Abstract
Rationale
Deficits in memory and attention are broadly acknowledged during psychosis; however, experiments on modeled psychosis often test working memory without systematic manipulation of attentional demands.
Objectives
The major research goal was discovering which neurobehavioral processes, attention, or memory contributed more to drug-provoked performance deficits.
Materials and methods
Rats were trained to perform operant ratio discrimination (RD) tasks wherein the number of presses at a rear-wall lever was discriminated using one of two front-wall levers. Effects from four psychotomimetic drugs, the serotonin agonist 2,5-dimethoxy-4-iodoamphetamine, the noncompetitive NMDA-glutamate receptor antagonist phencyclidine (PCP), and two CB1-selective cannabinoid agonists, WIN 55,512-2 and AM 411, were assessed using a signal detection analytical overlay to dissociate cognitive from noncognitive motor and motivational disruptions. Further methods allowed dissociation of attention compromises from mnemonic deficits.
Results
For each test compound, at least one dose elicited decreased RD accuracy without affecting response rates, and task difficulty was shown to be a crucial dictator of accuracy effect specificities. Effects from both PCP and WIN 55,512-2 biased animals to select the response lever conditioned for denser reinforcement. The same two drugs rendered peculiar response patterns in distracter light session components, considering light blinks were included to divert subjects’ attention away from task-relevant information. The response patterns determined during distracter components of PCP/WIN testing sessions, counterintuitively, suggest performance enhancement.
Conclusion
Comprehensive viewing of RD performance patterns after drug administration indicates that sustained attention and transient information management are significantly impaired during the drug-induced psychosis state, while selective attention is less affected.
Keywords: Animal model, Attention, Cannabinoids, Working memory, Discrimination, Psychostimulant, NMDA, Phencyclidine, Psychophysics, Schizophrenia
Introduction and research design
Studies to investigate human thought disorders are often initiated by drug administration to engender animal psychotomimesis. Investigators steering this research used the substantial literature base describing attentional and mnemonic compromise after psychotomimetic drug treatments (Yui et al. 2000; Bell 1965; Solowij and Michie 2007) as a point of departure and selected four psychotogenic drugs [an NMDA antagonist (phencyclidine: PCP), a serotonergic agonist (2,5-dimethoxy-4-iodoamphetamine: DOI), and two cannabinoid CB1 agonists (WIN 55,512-2: WIN and AM 411)] to model human psychosis. Although the organization of thought in nonhuman animals defies measurement, behavioral organization can be measured. Attentional and mnemonic aspects of drug-precipitated psychotomimetic states were measured by training rats to perform a ratio discrimination (RD) task. RD tasks demand careful sequencing of discrete lever presses (response string), after which RD-trained subjects must choose from simultaneously available response levers. In humans, NMDA antagonists, serotonin agonists, and cannabinoid agonists produce both sensorimotor gating deficits and cognitive impairment (Mathias et al. 2008; Negrete 1973).
Clinicians broadly acknowledge memory and attention disturbances in states of psychosis (Hemsley 1996; Jones et al. 1991). More pointedly, psychosis hampers the affected individual’s effortful focusing of thought (Holzman et al. 1978). Unfocused thought or unsustained attention can be modeled in laboratory animals by examining performance in a memory taxing task (McQuail and Burk 2006; Arnold et al. 2003). In contrast, compromises in selective attention manifest as distractibility (Green et al. 1992), which can be measured by monitoring changes in a trained lab animal’s performance when light flashes or intrusive tones are added to the conditioning environment (Burk 2004; McGaughy and Sarter 1995; Fries et al. 2008). The ultimate goal of the analysis of experimental effects in the present RD study is to illuminate the specific influences test compounds exert over selective relative to sustained attention. A search of current literature shows that several memory tests have been developed using rats, but effects from systematic manipulation of attentional demands are not generally scrutinized. This is surprising as human literature shows unequivocally that attentional load is a critical variable mediating the capacity of working memory (Kane et al. 2001; Kane and Engle 2003). To disentangle selective from sustained attention compromises, a compound RD schedule was utilized that entailed adjustments to programmed contingencies such that every 10 min (within 1-h sessions), flashing houselights were introduced or eliminated. It is useful to point out that both selective and sustained attention provide some filtering to regulate the capacity and efficiency of working memory (Baddeley 1986; Luck and Vecera 2002). To better determine experimentally imposed changes in rats’ attention or transient memory, research was divided to simultaneously measure drug effects in two subject groups. Psychotomimetic compounds were administered to groups designated as A- or as B-group rats. The RD-schedule designs were modified from procedures described by Moerschbaecher et al. (1984). Both A and B groups were conditioned to discriminate a high number from a low number under a multiple-schedule RD task, though the RD schedule utilized for the B group was simpler. The A group’s numeric ratio identified a high count as 30 lever presses (lp) and a low count as 5 lp. In comparison, the B group’s numeric ratio identified a high count of 30 lp and a low count of only 1 lp. At the outset of experiments, psychotomimetic treatment was expected to produce a larger attention/memory deficit in A rats performing the high-challenge RD task than in B rats performing the low-challenge RD task, even though matched doses of each test compound would be administered.
Although few investigators have used RD testing algorithms (e.g., Moerschbaecher et al. 1984; Branch 1974; Rosenberg and Woods 1975), alternate number discrimination tasks are frequently selected for behavioral characterization of drug-precipitated psychotomimesis (Platt 1973; Galbicka et al. 1994; Fetterman 1993; Willmore 2003; Mechner and Guevrekian 1962). When this type of experimental design is used, lever press number discernment is at the heart of performance accuracy. A preponderance of the data collected in number discrimination tests indicates that rats count rather than time the duration of lever press emissions in trial-specific behavioral strings (Willmore 2003). Not surprisingly, as number discrimination demands stimulus information processing, psychotomimetic drugs decrease accuracy. Moreover, discriminative acuity generally falls in response to drug doses lower than those associated with a lowering of response rates. Although the training procedure to condition rats to an RD schedule requires several months, response patterns in well-trained subjects are highly accurate. Hence, RD tasks provide a sensitive behavioral baseline, affording detection of both major and minor cognitive process changes. The principle goal in this research effort was to detect a putative change in attentional or mnemonic processing once the administered dose of a psychotomimetic test compound exceeded threshold. Accordingly, signal detection analysis was used, and the rate of trial completion was examined to separate out nonspecific performance disruptions, which may reflect treatment-induced alterations in basic sensory processing or motor integration.
Materials and methods
Subjects
Fourteen experimentally naïve and drug-naïve male Long Evans rats (Charles River Labs), weighing 280–320 g at the beginning of experiments, were subjects for this study. Animals, received at approximately 6 weeks of age, were housed individually with free access to water in a temperature-controlled room with 12-h light/dark exposure cycles. Rats were maintained at ~80% of their free-fed body weight by feedings given after daily training/testing sessions. Animals were segregated during the first week of experimentation into A-group (eight rats) and B-group (six rats) subjects. During the course of testing the sequence of compounds, three rats, two in the A-group and one in the B-group, either died or became too ill to perform on specific test dates. Therefore, the subject pool was lower for dose-effect curves determined late in these studies.
