figs. 7D, 7D′, and Fig. 2). NKCC1 was present in photoreceptor terminals at P10–P14, but not at P7, suggesting that the expression of NKCC1 might be closely correlated to rod synaptic development. Double-labeling of PSD95 and NKCC1 was performed on at lease six retinal sections per mouse and four mice per each age group from P1 to P21. The expression pattern of NKCC1 was consistent in the retinal sections at each postnatal age (P1–P21) tested.
Fig. 7.
Double labeling for NKCC1 and PSD95 in developing mouse retina from P7 to P21. (A) Single optical image from a doubled retinal section shows weak labeling of NKCC1 in the outer retina at P7. (A′) Superimposed images of PSD95 and NKCC1 labeling show the two antibodies labeled the pre- and post-synaptic sites in the OPL. (B an B′) Single image and the superimposed image of NKCC1 and PSD94 in the P10 mouse retina shows weak colabeling in photoreceptor terminals at this age. (C and C′) A faint band of NKCC1 labeling is present in the upper layer of OPL, colocalized with PSD95 in photoreceptor terminals. (D and D′) Photoreceptor terminals are NKCC1-positive at P21 as depicted in a double-labeled retinal section. The insets show higher magnifications of the OPL at different ages.
The distribution of NKCC1 in the post-synaptic areas of photoreceptors
Temporal expression of NKCC1 in developing HCs was examined by double-labeling for NKCC1 and calbindin. Weak Calbindin binding was present at P1 in retinal sections (data not shown). The colocalization of NKCC1 and calbindin was detected at P7 when NKCC1 staining first appeared in the OPL (see Fig. 6D). At this age, NKCC1 was also present in the dendrites of calbindin-positive HCs (Fig. 8A). By P10, overlapping NKCC1 and calbindin noticeably increased, not only on the processes, but also on the somatic membranes of HCs (see arrows, Fig. 8B). At P14, increased colocalization was detected on the somas and processes (Fig. 8C). By P21, the overlapping areas fully covered the processes and somatic membranes of HCs (Fig. 8D). Fig. 8E shows double-labeling NKCC1 and calbindin in retinal sections at P21 and the labeling pattern was similar to that in the adult mouse retina (see Fig. 3A)
Fig. 8.
Spatial and temporal localization of NKCC1 in HC development. HCs were double-labeled with NKCC1 and calbindin. Since NKCC1 was undetectable from P1 to P5, colocalization of NKCC1 and calbindin was examined at P7 and later. (A) Only faint labeling (yellow spots show over lapping areas) appears on the distal processes of calbindin-positive HCs at P7. (B) The size and intensity of the colocalization increased in the HC processes and NKCC1 labeling appearing on the somas of HCs at P10 (see arrows). (C) NKCC1 labeling extends to the somatic membranes of HCs at P14. (D) By P21, a great amount of NKCC1 labeling overlaps with calbindin on the somas and processes of HCs. (E) Double-labeling for NKCC1 and calbindin in the retina from a P21 mouse.
The expression of NKCC1 in developing rod-BCs was studied by double-labeling for PKC and NKCC1. Because both PKC and NKCC1 immunoreactivities were absent in the outer retinas at P1 to P5, we monitored any overlap of NKCC1 and PKC staining in the OPL at P7 and later. At P7, colocalization was detected in the OPL (Fig. 9A). The clear PKC-positive bipolar dendrites were observed at P10 when ON-bipolar dendrites formed a “triad” synaptic configuration in rod spherical terminals. Colocalization of NKCC1 and PKC immunoreactivity increased in the bipolar dendrites at this age (Fig. 9B). At P14, enhanced NKCC1 immunoreactivity on PKC-positive dendrites was present (Fig. 9C). By P21, more PKC-positive BCs were present and NKCC1 staining was strong in the dendrites of the BCs, particularly in the apical regions of the dendrites (Fig. 9D).
Fig. 9.
The distribution of NKCC1 in the dendrites of rod-BCs in development. (A) The scattering dual-labeling pattern is present on the dendrites of PKC-positive BCs at P7. (B, C, and D) Gradually, the labeling intensified and spread out on the dendrites from P10 to P21. However, no NKCC labeling is on the somas of the PKC-positive BCs.
The temporal and spatial patterns of NKCC1 in either calbindin-positive HCs or PKC-positive BCs were very consistent. The data were collected from at lease eight sections per mouse and three to five mice per each age group from P1 to P21.
