Abstract
Fibrinogen, 1 of 13 coagulation factors responsible for normal blood clotting, is synthesized by hepatocytes. Detailed roles of the orphan nuclear receptors regulating fibrinogen gene expression have not yet been fully elucidated. Here, we identified estrogen-related receptor gamma (ERRγ) as a novel transcriptional regulator of human fibrinogen gene expression. Overexpression of ERRγ specially increased fibrinogen expression in human hepatoma cell line. Cannabinoid receptor types 1(CB1R) agonist arachidonyl-2'-chloroethylamide (ACEA) up-regulated transcription of fibrinogen via induction of ERRγ, whereas knockdown of ERRγ attenuated fibrinogen expression. Deletion analyses of the fibrinogen γ (FGG) gene promoter and ChIP assays revealed binding sites of ERRγ on human fibrinogen γ gene promoter. Moreover, overexpression of ERRγ was sufficient to increase fibrinogen gene expression, whereas treatment with GSK5182, a selective inverse agonist of ERRγ led to its attenuation in cell culture. Finally, fibrinogen and ERRγ gene expression were elevated in liver tissue of obese patients suggesting a conservation of this mechanism. Overall, this study elucidates a molecular mechanism linking CB1R signaling, ERRγ expression and fibrinogen gene transcription. GSK5182 may have therapeutic potential to treat hyperfibrinogenemia.
Introduction
Obesity is frequently associated with elevated risk for cardiovascular disease (CVD) [1]. In addition, accumulating evidence indicates that elevated blood fibrinogen level is a risk factor for the development of CVD [2, 3]. Fibrinogen (Factor I) is a 340 kDa glycoprotein synthesized in liver by hepatocytes [4]. The three chains of fibrinogen (Aα, Bβ, and γ) are encoded by different genes, FGA, FGB, and FGG, respectively [5]. They form an elongated molecule that binds to a second identical molecule by disulfide bonds, forming the homodimeric fibrinogen molecule that circulates in the blood [6–8]. Hyperfibrinogenemia and hypofibrinolysis are associated with a hypercoagulable state that causes accumulation of fibrin, increasing the risk for thrombotic events and CVD [9]. In both adults and children, obesity is characterized by various derangements in key components of the hemostatic system, including the presence of hyperfibrinogenemia [10], which increases thrombus fibrin content, accelerates fibrin formation, and increases fibrin network stability. Hyperfibrinogenemia also increases thrombus resistance to tenecteplase-induced thrombolysis [11]. Down-regulation of fibrinogen expression may prevent CVD. The three fibrinogen genes are targets of several nuclear receptors. RAR-related orphan receptor alpha (RORα) regulates FGB expression in human hepatoma cells and mouse liver [12]. Endogenous bile acids are known as ligands for the nuclear receptor farnesoid X receptor (FXR), and all three fibrinogen subunits are induced by FXR in response to FXR ligands, suggesting that bile acids and FXR modulate fibrinolytic activity [13]. In addition, the nuclear receptor coactivator 2 (NCoA-2) is a positive regulator of FGB transcription, and sequestration of NCoA-2 by PPARα is a molecular mechanism by which PPARα agonists negatively regulate fibrinogen-β [14]. However, regulation of fibrinogen gene expression by members of the nuclear receptor superfamily remains largely uncharacterized.
The estrogen receptor–related receptor subfamily consists of three members, ERRα, β, and γ (NR3B1-3), which bind to both classic estrogen response elements (ERE) and extended half-site core sequences (TNAAGGTCA; ERR response element or ERRE) as either monomers or dimers [15]. Structural studies indicate that ERRγ is constitutively active in the absence of endogenous ligands, whereas small-molecule ligands can further activate or repress ERRγ transactivation [16–19]. The ligand-independent transcriptional activity of ERRγ depends on nuclear receptor co-regulators, such as NCoA-2, PGC-1α, receptor-interacting protein 140 (RIP140), and small heterodimer partner (SHP), and these co-regulators are involved in the regulation of liver metabolism [20–24]. ERRs are expressed in tissues with high metabolic demand and are regulated by the peripheral circadian clock in key metabolic tissues such as white and brown adipose tissues, muscle, and liver [25]. In a previous study, we showed that ERRγ regulates glucose metabolism by modulating phosphoenolpyruvate carboxykinase 1 (PEPCK) and glucose-6-phosphatase (G6Pase) gene expression, rate-limiting enzymes in glucose production [26, 27]. We also found that ERRγ participates in regulating pyruvate dehydrogenase kinase 4 (PDK4) gene expression and is a novel transcriptional regulator of phosphatidic acid phosphatase [28, 29]. Recently, we demonstrated that ERRγ is a transcriptional regulator of CYP7A1 gene expression and increases bile acid synthesis [30] and hepcidin expression [31]. We also showed that ERRγ controls hepatic CB1R–mediated CYP2E1 expression and oxidative liver injury upon alcohol use [32]. However, the role of ERRγ in liver metabolism is still not clear.
The endocannabinoid system comprises cannabinoid receptor types 1 and 2 (CB1R and CB2R). CB1R is expressed at high levels in the brain, but is also present at much lower concentrations in peripheral tissues, whereas CB2R is expressed predominantly in immune and hematopoietic cells [33]. Endocannabinoids (ECs) acting via CB1R play important roles in the control of body weight and energy homeostasis. Animal studies and clinical investigations in patient have shown that, in the obese state, the endocannabinoid system is hyperactivated because of impaired energy balance [34–36]. In obese or hyperglycemic type 2 diabetic patients, circulating levels of N-Arachidonoylethanolamine (AEA) and 2-Arachidonoylglycerol (2-AG) are elevated, and high levels of 2-AG are found in visceral adipose tissue [35, 37, 38]. Several studies indicate that inhibition of CB1R activity in peripheral tissues contributes to the metabolic benefits [36, 39], raising the possibility that selective targeting of peripheral CB1R could be used to treat metabolic syndrome. This concept is supported by recent studies, using a peripherally restricted neutral CB1R antagonist, AM6545[40], or a peripheral CB1R inverse agonist, JD5037 [41], in mice with high-fat diet–induced obesity (DIO). Our previous study revealed that activation of CB1R disrupts hepatic insulin receptor signaling via CREBH-mediated induction of Lipin1 gene expression [42]. In another study, we identified a novel mechanism of regulation of hepatic bile acid metabolism by alcohol via CB1R-mediated activation of ERRγ [30]. Together, these findings suggest that blocking the CB1R signaling pathway can restore hepatic metabolic homeostasis.
In this study, we identified the nuclear receptor ERRγ as a transcriptional regulator of hepatic fibrinogen gene expression. An increase in hepatic ERRγ gene expression led to the induction of fibrinogen, whereas ablation of hepatic ERRγ gene expression abolished this induction. Activation of hepatic CB1R signaling induced ERRγ-mediated transcription of the fibrinogen gene. GSK5182, a selective ERRγ inverse agonist, decreased ACEA-mediated induction of fibrinogen. Based on these findings, inhibition of the transcriptional activity of ERRγ by an inverse agonist may have the potential to ameliorate hyperfibrinogenemia.