Apparatus
Four experimental chambers (Lafayette Instr.), dimensions 21.5×21.5×28 cm, were used. Chambers had intelligence panels at the front wall with two symmetrically arranged retractable response levers, mounted 3.5 cm from the floor, and a central food cup. The rear wall included a central-mount lever, positioned 3.5 cm from the floor. Research Diet® (Natick, MA, USA) 45-mg rat pellets were delivered to the food cup as reinforcement. A 15-W house light above the chamber’s transparent ceiling provided constant ambient illumination.
Procedure
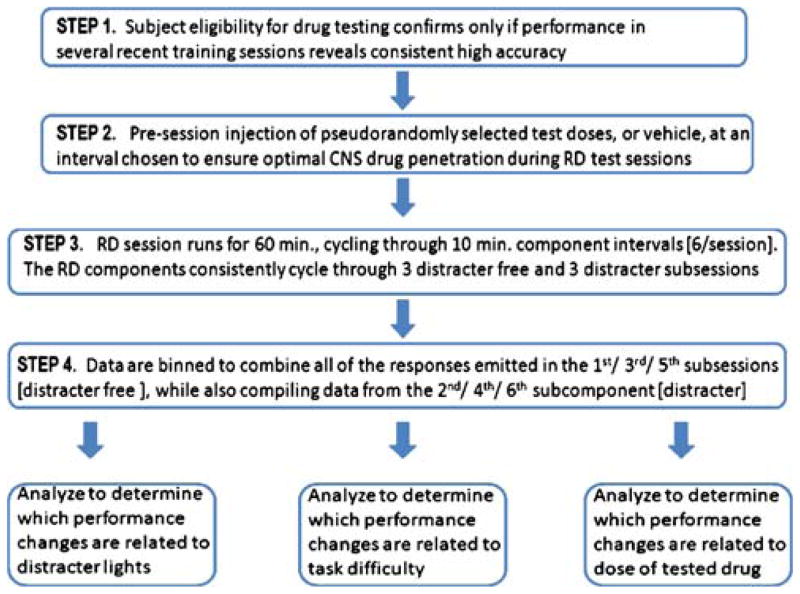
Experiments were performed using a multiple-component, discrete trial RD/RDdistract procedure. The procedural method was adapted from an RD method first described by Moerschbaecher et al. (1984). The FR discrimination procedure used here stipulates that rat subjects must emit lever presses on a rear-wall-mounted “work” lever. There was an equal probability of high FR stimulus values relative to low FR stimulus values on any given trial in RD sessions, as computer controls (ABET™) dictated random ratio presentation. Here, A-group rats were conditioned under a high-challenge RD schedule, while B-group rats were conditioned to perform under a low-challenge RD schedule. Experimenter adjustments of challenge levels effectuated with changes in the discrimination rule set. Thus, the A-group rats’ RD schedule was arranged with FR 30 versus FR 5 ratio discrimination, while B-group rats’ RD schedule was arranged with FR 30 versus FR 1 ratio discrimination. The completion of any high or low ratio turned off the stimulus above the rear lever, advancing trials through a 3-s pause, after which stimulus lights above the two front-wall mounted levers illuminated. If the completed ratio was high (i.e., FR 30), a response on the left front-wall lever was reinforced, whereas if the ratio completed was low (i.e., FR 5 or 1), a response on the right front-wall lever was reinforced. A correct response briefly extinguished the stimulus above each lever and produced the delivery of five sequential food pellets. Incorrect responses produced a 2-s timeout (TO), during which stimulus lights and the house light were off and responses had no programmed consequences. After either food delivery or TO, the stimulus light above the center lever illuminated, and new ratios presented for the subsequent trial, programmed with equal probability (i.e., noncorrection). Investigators trained RD subjects by successive approximation to emit full RD behavior chains (e.g., chain includes work-lever counting, pausing through a delay, and selecting the ratio-matched response lever). Investigators built the RD behavioral chain by training work-lever responses first. Rats were subsequently conditioned to pair high FR sequences at the work lever to left-mounted response manipulandae—while pairing low FR sequences to right-mounted response manipulandae. All RD sessions were run such that subjects were exposed to multiple RD/RDdistract subcomponents (visual distracter added during latter component). The length of all RD sessions was fixed at 60 min. Sessions always began with the RD component and cycled through 10 minute components as follows: RD → RDdistract → RD → RDdistract → RD → RDdistract. Rats were exposed to the RD schedules 7 days/week.
Distracter and distracter-free components
The frequency of house-light blinks was once per second. ABET programming tracked each 10-min subschedule interval; however, programming was set to avoid subschedule cycling until the trial in progress was completed. Data from the first, third, and fifth subcomponents in the compound schedule were collapsed, since these were all distracter free. Data from the second, fourth, and sixth subcomponents in the compound schedule were collapsed, since these were all distracter components.
Only those rats exhibiting highly accurate performance were tested with drugs. A low baseline rate of responding could also defer eligibility for drug testing. The two specific requirements for testability were correct (i.e., high- or low-ratio-paired) lever choice responses for 75% of trials in the most recent training session and that the number of trials completed in the most recent training session ≥25. Drug tests occurred approximately twice per week, with at least 48 h elapsing between drug administrations. Drugs were tested in the following order: PCP, DOI, WIN, AM 411. The order of dose presentations was randomized, and vehicle control tests were intermittent throughout the 4-month period of drug testing.
Drugs
PCP—Phencyclidine was obtained through NIDA (Rockville, MD, USA), dissolved in physiological saline, and injected s.c. at 1, 2, and 3 mg/kg doses 15 min prior to behavioral testing. DOI—The 5HT2 agonist, (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane, was obtained from RBI (Natick, MA, USA), saline-dissolved, and injected s.c. at 0.2, 0.4, and 0.6 mg/kg. AM411—The CB1-receptor-selective THC analogue compound was tested at 1, 3, and 5.3 mg/kg doses and was prepared for injection by a method adapted from Fenimore and Loy (1971). Briefly, AM 411 was dissolved in ethanol, and PVP (10%) was added to the AM 411/ethanol flask to produce a 3:1 mixture with excess PVP. Ethanol was evaporated, and the resultant residue was suspended in saline generating a 10 mg/ml AM 411 stock solution. WIN—This CB1-receptor-selective agonist was obtained from Sigma (Allentown, PA, USA). Each subject received WIN at 3.75, 5.25, and 10 mg/kg doses. The same solubilization procedure described for AM 411 was applied to generate WIN-injectable preparations
Data analysis
Analysis preface
Data generated in distract intervals of the compound schedule were collapsed, and analogous data were collapsed for all trials in the non-distract RD components. Recall that one of the central goals identified for this study is distinguishing RD choice differences when distracter lights are present. A second central goal is to identify differences between the A-group and the B-group animals’ performance, as this resolves the importance of task difficulty under our experimental conditions.
