Abstract
Cannabinoid CB1 inverse agonists such as rimonabant and AM 251 hold therapeutic promise as appetite suppressants, but the extent to which non-motivational factors contribute to their anorectic effects is not fully known. Examination of the behavioral satiety sequence (BSS) in rats, the orderly progression from eating to post-prandial grooming and then resting, has revealed that these compounds preserve the order of events but differ markedly from natural satiation. The most notable difference is that grooming (particularly scratching) is profoundly enhanced at anorectic doses, while eating and resting are diminished, raising the possibility that the anorectic effect is simply secondary to the grooming effect. In the current design, the neutral CB1 antagonist AM 4113, which has been found to lack some of the undesirable effects of AM 251, produced nearly identical effects on the BSS as AM 251. The possibility that competition from enhanced grooming could account for the anorectic effect of AM 4113 was examined by yoking the pattern of disruptions caused by grooming in the AM 4113-treated group to forced locomotion in a different group fed in a modified running wheel. This response competition did not significantly reduce food intake. It was concluded that AM 4113, a CB1 neutral antagonist, produces the same effects on the BSS as AM 251, but that response competition from enhanced grooming may not be a sufficient explanation for the anorectic effects of CB1 antagonists/inverse agonists.
Keywords: Cannabinoids, Feeding, Appetite, Motivation, Grooming, Satiety sequence, Rimonabant, Inverse agonist
1. Introduction
Compounds that block the CB1 cannabinoid receptor have generated a great deal of interest as putative appetite suppressants [2,5,6,8,11,21,26–28]. However, the mechanism by which these compounds reduce food intake is not entirely understood, and it is possible that the reduction in food intake seen with drugs such as the CB1 inverse agonists rimonabant (a.k.a. SR 141716A; [22,25]), AM 251 [12,21], AM 1387 [23], and the neutral antagonists O-2050 [11] and AM 4113 [3,7,28] may result at least in part from non-motivational actions. For instance, the CB1 inverse agonist AM 251 induces conditioned taste avoidance and conditioned gaping, both markers of nausea, at anorectic doses [22]. The neutral antagonist AM 4113 was later found to have no effect on conditioned gaping over a dose range that inhibited food intake [3,28]. This finding had two promising implications: first, CB1 neutral antagonists may lack the effect of nausea that has been shown with CB1 inverse agonists in rats [22] and humans [24]; and secondly, that the reduction in food intake produced by CB1 blockade is not likely to be due to nausea, as these appear to be dissociable mechanisms. It is therefore important to design novel CB1-acting compounds, such as neutral antagonists that lack many undesirable effects at anorectic doses.
In addition to such direct tests of alternative hypotheses, a critical assay of the putative appetite suppression of CB1 antagonists and inverse agonists is the systematic observation of the extent to which these compounds elicit behaviors similar to natural satiation. The behavioral satiety sequence (BSS) is an observational technique that examines feeding sessions for the presence of an orderly progression of predominant behaviors from eating to grooming, and finally to resting [15]. Manipulations such as prefeeding and dexfenfluramine decrease eating (which is the predominant behavior at the beginning of the feeding session irrespective of levels of satiety) while increasing post-prandial resting and decreasing the latency of onset of grooming and resting [15]. In other words, manipulations that increase satiety produce a leftward shift in the function of feeding, grooming, and resting over time. In contrast, other compounds, such as quinine [17] or MK-212 [14] inhibit food intake but disrupt the progression of feeding to grooming to resting. Thus, manipulations that induce satiety can be distinguished from those that simply reduce food intake. Recently, the CB1 inverse agonists rimonabant [29] and AM 251 [30] were found to preserve the overall order of the BSS, but with marked differences from naturally satiating manipulations such as prefeeding. In particular, the CB1 inverse agonists enhanced grooming, particularly scratching behavior, while time spent eating and resting decreased. As these effects were consistent with a disruption in behavior caused by grooming, it was proposed that the reductions in food intake seen with these compounds did not result from alterations in food motivation. Rather, it was suggested that, as eating, grooming, and resting are mutually exclusive activities (at least as operationally defined for the BSS), inhibition of food intake and resting is secondary to response competition from the enhanced grooming [29,30].
Experiments 1 and 2 were designed to test the BSS effects of the CB1 inverse agonist AM 251 and the CB1 neutral antagonist AM 4113, respectively. As CB1 neutral antagonists such as O-2050 and AM 4113 are known to reduce food intake [3,7,11,28], it is likely that blockade of endocannabinoid tone, rather than CB1 inverse agonist activity, is sufficient to suppress feeding. However, as CB1 antagonists may not produce effects such as nausea [28], it is possible that AM 4113 would not elicit the disruptions on the BSS that have been shown with AM 251 [30]. Lastly, Experiment 3 was designed to investigate the hypothesis that inhibition of food intake to the extent that has been observed with CB1 antagonists or inverse agonists can result from the induction of a mutually exclusive response. This was done by inducing behavioral disruptions (forced locomotion) during feeding sessions of noninjected animals that were yoked to patterns of grooming behavior in drug-treated subjects in Experiment 2.
2. Materials and methods
2.1. Subjects
A total of 24 adult male Sprague–Dawley rats (Hilltop Labs, Scottdale, PA) were used. Animals were doubly housed in opaque plastic tub cages and were maintained on a 12-h light/dark cycle (lights on at 08:00 h and lights off at 20:00 h). Experiments were conducted in the light part of the cycle, beginning at approximately 11:00 h. All subjects were drug-naïve at the start of their respective studies. Food restriction was put in place 24 h prior to feeding sessions. Although subjects’ weights were allowed to decrease to a minimum of 85% of free-feeding weight, body weight growth was observed during the experiments. Rats were fed Purina Rat Chow pellets ad lib in home cage except for a 24 h period prior to habituation and testing, when they received five pellets per cage per day. Water was available ad lib except during feeding sessions. All treatment and care were conducted as outlined in The Guide for the Care and Use of Laboratory Animals [16], and all protocols were approved and supervised by the Edinboro University Institutional Animal Care and Use Committee.
