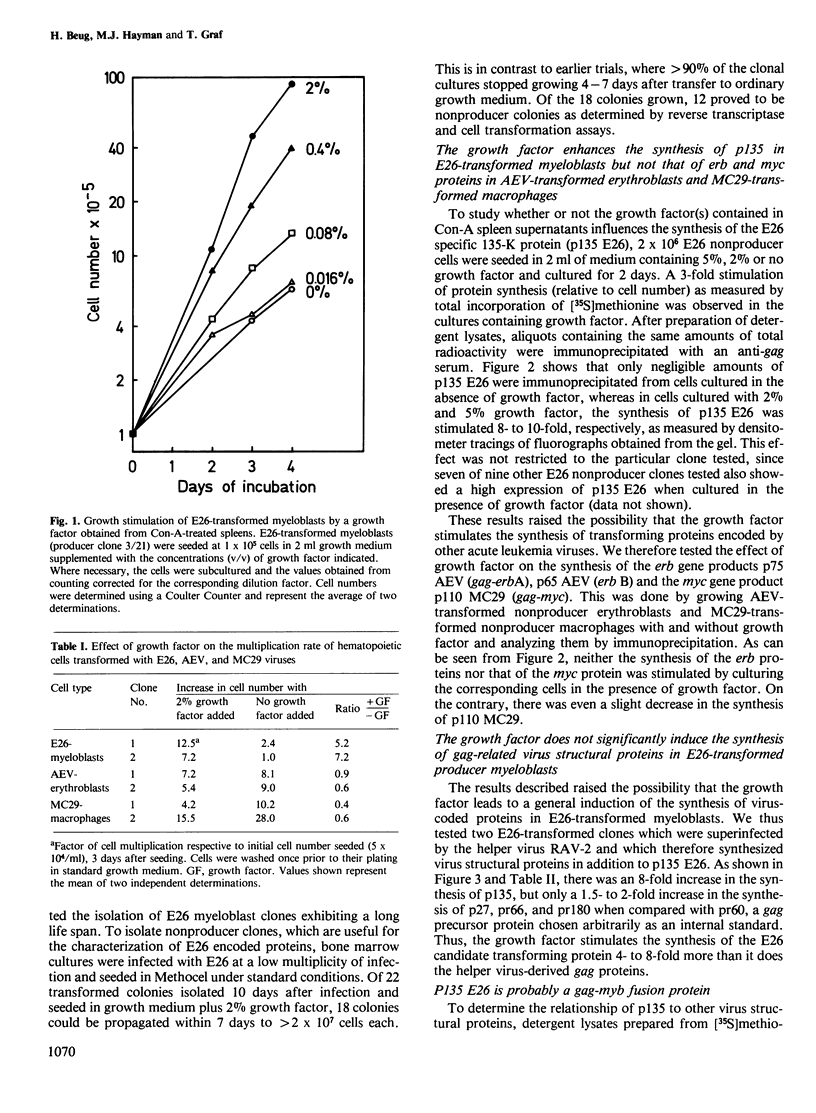
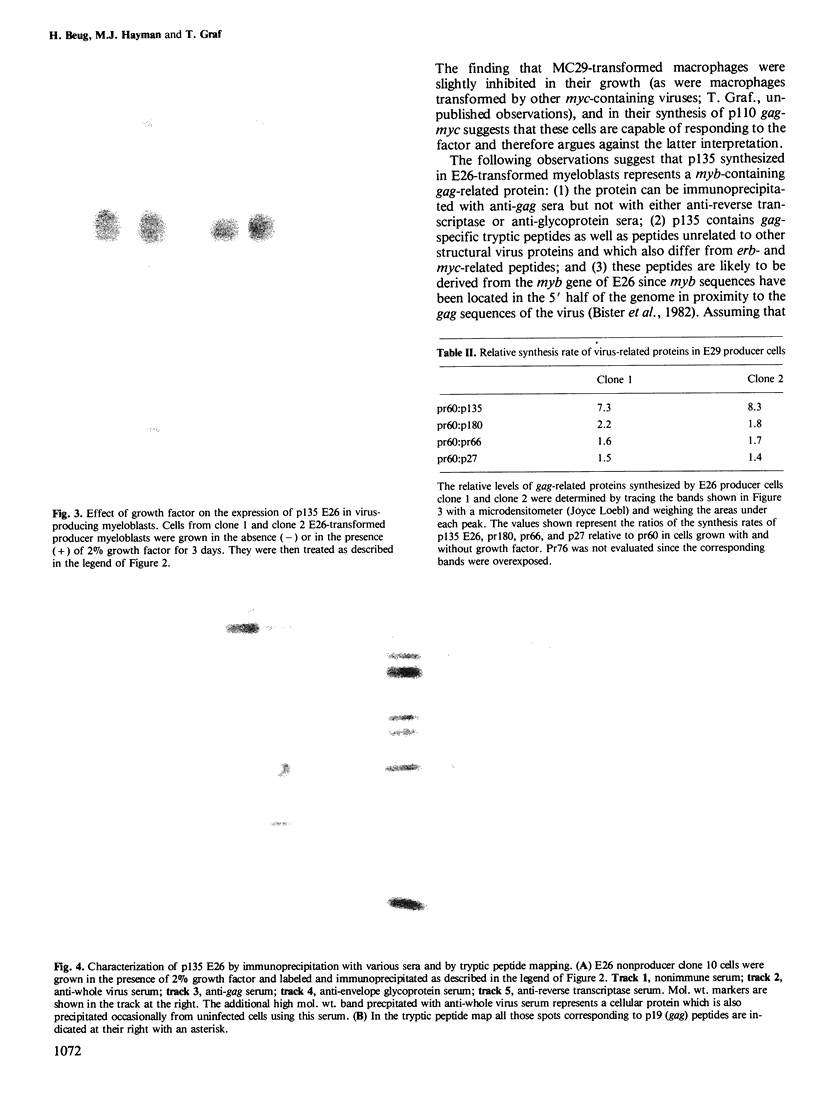
Abstract
Avian leukemia virus E26 contains the myb oncogene and transforms erythroid and myeloid hematopoietic cells in vivo and in vitro. E26-transformed nonproducer myeloblasts but not avian erythroleukemia virus (AEV)-transformed erythroblasts nor MC29-transformed macrophages were shown to be dependent for growth on factor(s) present in supernatants from Concanavalin A-stimulated chicken spleen cells. The same factor enhanced the synthesis of p135 E26, the candidate transforming protein of E26, but did not induce the synthesis of the transforming proteins of AEV and MC29 viruses nor that of helper virus-derived structural proteins. P135 E26 was shown to contain sequences related to the viral gag gene as well as sequences which may be related to the myb gene product. P135 E26 might constitute the first example of a viral onc protein whose synthesis is regulated directly or indirectly by an exogenous hematopoietic growth factor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Graf T., Hayman M. J. Production and characterization of antisera specific for the erb-portion of p75, the presumptive transforming protein of avian erythroblastosis virus. Virology. 1981 May;111(1):201–210. doi: 10.1016/0042-6822(81)90665-6. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bister K., Nunn M., Moscovici C., Perbal B., Baluda M., Duesberg P. H. Acute leukemia viruses E26 and avian myeloblastosis virus have related transformation-specific RNA sequences but different genetic structures, gene products, and oncogenic properties. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3677–3681. doi: 10.1073/pnas.79.12.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. Expression of endogenous avian myeloblastosis virus information in different chicken cells. J Virol. 1980 Oct;36(1):162–170. doi: 10.1128/jvi.36.1.162-170.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Beug H., von Kirchbach A., Hayman M. J. Three new types of viral oncogenes in defective avian leukemia viruses. II. Biological, genetic, and immunochemical evidence. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1225–1234. doi: 10.1101/sqb.1980.044.01.133. [DOI] [PubMed] [Google Scholar]