RD choice accuracy (traditional analysis)
Discriminative accuracy was determined by dividing the number of correct choices by the total number of choices in that type of schedule component—this quotient was examined as a percentage. These values are displayed, for high-count trials only, in Table 1. Accuracy data are segregated to tabulate means in distinct groups of rats (A group/B group), with data sorting to isolate distracter from distracter-free accuracies. For all means displayed in Table 1, an analogous standard error of the mean is indicated.
Table 1.
Effects of PCP, DOI, WIN, and AM 411 on accuracy in the high-count trials of RD sessions
| Drug | Dose (mg/kg) | Component Discrimination challenge level | High ratio accuracy | # observations | Discrimination challenge level | High ratio accuracy | # observations |
|---|---|---|---|---|---|---|---|
| PCP | A group | B group | |||||
| 30 versus 5 | 30 versus 1 | ||||||
| Vehicle | Distract | 95.3% (±2.3) | 7 | 97.4% (±2.4) | 6 | ||
| Regular | 94.0% (±3.0) | 7 | 96.8% (±2.0) | 6 | |||
| 1 | Distract | 82.4% (±9.2) | 8 | 91.1% (±5.4) | 5 | ||
| Regular | 72.5% (±11.7) | 8 | 88.1% (±5.4) | 5 | |||
| 2 | Distract | 53.1% (±8.0)a | 4 | 36.7% (±16.7)a | 6 | ||
| Regular | 37.1% (±13.8)a | 5 | 27.5% (±9.4)a | 6 | |||
| 3 | Distract | 72.2% (±14.5)a | 3 | 48.3% (±2.6)a | 3 | ||
| Regular | 50.0% (±28.9)a | 3 | 44.4% (±8.1)a | 3 | |||
| DOI | A group | B group | |||||
| 30 versus 5 | 30 versus 1 | ||||||
| Vehicle | Distract | 90.9% (±3.0) | 7 | 97.0% (±2.9) | 4 | ||
| Regular | 95.4% (±3.0) | 7 | 94.4% (±3.5) | 4 | |||
| 0.2 | Distract | 80.8% (±7.1) | 8 | 82.3% (±6.7) | 5 | ||
| Regular | 80.2% (±8.8) | 8 | 87.4% (±5.4) | 5 | |||
| 0.4 | Distract | 54.5% (±12.8) | 7 | 55.6% (±3.0) | 3 | ||
| Regular | 66.7% (±7.9) | 7 | 64.3% (±3.2) | 3 | |||
| 0.6 | Distract | 37.5% (±21.0)a | 5 | 50.0% (±5.0) | 2 | ||
| Regular | 36.1% (±16.5)a | 5 | 33.3% (±3.3) | 2 | |||
| WIN | A group | B group | |||||
| 30 versus 5 | 30 versus 1 | ||||||
| Vehicle | Distract | 96.2% (±2.6) | 7 | 95.8% (±4.0) | 3 | ||
| Regular | 86.6% (±1.1) | 7 | 100.0% (±0) | 3 | |||
| 3.8 | Distract | 91.3% (±2.6) | 7 | 100.0% (±0) | 3 | ||
| Regular | 77.2% (±3.4) | 7 | 82.8% (±8.1) | 3 | |||
| 5.3 | Distract | 75.6% (±15.5) | 6 | 73.2% (±8.7) | 3 | ||
| Regular | 66.5% (±13.5) | 6 | 81.0% (±13.3) | 3 | |||
| 10 | Distract | 74.6% (±15.5) | 6 | 71.4% (±71)b | 2 | ||
| Regular | 67.3% (±8.2) | 6 | 42.9% (±43)b | 2 | |||
| AM | A group | B group | |||||
| 411 | 30 versus 5 | 30 versus 1 | |||||
| Vehicle | Distract | 90.6% (±3.4) | 7 | 96.3% (±2.7) | 5 | ||
| Regular | 90.4% (±3.4) | 7 | 95.3% (±3.1) | 5 | |||
| 1 | Distract | 66.7% (±14.3) | 6 | 89.6% (±5.4) | 5 | ||
| Regular | 71.7% (±14.7) | 6 | 87.1% (±9.8) | 5 | |||
| 1.8 | Distract | 89.3% (±6.7) | 5 | 80.0% (±20.1) | 5 | ||
| Regular | 80.8% (±11.2) | 5 | 90.3% (±6.7) | 5 | |||
| 3 | Distract | 66.9% (±17.4) | 7 | 62.3% (±21.5) | 4 | ||
| Regular | 50.4% (±13.6) | 7 | 63.8% (±22) | 4 | |||
| 5.3 | Distract | 85.7% (±2.8) | 2 | 65.0% (±33.3) | 4 | ||
| Regular | 78.9% (±21.3) | 2 | 62.5% (±31.2) | 4 |
Accuracy is tabulated as a percentage and reflects the total number of correct high-count trials divided by the total number of high-count trials. Data shown are means and SEM. The number of rats tested in groups A and B, respectively, is designated under observation, although accuracy scores were excluded for sessions where fewer than five trials were observed
Significant difference from the vehicle value assessed by Duncan’s post hoc following a GLM assay to ascertain overall significance
Measured trend toward differing from vehicle (p=0.06) assessed by Duncan’s post hoc following a GLM assay to ascertain overall significance
Signal detection measures
The accuracy of choice behavior was also analyzed using signal detection analysis (SD) methods similar to those used in other complex discriminations (Willmore et al. 2002; Kirk et al. 1988; Watson and Blampied 1989; Tan et al. 1989; White et al. 1989; Poorheidari et al. 1998). For our SD measurements, response bias (log bias) and a bias-free measure of discriminability (log d, referred to as d′ in the present paper) were calculated using the following equation:
Chigh = correct ratio responses; Clow = correct low-ratio responses; Ehigh = incorrect high ratio responses; Elow = incorrect low-ratio responses. Response bias was calculated at each of the time delays using the following formula:
The group means for response bias were calculated on the absolute value of bias, thereby ignoring whether individual animals were biased to respond on the right- or the left-hand lever. If no errors were made in the session, the d′ and bias values became indeterminate. To prevent losing data because of indeterminate scores, 0.5 was added to the frequency of Chigh, Clow, Ehigh, and Elow (Stanhope et al. 1995; White and Alsop 1993). The accuracy, discriminability, and bias measures were analyzed with separate repeated measures analysis of variance (ANOVA), segregating distract from non-distract data sets and separating each drug. Individual session data on these measures were excluded for rats that completed less than five trials. Post hoc Duncan tests (p=0.05) compared subject performances at each dose to performance after vehicle injection. Total trial number was analyzed separately for each drug using a one-way, within-subject ANOVA (p=0.05) with Duncan post hoc testing. All of the statistical analyses were performed with SAS (Version 11; SAS Institute, Cary, NC, USA).