Discussion
The expression of NKCC is an underlying mechanism for GABA and glycine depolarizing responses in neurons. Using a specific antibody, we studied the spatial and temporal distribution of NKCC in adult and developing mouse retinas. The study reveals that NKCC1 is a major Cl− uptake transporter predominantly expressed in the pre- and post-synaptic regions of the OPL in the adult retina. The expression appeared at an early age of retinal development and gradually increased through the period of outer retinal development. Fig. 10B summarizes our findings and shows the time frame for expression of NKCC1 in the outer retina during development. NKCC1 was detected in the inner retinas in newborn mice, but the expression largely declined and was eliminated during the first week after birth. In adult mice, NKCC1 was barely detected in the inner retinas. The preferential distribution of NKCC1 in the outer retina implies that GABA and glycine might depolarize outer retinal neurons due to a high intracellular Cl− concentration created by NKCC1.
NKCC1 in the outer retinas in adult animals
One of the new findings in this study is that photoreceptor terminals are NKCC-positive in mouse retinas. The double-labeling results in Figs. 1B and 2A show that faint, diffuse staining was present in the upper layer of the OPL and colocalized with a synaptic density protein, PSD95, in photoreceptor terminals. This staining pattern has not been reported in other mammalian retinas in previous immunocytochemical studies (Vardi et al., 2000; Zhang et al., 2006a, 2006b). These previous studies used different NKCC1 antibodies and immunolabeling protocols than in our study. We pretreated the retinal sections with 1% SDS prior to performing the immunocytochemistry. Pretreatment (as suggested by the antibody manufacturer) might denature the NKCC protein but could assist in T4 antibody binding. We observed a non-specific pattern of NKCC staining in untreated retinal sections (no SDS) as well as in the NKCC1-deficient mouse retina. The specificity of the T4 antibody was calibrated in the NKCC1 deficient mouse retinas using Western Blots. The results indicate that the antibody is highly specific to NKCC1. The faint labeling in photoreceptor terminals was absent in the NKCC1-deficient mouse retinal sections (see Fig. 1), indicating that the labeling in photoreceptor terminals was specific. T4 antibody labeling in photoreceptors terminals was robust in all retinal sections tested.
The positive labeling of NKCC1 in photoreceptor terminals might indicate that the ECl would be positive to the dark resting potential in photoreceptors. According to the studies in amphibian retinas, the ECl in rods is around −20 mV, more positive than the dark potential that is usually around −40 mV (Miller & Dacheux, 1983; Thoreson et al., 2002; Thoreson & Bryson, 2004). We also observed T4 antibody labeling present in the amphibian photoreceptor terminals (unpublished data). The ECl in cones, which is around −45 mV to −35 mV, is more negative than that in rods according to studies in amphibian retinas (Kaneko & Tachibana, 1986; Thoreson & Bryson, 2004). Immunocytochemical localization of KCC2 was observed in cone terminals and somas in developing, but not in adult, ferret retinas (Zhang et al., 2006a, 2006b). However, photoreceptors were not labeled by KCC2 in mouse nor rat retinas (Vu et al., 2000). We postulate that NKCC1 might possibly be present in rods since they are the over whelming majority of photoreceptors in the rodent. Our immunocytochemical results suggest that GABA and glycine feedback might not produce an inhibition in the mouse photoreceptors.
In addition to photoreceptors, HCs appear to be another group of NKCC1-positive and KCC2-negative neurons in mouse retinas (Figs. 3A and 3B). The strong NKCC1 labeling in the OPL didn’t colocalize with calbindin-positive labeling in HCs, neither in the processes nor in the somas. This result is consistent with previous studies on rabbit and primate retinas (Vardi et al., 2000), but differs from a study in rat retinas in which both KCC2 and NKCC1 immunolabeling were present in the processes of HCs. The discrepancy might be due to species differences. In amphibian and rabbit retinas, GABA and glycine depolarize HCs because the ECl is about −20 mV (Stockton & Slaughter, 1991; Blanco et al., 1996; Shen, 2005; Varela et al., 2005a, 2005b). Since mouse retinal HCs possess GABA receptors (Feigenspan & Weiler, 2004), possibly, GABA depolarizes HCs due to a lack of KCC and expression of NKCC1 in the neurons.