Materials and methods
Clinical samples
Obese subjects were screened with physical examination for fatty liver (age range from 36 to 65 years with mean age of 56.06) in China from April to August 2011. BMI was calculated as weight (in kilograms) divided by the square of the height (in meters). Those who drank 140 or 70 g/week of alcohol (for men or women, respectively), at the time or in the previous 6 months of investigation were excluded from the study. HBV- or HCV-infected subjects were also excluded. For analyses of circulating fibrinogen levels, blood samples were collected from 30 obese patients with BMI >25 and 30 aged-matched normal subjects with BMI < 25. For analysis of hepatic ERRγ and fibrinogen mRNA expression, liver biopsy was performed in 16 obese subjects and 14 healthy subjects, who donated their partial livers for liver transplantation. Immunohistochemical staining was performed in liver tissue of 3 randomly selected patients with biopsy proven NASH. The baseline demographic characteristics of human subjects are described in S2 Table. Patient information was not revealed in this study, and the data were analyzed blindly. Acquisition and handling of patient materials were approved by the Ethical Committees of Shanghai Jiao Tong University School of Medicine and Medical Faculty of Mannheim at Heidelberg University (Az: 2007-011N-MA). We confirm that this study was conducted in accordance with the Declaration of Helsinki. None of the transplant donors were from a vulnerable population and all donors or next of kin provided written informed consent that was freely given.
Chemicals and plasmids
GSK5182 was synthesized as previously described with slight modifications[16]. ACEA and AM251 were purchased from Tocris Bioscience. The promoters of human FGA (-1.7 kb/+243 bp), FGB (-1.7 kb/+258 bp), and FGG (-1.5 kb/+233 bp, -1.2 kb/+233 bp, and -0.8 kb/+233 bp) were cloned into the XhoI/MluI sites of the PGL3-basic vector. These reporter plasmids were confirmed by DNA sequencing. Expression vectors for FLAG-ERRα, FLAG-ERRβ, and FLAG-ERRγ were described previously[27]. A mutation was introduced into the ERRE of the FGG promoter by site-directed mutagenesis (Stratagene, La Jolla, CA, USA), and the resultant construct (MT ERRE-luc) was confirmed by DNA sequencing.
Recombinant adenovirus
Ad-GFP, Ad-FLAG-ERRγ, Ad-USi and Ad-shERRγ were described previously [26]. All viruses were purified using CsCl2 or an Adeno-X maxi purification kit (Clontech, Palo Alto, CA, USA). Adenoviral infections in cells were described previously [30].
Cell culture and transient transfection assay
293T, HepG2 and Huh7 cells were maintained as described previously [28]. The cells were used for experiments at 80% confluence. Transient transfections were conducted as described previously [28]. Luciferase activity was normalized to β-galactosidase activity.
Measurement of fibrinogen level
Fibrinogen was extracted from cell culture medium and mouse blood, and its levels were determined using the Fibrinogen SimpleStep ELISA Kit (Abcam, Cambridge, MA, USA). Fibrinogen levels in human patients were analyzed by MILLIPLEX MAP Human Cardiovascular Disease (Acute Phase) Magnetic Bead Panel 3 (HCVD3MAG-67K, Milliplex Map, EMD Millipore, Billerica, MA, USA).
Real-time PCR and Western blot analysis
RT–PCR and western blot analysis were performed as described previously [28]. The following primary antibodies were used for the immunoblotting assay: β-actin (AbFrontier, Seoul, Korea), ERRγ (Perseus Proteomics, Tokyo, Japan), and fibrinogen (Dako, Carpinteria,CA, USA). All primer sequences are described in S1 Table.
Histological analysis
Liver tissues from patients with liver disease were fixed in 4% formaldehyde and embedded in paraffin. The slides were deparaffinized in xylene and rehydrated in a dilution series of graded ethanol to distilled water. Antigen retrieval was performed by microwave treatment in EDTA buffer (1 mmol/L, pH 8.0) for 10 minutes. The slides were incubated with 3% H2O2 for 30 minutes at room temperature. After washing with phosphate-buffered saline three times, slides were incubated with primary antibodies (1. ERRγ: Abcam; ab131593; 1:100 or 2. Fibrinogen: Abcam; ab58207; 1:100) at 4°C overnight. The next day, slides were washed with phosphate-buffered saline three times, followed by incubation with anti-mouse antibody HRP (Dako; 1:200) for 1 hour at room temperature. Color was developed with Diaminobenzidine (Sigma). Immunoreactivity was visualized under light microscopy.
ChIP assay
The ChIP assay was performed using a Chromatin Immunoprecipitation Assay Kit (Upstate Biotechnology, Lake Placid, NY, USA). Immunoprecipitation was performed using ERRγ antibody (Perseus Proteomics, Tokyo, Japan) or IgG (as a negative control). After recovering the DNA, polymerase chain reaction (PCR) was performed using primers encompassing the FGG promoter region. All primer sequences are described in S1 Table.
Statistics
Values are expressed as means ± standard error. Statistical significance was calculated using the unpaired Student’s t-test and one-way analysis of variance. Differences were considered significant at p<0.05.