Results
Baseline performance
Rats in the A group met criteria for drug testing (i.e., fully trained stable RD-schedule conformity) after 39 (±0.9) weeks, while rats in the B group met criteria after 36 (±2.3) weeks. High-count trials were selected over low-count trial accuracy levels to gauge drug influence on discrimination. Justifying the choice to analyze high trials, the high stimulus values (30 lp) cultivate more challenge, and counts within the 30 lp strings are difficult to discriminate. PCP was tested while animals in A and B groups were 46 weeks old, DOI was tested while A and B groups were 50 weeks old, WIN was tested while A and B groups were 54 weeks old, and AM 411 was tested while A and B groups were 59 weeks old. Vehicle-injection sessions were scheduled four times during the intervals allocated to test each drug. Subsequently, a single mean was tabulated for each performance value, and animals’ data were pooled to obtain an A- or B-group mean. No statistical change was noted when comparing vehicle-only measurements between the drug testing intervals, nor were differences evident in comparing distracter free to distracter components of vehicle sessions (Fig. 1).
Fig. 1.
Schematic diagram illustrates the major steps to initiate and execute behavioral pharmacology measurements in rodent RD experiments
PCP
Traditional accuracy determination
Dose effects on accuracy in high-count trials (Table 1) were in accord with plotted d′ effects (Fig. 2). A significant effect of PCP dose on incorrect high-RD accuracy percentages was found for the A-group [F (3, 12)=4.51, p=0.02] and the B-group [F (3, 12)=15.63, p=0.0003] animals (Table 1). RD sessions testing each dose of PCP revealed, by traditional rather than SD measurement, accuracy elevations with distraction lights blinking (Table 1).
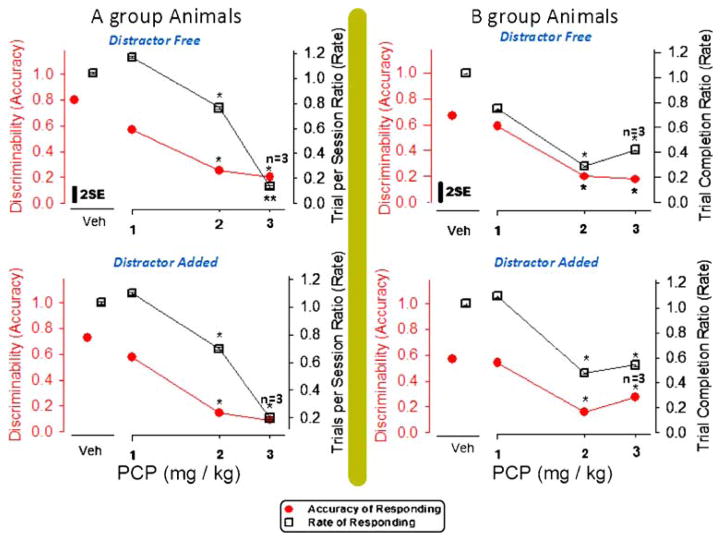
Fig. 2.
Effects of PCP in A-group rats (right panels) and in B-group rats (left panels) on the discriminability (d′) value measured in distracter-free components (upper panels) and counter measured in distracter-added components (lower panels) of an RD schedule. Variance in each panel is indicated by a bar representing 2×(√mean square error from GLM ÷ √N). *Significant difference from the vehicle value assessed by the Duncan method
SDT variables (accuracy and bias) and rate
The upper left plot in Fig. 2 shows group mean discriminability (accuracy) values for each of four dosing conditions in the PCP series of experiments for A-group animals. GLM analysis of the d′ values in the PCP-treated A-group animals revealed a significant dose effect relative to vehicle session d′ values [F (3, 12)=29.03, p=0.0001]. Post hoc tests showed d′ differences in the 2 and 3 mg/kg PCP test sessions. The right plotted graph in Fig. 2 (upper panel) shows mean d′ values for the B-group of animals, wherein a difference between PCP dosed and vehicle subjects was also revealed [F (3, 16)=6.87, p=0.007], and, as was true for A-group rats, the 2 and 3 mg/kg doses of PCP provoked d′ differences.
In the B-group discriminators, PCP affected bias scores (Table 2), and in both RD groups, PCP produced a dose-dependent decrease in the rate of trial completion. The impact PCP had on bias scores within B-group animals suggests a preference to press the right choice lever, which was conditioned as correct in low-count RD trials [F (3, 16)=6.87, p=0.007]. Post hoc testing showed the 2- and 3-mg/kg doses culpable for PCP bias score differences. In the A-group animals, bar pressing in sessions testing the 1 mg/kg dose was stimulated relative to sessions testing vehicle, while bar pressing in sessions testing 2 mg/kg was mildly decreased, and bar pressing in sessions testing the 3 mg/kg dose was drastically decreased [F (3, 28)=32.96, p=.0001]. Thus, the trial completion rate data point specifying responses in 2 mg/kg PCP testing sessions combines data from A-group rats that nearly ceased responding with A-group rats that continued avid responding. Also, this data point is positioned to indicate discriminability decrements at 2 mg/kg that exceed rate decrements (Fig. 2). In the B-group animals, the lever press rate fell more readily in all rats. The GLM analysis of data from PCP tests in B animals indicated trial completion during both the 2 and 3 mg/kg testing sessions was drastically lowered [F (3, 22)=8.96, p=0.0007; Fig. 2, right panel], and discriminability decrements during 2 mg/kg did not exceed rate decrements.
Table 2.