- Graf T., Oker-Blom N., Todorov T. G., Beug H. Transforming capacities and defectiveness of avian leukemia viruses OK10 and E 26. Virology. 1979 Dec;99(2):431–436. doi: 10.1016/0042-6822(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Graf T., von Kirchbach A., Beug H. Characterization of the hematopoietic target cells of AEV, MC29 and AMV avian leukemia viruses. Exp Cell Res. 1981 Feb;131(2):331–343. doi: 10.1016/0014-4827(81)90236-6. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Kitchener G., Graf T. Cells transformed by avian myelocytomatosis virus strain CMII contain a 90K gag-related protein. Virology. 1979 Oct 15;98(1):191–199. doi: 10.1016/0042-6822(79)90537-3. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Hayman M. J. Transforming proteins of avian retroviruses. J Gen Virol. 1981 Jan;52(Pt 1):1–14. doi: 10.1099/0022-1317-52-1-1. [DOI] [PubMed] [Google Scholar]
- Hayman M. J. Viral polyproteins in chick embryo fibroblasts infected with avian sarcoma leukosis viruses. Virology. 1978 Mar;85(1):241–252. doi: 10.1016/0042-6822(78)90428-2. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kitchener G., Hayman M. J. Comparative tryptic peptide mapping studies suggest a role in cell transformation for the gag-related protein of avian erythroblastosis virus and avian myelocytomatosis virus strains CMII and MC29. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1637–1641. doi: 10.1073/pnas.77.3.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Samarut J., Gazzolo L., Moscovici M. G. Myeloid and erythroid neoplastic responses to avian defective leukemia viruses in chickens and in quail. Virology. 1981 Sep;113(2):765–768. doi: 10.1016/0042-6822(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Rushlow K. E., Lautenberger J. A., Papas T. S., Baluda M. A., Perbal B., Chirikjian J. G., Reddy E. P. Nucleotide sequence of the transforming gene of avian myeloblastosis virus. Science. 1982 Jun 25;216(4553):1421–1423. doi: 10.1126/science.6283631. [DOI] [PubMed] [Google Scholar]
- Samarut J., Bouabdelli M. In vitro development of CFU-E and BFU-E in cultures of embryonic and post-embryonic chicken hematopoietic cells. J Cell Physiol. 1980 Dec;105(3):553–563. doi: 10.1002/jcp.1041050320. [DOI] [PubMed] [Google Scholar]
- Sotirov N. Histone H5 in the immature blood cells of chickens with leukosis induced by avian leukosis virus strain E26. J Natl Cancer Inst. 1981 Jun;66(6):1143–1149. doi: 10.1093/jnci/66.6.1143. [DOI] [PubMed] [Google Scholar]
- Stéhelin D., Saule S., Roussel M., Sergeant A., Lagrou C., Rommens C., Raes M. B. Three new types of viral oncogenes in defective avian leukemia viruses. I. Specific nucleotide sequences of cellular origin correlate with specific transformation. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1215–1223. doi: 10.1101/sqb.1980.044.01.132. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]