Abstract
Background and Objectives
Smoking rates are markedly higher among trauma-exposed individuals relative to non-trauma exposed individuals. Extant work suggests that both perceived stress and negative affect reduction smoking expectancies are independent mechanisms that link trauma-related symptoms and smoking. Yet, no work has examined perceived stress and negative affect reduction smoking expectancies as potential explanatory variables for the relation between trauma-related symptom severity and smoking in a sequential pathway model.
Methods
Thus, the present study utilized a sample of treatment-seeking, trauma-exposed smokers (n = 363; 49.0% female) to examine perceived stress and negative affect reduction expectancies for smoking as potential sequential explanatory variables linking trauma-related symptom severity and nicotine dependence, perceived barriers to smoking cessation, and severity of withdrawal-related problems and symptoms during past quit attempts.
Results
As hypothesized, perceived stress and negative affect reduction expectancies had a significant sequential indirect effect on trauma-related symptom severity and criterion variables.
Conclusions and Scientific Significance
Findings further elucidate the complex pathways through which trauma-related symptoms contribute to smoking behavior and cognitions, and highlight the importance of addressing perceived stress and negative affect reduction expectancies in smoking cessation programs among trauma-exposed individuals.
Keywords: Trauma, Tobacco, Perceived Stress, Smoking, Smoking Expectancies
Trauma exposure is alarmingly high among the general population.1 Trauma-exposed persons are at greater risk for developing nicotine dependence relative to non-trauma exposed persons. Compared to individuals not exposed to a traumatic event, trauma-exposed individuals (with or without psychopathology) are more likely to be smokers, smoke more heavily, have higher levels of nicotine dependence, and show poorer outcomes during quit attempts.2 Trauma-exposed smokers are also particularly motivated to smoke to reduce negative affect and expect that smoking will reduce it,3 perhaps owing to the fact that smoking appears to temporarily relieve distress among such persons.4 Despite the documented co-occurrence and clinically significant relations between trauma exposure and smoking, there is little understanding of the pathways underlying the trauma symptoms-smoking relation.
One mechanism linking trauma-related symptom severity and smoking is perceived stress.5 Perceived stress reflects perceptions of global life stress6 and is unique from negative affect because it taps into the stress appraisal process.7 Biologically, smoking alters stress management systems, including the hypothalamic-pituitary-adrenal axis and the autonomic nervous system, which subsequently contributes to smoking maintenance and more difficulty quitting.8 Among trauma-exposed smokers, perceived stress appears to be related to greater trauma-related symptom severity.5 Indeed, greater perceived stress serves as a linking mechanism between trauma-related symptom severity and problematic smoking cognition and behavior, including inflexibility/avoidance in the presence of aversive smoking-related thoughts, feelings, or internal sensations, perceived barriers to smoking cessation, and negative affect reduction smoking expectancies.5
In addition to perceived stress, expectancies that smoking will alleviate negative affective symptoms may play an important role in understanding complex pathways through which trauma-related symptom severity and perceived stress contributes to problematic smoking. In models linking perceived stress to smoking, negative affect reduction smoking expectancies appears to serve as an underlying mechanism9 and have been implicated as a secondary explanatory variable in pathways through which trauma-related symptoms contributes to more problematic smoking.10 Specifically, number of trauma types relate to smoking through a sequential pathway from negative affect to negative affect reduction smoking expectancies among trauma-exposed smokers.10 This empirical research provides further evidence that trauma-exposed smokers may use smoking as a means to manage stressful negative mood states, which in turn, reinforces expectancies that smoking can relieve negative affect. Yet, work has not yet examined the sequential pathways through which trauma-related symptom severity may contribute to greater perceived stress, which then leads to increased negative affect reduction smoking expectancies, and ultimately, more maladaptive smoking cognitions and behavior.
Trauma-exposed persons who experience greater levels of trauma-related symptom severity may be prone to higher levels of perceived stress.11 Drawing from self-regulation and coping theories for tobacco use,12,13 trauma-exposed smokers with higher levels of perceived stress may expect that smoking will lessen their experiential discomfort. Indeed, empirical evidence supports this theoretical model5. Thus, robust theoretical and empirical data demonstrate an effect of perceived stress on increased negative reduction smoking expectancies among trauma-exposed smokers. This pathway may subsequently relate to more problematic smoking behavior (e.g., greater nicotine dependence and quitting problems) among trauma-exposed smokers. In the context of this framework, the current study sought to examine the perceived stress and negative reduction smoking expectancies as sequential explanatory variables in the trauma-related symptom severity-smoking relation. It was hypothesized that, among adults, treatment-seeking trauma-exposed daily smokers trauma-related symptom severity would contribute to greater perceived stress. Subsequently, greater perceived stress would relate to increased expectancies that smoking would reduce negative affect, which would be associated with (1) greater nicotine dependence; (2) more perceived barriers to smoking cessation; and (3) more severe withdrawal-related problems and symptoms during past quit attempts.