Effects of PCP, DOI, WIN, and AM 411 on bias
| Drug | Dose (mg/kg) | Component | Discrimination challenge level | Bias (B″) | # observations | Discrimination challenge level | Bias (B″) | # observations |
|---|---|---|---|---|---|---|---|---|
| PCP | A group | B group | ||||||
| 30 versus 5 | 30 versus 1 | |||||||
| Vehicle | Distract | 0.13 (±0.04) | 7 | 0.12 (±0.04) | 6 | |||
| Regular | 0.11 (±0.03) | 7 | 0.19 (±0.08) | 6 | ||||
| 1 | Distract | 0.21 (±0.09) | 8 | 0.16 (±0.07) | 5 | |||
| Regular | 0.34 (±0.10) | 8 | 0.20 (±0.06) | 5 | ||||
| 2 | Distract | 0.32 (±0.11) | 4 | 0.43 (±0.07)a | 6 | |||
| Regular | 0.44 (±0.11) | 5 | 0.38 (±0.11)a | 6 | ||||
| 3 | Distract | 0.32 (±1.73) | 3 | 0.29 (±0.21)a | 3 | |||
| Regular | 0.08 (±0.08) | 3 | 0.38 (±0.18)a | 3 | ||||
| DOI | A group | B group | ||||||
| 30 versus 5 | 30 versus 1 | |||||||
| Vehicle | Distract | 0.12 (±0.44) | 7 | 0.15 (±0.05) | 3 | |||
| Regular | 0.13 (±0.06) | 7 | 0.13 (±0.08) | 4 | ||||
| 0.2 | Distract | 0.22 (±0.08) | 8 | 0.27 (±0.08) | 5 | |||
| Regular | 0.34 (±0.06) | 8 | 0.24 (±0.11) | 5 | ||||
| 0.4 | Distract | 0.41 (±0.12) | 7 | 0.31 (±0.28) | 3 | |||
| Regular | 0.20 (±0.07) | 7 | 0.33 (±0.14) | 3 | ||||
| 0.6 | Distract | 0.32 (±0.13) | 5 | 0.43 (±0.35) | 2 | |||
| Regular | 0.26 (±0.08) | 5 | 0.53 (±0.17) | 2 | ||||
| WIN | A group | B group | ||||||
| 30 versus 5 | 30 versus 1 | |||||||
| Vehicle | Distract | 0.14 (±0.05) | 7 | 0.08 (±0.01) | 3 | |||
| Regular | 0.19 (±0.05) | 7 | 0.16 (±0.03) | 3 | ||||
| 3.8 | Distract | 0.25 (±0.03) | 7 | 0.10 (±0.07) | 3 | |||
| Regular | 0.31 (±0.04) | 7 | 0.26 (±0.06) | 3 | ||||
| 5.3 | Distract | 0.26 (±0.08) | 6 | 0.20 (±0.14) | 3 | |||
| Regular | 0.38 (±0.09) | 6 | 0.31 (±0.03) | 3 | ||||
| 10 | Distract | 0.25 (±0.07) | 6 | 0.45 (±0.00)a | 2 | |||
| Regular | 0.23 (±0.06) | 6 | 0.66 (±0.00)a | 2 | ||||
| AM 411 | A group | B group | ||||||
| 30 versus 5 | 30 versus 1 | |||||||
| Vehicle | Distract | 0.14 (±0.05) | 7 | 0.12 (±0.04) | 5 | |||
| Regular | 0.15 (±0.05) | 7 | 0.20 (±0.08) | 5 | ||||
| 1 | Distract | 0.40 (±0.14) | 6 | 0.20 (±0.11) | 5 | |||
| Regular | 0.30 (±0.14) | 6 | 0.26 (±0.11) | 5 | ||||
| 1.8 | Distract | 0.22 (±0.09) | 5 | 0.38 (±0.16) | 5 | |||
| Regular | 0.19 (±0.10) | 5 | 0.45 (±0.20) | 5 | ||||
| 3 | Distract | 0.17 (±0.09) | 7 | 0.36 (±0.12) | 4 | |||
| Regular | 0.37 (±0.08) | 7 | 0.26 (±0.08) | 4 | ||||
| 5.3 | Distract | 0.21 (±0.09) | 2 | 0.33 (±0.11) | 4 | |||
| Regular | 0.23 (±0.18) | 2 | 0.26 (±0.09) | 4 |
Data shown are means and SEM. The number of rats tested in groups A and B, respectively, is designated under observation, although accuracy scores were excluded for sessions where fewer than five trials were observed
Significant difference from the vehicle value assessed by Duncan’s post hoc following a GLM assay to ascertain overall significance
DOI
Traditional accuracy determination
With examination of raw accuracy percentages, a dose-dependent increase in errors through all high-count trials was evident (Table 1). In the A-group of discriminators, this error proneness was statistically resolved [F (3, 16)=8.01, p=0.002]. The similar trend of error increases with dose elevations in B-group rats failed to attain significance [F (3, 5)=1.84]. Accuracy sharpening in distracter-added relative to distracter-free trials was not noted as it was with PCP.
SDT variables (accuracy and bias) and rate
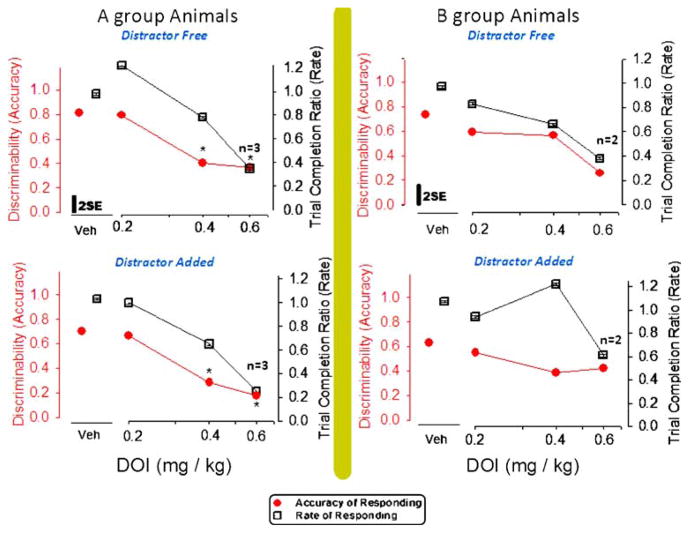
Figure 3 shows group average discriminability scores for the A-group animals (upper panel) without distracter lights on the left side of the page in three dose conditions after DOI administration. Similar data are plotted on the right side of this figure for the B-group animals from sessions with DOI pre-session injection. DOI testing sessions resolved differences between behavior observed in high-challenge and low-challenge RD tasks—in view of our finding that high difficulty was necessary to bring forth any statistical difference in discriminability. The plot oriented toward the left in Fig. 3 includes asterisks notating a statistically significant effect [F (3, 16)=9.32, p=0.0008], which was determined post hoc to arise from the 0.4- and 0.6-mg/kg test sessions. Unlike PCP, the 5HT receptor agonist DOI did not affect bias (Table 2). While DOI effects on trial number were significant for the A group [F (3, 16)=15.65, p=0.0001], attributable to the 0.6 mg/kg DOI dose, a trend indicated trials progressed more slowly in the B rats injected with DOI [F (3, 16)=2.55].
Fig. 3.
Effects of DOI in A-group rats (right panels) and in B-group rats (left panels) on the discriminability (d′) value measured in distracter-free components (upper panels) and counter measured in distracter-added components (lower panels) of an RD schedule. Variance in each panel is indicated by a bar representing 2×(√mean square error from GLM ÷ √N). *Significant difference from the vehicle value assessed by the Duncan method
WIN
Traditional accuracy determination
The percentage of high-count trials completed correctly during WIN testing sessions did not change significantly in the A group, but in B-group animals, a statistical trend promoting error in high-count trials was noted as doses elevated [F (3, 19)=5.65, p=0.06]. While accuracy measured in this traditional sense (percent correct) for the WIN testing series failed to attain significance, discriminability changes were detectable (see SDT variables below).
