Abstract
Cannabinoid CB1 antagonists are widely known to reduce motivation for food, but it is not known whether they induce satiety or reduce reward value of food. It may therefore be necessary to compare effects of altered satiety and reward food value in the same appetitive task, and determine whether CB1 antagonism produces a behavior pattern similar to either, both, or neither. A fine-grained analysis of fixed-ratio 10 (FR10) responding for palatable food initially included number and duration of, and between, all lever presses and food tray entries in order to differentiate the pattern of suppression of prefeeding from that caused by reducing the reward value of the pellets with quinine. Discriminant function analysis then determined that these manipulations were best differentiated by effects on tray entries, pellet retrieval latencies, and time of the first response. At 0.5 mg/kg, AM 6527 produced similar effects to reducing reward value, but at 1.0 and 4.0 mg/kg, effects were more similar to those when animals were satiated. We conclude that AM 6527 both reduced reward value and enhanced satiety, but as dose increased, effects on satiety became much more prominent. These findings contribute to knowledge about the behavioral processes affected by CB1 antagonism.
Keywords: Cannabinoid, motivation, multivariate analysis, obesity, reward
Introduction
Cannabinoid CB1 antagonists and inverse agonists reduce food intake in humans and other animals (McLaughlin, 2012; Sharma et al., 2015). Extensive analysis of this phenomenon has ruled out several undesirable explanations, including nausea/malaise (Sink et al., 2008), motor slowing (McLaughlin et al., 2005b), and response competition (Wright and Rodgers, 2013), strongly implicating aspects of appetitive motivation. Furthermore, clinical studies indicate self-reported increased likelihood of staying on a diet, and reduced appetite during treatment with the CB1 inverse agonist rimonabant (SR 141716A; Scheen et al., 2006).
In spite of these consistent findings on feeding and food-seeking behavior across species, the behavioral process behind the findings is not clear. Several lines of research suggest CB1 mediation of hedonic value of food, as well as of other reinforcers, including drugs of abuse. For instance, microanalysis of lick rate (Higgs et al., 2003; Sanchis-Segura et al., 2004) revealed rimonabant- and CB1 knockout-induced changes that matched the behavioral profile of animals licking for sucrose solution that was adulterated with quinine, which reduced its hedonic value (Davis and Levine, 1977). Similarly, in an analysis of the behavioral satiety sequence, the CB1 inverse agonist AM 251 and neutral antagonist AM 4113 reduced feeding at the beginning of the session, and resting at the end (Hodge et al., 2008), a behavioral profile also produced by quinine-adulterated food (Ishii et al., 2003a). AM 251 also enhanced aversive reactions to quinine and reduced THC-induced hedonic reaction to sucrose (Jarrett et al., 2007).
On the other hand, CB1 antagonists have also been found to inhibit intake of food regardless of apparent palatability (Madsen et al., 2009; McLaughlin et al., 2003, 2006; Verty et al., 2004), which is more consistent with the induction of satiety than reduced hedonic value. Similarly, in an analysis of lick rate for high-fat solution, rimonabant produced a behavioral profile more similar to prefed animals than to those provided with quinine-adulterated solution (Thornton-Jones et al., 2007). Moreover, the satiety hormone cholecystokinin may work in part by negatively modulating expression of CB1 receptors in gut afferents (Dockray, 2009), and resistance to leptin, which induces satiety, is reduced by CB1 inverse agonism (Tam et al., 2012). It is also possible that CB1 blockade produces its hypophagic effects via multiple mechanisms, as rimonabant had greater effects on a second-order schedule of responding for food that those seen in prefed animals, suggesting more effects than those on satiety alone (Thornton-Jones et al., 2005).
To resolve this conflict, we argue that it is necessary to compare the effects of satiety and reduced hedonic value on the same appetitive behavior, in the present study, food-maintained operant responding. Manipulating these processes is already known to reduce such responding (Ettinger and Staddon, 1983; Gill and Cain, 2011; Horstmann et al., 2015; Mathes et al., 2013), but we sought to analyze the rate and duration of operant lever pressing and food tray entries in order to create profiles of responding unique to either satiety or reduced hedonic value. Once established, response suppression caused by AM 6527 in the same subjects could be assessed simultaneously for the extent to which its effects better matched either nondrug manipulation. Similar microanalyses have been used to determine whether other response rate-altering manipulations affect behavior in similar or functionally distinct ways (Brown and Seiden, 1975; Carriero et al., 1998; Fowler et al., 1991; McLaughlin et al., 2010; Salamone et al., 1995; Varvel et al., 2002). We used AM 6527 because it is a newer neutral CB1 antagonist with oral bioavailability (Limebeer et al., 2010; Sink et al., 2009; Wills et al., 2014).