Results
Overexpression of ERRγ induces fibrinogen gene expression
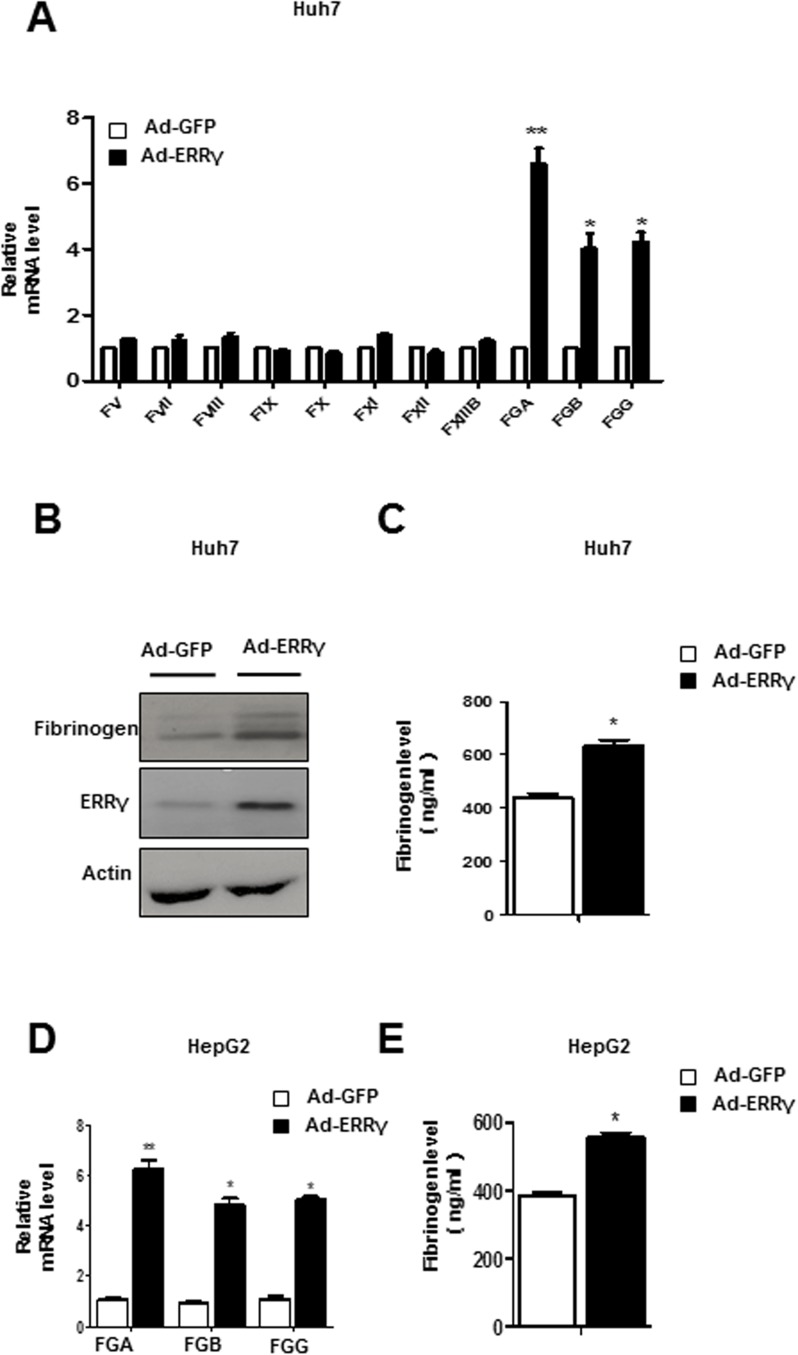
To define a functional link between ERRγ and coagulation factors gene expression, we overexpressed ERRγ using an adenovirus expressing ERRγ (Ad-ERRγ). Specifically, overexpression of ERRγ by Ad-ERRγ increased fibrinogen mRNA level in Huh7 and HepG2 cells (Fig 1A and 1D). A similar increase in fibrinogen protein level was also found in Ad-ERRγ infected Huh7 cell (Fig 1B). By contrast, overexpression of ERRγ had no significant effect on other coagulation factors in Huh7 cell line (Fig 1A). We also collected cell culture supernatant of Huh7 and HepG2 cells to measure levels of secreted fibrinogen, which were significantly increased upon overexpression of ERRγ (Fig 1C and 1E). Taken together, these results suggest that ERRγ can induce fibrinogen gene expression.
Fig 1. Overexpression of ERRγ induces fibrinogen gene expression.
(A-C) Huh7 cells were infected with Ad-GFP (control) or Ad-ERRγ. Total RNA and protein were isolated and used for qPCR (A) and western blot analyses (B). Cell culture media were collected to determine fibrinogen levels (C). Western blot images were cropped with a black cropping line. All gels for Western blot analysis were run under the same experimental conditions. Full uncropped blots are available as S1 Fig. (D-E) HepG2 cells were infected with Ad-GFP (control) or Ad-ERRγ. Total RNA were isolated and used for qPCR (D) Cell culture supernatants were collected to determine fibrinogen levels (E)* p<0.05, ** p<0.01. All data are representative of at least three independent experiments. Error bars show SEM.
Activation of the hepatic CB1 receptor induces fibrinogen expression
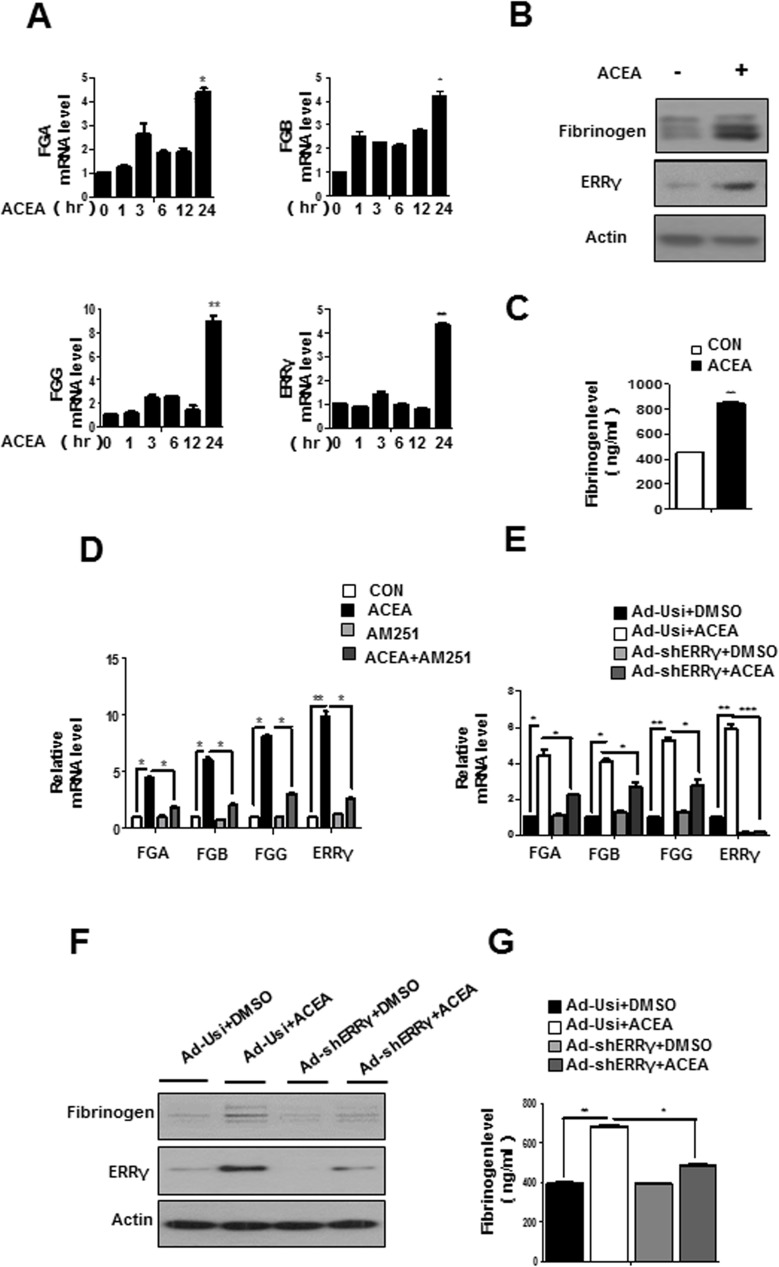
Knowing that the endocannabinoid system is hyperactivated in the obesity stage[35] and due to our previous study, where we showed that CB1R induces expression of ERRγ and its effect on modulating hepatic bile acid metabolism by alcohol [30], we speculated that CB1R might also involve in regulating fibrinogen expression in liver. We performed time course experiments to examine the induction of fibrinogen and ERRγ expression by ACEA, agonist of CB1R. ACEA significantly increased fibrinogen and ERRγ mRNA levels in Huh7 (Fig 2A), with maximal levels at 24 h. Consistent with the change in mRNA levels, fibrinogen and ERRγ protein levels were also elevated during ACEA treatment of Huh7 cells (Fig 2B). Moreover, ACEA treatment elevated fibrinogen level in the cell culture medium of Huh7 cells (Fig 2C).Then, AM251, a selective inverse agonist of CB1R, antagonized the effects of ACEA mediated induction of ERRγ and fibrinogen mRNA levels in Huh7 cells (Fig 2D). These results demonstrate that activation of the hepatic CB1 receptor increases ERRγ and fibrinogen expression at the mRNA and protein levels.