METHODS
Procedure
Data for the present study was collected during a large, multi-site randomized controlled clinical trial examining the efficacy of two smoking cessation interventions described in detail elsewhere.14 Participants were recruited at two sites (Vermont, Florida). Interested persons responding to community-based advertisements (e.g., flyers, newspaper ads, radio announcements) contacted the research team and were provided with a detailed description of the study via phone. Participants were then screened for initial eligibility, and if eligible, scheduled for an appointment at the University of Vermont or Florida State University; depending on which site they were recruited. After providing written informed consent, participants were interviewed using the SCID-I/NP and completed a computerized self-report assessment battery as well as biochemical verification of smoking status. Eligibility of the randomized controlled trial included (a) being between ages 18–65, (b) reporting smoking eight or more cigarettes per day, and (c) reporting motivation to quit rated as at least 5 or higher on a 10-point scale. Participants eligible for the larger trial were randomly assigned to one of two smoking cessation treatment: (a) Smoking Cessation Program or (b) Panic-Smoking Prevention Program. Participants were compensated $12.50 for completing the baseline visit and an additional $25 if they completed all treatment sessions. Data collection began in 2007 and concluded in 2014. The study protocol was approved by the Institutional Review Boards at the University of Vermont and Florida State University (clinicaltrials.gov # NCT01753141); all study procedures and treatment of human subjects were conducted in compliance with ethical standards of the American Psychological Association. The current study is based on secondary analyses of baseline (pre-treatment) data for a sub-set of the sample.
Participants
Participants were treatment-seeking, trauma-exposed, adult smokers recruited at two sites (Vermont and Florida) as part of a larger study designed to evaluate the efficacy of two smoking cessation interventions.14 Eligibility in the current study included (a) being between ages 18–65 and (b) having experienced at least one of the DSM-IV15 13 traumatic event types (e.g., “natural disaster,” “sexual or non-sexual assault by a stranger”), including an “other” category.16 Exclusion criteria included current suicidality and psychosis.
Measures
Demographics Questionnaire
Demographic information collected included gender, age, race, educational level, and marital status.
Structured Clinical Interview-Non Patient Version for DSM-IV (SCID-I/NP)
Diagnostic assessments of past year Axis I psychopathology were conducted using the SCID-I/NP.17 All SCID-I/NP interviews were administered by trained research assistants or doctoral-level staff and supervised by independent doctoral-level professionals. Interviews were audiotaped and the reliability of a random selection of 12.5% of interviews was checked for accuracy; no cases of (diagnostic coding) disagreement were noted.
Medical History Form
A medical history checklist was used to assess medical problems. As in past work,18,19 a composite variable was computed as an index of tobacco-related medical problems (labeled ‘Health’). Specifically, items participants indicated being diagnosed (heart problems, hypertension, respiratory disease, or asthma; all coded 0 [no] or 1 [yes]) were summed and a total score was created, with greater scores reflecting the occurrence of multiple markers of tobacco-related disease.
Smoking History Questionnaire (SHQ)
The SHQ20 is a self-report questionnaire used to assess smoking history, pattern, and problematic symptoms experienced during past quit attempts (e.g., “Think about your smoking during the last week, how many cigarettes did you smoke on an average day?”). The SHQ was used to describe the sample smoking history. Additionally, as is in past work,18 a mean composite score of severity of problem symptoms experienced during past quit attempts (e.g., nausea, headaches, irritability, anxiety) was derived and served as a criterion variable.
Posttraumatic Diagnostic Scale (PDS)
The PDS16 is a 49-item self-report instrument that assesses trauma exposure and the presence of posttraumatic stress symptoms (referred to as trauma-related symptoms in the current article) based on DSM-IV criteria.15 Respondents report if they have experienced any of 13 traumatic event types (e.g., “natural disaster,” “sexual or non-sexual assault by a stranger”), including an “other” category, and then indicate which was most disturbing. In the current study, only participants who reported at least one lifetime traumatic event were included. Participants report the frequency of 17 past-month PTSD symptoms for the most disturbing event endorsed according to a scale ranging from 0 (not at all/only once) to 3 (5 or more times a week/almost always). The PDS has evidenced excellent psychometric properties,21 including excellent internal consistency (α = .92) and good test-retest reliability (kappa = .74). The current study utilized the total score (α = .92).
Perceived Stress Scale (PSS)
The PSS7 is a 14-item scale that measures the degree to which situations in one's life are appraised as stressful during the past month on a 0 (never) to 4 (very often) scale. Item content reflects the degree to which respondents report experiencing life events as unpredictable, uncontrollable, and generally overloading (e.g., “How often have you felt that you were able to control the important things in your life?”). The PSS has good internal consistency (r = .84 – .86) and test-retest reliability (r = .85; Cohen et al., 1983). In the present study, the PSS total score demonstrated good internal consistency (α = .88).