The present study used stepwise multivariate discriminant function analysis of fixed-ratio 10 (FR10) responding for palatable 45 mg food pellets, and three manipulations administered within-subjects that were anticipated to reduce responding: AM 6527 (0.5–4.0 mg/kg); satiety (the prefeeding condition); and reduced hedonic value (the quinine condition).
Materials and methods
Subjects
Sixteen adult male Sprague-Dawley rats (Hilltop Labs, Scottdale, PA, USA) were used, and were pair-housed in plastic cages. Lights in the animal colony were on 08.00–20.00 h and all procedures were carried out at approximately 12.00 h. Upon arrival, animals were allowed ad lib access to lab chow, which was reduced to ~20 g per day after training began one week later, and then to ~5 g lab chow in addition to ~10–15 g operant pellets from daily operant sessions, approximately two weeks later when all body weights exceeded 250 g. This permitted weight gain (to approximately 350–400 g) during the course of the study, while incorporating enough food restriction to engender nearly 2000 responses per session. Water was freely available except during operant sessions. Animal protocols were approved by the Institutional Animal Care and Use Committee of Edinboro University of Pennsylvania (Protocol #20111), and were in accord with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011).
Materials
AM 6527 (Center for Drug Discovery, Northeastern University, Boston, MA, USA) is an orally-bioavailable pyrazole-3-car-boxamide analog of AM 251 with nanomolar CB1 receptor affinity and nearly hundred-fold preference for CB1 compared with CB2 (Sink et al., 2009). It was dissolved in DMSO, Tween-80 (both Fisher Scientific, Pittsburgh, PA, USA), and brought to volume in 0.9% saline, for a final concentration of 15%, 15%, and 70%, respectively. This solution also served as vehicle control. Injections were delivered via the i.p. route in a volume of 1.0 mL/kg, 30 min prior to operant test sessions. Pretreatment time and doses of 0.5, 1.0, and 4.0 mg/kg were selected based on previous research (Sink et al., 2009), as well as pilot studies to approximate the levels of response suppression produced by the other manipulations.
Palatable operant pellets (#F0042, Bio-Serv, Frenchtown, NJ, USA) served as the substrate of reinforcement. For the quinine conditions, these pellets were custom-formulated with quinine in concentrations of 0.4, 1.0, and 2.0 gquinine/kgpellet but were designed to maintain the color, shape, and odor of typical operant pellets. Concentrations were determined by extensive piloting to produce comparable response suppression to the other two manipulations.
Apparatus and procedure
Coulbourn Instruments (Whitehall, PA) operant boxes contained one response lever to the left of a centrally-mounted food tray. A computer running Windows XP controlled pellet delivery and recorded lever presses and tray entries via an infrared detector at the aperture of the food tray. Operant sessions lasted 30 min per day; rats were trained five days per week. Animals were given approximately one gram of operant pellets the evening before training began, to reduce neophobia, but were not exposed to the quinine operant pellets prior to testing. After one session of magazine training, animals began a CRF schedule; upon 100 responses in a session, this was changed to FR5, and then to FR10 upon a session with at least 400 responses on FR5. Test procedures began when no rat had more than three consecutive sessions of increased responding.
The testing regimen involved two test sessions per week (Tuesdays and Fridays), each preceded by two baseline training sessions; rats were therefore run six days per week but only submitted to test procedures twice per week, every three or four days. Analysis of training sessions on days following test procedures indicated no significant differences from pre-testing baselines.