Fig 2. Knockdown of ERRγ attenuates ACEA-mediated induction of fibrinogen.
(A) ACEA-mediated induction of fibrinogen expression. Huh7 cells were treated with ACEA (10 μM) for the indicated time periods. Total RNAs were extracted for qPCR analyses. (B-C) Huh7 cells were treated with ACEA (10 μM) for 24 h. Total protein was extracted for western blotting (B). Cell culture media were collected to determine fibrinogen levels (C). * p<0.05, ** p<0.01. All data are representative of at least three independent experiments. Error bars show SEM. (D) Huh7 cells were treatment with ACEA in the continued presence or absence of AM251 for 24 h. qPCR were performed to measure mRNA levels. * p<0.05. ** p<0.01. (E-G) qPCR (E) and western blot (F) analysis showing mRNA and protein levels of ERRγ, FGA, FGB, and FGG in Huh7 cells. Huh7 cells were infected with Ad-Usi or Ad-shERRγ for 48 h, followed by treatment with ACEA (10 μM). Cell culture media were collected to determine fibrinogen levels (G).Western blot images were cropped with a black cropping line. All gels for Western blot analysis were run under the same experimental conditions. Full uncropped blots are available as S1 and S2 Figs.
To functionally strengthen the link between CB1 receptor signaling and ERRγ in hepatic regulation of fibrinogen gene expression, we examined the effect of ERRγ knockdown by adenoviral overexpression of ERRγ shRNA (Ad-shERRγ). ACEA treatment increased ERRγ and fibrinogen mRNA levels in Huh7 cells, whereas fibrinogen expression was inhibited by Ad-shERRγ (Fig 2E). Consistent with the fibrinogen mRNA level, the ACEA-induced increase in the fibrinogen protein level in Huh7 cells was decreased by Ad-shERRγ (Fig 2F). The fibrinogen levels in cell culture medium were increased 1.5-fold after ACEA treatment, and this effect was significantly diminished following knockdown of ERRγ by Ad-shERRγ (Fig 2G). These results suggest that ERRγ acts downstream of ACEA to mediate induction of fibrinogen gene expression.
ERRγ activates the fibrinogen gene promoter
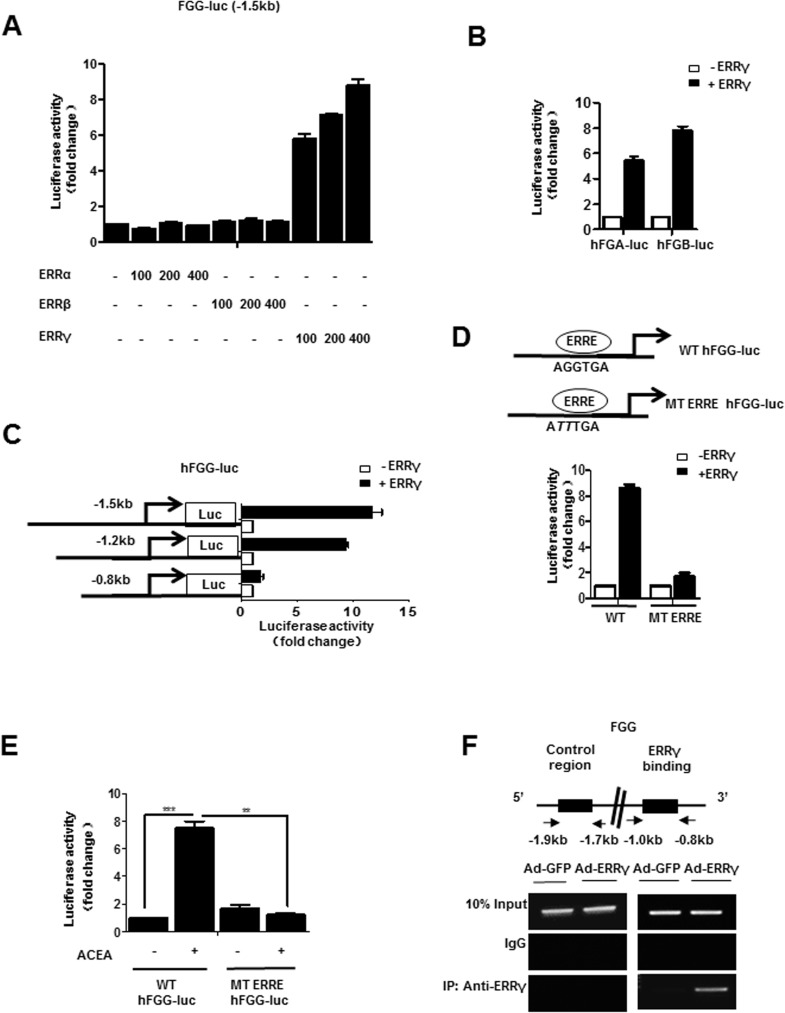
To determine the molecular mechanism by which ERRγ regulates fibrinogen gene transcription, we transfected the luciferase reporter construct driven by the human fibrinogen promoter into 293T cells. First, we examined the effect of the ERR subfamily on the human fibrinogen promoter. ERRγ specifically increased FGG promoter activity in a dose-dependent manner, whereas ERRα and ERRβ had no significant effect on the FGG promoter (Fig 3A). Further, co-transfection with ERRγ strongly induced FGA and FGB promoter activity (Fig 3B). In an approach using reporter assays with serial deletion constructs, we identified the DNA motif for the ERRγ effect to the region from -0.8 kb to -1.2 kb of the human FGG promoter, which upon deletion led to a marked decrease in ERRγ-mediated activation of the FGG promoter (Fig 3C). Moreover, close investigations of the human FGG promoter revealed a putative ERRγ binding motif (AGGTGA, as indicated by ERRE). To verify ERRγ binding site in the human FGG promoter, we performed transfection assays using the wild type and a promoter harboring a point mutation in the putative ERRγ binding site (ERRE). Activation of the human FGG promoter by ERRγ was significantly abolished by the ERRE mutation (Fig 3D). Human FGG promoter activity was increased more than 7-fold by ACEA treatment, whereas the ERRE-mutated promoter was unaffected (Fig 3E). These results suggest that ERRγ directly regulates fibrinogen gene transcription through the ERRE in the human promoter. Furthermore, binding of ERRγ to the endogenous FGG promoter was confirmed by ChIP assays with a specific antibody against ERRγ in Huh7 (Fig 3F). ERRγ was strongly recruited to the ERRE region of human FGG promoter, whereas no significant recruitment of ERRγ was observed in the control region. Overall, these results indicate that ERRγ directly binds and activates the fibrinogen gene promoter.