Our study supports previous results that an intracellular Cl− concentration gradient might be present in rod-BCs, because of NKCC1 in the dendrites and KCC2 in the terminals of the BCs. GABA inputs could evoke a dual-effect in rod-BCs: depolarizing the dendrites and hyperpolarizing the axonal terminals (Duebel et al., 2005; Varela et al., 2005a). Interestingly, we also found that KCC2 was present in PKC-positive rod-bipolar dendrites (see Fig. 4C). The presence of KCC2 in rod-bipolar dendrites is a fairly new discovery, since previous studies did not report KCC2 in rod-bipolar dendrites of rat, rabbit, or primate retinas (Vardi et al., 2000; Vu et al., 2000). These observations might serve as another example of species-dependent expression of Cl− cotransporters in retinal neurons. Electrophysiological studies suggest that KCC might be in the dendrites of ON-type cone bipolar cells of mouse retinas, since GABA hyperpolarizes cone-bipolar dendrites, including ON-type cone BCs (Satoh et al., 2001) This suggests that KCC might be expressed in the different groups of ON-bipolar dendrites in mouse retinas. GABAergic and glycinergic synapses on bipolar dendrites might mediate combined depolarizing and hyperpolarizing responses, dependent on the differential expression of NKCC and KCC.
Our results confirm previous findings that KCC2 is mainly expressed in the IPL of adult mouse retinas, corresponding to the inhibitory function of GABA and glycine in the IPL. NKCC1 was absent in the IPL in adult mouse retinas. Zhang et al. (2006a, 2006bb) reported T4 labeling in the IPL of adult mouse retinas, but they included the caveat that the labeling may not be specific in the inner retina.
The expression of NKCC1 in the distal retinal synaptic development
The morphological description of mouse retinal development has been well documented (Blanks et al., 1974; Rich et al., 1997; Sherry et al., 2003) allowing us to study the temporal expression pattern of NKCC1 at different stages of retinal development and to assess the role of GABA and glycine in neuronal development. Our study indicates that NKCC1 immunoreactivity was present in the IPL and neuroblastic cells in P1 retinas, but was diminished by the end of the first postnatal week. NKCC1 was detected in the OPL at P7 with increased labeling as photoreceptor terminal differentiation progressed including dendritic outgrowth of HCs and rod-BCs. Thus expression of NKCC1 in the outer retina could be correlated to dendritic growth and synaptic formation of photo-receptors and second-order neurons.
Previous studies of rat retinal development reported barely detectable KCC2 expression at birth but evidence in the inner retina at P3 (when KCC2 expression is still absent in the outer retinas). KCC2 labeling appears for the first time in the outer retina at P7 (Vu et al., 2000). This might suggest that NKCC1 expression declines while KCC2 expression increases in the inner retina during the first postnatal week, whereas in the outer retina, both NKCC1 and KCC2 are simultaneously expressed by P7. Possibly, P7 is a critical time for developing GABAergic synaptic functions in outer retinal neurons since the ECl in local areas are largely related to expression of NKCC1 and KCC2. Synaptic formation in the inner retina is initiated in NKCC1-expressed conditions, and is completed later in the presence of KCC2 when NKCC1 is absent (Vu et al., 2000). Developmental expression of NKCC1 suggests different roles of GABA and glycine in synaptic formation in the outer retina compared to the inner retina.
Since cones in the mouse retina start synaptogenesis at P4–P5, three to four days earlier than rods, allowing the study of NKCC1 in a time-dependent manner in rod and cone development. Here we report an NKCC1 immunoreactive band appeared first in the outer retina at P7, after synaptogenesis has already started in cones. At P10, after rods synaptogenesis has begun, a substantial level of NKCC1 was present in the OPL; however, most of the binding was colocalized with calbindin and PKC in HCs and BCs, with only a weak colocalization of NKCC1 and PSD95 present in photoreceptor terminals. Until mouse eyes open and synaptogeneis is completed, a diffuse pattern of NKCC1 staining could be observed in the upper layer of the OPL and overlapped with PSD95 in photoreceptor terminals. At P21, NKCC1 was established in the outer retina and maintained throughout adulthood. These results suggest that accumulating intracellular Cl− might have occurred when photoreceptor synapses were formed. However, we do not know whether KCC2 is transiently expressed in photoreceptors during development, as suggested in ferret retinas (Zhang et al., 2006a, 2006b).
Synaptic formation in processes and dendrites of second-order neurons was highly related to the expression of NKCC1. At P7, NKCC1 binding was observed on the calbindin-positive HC dendrites when HCs make “dyad” contacts to cones at the ribbon synapse, suggesting that the expression of NKCC is associated with the formation of synaptic complexes between HCs and cones. The same phenomenon was also observed in the formation of synaptic contacts between rods and rod-bipolar dendrites. When rods initiate synaptic contact with rod-bipolar dendrites at P10, expression of NKCC1 increases in bipolar dendrites. These results indicate that the expression of NKCC1 in second-order neurons is concomitant with the formation of synaptic complexes with photoreceptor terminals. The expression pattern of NKCC1 in mouse outer retinal development suggests an alternative mechanism requiring high intracellular Cl− in neuronal synaptic formation.