Smoking Consequences Questionnaire (SCQ)
The SCQ22 is a 50-item self-report measure that assesses tobacco use outcome expectancies believed to underlie smoking motivation on a Likert-type scale, ranging from 0 (completely unlikely) to 9 (completely likely). The measure consists of four key subscales: Positive Reinforcement/Sensory Satisfaction (PR; 15 items), Negative Consequences (18 items), Appetite-Weight Control (AWC; 5 items), and Negative Reinforcement/Negative Affect Reduction (NR; 12 items). The entire measure and its factors exhibit good psychometric properties.22,23 In the present study, we utilized the positive expectancy subscales (i.e., SCQ-PR, SCQ-AWC, and SCQ-NR) given theoretical and empirical evidence for their association with problematic use.24–30 SCQ-NR served as an explanatory variable and the two other positive expectancies were covariates in all analyses. Utilized SCQ subscales demonstrated excellent internal consistency (SCQ-PR: α = .88; SCQ-AWC: α = .91; SCQ-NR: α = .94).
Fagerström Test for Nicotine Dependence (FTND)
The FTND31 is a 6-item scale that assesses gradations in tobacco dependence. Scores range from 0–10, with higher scores reflecting high levels of physiological dependence on nicotine. The FTND has adequate internal consistency, positive associations with key smoking variables (e.g., saliva cotinine), and high test-retest reliability.32,33 The FTND total score was included as a criterion variable in the present study. The FTND demonstrated typical-range internal consistency among the present study sample (α = .59).
Barriers to Cessation Scale (BCS)
The BCS assessed barriers, or specific stressors, associated with smoking cessation.34 The BCS is a 19-item measure on which respondents indicate, on a 4-point Likert-style scale (0 [not a barrier] to 3 [large barrier]), the extent to which they identify with each of the listed barriers to cessation. The BCS maintains good internal consistency regarding the total score, and good content and predictive validity of the measure.34 The total score was utilized (α = .89).
Positive and Negative Affect Scale (PANAS)
The PANAS35 measured the extent to which participants generally experience 20 different feelings and emotions on a scale ranging from 1 (Very slightly or not at all) to 5 (Extremely). The measure yields two factors, negative and positive affectivity, and has strong documented psychometric properties.35 The PANAS negative affectivity subscale (PANAS-NA; 10 items) was utilized in the present study (α = .90).
Analytic Strategy
Analyses were conducted using the SPSS PROCESS macro, designed to test conditional process models that utilize an ordinary least squares-based path analytical framework to test for both direct and indirect effects.36 Standard errors of indirect effects estimated using bootstrapping is a recommended approach when data distribution is non-normal or unknown.37,38 Models included trauma-related symptom severity (PDS) as the predictor and perceived stress (PSS) and negative affect reduction smoking expectancies (SCQ-NR) as sequential explanatory variables. A test of serial mediation (e.g., sequential mediation) was utilized to examine the proposed causal chain.39 The indirect effect through both mediators is calculated by multiplying coefficients from (1) the path from the predictor to the first mediator (path a1), (2) the path from the first mediator to the second mediator (path d21), and (3) the path from the second predictor to the outcome (path b2) and is represented by a1d21b2. The completely standardized indirect effects were used as indicators of effects size.40 Covariates included gender, tobacco-related illness, PANAS-NA, and other positive smoking outcome expectancies (i.e., SCQ-PR and SCQ-AWC). Three independent models were conducted with nicotine dependence (FTND; Model 1), perceived barriers to cessation (BCS; Model 2), and severity of problems experienced while trying to quit (Quit Problems; Model 3) as criterion variables. All models were subjected to 10,000 bootstrap re-samplings and a 95-percentile confidence interval (CI) was estimated.36
RESULTS
Descriptives
Of the 570 treatment-seeking, adult smokers who provided baseline data on trauma exposure, 131 did not report having experienced at least one lifetime traumatic event, 9 were identified as multivariate outliers, and 67 participants had incomplete data; these 207 cases were excluded from further analyses. The final sample consisted of 363 (49.0% female; Mage = 37.91; SD = 13.31) treatment-seeking, trauma-exposed, adult smokers.
Participants were primarily White (86.5%) with an average daily smoking rate of 17.53 (SD = 9.50) cigarettes per day, for an average of 19.66 years (SD = 13.21), and a moderate level of tobacco dependence (Fagerström Test for Nicotine Dependence: M = 5.24, SD = 2.30).31 Participants indicated the following traumatic event as most disturbing in their lifetime: accident (25.3%), sexual assault/someone you know (9.9%), non-sexual assault/someone you know (9.4%), life threatening illness (9.4%), disaster (8.8%), non-sexual assault/stranger (8.0%), sexual contact under 18 with someone 5+ years older (5.0%), imprisonment (4.4%), sexual assault/stranger (4.4%), combat (1.7%), torture (1.4%), and other event (12.1%).