The experimental design used two (3 × 4) factors that were varied within-subjects, because pilot data indicated that discriminant function analysis (DFA) was sensitive to idiosyncratic differences between subjects that would not be an issue in a within-subjects design. The first factor, treatment, included AM 6527, prefeeding, and quinine conditions, and was administered by dividing the 16 animals into three cohorts of 8, 4, and 4, each receiving a different treatment on every test day in order to randomize treatment order. Within each cohort, four levels (control, low, medium, and high) of each treatment were administered in a pseudorandomized, counterbalanced fashion to ensure no order effects. Testing continued until all animals were exposed to all 12 (i.e., 3 × 4) combinations of treatment and level; with two tests per week, testing took six weeks. Levels of AM 6527 were the doses as described above. The prefeeding treatment was administered by providing operant pellets in small metal containers 3 h before session in quantities of 0.0, 4.5, 9.0, and 18.0 g per rat, with containers removed 5 min prior to test. The quinine treatment was administered within the pellets as described above and placed in the operant chamber pellet hopper prior to test session. For the quinine manipulation, unadulterated operant pellets served as the control condition (i.e., a 0.0 g/kg quinine condition).
Statistical analysis
All analyses were performed in SPSS 21. Overall responding (i.e., number of responses in each 30-min session) was analyzed using a 3 × 4 (treatment × level) repeated-measures analysis of variance (ANOVA). An interaction in this test would permit 3 × 4 repeated-measures ANOVA of variables within the operant session that could potentially discriminate between Treatments, as has been done previously (McLaughlin et al., 2005a, 2010). Significant interactions were followed by simple main effects of level; where these were significant, non-orthogonal planned comparisons (Keppel and Wickens, 2004) between Control and each of the three other Levels were used as a post hoc test.
Ten behavioral measures were recorded that did not overlap with each other in time, but together accounted for all recordable activity within the operant session. First response time (TFR) and last response time (TLR) were measured in seconds from the start of each session. Response duration (RD) and tray duration (TD) were both measured in milliseconds. To ensure normality for analysis, RDs over 800 ms and TDs over 1000 ms were capped by being recorded as those respective values. For these duration measures, caps were placed at the approximate point that limited the most extreme 2% of values. Empty tray entries (ETEs) were defined as food tray entries excluding those to obtain reinforcers (which would simply be an outcome of overall response rate). Length of post-reinforcement pauses (PRP, in s) was capped at 30 s, similar to other duration measures. Inter-response time (IRT, in ms) excluded responses resulting in reinforcement. As has been done previously, IRTs were limited in time to distinguish from longer pauses in responding (McLaughlin et al., 2005b, 2010); IRTs longer than 500 ms were recorded as response pause length (PL, in s), which was capped at 30 s.
Retrieval latency (RLat, in ms), or time to retrieve the reinforcer after the final response in a burst, was capped at 1000 ms. During training, but prior to analysis, it was discovered that animals often made more than the required ratio of 10 responses during each response burst; therefore, the measure responses after pellet was compared across conditions as well.
Univariate ANOVA was followed by DFA, including as possible predictors any measure that produced significant interactions of Treatment and Level. Use of only predictors with significant interactions was done to lessen the possibility of treatments being discriminated by a function to which too many predictors were overfit; furthermore, a stepwise procedure was used in which predictors were entered or removed according to maximum reduction in Wilks’ λ at each step. Entry criterion was p < .15, and predictors were removed if p > .20, as has been recommended (Costanza and Afifi, 1979).
DFA creates one or more functions (assessed via chi-square with α = .05) that describe the strongest relationship between the predictors and treatments; that is, to determine whether the pattern of response suppression under prefeeding was distinguishable from that caused by quinine. The central question was whether AM 6527 caused animals to respond in a manner more consistent with satiety or reduced hedonic value. This was answered in two ways. First, we performed one-way ANOVA on discriminant scores (i.e., scores resulting when discriminant functions, which were linear combinations of significant predictors, were solved for each case in the data set) to determine whether there were differences between treatments, followed by Tukey’s HSD. Secondly, individual AM 6527 cases were classified according to the function(s) as being either prefeeding-like or quinine-like. These classifications were assessed with a chi-square goodness-of-fit test, which tested the null hypothesis that cases were evenly distributed and therefore could not be classified as either prefeeding-like or quinine-like.
DFA was first performed on all prefeeding and quinine cases, collapsed across level, in which number of responses in a session was lower than the minimum value found in the control conditions. This was done so that cases were included only if they exhibited some degree of reduced responding, and was termed the overall analysis. Following this, analyses were performed at each of the low, medium, and high levels in order to observe the emergence of dose-dependent group differences. Only the predictors retained in the overall stepwise analysis were used in these analyses by level. No cutoff of response values was employed in these analyses.