2.2. Drugs
AM 251 and AM 4113 were synthesized at the Center for Drug Discovery (Northeastern University, Boston, MA) and dissolved in dimethyl sulfoxide (DMSO; Fisher Scientific) at a concentration of 80 mg/ml. These solutions were then suspended in Tween-80 (Fisher Scientific) and brought to volume with 0.9% saline, for a final concentration of 1:1:8 DMSO:Tween-80:saline. This solution was also used as the vehicle treatment. Doses of 2.0, 4.0, and 8.0 mg/kg were prepared via serial dilutions for both compounds. Drugs, along with vehicle control, were administered via the i.p. route (1 mg/ml solution) 30 min prior to weekly test sessions. Injection regimen was a within-subjects design with pseudorandom order of treatment such that each rat received all four treatment conditions (drug doses plus vehicle control) in a unique order. Doses, pretreatment times, and the injection regimen were based on previous findings [21,22,28].
2.3. Apparatus
For Experiments 1 and 2 (BSS), two observation chambers (internal dimensions 30 cm × 25 cm × 22 cm) were set up side-by-side and angled slightly toward a camera to facilitate scoring of observations later for pairs of cagemates simultaneously. The two chambers were modified from operant boxes (Lehigh Valley) with two stainless steel sides, and two sides and a ceiling made of Plexiglas. The metal grid floor of the observation chamber was covered with a finished particleboard surface to facilitate resting. Levers were retracted and subjects had no experience in operant conditioning. A wireless camera (Cop Security) was directed at one side of the observation chambers to record the behaviors of the subjects during testing. In addition, a mirror was placed at an angle behind each observation chamber opposite the camera to assist with the scoring of behaviors. The camera sent a continuous feed to an adjacent room containing an antenna receiver unit connected to a 27 in. television and videocassette recorder, which recorded testing sessions. This recording system was used over live observation because pilot experiments suggested that the presence of observers often disrupted feeding and resting.
For Experiment 3, a running wheel apparatus (Wahmann) was modified such that two wheels were mounted concentrically and open toward each other, to create a feeding chamber (36 cm diameter by 22 cm depth) that allowed modest amounts of lateral movement, similar to the feeding apparatus used in Experiments 1 and 2. The radial surface was made of wire mesh, while the sides were of polished steel. Metal clips were placed at the top so that the wheel could be locked when necessary (such as in baseline recording sessions; see description of Experiment 3), and cardboard notches were attached that produced a soft audible click once per second such that experimenters could turn the wheel at constant velocity and for precise periods of time while watching a timer. Twenty of these cardboard notches were placed on the radial surface of the wheel so that the wheel would be turned manually at 3 rpm. Pilot studies indicated that this velocity was just sufficient to disrupt food intake. A stainless steel drop pan below collected spillage.
2.4. Experiments 1 and 2 (BSS)
Separate groups of subjects were used for AM 251 (Experiment 1; n = 8) and AM 4113 (Experiment 2; n = 8). Subjects were habituated in observation chambers with approximately 15–20 g of Purina Rat Chow (which also served as the test food in all experiments) for five consecutive unrecorded habituation sessions in which subjects were monitored remotely via camera feed. Monitoring of habituation indicated that 40 min sessions were just sufficient to engender the full sequence of feeding, grooming, and resting; therefore 50 min sessions were employed for the remainder of all studies. Beginning the following week, and once per week for the remainder of the study, rats were given one 50 min habituation session, followed the next day by a test session. For both the weekly habituation and test sessions, food was pre-weighed in amounts ranging from 15 to 20 g; enough food was allowed to ensure that animals always had ad lib access in the test chamber. Water was not available in the test cages; extensive piloting revealed only minimal water intake: only 3 of 16 pilot animals exhibited drinking bouts, which occurred in five or fewer 10 s intervals in the entire session. Furthermore, drinking was unaffected by CB1 inverse agonists. On test days (usually Fridays), subjects were injected i.p. in the colony room 30 min prior to testing. Tape recording began and pre-weighed chow was placed in each chamber just before subjects were placed therein. Following the test period, all subjects were returned to the homecage, and food and spillage were collected and reweighed, and the amounts were recorded.
2.5. Experiment 3 (Yoked response competition)
Following Experiments 1 and 2, a new group of rats (n = 8) were habituated in the running wheel apparatus. As with the BSS experiments, pre-weighed food was introduced to the test apparatus just before the subjects were put in. During each 50-min habituation session, the wheel was locked to prevent rotation. As subjects consumed relatively high amounts (typically >8 g) from the very first habituation session, only three such sessions were used. For testing, a yoked procedure was employed in which the time and duration of grooming (including fur cleaning, scratching, biting, forepaw flutter, and wet dog shakes) was taken from each session in Experiment 2. This was done by manually “time-stamping” the beginning and end of every grooming bout or instance of any of the five components of grooming listed above. In each session, experimenters blind to treatment were instructed to turn the wheel at times and durations identical to those for one yoked session in Experiment 2, with the exception that each movement of the wheel was a minimum of 5 s (i.e., a quarter turn). This rule was instituted to assure that all movements of the wheel disrupted feeding, and experimenters confirmed that each scheduled turn produced an overt disruption of any feeding. Experiment 3 was comprised of five test sessions. For each of the eight animals, four of the sessions were yoked to the four AM 4113 sessions of one subject from Experiment 2; therefore, each rat received bouts of disruption in behavior that were identical in duration and number that exhibited by one subject at each dose (2.0, 4.0, 8.0 mg/kg, and vehicle) of AM 4113. Additionally, subjects received these yoked sessions in an order identical to that used in Experiment 2, with the exception of baseline sessions. These baseline sessions were pseudorandomly interspersed within the regimen of yoked sessions and simply consisted of locking the wheel (preventing rotation) during a 50 min feeding session; experimenters were instructed to be present in the room during these sessions. The baseline sessions were necessitated by the fact that uninjected subjects may still experience response competition (such as from grooming) over and above that induced by turning the running wheel; even sessions yoked to the vehicle condition in Experiment 2 necessitated a small amount of disruption by turning the wheel. These sessions therefore measured feeding in the absence of any programmed response competition. No subjects were yoked to performances in Experiment 1 (AM 251), because data analyses (see Section 3) indicated that AM 4113 induced feeding suppression and grooming of greater magnitude at each dose.