Fig 3. ERRγ directly regulates fibrinogen gene transcription.
(A) ERRγ-specific induction of human FGG promoter activity. 293T cells were transfected with vectors expressing human FGG-luc and ERRα, ERRβ, and ERRγ. (B) ERRγ induced human FGA and FGB promoters. 293T cells were transfected with vectors expressing human FGA-luc or FGB-luc and ERRγ. (C) Mapping of the human FGG promoter. 293T cells were transfected with deletion constructs of hFGG-luc and ERRγ. (D) ERRE-dependent activation of the human FGG promoter. 293T cells were transiently transfected with pCDNA3-FLAG-ERRγ, hFGG-luc (WT), hFGG-Luc (MT ERRE). (E) ERRE is required for ACEA-mediated activation of the human FGG promoter. Huh7 cells were transfected with vectors expressing hFGG-luc (WT) or hFGG-luc (MT ERRE) and treated with ACEA (10 μM) at 36 h post transfection. Experiments in A-E were conducted in triplicate, and data are expressed as fold activation relative to the control. * p<0.05, ** p<0.01, *** p<0.001. (F) ChIP assay showing occupancy of the ERRE from the human FGG promoter by ERRγ. Huh7 cells were infected with Ad-GFP or Ad-ERRγ for 48 h. Input represents 10% of purified DNA in each sample. Cell extracts were immunoprecipitated with IgG or ERRγ antibody, and purified DNA samples were employed for PCR with primers encompassing the ERRE (-1.0 kb to -0.8 kb) and a distal site (-1.9 kb to -1.7 kb) of the FGG gene promoter. Error bars show SEM. The gel images were cropped with a black cropping line. Full uncropped gels are available as S3 Fig. All gels for ChIP analysis were run under the same experimental conditions.
An inverse agonist of ERRγ inhibits fibrinogen gene expression in human hepatoma cell
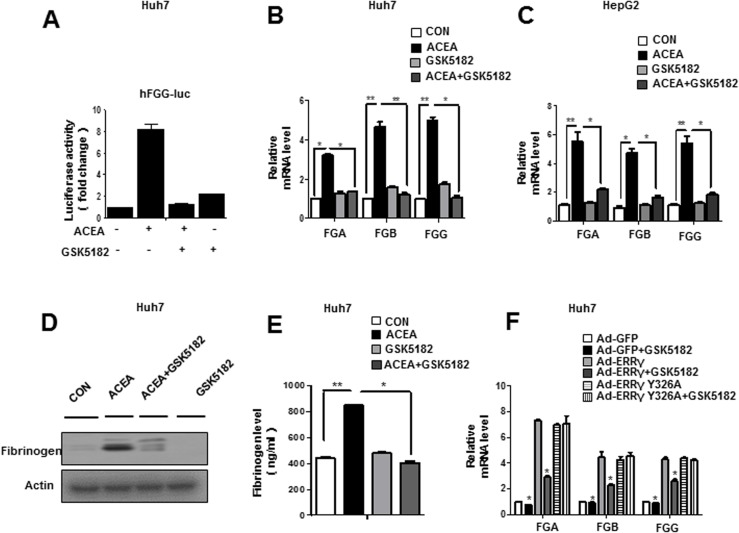
GSK5182, an ERRγ inverse agonist, has been used to selectively inhibit transactivation byERRγ[16]. To further clarify the role of ERRγ in CB1R-mediated induction of fibrinogen gene expression, cells were transfected with the fibrinogen promoter reporter construct and treated with ACEA in the presence or absence of GSK5182. ACEA-activated human FGG promoter activity (Fig 4A) and fibrinogen gene expression (Fig 4B and 4C) were inhibited by GSK5182 in Huh7 and HepG2 cells. Consistent with the change in fibrinogen mRNA level, ACEA-induced fibrinogen protein levels were also significantly decreased by GSK5182 in Huh7 (Fig 4D). In addition, fibrinogen levels in culture medium were dramatically increased after ACEA treatment, and this increase was significantly attenuated by GSK5182 treatment of Huh7 (Fig 4E). Finally, ERRγ-mediated induction of fibrinogen mRNA was significantly decreased by GSK5182 treatment in Huh7 cells (Fig 4F). Our previous results suggest that GSK5182 cannot bind to the ERRγ Y326A mutant [28]. Here, the effect of GSK5182 was abolished in the ERRγ Y326A mutant suggesting that GSK5182 specifically inhibits ERRγ transcriptional activity, eventually leading to reduced fibrinogen expression (Fig 4F). Taken together, these results indicate that inactivation of ERRγ with the inverse agonist GSK5182 decreases CB1 receptor–mediated fibrinogen gene expression.
Fig 4. Inverse agonist of ERRγ inhibits ACEA-mediated fibrinogen gene expression in Huh7 cells.
(A) GSK5182 decreased ACEA-mediated FGG promoter activity. Huh7 cells were transfected with vectors expression hFGG-luc, and then treated with ACEA (10 μM) and/or GSK5182 (10 μM). (B-E) GSK5182 inhibited ACEA-mediated fibrinogen expression and secretion in human hepatoma cell line. Huh7 and HepG2 cells were treated with ACEA (10 μM) for 12 h. The cell culture medium was replaced, and GSK5182 (10 μM) was added for the final 24 h. Total mRNA and protein were extracted for qPCR (B-C) and western blot analyses (D). Cell culture media were collected to determine fibrinogen levels in Huh7 cells (E). (F) GSK5182 specifically inhibits ERRγ transcriptional activity. Huh7 cells were infected with Ad-GFP, Ad-ERRγ, or Ad-ERRγ Y326A and then treated with GSK5182 (10 μM) for 24 h. Fibrinogen mRNA levels were analyzed by qPCR. * p<0.05, ** p<0.01. All data are representative of at least three independent experiments. Western blot images were cropped with a black cropping line. All gels for Western blot analysis were run under the same experimental conditions. Full uncropped blots are available as S2 Fig. Error bars show SEM.