Of the sample, 46.1% met criteria for at least one current (past year) psychological disorder which included: social anxiety disorder (11.0%), generalized anxiety disorder (5.5%), major depressive disorder (4.4%), posttraumatic stress disorder (3.9%), alcohol use disorder (3.6%), specific phobia (3.4%), cannabis use disorder (3.1%), panic disorder with or without agoraphobia (2.5%), dysthymia (1.9%), anxiety disorder not otherwise specified (1.7%), obsessive-compulsive disorder (1.4%), non-alcohol substance dependence (0.9%), bipolar disorder (0.6%), depressive disorder not otherwise specified (0.6%), anorexia nervosa (0.3%), polysubstance dependence (0.3%), and disorder not otherwise specified (1.4%). Table 1 reports sample characteristics.
Table 1.
Participant characteristics
| M(SD)/N[%] | |
|---|---|
| Age | 37.91 (13.31) |
| Gender | |
| Male | 185 [51.0] |
| Female | 178 [49.0] |
| Race/ethnicity | |
| White | 314 [86.5] |
| Black Non-Hispanic | 23 [6.3] |
| Black Hispanic | 1 [0.3] |
| Hispanic | 12 [3.3] |
| Asian | 4 [1.1] |
| Other | 9 [2.5] |
| Education Completed | |
| Less than high school | 17 [4.7] |
| High school graduate or equivalent | 68 [18.7] |
| Some college | 134 [36.9] |
| Associates degree | 40 [11.0] |
| Bachelor degree | 53 [14.6] |
| Some graduate or professional school | 23 [6.3] |
| Graduate or professional school | 28 [7.7] |
| Marital Status | |
| Married or living with someone | 128 [35.3] |
| Widowed | 8 [2.2] |
| Separated | 13 [3.6] |
| Divorced or annulled | 67 [18.5] |
| Never married | 147 [40.5] |
| Smoking Rate | 17.53 (9.50) |
| Health | 0.38 (0.63) |
| PANAS-NA | 18.92 (6.94) |
| SCQ-PR | 5.73 (1.46) |
| SCQ-AW | 4.24 (2.34) |
| PDS | 7.56 (9.07) |
| PSS | 24.39 (7.87) |
| SCQ-NR | 5.73 (1.81) |
| FTND | 5.24 (2.29) |
| BCS | 24.86 (10.92) |
| Quit Problems | 2.08 (0.67) |
Note. N = 363; M(SD): Mean (Standard Deviation); Health (Medical History Form); PANAS-NA (Positive and Negative Affect Scale-Negative Affect subscale35); SCQ-PR (Smoking Consequences Questionnaire-Positive Reinforcement subscale22); SCQ-AW (Smoking Consequences Questionnaire-Appetite/Weight Control subscale22); PDS (Posttraumatic Diagnostic Scale16); PSS (Perceived Stress Scale7); SCQ-NR (Smoking Consequences Questionnaire Negative Reinforcement/Negative Affect Reduction Subscale22); FTND (Fagerström Test for Nicotine Dependence31); BCS (Barriers to Cessation34); Quit Problems (Smoking History Questionnaire20).
Zero-Order Correlations
The PDS was positively and significantly correlated with PSS (r = .45, p < .001) and SCQ-NR (r = .24, p < .001). Both PSS and SCQ-NR were positively and significantly correlated with each other (r =.44, p < .001). PSS was positively correlated with BCS (r = .39, p < .001) and Quit Problems (r =.38, p < .001). SCQ-NR was positively correlated with FTND (r =.19, p < .001), BCS (r = .51, p < .001), and Quit Problems (r = .45, p < .001). PDS was positively correlated with BCS (r = .23, p < .001) and Quit Problems (r = .46, p < .001). All criterion variables significantly correlated with one another (r = .18–.57, p < .001). Bivariate correlations are presented in Table 2.
Table 2.
Correlations among variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Gendera | -- | ||||||||||
| 2. | Healtha | −.02 | -- | |||||||||
| 3. | PANAS-NRa | .18*** | .02 | -- | ||||||||
| 4. | SCQ-PRa | .06 | −.10 | .20*** | -- | |||||||
| 5. | SCQ-AWa | .26*** | .02 | .14** | .28*** | -- | ||||||
| 6. | PDSb | .20*** | −.09 | .49*** | .14** | .04 | -- | |||||
| 7. | PSSc | .19*** | .01 | .66*** | .17** | .25*** | .45*** | -- | ||||
| 8. | SCQ-NRc | .21*** | −.08 | .40*** | .56*** | .42*** | .24*** | .44*** | -- | |||
| 9. | FTNDd | −.04 | −.02 | .03 | .15** | .11* | .08 | .10 | .19*** | -- | ||
| 10. | Barriers to Cessationd | .23*** | .01 | .37*** | .46*** | .27*** | .23*** | .39*** | .51*** | .18*** | -- | |
| 11. | Quit Problemsd | .30*** | .04 | . 41*** | .36*** | .32*** | .45*** | .38*** | .45*** | .20*** | .57*** | -- |
Note. N = 363
p < .001,
p < .01,
p < .05.