2.6. Measures
For all three Experiments, food intake was defined as the difference between pre- and post-session food weights, including spillage. Food intake was the only measure used in Experiment 3. After BSS sessions (Experiments 1 and 2), video-taped sessions were scored by observers blind to treatment and trained to >97% agreement in tallying BSS behaviors. Observers scored each session by tallying eight all-encompassing and mutually exclusive behaviors: feeding, activity, resting, fur cleaning, biting, scratching, paw flutter, and wet dog shakes. Unlike previous studies [15], activity was not divided into rearing, sniffing and locomotion because extensive piloting suggested that the proportion of these behaviors was unaffected by CB1 inverse agonism. In contrast, grooming was divided into fur cleaning, biting, scratching, forepaw flutter, and wet dog shakes based on pilot studies suggesting enhancement of grooming. Eating was defined as gnawing or subsequent chewing of food pellets. Activity was defined as locomotion, sniffing, or rearing. Resting comprised any inactivity, including sleeping. Fur cleaning was defined as licking and palpation of the fur in stereotyped fashion. Biting referred to gnawing (with rapid jaw deflection) of the fur, paws, or tail. Scratching was defined as rapid flutter of the hindpaws toward the fur. Forepaw flutter comprised high-frequency vibration of the forepaw(s). Lastly, wet dog shakes indicated a shiver or vibration of the trunk around the longitudinal axis. These latter five behaviors were combined into grooming for the purpose of BSS analyses. Score sheets divided each session into 300 intervals of 10 s each, and observers were instructed to tally the occurrence of any of the behaviors listed above; thus each interval could have one to eight behaviors tallied. In subsequent analysis using Microsoft Excel, each interval was arbitrarily given a value of one; for instance, if only one of the eight behaviors was noted in an interval, it would receive a value of one, whereas if four different behaviors were observed in a single interval, a value of 0.25 would be assigned to each. These values were summed over ten, 5-min bins. For each behavior, the counts in these 5-min bins ranged from 0 (none of the behavior noted) to 30 (appeared continuously to the exclusion of all other behavior). These counts served as the primary measure of the progression of BSS behaviors.
2.7. Analysis
Data were analyzed in SPSS 15.0. Food intake was analyzed by one-way ANOVA with repeated measures on the dose (Experiments 1 and 2) or yoked treatment (Experiment 3) factor. Significant F ratios in this and all other one-way ANOVAs were further analyzed by nonorthogonal planned comparisons which were performed as post hoc analyses. The number of comparisons was restricted to k−1, where k is the number of treatment conditions [20]; where a main effect of dose was found, each dose was compared to the vehicle condition.
The BSS was analyzed in several ways. First, two-way 4×10 ANOVAs were performed with dose and bin as repeated-measures factors. Significant main effects of dose were interpreted as drug treatment-induced changes in the overall magnitude of a behavior, while dose × bin interactions were taken to mean that drug treatment affected the emergence or decline of a behavior over the session, without necessarily affecting its overall magnitude. Where significant dose × bin interactions were found for eating or resting, followup one-way ANOVAs were performed for the dose factor for each bin. No post hoc analyses of grooming were planned because a multi-tonic effect was expected (rather than an increase or decrease), which would make interpretation of followup analyses difficult. Within each bin, where significant dose effects were found for eating or resting, planned comparisons were used to analyze the effect of drug. Again, where a main effect of dose was found, each dose was compared to the vehicle condition. It was predicted that, if the BSS were preserved, dose-dependent effects on eating and resting would occur at the start and end of the session, respectively. In order to determine whether AM 251 or AM 4113 affected the pattern of grooming, components of grooming (fur cleaning, biting, scratching, forepaw flutter, and wet dog shakes) were also expressed as a percentage of total grooming and analyzed via one-way ANOVA.
3. Results
As expected, AM 251 dose-dependently decreased food intake (F(3,21) = 3.22; P < .05); the mean intake levels can be seen in Table 1. Planned comparisons indicated that the 8.0 mg/kg dose was significantly different from vehicle.
Table 1.
Effects of the CB1 inverse agonist AM 251 on mean session counts of behaviors associated with satiety
| Dose (mg/kg)
|
||||
|---|---|---|---|---|
| Vehicle | 2.0 | 4.0 | 8.0 | |
| Food intake (g)* | 7.13 (0.59) | 5.99 (0.80) | 4.73 (0.70) | 3.771 (0.97)* |
| Eating (counts) | 125.62 (11.60) | 93.95 (16.15) | 87.85 (19.92) | 56.60 (15.58) |
| Resting (counts) | 38.86 (9.94) | 49.79 (7.79) | 33.62 (10.72) | 45.27 (20.16) |
| Activity (counts)** | 134.63 (6.37) | 101.89 (10.06)* | 109.63 (12.88) | 79.74 (11.52)** |
| Fur cleaning (counts)* | 9.24 (1.65) | 26.55 (6.73) | 33.77 (9.89)* | 45.06 (9.42)** |
| Biting (counts)* | 1.17 (0.49) | 7.91 (3.18) | 10.99 (5.01) | 22.78 (7.20)** |
| Scratching (counts)** | 2.06 (0.83) | 18.526 (7.65) | 22.95 (8.90) | 47.85 (10.41)** |
| Forepaw flutter (counts) | 0.47 (0.23) | 0.40 (0.18) | 0.06 (0.04) | 0.81 (0.48) |
| Wet dog shakes (counts) | 0.13 (0.08) | 0.79 (0.52) | 0.88 (0.40) | 1.61 (0.63) |
| Total grooming (counts)* | 13.06 (2.17) | 54.18 (17.20) | 68.65 (21.36) | 118.11 (25.17)** |
Significant dose effects for each measure were followed by planned comparisons; indicated group means were significantly different from the vehicle condition
P < .05;
P < .01).