Close scrutiny of WIN-induced changes in subject performance, in both RD groups, reveals WIN-provoked increases in accuracy in 30 lp ratio trials during session components which included rhythmic light flashes (Table 1). By GLM, significance of this Subject × Component interaction was ascertained in the A group [F (6, 32)=37.17, p=0.0009]. Recall that a similar drug action was reported for PCP. Investigators conceive that this effect could arise from subjects pacing work-lever responses to accord with exogenously presented stimuli. Alternatively, this effect could arise as an interaction between psychotomimetic drugs and subject adaptability in a rapidly (i.e., every 10 min) changing test environment.
SDT variables (accuracy and bias) and rate
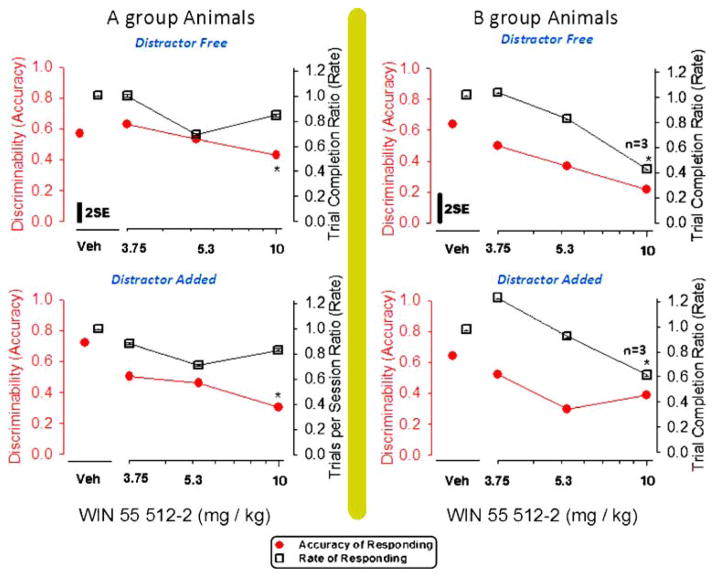
Figure 4 illustrates the discriminability effects with pre-session dosing of WIN in the A-group animals (left plot) and in the B-group animals (right plot). Evidently, a high level of task difficulty was necessary to bring forth truly significant differences in trained subjects’ detection of stimuli. Accordingly, the plot oriented toward the left in Fig. 4 has asterisks indicating differences [F (3, 16)=7.91, p=0.0018], which were determined post hoc to arise from 10-mg/kg test sessions, while a similar plot shows that B-group animals’ performance lacked discriminability differences. Like PCP, WIN affected bias, and did so exclusively in the low-challenge RD group [F (3, 19)= 10.73, p=0.02]; this effect was determined by post hoc testing to be attributable to the 10-mg/kg dose. Score changes were consistent with biasing after PCP administrations (preference for right choice response lever). Therefore, high doses of both PCP and WIN caused the B group of RD-schedule-controlled animals to respond as though low-count stimuli had repeatedly been presented through a series of session trials. WIN had effects on the trial number in test sessions, with differences resolved in the B group [F (3, 11)=5.11, p=0.028] during the 10-mg/kg dosing session. No systematic change could be detected in the number of trials per session when the A-group rats were treated with WIN [F (3, 23)=1.65].
Fig. 4.
Effects of WIN 55,512-2 in A-group rats (right panels) and in B-group rats (left panels) on the discriminability (d′) value measured in distracter-free components (upper panels) and counter measured in distracter-added components (lower panels) of an RD schedule. Variance in each panel is indicated by a bar representing 2×(√mean square error from GLM ÷ √N). * Significant difference from the vehicle value assessed by the Duncan method
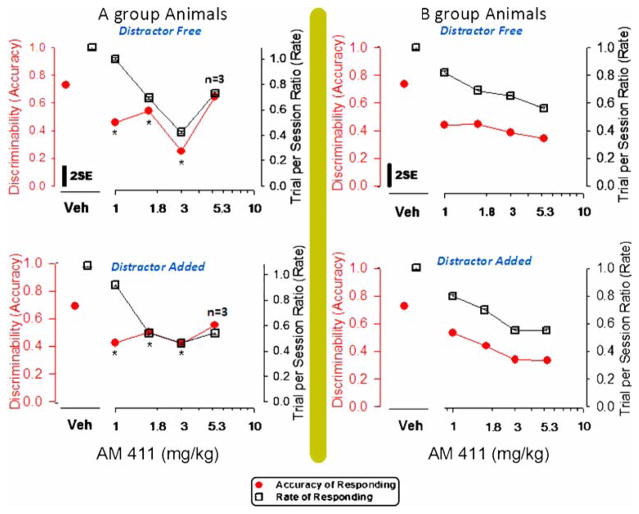
AM 411
Traditional accuracy determination
No distinguishable differences in the number of correct high-count trials were determined in sessions testing AM 411 in either the A-group animals [F (3, 22)=1.70] or the B-group animals [F (3, 22)=0.73]. Also, in contrast to trends identified for PCP and WIN, no systematic influence from blinking house-light additions prompted or “paced” rat responses.
SDT (accuracy and bias) and rate
Figure 5 illustrates the effects of AM 411 on discriminative behavioral endpoints. AM 411 affected the accuracy of discriminative responses at three doses in the high-challenge RD group [F (3, 22)=8.78, p=0.0007]. A memory-specific (i.e., sustained attention or information processing) compromise can be interpreted from data collected in the sessions testing a low dose of AM 411 (1 mg/kg), since d′ values were decreased, while the rate of trial completion remained stable. However, an alternate impression of accuracy effects emerges in noting that no dose-related performance changes are revealed through the traditional percent accuracy measurements (Table 1).
Fig. 5.
Effects of AM 411 in A-group rats (right panels) and in B-group rats (left panels) on the discriminability (d′) value measured in distracter-free components (upper panels) and counter measured in distracter-added components (lower panels) of an RD schedule. Variance in each panel is indicated by a bar representing 2×(√mean square error from GLM ÷ √N). *Significant difference from the vehicle value assessed by the Duncan method
Interpretation and discussion
Sustained attention and transient memory
Founding theories on attention stipulate sustained attention as a process reflecting an examined subject’s alert receptivity to information—a process by which conscious effort is expended to focalize relevant information (Posner and Snyder 1975; Moray 1969; Davies and Parasuraman 1982). By this definition, it is reasonable to declare that sustained attention guides a rat’s progression through RD sessions, and sustained attention was compromised by all four test compounds. Even with granting a support influence from sustained attention, however, many aspects of animal cognition are noted for subjects to perform RD. Indeed, transient or working memory processes are inextricably linked to sustained attention processes (Hulse et al. 1978). From the overarching “cognitive” perspective, our analysis of memory effects after doses of each compound unquestionably reveals selective (i.e., rate-independent) and dose-related d′ decrements for all test drugs (Figs. 2 through 5). Moreover, the data in Table 1 indicate a dose-ordered drop in the percentage of correct RD trials for three test compounds (AM 411 is unique in its failure to propagate uniform accuracy changes). It is interesting to note that for three of four test compounds (DOI, WIN, AM 411), a high-challenge RD task (30 lp versus 5 lp) was necessary to discern a statistically significant change in cognitive behavioral indicators in sessions testing nonsedative doses of such drugs.