Obese patients present with increased ERRγ and fibrinogen gene expression
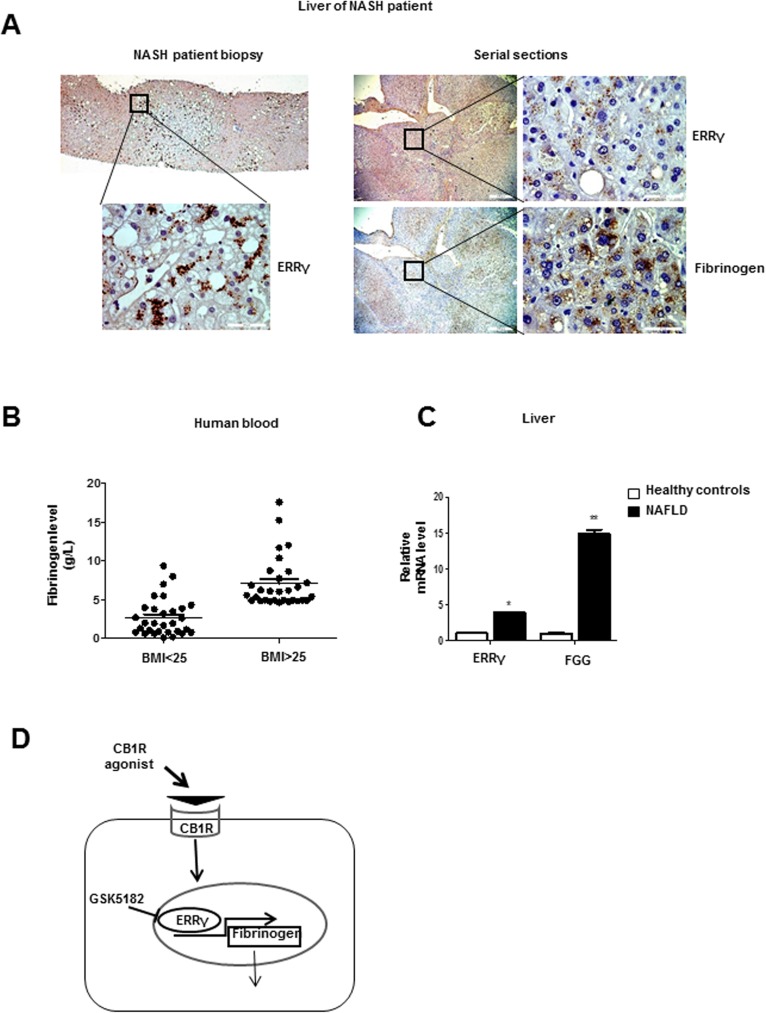
To translate the findings in vitro to human patients with fatty liver disease, we assessed ERRγ expression by immunohistochemistry and found a significant positive staining in three out of three nonalcoholic steatohepatitis (NASH) patients, where one representative result is shown (Fig 5A left). Analyses of serial sections from steatotic liver areas in addition indicate coregulation of ERRγ and Fibrinogen protein expression (Fig 5A right). To further investigate the association between obesity and fibrinogen levels, we analyzed liver tissues and blood samples from a larger cohort of patients with fatty liver disease and controls. Blood fibrinogen concentrations were higher in overweight subjects (BMI>25) than in subjects with normal weight (BMI<25) (Fig 5B). Furthermore, expression levels of ERRγ and FGG mRNAs were significantly higher in subjects with nonalcoholic fatty liver disease (NAFLD) than in those without NAFLD (Fig 5C). These results suggest that increased fibrinogen levels are related to overexpression of ERRγ in obese subjects.
Fig 5. Patients with NAFLD/NASH exhibit elevated ERRγ and fibrinogen expression in the liver.
(A) Left: representative immunohistochemistry results for ERRγ expression in liver tissue, as identically found in all three analysed patients with NASH. Right: serial sections of steatotic liver tissue showing colocalization of ERRγ and Fibrinogen staining. (B) Fibrinogen levels in blood of healthy controls and overweight patients. (C) qPCR analysis showing mRNA levels of hepatic fibrinogen gamma and ERRγ in liver tissue of healthy controls and patients with NAFLD. * p<0.05, ** p<0.01. (D) Proposed model for CB1 receptor–mediated induction of fibrinogen gene expression via ERRγ. Activation of hepatic CB1 receptor increases ERRγ gene expression, which in turn leads to fibrinogen expression causing hyperfibrinogenemia. GSK5182, an ERRγ inverse agonist, inhibits CB1 receptor–mediated fibrinogen gene expression.
Discussion
We focused our studies about regulation of fibrinogen expression on the fibrinogen γ-chain gene, because findings from most animal and cell culture studies indicate that the fibrinogen γ-chain gene is expressed at higher levels than the α- and β-chains. However, we cannot exclude the possibility that, under certain experimental conditions or in different species, regulation of fibrinogen α and β genes is equally important. For example, we observed that fibrinogen-α mRNA level was higher than the beta- and γ-chains when ERRγ was overexpressed in Huh7 cells (Fig 1A). However, ACEA treatment showed the strongest induction of fibrinogen-γ mRNA level in Huh7 (Fig 2A). Although Dufour et al. demonstrated that ERRα and ERRγ have common direct target genes, our results show that the fibrinogen promoter is activated by ERRγ, but not ERRα or ERRβ (Fig 3A), highlighting the functional complexity of the transcriptional signature of the ERR subfamily. Besides, a close investigation of FGA and FGB promoters revealed potential ERRγ binding sites as found in the FGG promoter, which could be more thoroughly investigated in future research.
Recent reports indicate that hepatic CB1 receptors play a major role in the obesity-related, weight-independent component of insulin resistance [39, 43]. Further, another study found that fibrinogen level correlates with insulin [44]. These indicate that the fibrinogen level have effects on blood glucose and diabetes. In addition, a previous study found that fibrinogen levels are significantly higher in obese 6-month-old Zucker rats than in lean age-matched controls, and that treatment with the CB1R antagonist significantly reduced fibrinogen binding in old obese Zucker rats[45]. In this study, our results show for the first time that activation of hepatic CB1R can induce fibrinogen expression via ERRγ in human hepatoma cell lines, and that knockdown ERRγ by Ad-shERRγ or the inverse agonist GSK5182 can inhibit ACEA-mediated induction of fibrinogen gene expression (Fig 4). These suggest that ERRγ-mediated induction of fibrinogen gene expression may be conserved in different species.
Therapeutic reduction of fibrinogen has attracted research attention since the causal link between plasma fibrinogen levels and the risk of CVD is well established, and several options for achieving this aim have been reported. Fibrinogen levels can be modulated by changes in lifestyle, of which smoking cessation is by far the most effective. Weight or stress reduction or an increase in regular physical activity may also be effective. Dietary changes appear to have a weaker effect, although regular, moderate alcohol consumption may result in a small reduction [46]. Many oral drugs decrease fibrinogen levels. Among them, the fibrates are most effective (e.g., bezafibrate reduces elevated fibrinogen by as much as 40%, and ticlopidine by about 15%) [47]. GSK5182, which is currently one of the leading ERRγ inverse agonists, shows promise for the development of drugs targeting ERRγ-related diseases. This study demonstrated that fibrinogen gene expression was reduced in a GSK5182-treated model, suggesting that this compound also provides a platform for the treatment of hyperfibrinogenemia (Fig 4).