Fig. 1 shows the three main behaviors of the BSS (A: feeding, B: grooming [including fur cleaning, biting, scratching, forepaw flutter, and wet dog shakes], and C: resting) at each dose of AM 251. Each measure was subject to a two-way ANOVA of the dose and bin factors. Somewhat surprisingly, in spite of the dose-dependent decrease in intake, AM 251 did not significantly alter feeding counts (F(3,21) = 2.61; n.s.); however, a significant main effect of bin (F(9,63) = 28.41; P < .001) was found, as was a significant dose × bin interaction (F(27,189) = 1.64; P < .05). This suggests that feeding decreased over the course of the session, and that this pattern was altered by AM 251. As a significant interaction was found for feeding, one-way ANOVAs with repeated measures on the dose factor were performed on each of the 10 bins. Using these analyses, main effects of dose were found in the first three bins (comprising the first 15 min of the session), as well as bin 5. Planned comparisons were used in post hoc analysis; the doses that significantly suppressed feeding relative to vehicle are shown in Fig. 1(A).
Fig. 1.
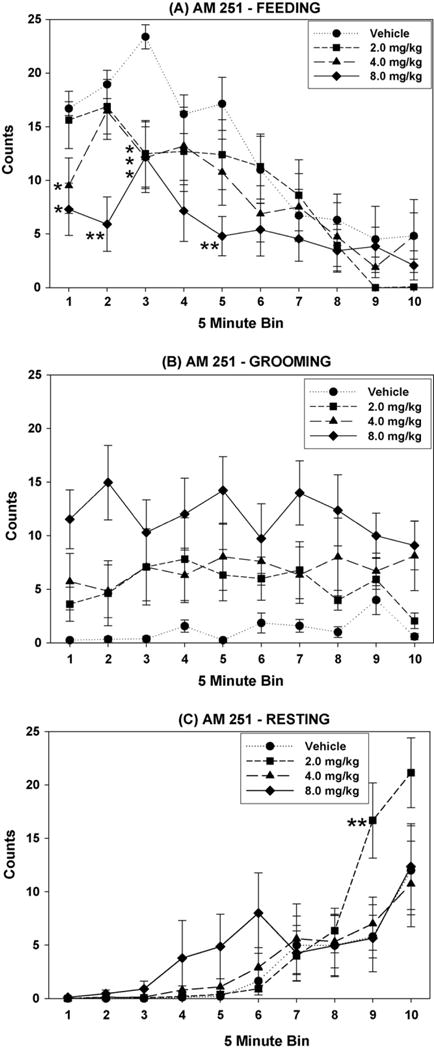
Effects of the CB1 inverse agonist AM 251 are shown on the three primary behaviors of the behavioral satiety sequence (BSS), feeding (A), grooming (B), and resting (C). Dose × bin interactions are noted for feeding (A) and resting (C), and a significant dose effect of grooming was found (B). Analysis of the dose factor in each bin revealed dose-dependent decreases in feeding in bins 1, 2, 3, and 5, and an increase in resting in bin 9 (*P < .05, **P < .01 difference from vehicle via planned comparisons).
In contrast to eating, a main effect of grooming was found for the factor of dose (Fig. 1(B); F(3,21) = 4.67; P < .05), and planned comparisons indicated that grooming was significantly greater at the 8.0 mg/kg dose than at the vehicle dose. Neither a main effect of bin (F(9,63) = 0.77; n.s.), nor a significant interaction of dose and bin (F(27,189) = 1.03; n.s.) were found for the measure of grooming.
As with feeding, no dose effect was found for the resting measure (F(3,21) = 0.70; n.s.), while the main effect of bin was significant (F(9,63) = 22.79; P < .001). A dose × bin interaction was also found for resting (F(27,189) = 1.99; P < .01), indicating that while AM 251 did not enhance post-prandial resting overall, the significant pattern of emergence in resting over time was altered. The significant interaction also permitted analysis of main effects of dose within each bin; in this analysis, a dose effect was only found within bin 9. Fig. 1(C) shows that the 2.0 mg/kg dose significantly enhanced resting relative to vehicle. As seen in Table 1, counts of activity (which included all non-BSS behaviors) were decreased by AM 251 (F(3,21) = 4.64; P < .05). Planned comparisons revealed differences from vehicle levels of activity at doses of 2.0 (P < .05) and 8.0 (P < .01), but not 4.0 mg/kg. Analysis of the main effect of bin yielded a significant result (F(9,63) = 4.41; P < .001), but that of the dose × bin interaction did not (F(27,189) = 1.48; n.s.).
As can be seen in Table 1, fur cleaning (F(3,21) = 3.394; P < .05) was dose-dependently increased. Both the 4.0 and 8.0 mg/kg doses were different from vehicle, according to planned comparisons. For the biting measure, a significant effect of dose (F(3,21) = 3.66; P < .05) was found. Analyses using planned comparisons revealed a difference between 8.0 mg/kg AM 251 and vehicle (P = .01). Scratching counts were also dose-dependently enhanced (F(3,21) = 5.27; P < .01); as with the biting measure, the 8.0 mg/kg dose was significantly different from vehicle (P < .01). AM 251 did not produce a significant dose effect for either forepaw flutter (F(3,21) = 1.08; n.s.) or wet dog shakes (F(3,21) = 1.72; n.s.). No significant effects were found for the main effect of bin, nor for the dose × bin interaction, for any grooming measure. To further analyze dose-related alterations in grooming patterns, the five component behaviors were converted to a percentage of all grooming behaviors in each session, and analyzed with one-way ANOVA. These analyses (Fig. 2) revealed that counts of fur cleaning (F(3,21) = 4.00; P < .05) decreased, while scratching increased (F(3,21) = 5.51; P < .01), with differences from vehicle apparent at the 8.0 mg/kg dose for both measures. Furthermore, an increase in biting fell just short of significance (F(3,21) = 2.87; P = .06), but a linear dose-related trend was found (F(1,7) = 13.05; P < .005). While raw counts of forepaw flutter were not affected by AM 251, the proportion of this behavior significantly decreased (F(3,21) = 3.50; P < .05), and all doses were significantly lower than the vehicle condition. Analysis of wet dog shakes (F(3,21) = 0.40; n.s.) as a percentage of grooming produced nonsignificant results.