Covariate;
Predictor;
Explanatory Variable;
Criterion Variable
Gender: 1 (Male) and 2 (Female); Health (Medical History Form); PANAS-NA (Positive and Negative Affect Scale-Negative Affect subscale35); SCQ-PR (Smoking Consequences Questionnaire-Positive Reinforcement subscale22); SCQ-AW (Smoking Consequences Questionnaire-Appetite/Weight Control subscale22); PDS (Posttraumatic Diagnostic Scale16); PSS (Perceived Stress Scale7); SCQ-NR (Smoking Consequences Questionnaire Negative Reinforcement/Negative Affect Reduction Subscale22); FTND (Fagerström Test for Nicotine Dependence31); BCS (Barriers to Cessation34); Quit Problems (Smoking History Questionnaire20).
Regression Analyses
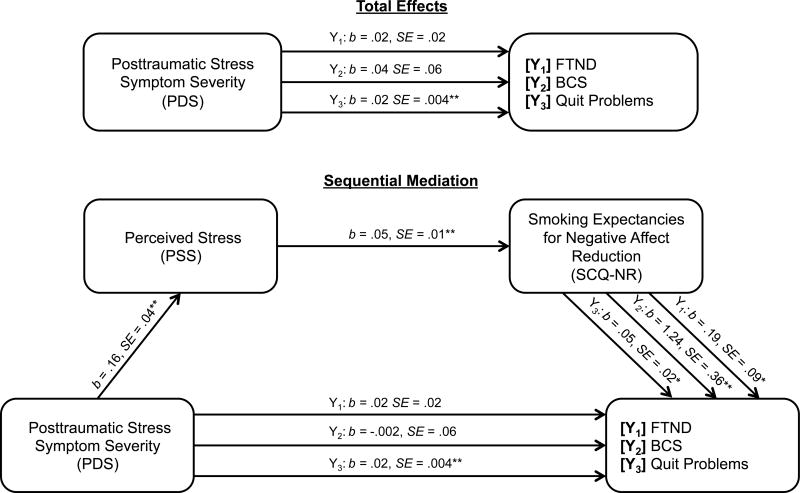
For all models, PDS significantly predicted PSS (path a1: b = .16, SE = .04, CI95% = .08, .23) and PSS significantly predicted SCQ-NR (path d21: b = .05, SE = .01, CI95% = .03, .07). SCQ-NR significantly predicted each criterion variable (paths b2: FTND: b = .19, SE = .09, CI95% = .01, .37; BCS: b = 1.24, SE = .36, CI95% = .54, 1.94; Quit Problems: b = .05, SE = .02, CI95% = .01, .09). Next, the proposed indirect effect models with both mediators were examined. Please see Figure 1 for a visual presentation of results.
Figure 1. Proposed model: Perceived stress (PSS) and negative affect reduction smoking expectancies (SCQ-NR) as potential explanatory variables for the effect of posttraumatic stress symptom severity on smoking criterion variables.
Note: N = 363 for analyses of models Y1–Y3. ** p < .001, * p < .05. Predictor: PDS (Posttraumatic Diagnostic Scale16); Mediator1: PSS (Perceived Stress Scale7); Mediator2: SCQ-NR (Smoking Consequences Questionnaire Negative Reinforcement/Negative Affect Reduction Subscale22); Y1: FTND (Fagerström Test for Nicotine Dependence31); Y2: BCS (Barriers to Cessation34); Y3: Quit Problems (Smoking History Questionnaire20). Covariates included gender, health, Positive and Negative Affect Scale-Negative Affect subscale,35 Smoking Consequences Questionnaire-Positive Reinforcement subscale,22 and Smoking Consequences Questionnaire-Appetite/Weight Control subscale22.
Model 1 tested the indirect association between PDS and FTND through PSS and SCQ-NR. The proposed model with both explanatory variables accounted for significant variance in FTND (R2 = .07, F[8, 354] = 2.61, p = .01). The sequential indirect effect was significant (a1d21b2 = .002, SE = .001, CI95% = .0001 to .004). The standardized serial indirect effect indicated a small effect size for this model (a1d21b2 = .005, SE = .003, CI95% = .001, .014). Examination of indirect effect suggested that PDS relates to greater nicotine dependence indirectly through the sequential effect of greater perceived stress and greater negative affect reduction smoking expectancies.
Model 2 tested the indirect association between PDS and BCS through PSS and SCQ-NR. The proposed model with both explanatory variables accounted for significant variance in BCS (R2 = .38, F[8, 354] = 26.56, p < .001). The sequential indirect effect was significant (a1d21b2 = .01, SE = .005, CI95% = .003, .023). The standardized serial indirect effect indicated a small effect size for this model (a1d21b2 = .008, SE = .004, CI95% = .003, .020). Examination of indirect effects suggested that PDS relates to greater BCS indirectly through the sequential effect of greater perceived stress and negative affect reduction smoking expectancies.
Model 3 tested the indirect association between PDS and quit problems through PSS and SCQ-NR. The proposed model with both explanatory variables was significant (R2 = .410, F[8, 354] = 29.84, p < .001). The sequential indirect effect was significant (a1d21b2 = .004, SE = .0002, CI95% = .0001, .001). The standardized indirect effect indicated a small effect size for this model (a1d21b2 = .006, SE = .003, CI95% = .001, .016). Examination of indirect effects suggested that PDS related to quit problems indirectly through the sequential effect of greater perceived stress and greater negative affect reduction smoking expectancies.