The purpose of excluding AM 6527 data from the DFA was to find measures that best distinguished between response suppression caused by satiety from that caused by reduced hedonic value, and then to force AM 6527 to be classified as one or the other. In so doing, we sought to reveal and distinguish phenotypes of satiety and reduced hedonic value that were not influenced by drug effects. On the other hand, initially including AM 6527 with the other treatments in the DFA would also determine the extent to which drug effects were different from both nondrug treatments. To do this, and also to confirm results of the overall analysis, an analysis was then run that included AM 6527 cases (also collapsed across level) in creation of the function(s). Results were then analyzed as described above, except that cases could now be classified as AM 6527-like.
Results
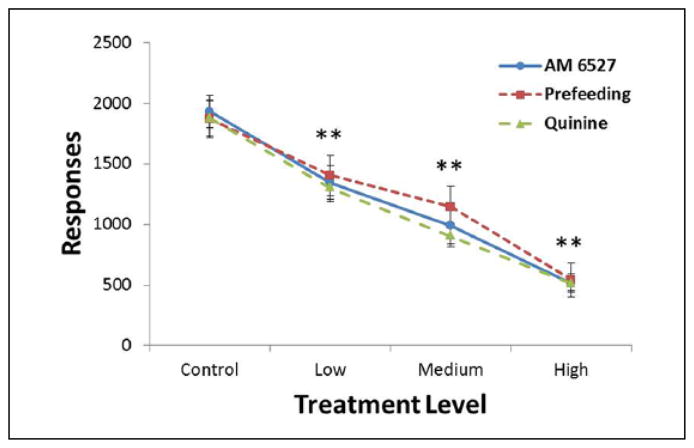
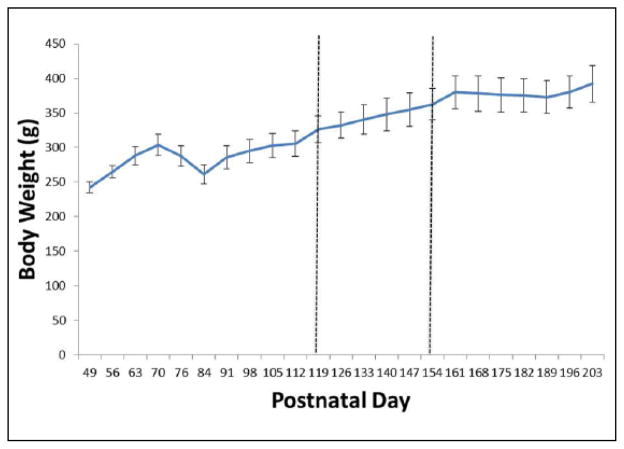
As shown in Figure 1, all three treatments significantly suppressed responding in a linear fashion, with a main effect of level (F(3,45) = 119.6, p < .001) and post hoc testing revealing significant differences from each level and all other levels. Neither the main effect of treatment (F(2,30) = 0.6, p = .585) nor the interaction of treatment and level (F(6,90) = 0.4, p = .885) were significant. This analysis confirmed, first, that there was overall a variable amount of response suppression on which to perform further analysis, and second, that all three manipulations had similar suppression of food-motivated behavior. Figure 2 shows average body weights throughout the study, and for one month after, when animals ceased operant sessions and were fed 15–20 g lab chow daily. Body weight growth was modest and steady throughout the study, although food restriction limited weights, relative to typical male Sprague-Dawley animals from this vendor (Hilltop Lab Animals Inc., n.d.). The decrease between post-natal days (PND) 70 and 84 is likely due to the gradual transition from CRF to FR10, beginning PND 67. As animals only received one dose of AM 6527 approximately every 10 days, it was not expected that weight would decrease during the study period.
Figure 1.
FR10 responding for food pellets is suppressed to a similar extent by all three treatments at the low (0.5 mg/kg AM 6527, 4.5 g pellets prefeeding, 0.4 g/kg quinine), medium (1.0 mg/kg AM 6527, 9 g pellets prefeeding, 1.0 g/kg quinine), and high (4.0 mg/kg AM 6527, 18 g pellets prefeeding, 2.0 g/kg quinine) levels. These comparable effects were necessary for interpreting changes in the microstructure of lever pressing. **p < .01 difference from control condition.
Figure 2.
Body weights. Vertical lines define the start and end of the six-week test period. Animals were weighed daily, but for clarity, data shown were recorded every Friday during the experiment.