Fig. 2.
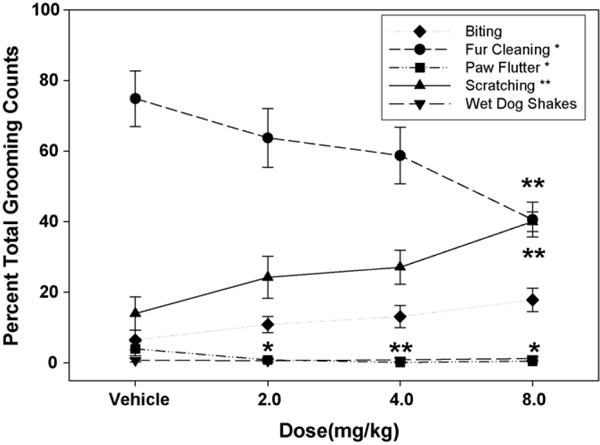
Analysis of grooming components indicated a dose-dependent change in the profile of grooming with AM 251. Each component is expressed as a percentage of the total. Fur cleaning decreased, while scratching increased (*P < .05, **P < .01 difference from vehicle via planned comparisons). One-way ANOVA did not yield a dose effect of biting, but a significant linear trend was found.
In Experiment 2, AM 4113 suppressed food intake (F(3,21) = 17.925; P < .001), as can be seen in Table 2. Planned comparisons revealed that the 2.0, 4.0, and 8.0 mg/kg doses produced significantly less consumption than vehicle (P’s < .01).
Table 2.
Effects of the CB1 antagonist AM 4113 on mean session counts of behaviors associated with satiety
| Dose (mg/kg)
|
||||
|---|---|---|---|---|
| Vehicle | 2.0 | 4.0 | 8.0 | |
| Food intake (g)** | 6.50 (1.27) | 3.91 (0.49)** | 1.94 (0.62)** | 1.23 (0.26)** |
| Eating (counts)** | 102.40 (21.38) | 55.89 (11.31)** | 31.65 (6.91)** | 34.30 (9.19)** |
| Resting (counts) | 58.96 (30.13) | 48.81 (12.63) | 53.44 (22.41) | 49.09 (18.16) |
| Activity (counts) | 123.88 (16.30) | 89.96 (12.94) | 90.73 (15.46) | 84.53 (8.80) |
| Fur cleaning (counts)** | 12.04 (3.23) | 47.87 (8.69)** | 45.464 (7.05)** | 55.17 (9.68)** |
| Biting (counts)** | 0.90 (0.37) | 17.70 (5.64)** | 24.31 (5.40)** | 23.35 (4.47)** |
| Scratching (counts)** | 1.73 (0.67) | 40.89 (7.68)** | 53.16 (8.79)** | 50.96 (8.08)** |
| Forepaw flutter (counts) | 0.06 (0.06) | 0.60 (0.30) | 0.89 (0.41) | 0.41 (0.14) |
| Wet dog shakes (counts)** | 0.06 (0.06) | 1.728 (0.45)** | 2.16 (0.61)** | 1.74 (0.42)** |
| Total grooming (counts)** | 14.80 (3.55) | 108.78 (20.77)** | 125.98 (19.01)** | 131.63 (19.77)** |
Asterisks following measures indicate significant dose effects; asterisks following group means indicate a significant difference with the vehicle condition via planned comparisons
P < .01
As seen in Fig. 3(A), two-way dose × bin ANOVA indicated significant main effects of dose (F(3,21) = 10.05; P < .001), bin (F(9,63) = 2.92; P < .01), and a significant interaction (F(27,189) = 2.38; P < .001) for the measure of eating. Subsequent analysis of the dose factor suggested significantly fewer eating counts at the 2.0, 4.0, and 8.0 mg/kg doses, compared with vehicle. Tests of the simple dose effects at each bin revealed significant differences in bins 1–4 (representing the first 20 min of the session), as well as in bin 7. Fig. 3(A) shows the results of followup analyses of significant main effects of dose, in which eating counts for the each dose of AM 4113 were compared to those in the vehicle condition.
Fig. 3.
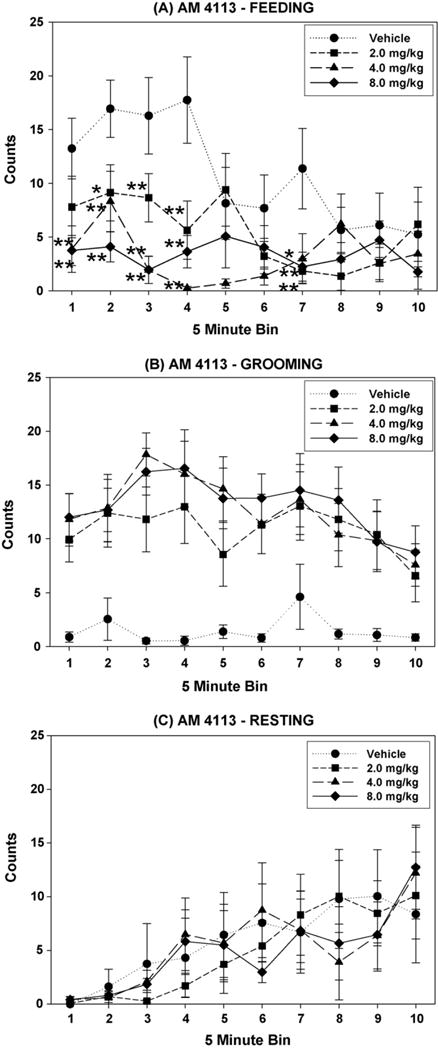
Effects of the CB1 antagonist AM 4113 are shown for the progression of feeding (A), grooming (B), and resting (C) on the BSS. A main effect of dose and dose × bin interaction were found for feeding, and dose-dependent decreases in feeding are found in bins 1–4 and 7 (*P < .05, **P < .01 difference from vehicle via planned comparisons). Grooming (B) was significantly enhanced, but resting (C) was unaffected.