Specificity Analyses
To further strengthen interpretation of results, three alternative models were tested with the two proposed explanatory variables reversed; specifically, models were tested that examined the pathway from PDS to criterion variables through the indirect, sequential pathway from SCQ-NR to PSS. Tests of the indirect effects in these models were estimated based on 10,000 bootstrap re-samples. Results from these alternative, serial mediation models were non-significant (FTND: a1d21b2 = .001, SE = .0002, CI95% = −.0002, .001; BCS: a1d21b2 = .001, SE = .002, CI95% = −.002, .006; Quit Problems: a1d21b2 = <.001, SE = <.001, CI95% = −.0001, .0001).1
Discussion
As hypothesized, a chain pathway was observed among trauma-exposed smokers whereby greater severity in trauma-related symptoms was associated with perceived stress, which subsequently related to greater negative affect reduction expectancies for smoking and ultimately greater nicotine dependence, greater perceived barriers to cessation, and more severe withdrawal-related problems and symptoms during past quit attempts. The observed effects were evident above and beyond the variance accounted for by gender, tobacco-related medical problems, the propensity to experience negative affect, and other positive outcome expectancies. Although the present research design does not permit explication of the temporal ordering of the observed associations, confidence in the observations was strengthened by evaluating an alternative model in which the two proposed explanatory variables were reversed; all alternative models were non-significant. These data are in line with the perspective that trauma-related symptom severity may be related to the studied smoking dependent variables through a pathway from perceived stress to negative reinforcement expectancies for smoking.
The present findings broaden current conceptual understanding of pathways through which trauma-related symptoms contribute to smoking. While past work has provided evidence for trauma-related symptoms and perceived stress5 and perceived stress and negative affect reduction expectancies for smoking9 separately, the current study broadens this corpus of work by jointly examining the sequential path from trauma-related symptom severity to perceived stress to negative affect reduction expectancies for smoking, and finally to smoking. Thus, this initial work elucidates a conceptual and empirically supported pathway that explains, in part, the relation between trauma-related symptoms and maladaptive smoking behavior and cognition. Indeed, two unique barriers were identified that sequential contribute to factors that may impede quit success. Although small effect sizes were observed for the studied relations, this work serves as a functional springboard that can be used to inform future research and direct clinical application for addressing the unique needs of trauma-exposed smokers. In light of the high co-occurrence of trauma-exposure and smoking,2 elucidating associative patterns through which trauma-related symptom severity contributes to smoking has a high degree of theoretical and clinical significance.
Clinically, the present data suggest that smoking cessation programs for trauma-exposed individuals may benefit from stress management psychoeducation and skills training that integrates the impact of trauma-related symptoms on perceived stress. Additionally, it may be advisable to address negative affect reduction smoking outcome expectancies among trauma-exposed smokers with elevated perceived stress to facilitate changes in smoking behavior. Although additional research is needed, smoking cessation programs for trauma-exposed individuals that incorporate stress management skills training and challenge negative affect reduction outcome expectancies may be more efficacious over standard smoking cessation programs. Trauma-exposed smokers may benefit by targeting specific life stressors, including work, interpersonal, and familial stressors. Targeting perceived stress, or general stressors, may subsequently facilitate changes in smoking cognitions and behavior, including negative affect reduction smoking outcome expectancies. By addressing this chain response of symptoms related concurrently to trauma-related symptoms and smoking, treatment seeking trauma-exposed smokers may be more inclined to evaluate and change factors related to nicotine dependence, cognitions about perceived barriers to cessation, and perceived severity of withdrawal-related problems and symptoms experienced in future quit attempts.
There are a number of study limitations. First, the cross-sectional nature of the study design does not allow for testing of temporal sequencing. Future studies should examine these relations prospectively. Second, our sample consisted of primarily White, community-recruited, treatment-seeking, trauma-exposed daily cigarette smokers with a moderate smoking rate. Future studies may benefit by sampling ethnically diverse, lighter and heavier smokers to ensure the generalizability of the results to the general smoking population. Third, the current study focused on all smokers who endorsed having experienced at least one traumatic event defined on the PDS. Although the majority of the sample reported that the traumatic event involved actual or threatened death or serious injury or a threat to the physical integrity of self or others, or responded with feeling of helplessness or terror, fourteen of the included participants did not endorse that the traumatic event they experienced was accompanied by actual or threatened death or serious injury or a threat to the physical integrity of self or others, or responding with feeling of helplessness or terror. In light of recent changes to DSM-defined trauma (see DSM-541), future research may benefit from examining current pathways in a sample of trauma-exposed smokers who only indicate actual or threatened death or serious injury (i.e., report DSM-5 criterion A for trauma). Similarly, we did not have sufficient data to complete analyses on smokers with posttraumatic stress disorder (PTSD) and the observed level of self-reported trauma-related symptoms, as noted earlier, was in the mild range. This limitation restricts the generalizability of current findings to smokers with PTSD and/or smokers with higher level of trauma-related symptoms. To further gauge the clinical significance of the current findings, it would be important for future work to replicate this model with smokers who meet criteria of PTSD and/or smokers who endorse higher levels of trauma-related symptoms. Finally, clinical implications of the current work, although promising, should be interpreted with caution given the small effect sizes. As an important next step in this line of inquiry, it would be beneficial for future work to examine the cost-effectiveness of an integrated stress-smoking cessation treatment developed to address the unique needs of trauma-exposed smokers.