Acknowledgments
We would like to thank Drs. K.W. Yau and F. Müller for the generous gifts of NKCC1-deficient mouse retinal samples and the HCN4 antibody and Dr. J. Blanks for critically reading and editing the manuscript. This work was supported by National Institutes of Health research grant EY14161 (WS).
References
- Balkema GW, Rizkalla R. Ultrastructural localization of a synaptic ribbon protein recognized by antibody B16. Journal of Neurocytology. 1996;25:565–571. doi: 10.1007/BF02284824. [DOI] [PubMed] [Google Scholar]
- Beltran WA, Rohrer H, Aguirre GD. Immunolocalization of ciliary neurotrophic factor receptor alpha (CNTFRalpha) in mammalian photoreceptor cells. Molecular Vision. 2005;11:232–244. [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: The nature of the nurture. Nature Reviews Neurosciences. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Blanco R, Vaquero CF, de la Villa P. The effects of GABA and glycine on horizontal cells of the rabbit retina. Vision Research. 1996;36:3987–3995. doi: 10.1016/s0042-6989(96)00145-9. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Adinolfi AM, Lolley RN. Synaptogenesis in the photoreceptor terminal of the mouse retina. Journal of Comparative Neurology. 1974;156:81–93. doi: 10.1002/cne.901560107. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: An excitatory transmitter in early postnatal life. Trends in Neuroscience. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl− co-transporter. Nature Genetics. 1999;22:192–195. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- Duebel J, Haverkamp S, Schleich W, Feng G, Augustine G, Kuner T, Euler T. Two-photon imagining reveals somatodendritic chloride gradient in retinal On-type bipolar cells expressing the biosensor clomeleon. Neuron. 2005;49:81–94. doi: 10.1016/j.neuron.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Janssen-Bienhold U, Hormuzdi S, Monyer H, Degen J, Sohl G, Willecke K, Ammermuller J, Weiler R. Expression of connexin36 in cone pedicles and OFF-cone bipolar cells of the mouse retina. Journal of Neuroscience. 2004;24:3325–3334. doi: 10.1523/JNEUROSCI.5598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Weiler R. Electrophysiological properties of mouse horizontal cell GABAA receptors. Journal of Neurophysiology. 2004;92:2789–2801. doi: 10.1152/jn.00284.2004. [DOI] [PubMed] [Google Scholar]
- Gavrikov KE, Nilson JE, Dmitriev AV, Zucker CL, Mangel SC. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proceedings of the National Academy of Sciences USA. 2006;103:18793–18798. doi: 10.1073/pnas.0604551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, Forbush B., III The Na-K-Cl cotransporters. Journal of Bioenergetics and Biomembranes. 1998;30:161–172. doi: 10.1023/a:1020521308985. [DOI] [PubMed] [Google Scholar]
- Jang IS, Jeong HJ, Akaike N. Contribution of the Na-K-Cl cotransporter on GABA(A) receptor-mediated presynaptic depolarization in excitatory nerve terminals. Journal of Neuroscience. 2001;21:5962–5972. doi: 10.1523/JNEUROSCI.21-16-05962.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellali A, Stussi-Garaud C, Gasnier B, Rendon A, Sahel JA, Dreyfus H, Picaud S. Cellular localization of the vesicular inhibitory amino acid transporter in the mouse and human retina. Journal of Comparative Neurology. 2002;449:76–87. doi: 10.1002/cne.10272. [DOI] [PubMed] [Google Scholar]
- Kakazu Y, Akaike N, Komiyama S, Nabekura J. Regulation of intracellular chloride by cotransporters in developing lateral superior olive neurons. Journal of Neuroscience. 1999;19:2843–2851. doi: 10.1523/JNEUROSCI.19-08-02843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. Journal of Physiology. 1986;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. Journal of Neuroscience. 2004;24:7931–7938. doi: 10.1523/JNEUROSCI.2115-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, Wehrle R, Alvarez-Leefmans FJ, Gasnier B, Sotelo C. Postnatal maturation of Na, K, 2Cl− cotransporter’s expression and inhibitory synaptogenesis in the rat hippocampus: An immunocytochemical analysis. European Journal of Neuroscience. 2002;15:233–245. doi: 10.1046/j.0953-816x.2001.01854.x. [DOI] [PubMed] [Google Scholar]
- Mataruga A, Kremmer E, Müller F. Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. Journal of Comparative Neurology. 2007;502:1123–1137. doi: 10.1002/cne.21367. [DOI] [PubMed] [Google Scholar]
- Miller RF, Dacheux RF. Intracellular chloride in retinal neurons: measurement and meaning. Vision Research. 1983;23:399–411. doi: 10.1016/0042-6989(83)90087-1. [DOI] [PubMed] [Google Scholar]