As with AM 251, grooming was dose-dependently enhanced by AM 4113 (Fig. 3(B); F(3,21) = 10.65; P < .001). Planned comparisons indicated a significant enhancement of grooming between the 2.0, 4.0, and 8.0 mg/kg doses, and the vehicle condition. A bin effect was also found (F(9,63) = 3.08; P < .01), but not an interaction (F(27,189) = 0.83; n.s.).
As shown in Fig. 3(C), no significant dose effect of resting was found (F(3,21) = 0.05; n.s.). A significant overall effect of bin was found (F(9,63) = 9.10; P < .001). As there was no interaction between dose and bin (F(27,189) = 0.67; n.s.), analyses of dose effects were not performed. Analysis of activity yielded a significant main effect of bin (F(9,63) = 2.76; P < .01), but no effect of dose (F(3,21) = 1.54; n.s.), and no dose × bin interaction (F(27,189) = 1.27; n.s.).
As seen in Table 2, fur cleaning (F(3,21) = 7.96; P < .001), biting (F(3,21) = 7.14; P < .005), scratching (F(3,21) = 10.82; P < .001), and wet dog shakes (F(3,21) = 4.52; P < .05) were dose-dependently enhanced by AM 4113, and for all behaviors all dose levels were significantly different from vehicle, according to planned comparisons. No significant effects were found for paw flutter (F(3,21) = 1.89; n.s.). There was a significant main effect of bin (F(9,63) = 5.62; P < .001) and a dose × bin interaction (F(27,189) = 1.70; P < .05) for scratching; no other grooming component produced bin effects or an interaction. As seen in Fig. 4, AM 4113 decreased fur cleaning as a percentage of all grooming counts (F(3,21) = 15.02; P < .001). All doses of AM 4113 produced a significant decrease compared with vehicle, according to planned comparisons. Compensatory increases are seen in biting (F(3,21) = 4.23; P < .05), scratching (F(3,21) = 14.53; P < .001), and wet dog shakes (F(3,21) = 6.59; P < .005), but not forepaw flutter (F(3,21) = 0.89; n.s.). For the biting, scratching, and wet dog shakes measures, all three doses of AM 4113 significantly enhanced behavior, even as a percentage of grooming (P’s < .05).
Fig. 4.
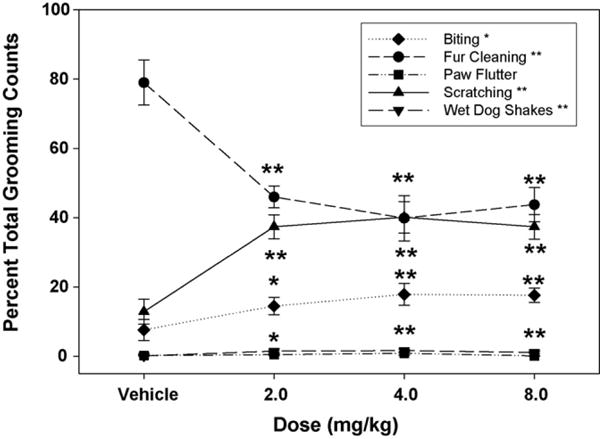
AM 4113 decreases the predominance of fur cleaning as a percentage of all grooming behaviors. Significant compensatory increases are found in biting, scratching, and wet dog shakes (*P < .05, **P < .01 difference from vehicle condition via planned comparisons).
Lastly, Fig. 5 shows mean food intake at each level of yoked behavioral disruption in Experiment 3. Baseline performance was defined as a feeding session with no programmed interruptions, while the other four conditions correspond to wheel running in uninjected rats that was yoked to grooming behavior at each dose level of AM 4113 in Experiment 2. Food intake was not affected by this manipulation (F(4,28) = 1.13; n.s.).
Fig. 5.
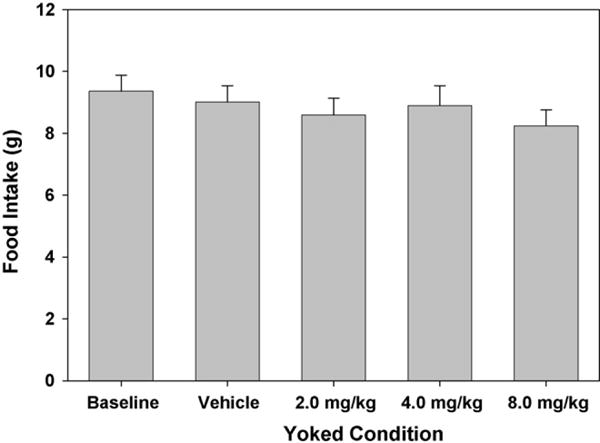
Response competition with eating was manipulated by forcing locomotion in a modified running wheel at identical times and durations to grooming recorded in the AM 4113 study (Experiment 2). The Baseline condition was not yoked to Experiment 2 and consisted of uninterrupted feeding sessions. Response competition did not significantly inhibit food intake.