Overall, the present study serves as an initial investigation into a complex pathway among trauma-related symptom severity, perceived stress, negative affect reduction smoking outcome expectancies, and a relatively wide range of clinically significant smoking processes with adult treatment-seeking, trauma-exposed smokers. The findings suggest perceived stress and negative affect reduction smoking outcome expectancies may sequentially represent a possible explanatory pathway in the relation between trauma-related symptom severity and certain smoking behavior. Future research should focus on understanding these relations among those with PTSD as well as how these relations may differentially relate to smoking across specific PSTD symptom clusters. Additionally, future work is needed to better understand the extent to which trauma-exposed smokers with trauma-related symptoms may benefit from clinically addressing perceived stress and negative affect reduction smoking outcome expectancies during smoking cessation treatment. Similarly, it may be clinically informative for future work to compare and contrast patterns of findings across trauma-exposed smokers who meet DSM-5 criterion A trauma and those that have been exposed to a potentially traumatic event, but do not perceive actual or threatened death or serious injury. Indeed, these smokers may be a particularly resilient subset of the smoking population and understanding their unique characteristics may further elucidate protective factors among smokers who experience a potentially traumatic event. Lastly, to bolster further support for the current findings, future work could build upon this initial investigation by evaluating negative affect reduction smoking expectancies as a potential explanatory variable for the effect of perceived stress on maladaptive smoking behavior. This work may be particularly informative when it includes a larger and perhaps more generalizable sample of smokers, including those who have and have not experienced a traumatic event.
Acknowledgments
Grant Support: This work was supported by a National Institute of Health grant awarded to Drs. Michael J. Zvolensky and Norman B. Schmidt (R01-MH076629-01A1).
Footnotes
We examined the tested models controlling for time since trauma. The overall pattern of findings, including results from hypothesized models and alternative models, remained consistent with currently reported findings. Specifically, significant sequential indirect effects were observed for proposed models when controlling for time since trauma exposure (FTND: a1d21b2 = .001, SE = .001, CI95% = .0002, .004; BCS: a1d21b2 = .009, SE = .004, CI95% = .003, .021; Quit Problems: a1d21b2 = .0004, SE = .0002, CI95% = .0001, .001) and non-significant effects were observed across alternative models when controlling for time since trauma (FTND: a1d21b2 = <.001, SE = .0002, CI95% = −.0002, .001; BCS: a1d21b2 = .001, SE = .002, CI95% = −.002, .005; Quit Problems: a1d21b2 = <.001, SE = <.001, CI95% = −.0002, <.001).
References
- 1.Frans Ö, Rimmö PA, Åberg L, Fredrikson M. Trauma exposure and post- traumatic stress disorder in the general population. Acta Psychiatr Scand. 2005;111(4):291–290. doi: 10.1111/j.1600-0447.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 2.Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: A critical review of the empirical literature. Clin Psychol Rev. 2007;27(1):14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calhoun PS, Levin HF, Dedert EA, Johnson Y, Beckham JC. The relationship between posttraumatic stress disorder and smoking outcome expectancies among US military veterans who served since September 11, 2001. J Trauma Stress. 2011;24(3):303–308. doi: 10.1002/jts.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckham JC, Feldman ME, Vrana SR, et al. Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: a preliminary study. Exp Clin Psychopharmacol. 2005;13(3):219. doi: 10.1037/1064-1297.13.3.219. [DOI] [PubMed] [Google Scholar]
- 5.Garey L, Bakhshaie J, Vujanovic AA, Reitzel LR, Schmidt NB, Zvolensky MJ. Posttraumatic stress symptom severity and cognitive-based smoking processes among trauma-exposed treatment-seeking smokers: The role of perceived stress. Addict Behav. doi: 10.1016/j.addbeh.2016.03.038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409. doi: 10.1037//0022-006x.60.3.409. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983:385–396. [PubMed] [Google Scholar]
- 8.Richards JM, Stipelman BA, Bornovalova MA, Daughters SB, Sinha R, Lejuez C. Biological mechanisms underlying the relationship between stress and smoking: state of the science and directions for future work. Biol Psychol. 2011;88(1):1–12. doi: 10.1016/j.biopsycho.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robles Z, Garey L, Hogan J, Bakhshaie J, Schmidt NB, Zvolensky MJ. Examining an underlying mechanism between perceived stress and smoking cessation-related outcomes. Addict Behav. 2016;58:149–154. doi: 10.1016/j.addbeh.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farris SG, Zvolensky MJ, Beckham JC, Vujanovic AA, Schmidt NB. Trauma exposure and cigarette smoking: The impact of negative affect and affect-regulatory smoking motives. J Addict Dis. 2014;33(4):354–365. doi: 10.1080/10550887.2014.969622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman SM, Paliwal P, Sinha R. Childhood maltreatment, perceived stress, and stress-related coping in recently abstinent cocaine dependent adults. Psychology of Addictive Behaviors. 2007;21(2):233. doi: 10.1037/0893-164X.21.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrams DB, Niaura RS. Social learning theory. In: Blane HT, Leonard KE, editors. Psychological Theories of Drinking and Alcoholism. New York. New York: Guilford Press; 1987. pp. 131–178. [Google Scholar]