Selective attention and distraction stimuli
Selective attention guides an organism to select certain information amongst many available signals. Treatment of the selected stimulus is special, as this piece of information is more likely to affect awareness, memory, and behavior. Performance during distracter components of the compound RD schedules reflects selective attention, because schedule contingencies require subjects to attend to number stimuli, while ignoring light flashes. Performance during distracter components secondarily reflects attentional orienting, because hour-long RD sessions are divided to intermittently include house-light flashes. Poor performance was expected during the distracter components when subjects were acutely influenced by psychotomimetic drugs, as this alteration in stimulus set was anticipated to sway subjects’ attention away from counting. Contrary to this expectation, the data from two drug testing sessions, those testing PCP and WIN, indicate that the efficacy of number discriminations was elevated. A reasonable or likely interpretation of this performance enhancement is that the effect derives from the tonic rhythmicity of light blinks.
Pharmacologic effects
Human psychoses involve complex alterations in several neurotransmitter and receptor systems. For this reason, psychosis can be elicited by drugs from at least four major pharmacologic categories. In animals, the same drugs induce psychotomimetic state syndromes, which are comprised of species-dependent psychotomimetic trademark behaviors such as stereotypy, hyperactivity, skewed sensory gating, and, most relevant to the present study, information processing compromises.
PCP, a noncompetitive NMDA-glutamate receptor blocker, produced patterns of rat behavior indicative of intoxication. Sustaining this claim, over-trained rat subjects behaved variably during PCP test sessions (e.g., drastic bidirectional rate changes in some animals, right lever biasing, or gross perseveration of inaccuracy). Focusing next on the second test compound, DOI, which is a 5HT 2a/2c agonist possessing hallucinogenic properties in humans. By much alternate experimental readout, DOI’s categorization as a propsychotic drug is supported. For example, DOI was shown to impair rat sensorimotor/sensory gating response in the prepulse inhibition of acoustic startle test (Sipes and Geyer 1994). Data collected in our RD tests with DOI extend, and are consistent with, previous in vivo tests.
Chronic cannabis use can offset schizophreniform states, inducing patients to suffer memory dysfunction, perceptual distortion, and hallucination (Martin et al. 2003; Skosnik et al. 2001). Cannabis can additionally provoke relapse and exacerbate symptoms in psychiatric patients (Johns 2002). The psychoneural effects of cannabis are presumed to be CB1-receptor-mediated, because these signs and symptoms reverse when the CB1-receptor-selective antagonist SR 141716A is given prior to marijuana or other cannabinoid compounds (Huestis et al. 2001). Against this experimental backdrop, rate-independent discriminability changes were expected in RD experiments, especially in sessions measuring high WIN and AM 411 doses. Then again, close analysis and efforts to compare between cannabinoid compounds effects show that AM 411 effects were less profound than WIN effects. The strongest data offering support to this contention are found by examining accuracy values in Table 1. While some changes in performance can be linked to AM 411 dose, no clear systematic, dose-dependent trend is discernible from measures shown in Table 1. Furthermore, AM 411 did not give rise to a lever-biasing effect and did not produce paced responding during exposure to distracter lights.
In closing, two major research findings emerged in these studies. First, the RD tests demonstrate that psychotomimetic drugs provoke impairments in sustained attention and information processing by administering low or moderate doses, while high doses are required to suppress response rate. Second, the imposed light blink distracters were not effective in swaying subjects’ attention from task-relevant activities. The paper finishes with commentary, as this may assist new research design. Of the many research papers available from authors targeting a clarification of psychotomimetic drug effects, a truly clear concluding statement only appeared on papers where task difficulty was high. It appears that behavioral scientists, at least those interested in this family of drug agents, will need to select models commensurate with the neural disrupt these drugs impose.
Acknowledgments
The authors extend thanks to J. Edward Moreton, Ph.D., for the University of Maryland School of Pharmacy, for technical and conceptual contributions to this research effort.
Contributor Information
C. B. Willmore, Harding University College of Pharmacy, Searcy, AR 72149, USA. University of Maryland School of Pharmacy, Baltimore, MD 21201, USA. Raabe College of Pharmacy, Ohio Northern University, Ada, OH 45810, USA
D. M. Krall, Raabe College of Pharmacy, Ohio Northern University, Ada, OH 45810, USA
F. M. Spears, Harding University College of Pharmacy, Searcy, AR 72149, USA
A. Makriyannis, Center for Drug Discovery, Northeastern University, Boston, MA, USA
G. I. Elmer, Department of Psychiatry, University of Maryland School of Medicine; Maryland Psychiatric Research Institute, Baltimore, MD, USA
References
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Bruno JP, Sarter M. Assessment of sustained and divided attention in rats. Curr Protoc Neurosci. 2003;Chapter 8(Unit 8):5E. doi: 10.1002/0471142301.ns0805es22. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford; New York: 1986. [Google Scholar]
- Bell DS. Comparison of amphetamine psychosis and schizophrenia. Br J Psychiatry. 1965;111:701–707. doi: 10.1192/bjp.111.477.701. [DOI] [PubMed] [Google Scholar]
- Branch M. Behavior as a stimulus: joint effects of d-amphetamine and pentobarbital. J Pharmacol Exp Ther. 1974;189:33–41. [PubMed] [Google Scholar]
- Burk J. Introduction of a retention interval in a sustained attention task in rats: effects of a visual distracter and increasing the inter-trial interval. Behav Proc. 2004;67:521–531. doi: 10.1016/j.beproc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Davies D, Parasuraman R. The psychology of vigilance. Academic; New York: 1982. [Google Scholar]
- Fenimore DC, Loy PR. Injectible dispersion of delta 9-tetrahydrocannabinol in saline using polyvinylpyrrolidone. J Pharm Pharmacol. 1971;23:310. doi: 10.1111/j.2042-7158.1971.tb08667.x. [DOI] [PubMed] [Google Scholar]