4. Discussion
AM 251 and AM 4113 reduced food intake and appeared to affect the BSS similarly. The satiety sequence was generally preserved by both drugs (i.e., a peak in eating was observed before a peak in resting), with the possible exception of the 8.0 mg/kg dose of AM 4113. For both compounds, ANOVA of the main effect of dose in each bin revealed dose-dependent reductions in feeding during the earlier (but not later) bins (Figs. 1 and 3). For AM 251, post hoc analysis of the main effect of dose indicated that only 8.0 mg/kg suppressed food intake; however, analyses of the dose effects within each bin suggested that bin 1 was affected by 4.0 as well as 8.0 mg/kg, and bin 3 (in which feeding was maximal in the vehicle condition) was affected by all three doses (Fig. 1(A)). While neither drug produced a significant change in resting, main effects of bin were found, and followup analyses (not shown) revealed main effects of bin at every dose level for both drugs, indicating that resting counts increased as session progressed. A significant dose × bin interaction was found for AM 251-treated animals, and post hoc analysis revealed that 2.0 mg/kg enhanced resting in bin 9 (P < .01), as seen in Fig. 1(C). Taken together, 2.0 mg/kg AM 251 appears to decrease latency to rest (consistent with behavioral satiety), but this pattern does not occur at higher doses. It is noteworthy that this potential disruption in resting occurred at the dose (8.0 mg/kg) that suppressed food intake, although as indicated, 2.0 and 4.0 mg/kg significantly reduced intake in at least one of the early bins. While a significant bin effect was found in the AM 4113 group, and resting increased in a time-dependent fashion, resting counts did not increase by dose as feeding counts decreased. This is contrary to the behavioral profile of satiety [15] and may indicate disrupted resting. As AM 251 enhanced grooming and did not increase resting at the dose that suppressed food intake, the differences between the effects of the two drugs is likely due to potency rather than qualitative differences in their actions (however, it should be noted that past reports (e.g., [21,22]) have found significant reductions in intake with lower doses of AM 251). This is in contrast with the effects of these drugs on taste reactivity, a marker of nausea. In that type of procedure, AM 251, but not AM 4113, induced conditioned gaping and chin rubbing at anorectic doses [22,28], suggesting that inhibition of food intake is dissociable from induction of nausea, and not likely a secondary effect of nausea. In the current design, the disruption in resting occurred in both compounds at anorectic doses.
Another, possibly related, similarity between the two compounds is the marked effects on grooming, particularly scratching and biting behavior. This is consistent with previous reports on the CB1 inverse agonists SR 141716A [29] and AM 251 [30], but the current paper is believed to be the first report of these effects by a CB1 neutral antagonist that has been demonstrated to lack intrinsic activity [3,7,28]. For both compounds, fur cleaning, biting, and scratching are increased and predominate over eating and resting at anorectic doses. Wet dog shakes were significantly increased by AM 4113, although they were a low-incidence behavior even at high doses. Moreover, as can be seen in Figs. 2 and 4, AM 251 and AM 4113 respectively altered the profile of grooming. Fur cleaning, the predominant part of grooming in vehicle-treated and noninjected (data not shown) animals, dose-dependently gave way to scratching, and to a lesser extent, biting. This altered pattern of behaviors is likely to be distinguishable from the effects of dopamine D1 agonists [1,9], which reduce food intake but enhance stereotyped grooming (i.e., orderly grooming in which fur cleaning and palpation remain predominant; [4]).
As has been suggested [29,30], it is possible that AM 251 and AM 4113 do not suppress appetite over the doses tested, but simply induce a great deal of grooming behavior, with which food intake is mutually exclusive. Indeed, normal intake of solid food (e.g., rat chow) typically involves an upright posture in which rats balance on the hindpaws while grasping and turning food pellets with the forepaws [22]. It is possible that competing responses could easily disrupt a feeding sequence, even in food-restricted animals. This response competition hypothesis is bolstered by the fact that grooming appears to be enhanced at or possibly below anorectic doses of AM 251 and AM 4113. Additionally, while the BSS was preserved, manipulations that induce satiety increase resting as eating decreases [15], while AM 251 and AM 4113 did not affect, and in some cases decreased, resting. Experiment 3 was designed to test whether direct response competition (i.e., forced locomotion in a running wheel) was sufficient to produce an inhibition in food intake of the magnitude of that produced by AM 4113. Disruption from forced locomotion in this experiment was modeled after the AM 4113 study; of the two compounds tested, AM 4113 produced the greater magnitude of effect on food intake reduction and induction of grooming at each dose level. It was therefore considered reasonable that findings would apply to the effects of AM 251 as well. Response competition via wheel turning was not found to inhibit food intake (Fig. 5). Observers blind to the yoked drug treatment were trained to ensure that each wheel turn disrupted ongoing feeding, and that rats did not eat during the specified times, yet it appeared that the food-restricted rats simply compensated for the frequent, sometimes long-lasting disruption, possibly by increasing the rate of intake, or by reducing time spent in other, unmeasured behaviors. It can therefore be concluded that simple response competition of the frequency and duration induced by AM 4113 does not necessarily account for most or all of the reduction in food intake. It is also known that excessive grooming (or its as yet-undetermined cause) likely does not interfere with feeding-related motor control, as anorectic doses of AM 251 produce no alterations to feeding rate or forepaw usage during feeding [22]. Lastly, reports of reduced appetite are also found in clinical trials [24], suggesting that rimonabant reduces motivation for food, at least in humans, and this is likely to account for much of the reduction in food intake.