- 13.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt NB, Raines AM, Allan NP, Zvolensky MJ. Anxiety sensitivity risk reduction in smokers: A randomized control trial examining effects on panic. Behav Res Ther. 2016;77:138–146. doi: 10.1016/j.brat.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association A. Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. Arlington, VA: American Psychiatric Publishing; 2000. [Google Scholar]
- 16.Foa EB. Post-traumatic stress diagnostic scale (PDS) Minneapolis: National Computer Systems; 1995. [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for Axis I DSM-IV disorders. New York: Biometrics Research; 1994. [Google Scholar]
- 18.Buckner JD, Farris SG, Zvolensky MJ, et al. Dysphoria and smoking among treatment seeking smokers: the role of smoking-related inflexibility/avoidance. American Journal Of Drug & Alcohol Abuse. 2015;41(1):45–51. doi: 10.3109/00952990.2014.927472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farris SG, Zvolensky MJ, Blalock JA, Schmidt NB. Negative affect and smoking motives sequentially mediate the effect of panic attacks on tobacco-relevant processes. Am J Drug Alcohol Abuse. 2014;40(3):230–239. doi: 10.3109/00952990.2014.891038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111(1):180. [PubMed] [Google Scholar]
- 21.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Psychological Assessment. 1997;9(4):445. [Google Scholar]
- 22.Brandon TH, Baker TB. The smoking consequences questionnaire: The subjective expected utility of smoking in college students. J Consult Clin Psychol. 1991;3(3):484. [Google Scholar]
- 23.Downey KK, Kilbey MM. Relationship between nicotine and alcohol expectancies and substance dependence. Exp Clin Psychopharmacol. 1995;3(2):174. [Google Scholar]
- 24.Ahijevych K, Wewers ME. Factors associated with nicotine dependence among African American women cigarette smokers. Research in nursing & health. 1993;16(4):283–292. doi: 10.1002/nur.4770160407. [DOI] [PubMed] [Google Scholar]
- 25.Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire-Adult: Measurement of smoking outcome expectancies of experienced smokers. Psychological Assessment. 1995;7(4):484. [Google Scholar]
- 26.Downey KK, Kilbey MM. Relationship between nicotine and alcohol expectancies and substance dependence. Experimental and Clinical Psychopharmacology. 1995;3(2):174. [Google Scholar]
- 27.Brandon TH, Juliano LM, Copeland AL. Expectancies for tobacco smoking. 1999 [Google Scholar]
- 28.Goldman MS, Brown SA, Christiansen BA. Expectancy theory-thinking about drinking. In: Blane, Leonard KE, editors. Psychological theories of drinking and alcoholism. New York, NY: Guilford Publications; 1987. p. 181. [Google Scholar]
- 29.Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of abnormal psychology. 1988;97(2):133. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- 30.Marlatt GA, Donovan DM. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. Guilford Press; 2005. [Google Scholar]
- 31.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction to Alcohol and Other Drugs. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 32.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 33.Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerstrom tolerance questionnaire and the Fagerstrom test for nicotine dependence. Addict Behav. 1994;19(1):33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 34.Macnee CL, Talsma A. Development and testing of the barriers to cessation scale. Nurs Res. 1995;44(4):214–219. [PubMed] [Google Scholar]
- 35.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 36.Hayes A. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Methodology in the social sciences. New York, NY: The Guilford Press; 2013. [Google Scholar]
- 37.Kelley K. The effects of nonnormal distributions on confidence intervals around the standardized mean difference: Bootstrap and parametric confidence intervals. Educational and Psychological Measurement. 2005;65(1):51–69. [Google Scholar]
- 38.Kirby KN, Gerlanc D. BootES: An R package for bootstrap confidence intervals on effect sizes. Behavior Research Methods. 2013;45(4):905–927. doi: 10.3758/s13428-013-0330-5. [DOI] [PubMed] [Google Scholar]
- 39.Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. 2012 [Google Scholar]
- 40.Preacher KJ, Kelley K. Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods. 2011;16(2):93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- 41.Association AP. DSM 5. American Psychiatric Association; 2013. [Google Scholar]