NAFLD has been traditionally regarded as the consequence of a high-fat western diet and sedentary lifestyle[48, 49]. CVD still is one major cause of mortality in NAFLD patients[48, 50, 51]. Recent research shows that of 976 inpatients and 4,742 outpatients with NAFLD, 30% had CVD or metabolic syndrome conditions and 12% presented with cirrhosis [50]. In an attempt to directly link the phenotype of NAFLD to CVD risk factors, Yeung and coworkers used metabolic nutrient overload in hepatoblastoma C3A cells and identified up-regulation of all three fibrinogen component subunits of the coagulation cascade as critical molecular link [51]. In the present study, we confirmed elevated expression of fibrinogen in liver tissue from patients with NAFLD, and that the concentration of fibrinogen correlated with BMI (Fig 5B). This is consistent with the findings of the Ditschuneit group [52], who demonstrated that body weight gain increases the fibrinogen concentration and extremely overweight patients have higher fibrinogen levels, whereas weight loss is correlated with lower fibrinogen levels.
Overall, our results reveal that ERRγ plays a significant role in CB1 receptor–mediated fibrinogen gene expression. ERRγ binds to ERRE and activates human fibrinogen gene transcription. GSK5182, an ERRγ inverse agonist, resulted in a marked reduction of ERRγ-induced fibrinogen gene expression (Fig 5D). The CB1R signaling pathway is activated following induction and activation of ERRγ, which plays a significant role in up-regulating fibrinogen. Therefore, targeting ERRγ represents a promising therapeutic strategy for ameliorating hyperfibrinogenemia and therewith is expected to reduce CVD risk in fatty liver patients.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank our laboratory members for cooperation and discussion related to this work. We thank Dr. David D. Moore and Dr. Seok-Yong Choi for their careful and critical reading of this work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Creative Research Initiatives Grant (20110018305) through the National Research Foundation of Korea (NRF) funded by the Korean government (Ministry of Science, ICT, & Future Planning); and Federal Ministry of Education and Research grant LiSyM (S.D., Grant PTJ-FKZ: 031L0043). B.D. was supported by a German Egyptian Research Long-Term Scholarship of DAAD/Egypt government.
References
- 1.Stamler J. Overweight, hypertension, hypercholesterolemia and coronary heart disease. Medical Complications of Obesity. 1979:191–216. [Google Scholar]
- 2.Stone MC, Thorp JM. Plasma fibrinogen—a major coronary risk factor. J R Coll Gen Pract. 1985;35(281):565–9. ; PubMed Central PMCID: PMC1961456. [PMC free article] [PubMed] [Google Scholar]
- 3.Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984;311(8):501–5. doi: 10.1056/NEJM198408233110804 . [DOI] [PubMed] [Google Scholar]
- 4.Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–9. doi: 10.1016/S0065-3233(05)70008-5 [DOI] [PubMed] [Google Scholar]
- 5.Tennent GA, Brennan SO, Stangou AJ, O'Grady J, Hawkins PN, Pepys MB. Human plasma fibrinogen is synthesized in the liver. Blood. 2007;109(5):1971–4. doi: 10.1182/blood-2006-08-040956 [DOI] [PubMed] [Google Scholar]
- 6.Fuss C, Palmaz JC, Sprague EA. Fibrinogen: structure, function, and surface interactions. J Vasc Interv Radiol. 2001;12(6):677–82. . [DOI] [PubMed] [Google Scholar]
- 7.Hall CE, Slayter HS. The fibrinogen molecule: its size, shape, and mode of polymerization. J Biophys Biochem Cytol. 1959;5(1):11–6. ; PubMed Central PMCID: PMC2224630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller LL, Bly CG, Watson ML, Bale WF. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J Exp Med. 1951;94(5):431–53. ; PubMed Central PMCID: PMC2180336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollman JM, Pandi L, Sawaya MR, Riley M, Doolittle RF. Crystal structure of human fibrinogen. Biochemistry. 2009;48(18):3877–86. doi: 10.1021/bi802205g . [DOI] [PubMed] [Google Scholar]
- 10.Doolittle RF, Spraggon G, Everse SJ. Three-dimensional structural studies on fragments of fibrinogen and fibrin. Curr Opin Struct Biol. 1998;8(6):792–8. . [DOI] [PubMed] [Google Scholar]
- 11.Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117(18):4953–63. doi: 10.1182/blood-2010-11-316885 ; PubMed Central PMCID: PMC3100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauvet C, Bois-Joyeux B, Fontaine C, Gervois P, Bernard MA, Staels B, et al. The gene encoding fibrinogen-beta is a target for retinoic acid receptor-related orphan receptor alpha. Mol Endocrinol. 2005;19(10):2517–26. doi: 10.1210/me.2005-0153 [DOI] [PubMed] [Google Scholar]
- 13.Anisfeld AM, Kast-Woelbern HR, Lee H, Zhang Y, Lee FY, Edwards PA. Activation of the nuclear receptor FXR induces fibrinogen expression: a new role for bile acid signaling. J Lipid Res. 2005;46(3):458–68. doi: 10.1194/jlr.M400292-JLR200 . [DOI] [PubMed] [Google Scholar]
- 14.Gervois P, Vu-Dac N, Kleemann R, Kockx M, Dubois G, Laine B, et al. Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor alpha agonists via inhibition of CCAAT box/enhancer-binding protein beta. J Biol Chem. 2001;276(36):33471–7. doi: 10.1074/jbc.M102839200 . [DOI] [PubMed] [Google Scholar]
- 15.Razzaque MA, Masuda N, Maeda Y, Endo Y, Tsukamoto T, Osumi T. Estrogen receptor-related receptor gamma has an exceptionally broad specificity of DNA sequence recognition. Gene. 2004;340(2):275–82. doi: 10.1016/j.gene.2004.07.010 . [DOI] [PubMed] [Google Scholar]
- 16.Chao EY, Collins JL, Gaillard S, Miller AB, Wang L, Orband-Miller LA, et al. Structure-guided synthesis of tamoxifen analogs with improved selectivity for the orphan ERRgamma. Bioorg Med Chem Lett. 2006;16(4):821–4. doi: 10.1016/j.bmcl.2005.11.030 . [DOI] [PubMed] [Google Scholar]
- 17.Coward P, Lee D, Hull MV, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc Natl Acad Sci U S A. 2001;98(15):8880–4. doi: 10.1073/pnas.151244398 ; PubMed Central PMCID: PMC37529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greschik H, Wurtz JM, Sanglier S, Bourguet W, van Dorsselaer A, Moras D, et al. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol Cell. 2002;9(2):303–13. . [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Zuercher WJ, Consler TG, Lambert MH, Miller AB, Orband-Miller LA, et al. X-ray crystal structures of the estrogen-related receptor-gamma ligand binding domain in three functional states reveal the molecular basis of small molecule regulation. J Biol Chem. 2006;281(49):37773–81. doi: 10.1074/jbc.M608410200 . [DOI] [PubMed] [Google Scholar]
- 20.Hentschke M, Susens U, Borgmeyer U. PGC-1 and PERC, coactivators of the estrogen receptor-related receptor gamma. Biochem Biophys Res Commun. 2002;299(5):872–9. . [DOI] [PubMed] [Google Scholar]