Nevertheless, the patterns of BSS behaviors were altered by both drugs in a manner that is quite different from natural satiation, and interference from excessive grooming may affect other measures than feeding. It is possible that this effect explains why resting was not dose-dependently increased, as is found in satiated animals; blind observers reported anecdotally that the scratching often abruptly ended resting bouts, but not eating bouts. Response competition from grooming may also explain the reduction in activity, and reduced locomotion in other designs [18,22,28]. However, it should be noted that AM 4113 did not significantly reduce activity counts in spite of being the more potent of the two drugs on all grooming measures, suggesting that reductions in locomotion seen with CB1 antagonism [28] may not simply be secondary to response competition from grooming. Regardless of the extent to which the induction of grooming interferes with other behaviors, it may represent a notable side effect of CB1 receptor blockade, although it is unclear how (or whether) it would manifest as a side effect in humans undergoing pharmacotherapy targeting the CB1 receptor. Further inquiries into the nature of the grooming effect are therefore necessary to determine if this effect can be uncoupled from the anorectic effect. For example, if the site of action of grooming is central while that of the feeding effect and weight loss is peripheral [13], it would be worthwhile to develop CB1 antagonists with limited ability to cross the blood–brain barrier. While the striatum, which exhibits extremely dense CB1 binding [10] has been suggested as a potential site for the grooming effect [30], peripheral irritation is also a possible explanation [19]. The current paper also indicates that, as both grooming and feeding were affected by the neutral antagonist AM 4113, it is possible that tonic endocannabinoid release is normally involved in the suppression of scratching behavior in rats, as well as regulation of food intake.
Acknowledgments
This work was supported by EUP Senate research grants to PJM. The authors gratefully acknowledge the technical assistance of Dr. Charles Edwards.
References
- 1.Al-Naser HA, Cooper SJ. A-68930, a novel, potent dopamine D1 receptor agonist: a microstructural analysis of its effects on feeding and other behaviour in the rat. Behav Pharm. 1994;5:210–8. [PubMed] [Google Scholar]
- 2.Arnone M, Maruani J, Chaperon F, Thiebot M-H, Poncelot M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 3.Bergman J, Delatte MS, Paronis CA, Vemuri K, Thakur GA, Makriyannis A. Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol Behav. 2008;93(4–5):666–70. doi: 10.1016/j.physbeh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge KC, Aldridge JW. Super-stereotypy I: enhancement of a complex movement sequence by systemic dopamine D1 agonists. Synapse. 2000;37(3):194–204. doi: 10.1002/1098-2396(20000901)37:3<194::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 5.Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB)1 receptor antagonist, AM251, causes a sustained reduction of daily food intake on the rat. Physiol Behav. 2004;82(5):863–9. doi: 10.1016/j.physbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Chambers AP, Koopmans HS, Pittman QJ, Sharkey KA. AM251 produces sustained reductions in food intake and body weight that are resistant to tolerance and conditioned taste aversion. Br J Pharmacol. 2006;147:109–16. doi: 10.1038/sj.bjp.0706439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, et al. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293(6):R2185–93. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- 8.Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR141716A. Life Sci. 1998;63:113–7. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- 9.Cooper SJ, Francis J, Rusk IN. The anorectic effect of SK&F 38393, a selective dopamine D1 receptor agonist: a microstructural analysis of feeding and related behaviour. Psychopharmacology (Berl) 1990;100(2):182–7. doi: 10.1007/BF02244403. [DOI] [PubMed] [Google Scholar]
- 10.Egertová M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1. J Comp Neurol. 2000;422(2):159–71. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530(1/2):103–6. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 123I-labeled AM 251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–8. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- 13.Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–7. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halford JCG, Lawton CL, Blundell JE. The 5-HT2 receptor agonist MK-212 reduced food intake and increases resting but prevents the behavioural satiety sequence. Pharmacol Biochem Behav. 1997;56(1):41–6. doi: 10.1016/S0091-3057(96)00152-9. [DOI] [PubMed] [Google Scholar]
- 15.Halford JCG, Wanninayake SCD, Blundell JE. Behavioral satiety sequence (BSS) for the diagnosis of drug action on food intake. Pharmacol Biochem Behav. 1998;61(2):159–68. doi: 10.1016/s0091-3057(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 17.Ishii Y, Blundell JE, Halford JC, Rodgers RJ. Palatability, food intake and the behavioural satiety sequence in male rats. Physiol Behav. 2003;80(1):37–47. doi: 10.1016/s0031-9384(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 18.Järbe TUC, Andrzejewski ME, Di Patrizio NV. Interactions between the CB1 receptor agonist Δ9-THC and the CB1 receptor antagonist SR-141716 in rats: Open field revisited. Pharmacol Biochem Behav. 2002;73(4):911–9. doi: 10.1016/s0091-3057(02)00938-3. [DOI] [PubMed] [Google Scholar]
- 19.Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316(5830):1494–7. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- 20.Keppel G, Wickens TD. Design and analysis: a researcher’s handbook. 4th. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. [Google Scholar]
- 21.McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 Antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14(8):583–8. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salam-one JD. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology (Berl) 2005;180(2):286–93. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin PJ, Quan L, Wood JT, Wisniecki A, Winston KM, Swezey LA, et al. Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM 1387. Pharmacol Biochem Behav. 2006;83(3):396–402. doi: 10.1016/j.pbb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Pi-Sunyer FX, et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295(7):761–75. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, et al. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–4. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 26.Rutkowska M. The effect of AM 251, a cannabinoid CB1 receptor antagonist, on food intake in rats. Acta Pol Pharm. 2004;61(5):401–3. [PubMed] [Google Scholar]
- 27.Salamone JD, McLaughlin PJ, Sink KS, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: Effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91(4):383–8. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, et al. The novel cannabinoid CB(1) receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33(4):946–55. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tallett AJ, Blundell JE, Rogers RJ. Grooming scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology (Berl) 2007;195(1):27–39. doi: 10.1007/s00213-007-0880-2. [DOI] [PubMed] [Google Scholar]
- 30.Tallett AJ, Blundell JE, Rogers RJ. Acute anorectic response to cannabinoid CB1 receptor antagonist/inverse agonist AM 251 in rats: indirect behavioural mediation. Behav Pharmacol. 2007;18:591–600. doi: 10.1097/FBP.0b013e3282eff0a9. [DOI] [PubMed] [Google Scholar]