- Fetterman JG. Numerosity discrimination: both time and number matter. J Exp Psychol Anim Behav Proc. 1993;19:149–164. [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbicka G, Kautz MA, Jagers T. Behavioral effects of enantiomers of dizocilpine under two “counting” procedures in rats. Pharmacol Biochem Behav. 1994;49:943–948. doi: 10.1016/0091-3057(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gaier DJ. Sustained and selective attention in schizophrenia. Prog Exp Pers Psychopathol Res. 1992;15:290–313. [PubMed] [Google Scholar]
- Hemsley DR. Schizophrenia. A cognitive model and its implications for psychological intervention. Behav Modif. 1996;20:139–169. doi: 10.1177/01454455960202001. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Levy DL, Proctor LR. The several qualities of attention in schizophrenia. J Psychiatr Res. 1978;14:99–110. doi: 10.1016/0022-3956(78)90012-2. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Hulse S, Fowler H, Honig W. Cognitive processes in animal behavior. Earlbaum; Hillsdale: 1978. [Google Scholar]
- Johns A. From dead drunk to dead drunks—medico-legal aspects of substance misuse, violence and mental illness. Med Leg J. 2002;70:108–125. doi: 10.1258/rsmmlj.70.3.108. [DOI] [PubMed] [Google Scholar]
- Jones SH, Hemsley DR, Gray JA. Contextual effects on choice reaction time and accuracy in acute and chronic schizophrenics. Impairment in selective attention or in the influence of prior learning? Br J Psychiatry. 1991;159:415–421. doi: 10.1192/bjp.159.3.415. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kane M, Conway A, Bleckley M, Engle R. A controlled attention view of working memory capacity. J Exp Psychol. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kirk RC, White KG, McNaughton N. Low dose scopolamine affects discriminability but not rate of forgetting in delayed conditional discrimination. Psychopharmacology (Berl) 1988;96:541–546. doi: 10.1007/BF02180037. [DOI] [PubMed] [Google Scholar]
- Luck S, Vecera S. Attention. In: Pashler H, Yantis S, editors. Stephens handbook of experimental psychology. Wiley; New York: 2002. pp. 235–286. [Google Scholar]
- Martin RS, Secchi RL, Sung E, Lemaire M, Bonhaus DW, Hedley LR, Lowe DA. Effects of cannabinoid receptor ligands on psychosis-relevant behavior models in the rat. Psychopharmacology (Berl) 2003;165:128–135. doi: 10.1007/s00213-002-1240-x. [DOI] [PubMed] [Google Scholar]
- Mathias S, Lubman DI, Hides L. Substance-induced psychosis: a diagnostic conundrum. J Clin Psych. 2008;69:358–367. doi: 10.4088/jcp.v69n0304. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Burk JA. Evaluation of muscarinic and nicotinic receptor antagonists on attention and working memory. Pharmacol Biochem Behav. 2006;85:796–803. doi: 10.1016/j.pbb.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Mechner F, Guevrekian L. Effects of deprivation upon counting and timing in rats. J Exp Anal Behav. 1962;5:463. doi: 10.1901/jeab.1962.5-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerschbaecher JM, Mastropaolo J, Winsauer PJ, Thompson DM. Effects of opioids on accuracy of a fixed-ratio discrimination in monkeys and rats. J Pharmacol Exp Ther. 1984;230:541–549. [PubMed] [Google Scholar]
- Moray N. Attention: selective processes in vision and hearing. Academic; New York: 1969. [Google Scholar]
- Negrete JC. Relative value of various etiological factors in short lasting, adverse psychological reactions to cannabis smoking. Br J Addict Alcohol Other Drugs. 1973;68:221–229. doi: 10.1111/j.1360-0443.1973.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Platt JR. Percentile reinforcement: paradigms for experimental analysis of response shaping. In: Bower GH, editor. The psychology of learning and motivation. Academic; New York: 1973. pp. 271–296. [Google Scholar]
- Poorheidari G, Stanhope KJ, Pratt JA. Effects of the potassium channel blockers, apamin and 4-aminopyridine, on scopolamine-induced deficits in the delayed matching to position task in rats: a comparison with the cholinesterase inhibitor E2020. Psychopharmacology (Berl) 1998;135:242–255. doi: 10.1007/s002130050506. [DOI] [PubMed] [Google Scholar]
- Posner M, Snyder C. Attention and cognitive control. In: Solso R, editor. Information processing in cognition: The Loyola Symposium. Erlbaum; Hillsdale: 1975. pp. 55–85. [Google Scholar]
- Rosenberg J, Woods J. Effects of pentobarbial on fixed ratio discrimination. Bull Psych Soc. 1975;5:33–35. [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Spatz-Glenn L, Park S. Cannabis use is associated with schizotypy and attentional disinhibition. Schizophr Res. 2001;48:83–92. doi: 10.1016/s0920-9964(00)00132-8. [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT. Cannabis and cognitive dysfunction: parallels with endophenotypes of schizophrenia? J Psychiatry Neurosci. 2007;32:30–52. [PMC free article] [PubMed] [Google Scholar]
- Stanhope KJ, McLenachan AP, Dourish CT. Dissociation between cognitive and motor/motivational deficits in the delayed matching to position test: effects of scopolamine, 8-OH-DPAT and EAA antagonists. Psychopharmacology (Berl) 1995;122:268–280. doi: 10.1007/BF02246548. [DOI] [PubMed] [Google Scholar]
- Tan S, Kirk RC, Abraham WC, McNaughton N. Effects of the NMDA antagonists CPP and MK-801 on delayed conditional discrimination. Psychopharmacology (Berl) 1989;98:556–560. doi: 10.1007/BF00441959. [DOI] [PubMed] [Google Scholar]
- Watson JE, Blampied NM. Quantification of the effects of chlorpromazine on performance under delayed matching to sample in pigeons. J Exp Anal Behav. 1989;51:317–328. doi: 10.1901/jeab.1989.51-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Alsop G. Cognition in birds. In: Saghal A, editor. Behavioral neuroscience, a practical approach. Oxford University Press; London: 1993. pp. 137–147. [Google Scholar]
- White KG, McCarthy D, Fantino E. Cognition and behavior analysis. J Exp Anal Behav. 1989;52:197–198. doi: 10.1901/jeab.1989.52-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore CB. The cognitive effect profiles of NMDA receptor modulating drugs are resolvable if stimulus complexity is varied in a number discernment task. Behav Cogn Neurosci Rev. 2003;2:130–147. doi: 10.1177/1534582303255858. [DOI] [PubMed] [Google Scholar]
- Willmore CB, Bespalov AY, Beardsley PM. Site-selective N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-iso-xazolepropionate antagonists produce distinct effects in rats performing complex discriminations. Neurobiol Learn Mem. 2002;78:347–364. doi: 10.1006/nlme.2002.4077. [DOI] [PubMed] [Google Scholar]
- Yui K, Ikemoto S, Ishiguro T, Goto K. Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci. 2000;914:1–12. doi: 10.1111/j.1749-6632.2000.tb05178.x. [DOI] [PubMed] [Google Scholar]