- 21.Herzog B, Hallberg M, Seth A, Woods A, White R, Parker MG. The nuclear receptor cofactor, receptor-interacting protein 140, is required for the regulation of hepatic lipid and glucose metabolism by liver X receptor. Mol Endocrinol. 2007;21(11):2687–97. doi: 10.1210/me.2007-0213 ; PubMed Central PMCID: PMC2140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong H, Yang L, Stallcup MR. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem. 1999;274(32):22618–26. . [DOI] [PubMed] [Google Scholar]
- 23.Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277(43):40265–74. doi: 10.1074/jbc.M206324200 . [DOI] [PubMed] [Google Scholar]
- 24.Sanyal S, Kim JY, Kim HJ, Takeda J, Lee YK, Moore DD, et al. Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J Biol Chem. 2002;277(3):1739–48. doi: 10.1074/jbc.M106140200 . [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126(4):801–10. doi: 10.1016/j.cell.2006.06.050 . [DOI] [PubMed] [Google Scholar]
- 26.Kim DK, Gang GT, Ryu D, Koh M, Kim YN, Kim SS, et al. Inverse agonist of nuclear receptor ERRgamma mediates antidiabetic effect through inhibition of hepatic gluconeogenesis. Diabetes. 2013;62(9):3093–102. doi: 10.2337/db12-0946 ; PubMed Central PMCID: PMC3749343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DK, Ryu D, Koh M, Lee MW, Lim D, Kim MJ, et al. Orphan nuclear receptor estrogen-related receptor gamma (ERRgamma) is key regulator of hepatic gluconeogenesis. J Biol Chem. 2012;287(26):21628–39. doi: 10.1074/jbc.M111.315168 ; PubMed Central PMCID: PMC3381127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DK, Kim JR, Koh M, Kim YD, Lee JM, Chanda D, et al. Estrogen-related receptor gamma (ERRgamma) is a novel transcriptional regulator of phosphatidic acid phosphatase, LIPIN1, and inhibits hepatic insulin signaling. J Biol Chem. 2011;286(44):38035–42. doi: 10.1074/jbc.M111.250613 ; PubMed Central PMCID: PMC3207427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Kim EJ, Kim DK, Lee JM, Park SB, Lee IK, et al. Hypoxia induces PDK4 gene expression through induction of the orphan nuclear receptor ERRgamma. PLoS One. 2012;7(9):e46324 doi: 10.1371/journal.pone.0046324 ; PubMed Central PMCID: PMC3457976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Kim DK, Lee JM, Park SB, Jeong WI, Kim SH, et al. Orphan nuclear receptor oestrogen-related receptor gamma (ERRgamma) plays a key role in hepatic cannabinoid receptor type 1-mediated induction of CYP7A1 gene expression. Biochem J. 2015;470(2):181–93. doi: 10.1042/BJ20141494 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DK, Jeong JH, Lee JM, Kim KS, Park SH, Kim YD, et al. Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat Med. 2014;20(4):419–24. doi: 10.1038/nm.3483 . [DOI] [PubMed] [Google Scholar]
- 32.Kim DK, Kim YH, Jang HH, Park J, Kim JR, Koh M, et al. Estrogen-related receptor gamma controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut. 2013;62(7):1044–54. doi: 10.1136/gutjnl-2012-303347 ; PubMed Central PMCID: PMC3812689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2 ; PubMed Central PMCID: PMC2241751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–5. doi: 10.1038/35071088 . [DOI] [PubMed] [Google Scholar]
- 35.Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91(8):3171–80. doi: 10.1210/jc.2005-2679 . [DOI] [PubMed] [Google Scholar]
- 36.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115(5):1298–305. doi: 10.1172/JCI23057 ; PubMed Central PMCID: PMC1087161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55(11):3053–60. doi: 10.2337/db06-0812 ; PubMed Central PMCID: PMC2228260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54(10):2838–43. ; PubMed Central PMCID: PMC2228268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118(9):3160–9. doi: 10.1172/JCI34827 ; PubMed Central PMCID: PMC2491458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120(8):2953–66. doi: 10.1172/JCI42551 ; PubMed Central PMCID: PMC2912197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16(2):167–79. doi: 10.1016/j.cmet.2012.07.002 ; PubMed Central PMCID: PMC3832894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chanda D, Kim YH, Kim DK, Lee MW, Lee SY, Park TS, et al. Activation of cannabinoid receptor type 1 (Cb1r) disrupts hepatic insulin receptor signaling via cyclic AMP-response element-binding protein H (Crebh)-mediated induction of Lipin1 gene. J Biol Chem. 2012;287(45):38041–9. doi: 10.1074/jbc.M112.377978 ; PubMed Central PMCID: PMC3488074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C. Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes. 2011;60(4):1055–62. doi: 10.2337/db10-0962 ; PubMed Central PMCID: PMC3064079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raynaud E, Perez-Martin A, Brun J, Aissa-Benhaddad A, Fedou C, Mercier J. Relationships between fibrinogen and insulin resistance. Atherosclerosis. 2000;150(2):365–70. . [DOI] [PubMed] [Google Scholar]
- 45.Schafer A, Pfrang J, Neumuller J, Fiedler S, Ertl G, Bauersachs J. The cannabinoid receptor-1 antagonist rimonabant inhibits platelet activation and reduces pro-inflammatory chemokines and leukocytes in Zucker rats. Br J Pharmacol. 2008;154(5):1047–54. doi: 10.1038/bjp.2008.158 ; PubMed Central PMCID: PMC2451057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Folsom AR. Epidemiology of fibrinogen. Eur Heart J. 1995;16 Suppl A:21–3; discussion 3–4. . [DOI] [PubMed] [Google Scholar]
- 47.Kockx M, Gervois PP, Poulain P, Derudas B, Peters JM, Gonzalez FJ, et al. Fibrates suppress fibrinogen gene expression in rodents via activation of the peroxisome proliferator-activated receptor-alpha. Blood. 1999;93(9):2991–8. . [PubMed] [Google Scholar]
- 48.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466 . [DOI] [PubMed] [Google Scholar]
- 49.Feldstein AE. Novel Insights into the Pathophysiology of Nonalcoholic Fatty Liver Disease. Seminars In Liver Disease. 2010;30(4):391–401. doi: 10.1055/s-0030-1267539 [DOI] [PubMed] [Google Scholar]
- 50.Sayiner M, Otgonsuren M, Cable R, Younossi I, Afendy M, Golabi P, et al. Variables Associated With Inpatient and Outpatient Resource Utilization Among Medicare Beneficiaries With Nonalcoholic Fatty Liver Disease With or Without Cirrhosis. J Clin Gastroenterol. 2016. doi: 10.1097/MCG.0000000000000567 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeung ENW, Treskes P, Martin SF, Manning JR, Dunbar DR, Rogers SM, et al. Fibrinogen production is enhanced in an in-vitro model of non-alcoholic fatty liver disease: an isolated risk factor for cardiovascular events? (vol 14, 86, 2015). Lipids In Health And Disease. 2016;15 doi: 10.1186/S12944-016-0290-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ditschuneit HH, Flechtner-Mors M, Adler G. Fibrinogen in obesity before and after weight reduction. Obes Res. 1995;3(1):43–8. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.