- Müller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Kaupp UB. HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. European Journal of Neuroscience. 2003;17:2084–2096. doi: 10.1046/j.1460-9568.2003.02634.x. [DOI] [PubMed] [Google Scholar]
- Reisert J, Lai J, Yau KW, Bradley J. Mechanism of the excitatory Cl− response in mouse olfactory receptor neurons. Neuron. 2005;45:553–561. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich KA, Zhan Y, Blanks JC. Migration and synaptogenesis of cone photoreceptors in the developing mouse retina. Journal of Comparative Neurology. 1997;388:47–63. [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiology Review. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Satoh H, Kaneda M, Kaneko A. Intracellular chloride concentration is higher in rod bipolar cells than in cone bipolar cells of the mouse retina. Neuroscience Letters. 2001;310:161–164. doi: 10.1016/s0304-3940(01)02120-6. [DOI] [PubMed] [Google Scholar]
- Sharma RK, O’Leary TE, Fields CM, Johnson DA. Development of the outer retina in the mouse. Brain Research. Developmental Brain Research. 2003;145:93–105. doi: 10.1016/s0165-3806(03)00217-7. [DOI] [PubMed] [Google Scholar]
- Shen W. Repetitive light stimulation inducing glycine receptor plasticity in the retinal neurons. Journal of Neurophysiology. 2005;94:2231–2238. doi: 10.1152/jn.01099.2004. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Wang MM, Bates J, Frishman LJ. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. Journal of Comparative Neurology. 2003;465:480–498. doi: 10.1002/cne.10838. [DOI] [PubMed] [Google Scholar]
- Stockton RA, Slaughter MM. Depolarizing actions of GABA and glycine on amphibian retinal horizontal cells. Journal of Neurophysiology. 1991;65:680–692. doi: 10.1152/jn.1991.65.3.680. [DOI] [PubMed] [Google Scholar]
- Sun D, Murali SG. Na+-K+-2Cl− cotransporter in immature cortical neurons: A role in intracellular Cl− regulation. Journal of Neurophysiology. 1999;81:1939–1948. doi: 10.1152/jn.1999.81.4.1939. [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. Journal of Neuroscience. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Bryson EJ. Chloride equilibrium potential in salamander cones. BMC Neuroscience. 2004;5:53. doi: 10.1186/1471-2202-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Stella SL, Jr, Bryson EI, Clements J, Witkovsky P. D2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Visual Neuroscience. 2002;19:235–247. doi: 10.1017/s0952523802192017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. Journal of Neuroscience. 2000;20:7657–7663. doi: 10.1523/JNEUROSCI.20-20-07657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Blanco R, de la Villa P. Depolarizing effect of GABA in rod bipolar cells of the mouse retina. Vision Research. 2005a;45:2659–2667. doi: 10.1016/j.visres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Varela C, Rivera L, Blanco R, de la Villa P. Depolarizing effect of GABA in horizontal cells of the rabbit retina. Neuroscience Research. 2005b;53:257–264. doi: 10.1016/j.neures.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Vu TQ, Payne JA, Copenhagen DR. Localization and developmental expression patterns of the neuronal K-Cl cotransporter (KCC2) in the rat retina. Journal of Neuroscience. 2000;20:1414–1423. doi: 10.1523/JNEUROSCI.20-04-01414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MM, Janz R, Belizaire R, Frishman LJ, Sherry DM. Differential distribution and developmental expression of synaptic vesicle protein 2 isoforms in the mouse retina. Journal of Compartive Neurology. 2003;460:106–122. doi: 10.1002/cne.10636. [DOI] [PubMed] [Google Scholar]
- Wässle H, Yamashita M, Greferath U, Grünert U, Müller F. The rod bipolar cell of the mammalian retina. Visual Neuroscience. 1991;7:99–112. doi: 10.1017/s095252380001097x. [DOI] [PubMed] [Google Scholar]
- Yan Y, Dempsey RJ, Flemmer A, Forbush B, Sun D. Inhibition of Na(+)-K(+)-Cl(−) cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Research. 2003;961:22–31. doi: 10.1016/s0006-8993(02)03832-5. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Fina ME, Vardi N. Regulation of KCC2 and NKCC during development: Membrane insertion and differences between cell types. Journal of Comparative Neurology. 2006a;499:132–143. doi: 10.1002/cne.21100. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Pathak HR, Coulter DA, Freed MA, Vardi N. Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. Journal of Neurophysiology. 2006b;95:2404–2416. doi: 10.1152/jn.00578.2005. [DOI] [PubMed] [Google Scholar]