Analysis of individual measures of food-motivated responding
Time of both first (TFR; F(6,90) = 2.4, p = .036) and last (TLR; F(6,90) = 5.4, p < .001) response produced significant interactions, suggesting that the three manipulations caused rats to begin and finish responding at different times. Table 1 suggests that, when sated, animals began to respond later and finished earlier, while quinine did so to a lesser extent. The main effect of dose for AM 6527 was not significant for TFR, likely due to increasing variability at higher doses; however, cases with the highest TFR values in the 4.0 mg/kg group were eliminated as outliers in the DFA. Significant main effects of treatment (F(2,30) = 5.6, p =.009; F(2,30) = 5.9, p =.007) and level (F(3,45) = 12.4, p < .001; F(3,45) = 12.1, p < .001) were also found for TFR and TLR, respectively. Response duration (RD) increased (F(3,45) = 52.1, p < .001), and tray duration (TD) decreased (F(3,45) = 8.2, p < .001), by level, but not by treatment (F(2,30) = 2.7, p = .080; F(2,30) = 0.8, p = .470, respectively), nor did treatment and level interact (F(6,90) = 1.2, p = .310; F(6,90) = 0.4, p = .900), indicating that RD and TD were similarly affected by all treatments.
Table 1.
All measures of operant performance. Simple main effects of level were performed for measures yielding a treatment × level interaction.
| Measure | Treatment | Control | Low | Medium | High |
|---|---|---|---|---|---|
| First response (s) | AM 6527 | 6.2 (8.7) | 9.0 (9.1) | 15.0 (32.0) | 197.9 (385.6) |
| Prefeeding†† | 2.0 (2.9) | 27.7 (28.6)** | 25.5 (29.4)** | 127.3 (225.8)** | |
| Quinine†† | 4.8 (5.4) | 13.3 (22.2) | 9.5 (20.8) | 34.0 (34.3) | |
| Last response (s) | AM 6527 | 1772.3 (50.2) | 1705.7 (124.6) | 1675.8 (215.0) | 1669.1 (227.6) |
| Prefeeding†† | 1696.6 (165.9) | 1756.9 (84.1) | 1620.7 (195.2) | 1307.8 (408.8)* | |
| Quinine | 1783.8 (23.5) | 1674.7 (224.8) | 1709.5 (130.8) | 1686.9 (118.8) | |
| Response duration (ms) | AM 6527 | 194.6 (58.9) | 205.2 (65.2) | 224.8 (62.7) | 250.5 (89.8) |
| Prefeeding | 206.1 (59.3) | 220.6 (74.3) | 222.6 (78.7) | 297.4 (130.74) | |
| Quinine | 198.3 (58.7) | 209.4 (52.7) | 209.1 (51.9) | 270.9 (61.6) | |
| Tray duration (ms) | AM 6527 | 586.7 (72.9) | 581.1 (77.1) | 573.4 (87.2) | 518.3 (92.4) |
| Prefeeding | 571.7 (94.2) | 573.9 (89.5) | 578.7 (85.0) | 508.4 (95.9) | |
| Quinine | 566.8 (71.7) | 570.1 (91.7) | 551.7 (59.4) | 519.6 (82.1) | |
| Empty tray entries | AM 6527†† | 97.3 (16.4) | 73.5 (12.7) | 73.8 (9.9) | 59.3 (10.7) |
| Prefeeding†† | 96.5 (14.5) | 73.7 (10.3) | 61.0 (9.6) | 45.1 (9.4)** | |
| Quinine†† | 107.4 (58.6) | 93.9 (50.1) | 91.8 (36.3) | 128.4 (53.2) | |
| Post-reinforcement pause (s) | AM 6527†† | 2.2 (1.0) | 3.3 (1.4) | 4.1 (1.7)** | 7.0 (4.2)** |
| Prefeeding†† | 2.3 (1.1) | 3.1 (1.4) | 3.6 (2.1)* | 4.8 (3.3)** | |
| Quinine†† | 2.4 (1.0) | 3.5 (1.5)* | 5.4 (1.8)** | 4.3 (2.2)** | |
| Inter-response time (ms) | AM 6527†† | 121.2 (29.7) | 119.5 (23.9) | 128.6 (32.3) | 141.6 (30.1)** |
| Prefeeding | 114.9 (25.9) | 123.2 (24.2) | 123.0 (30.1) | 125.6 (40.1) | |
| Quinine | 121.3 (23.4) | 121.3 (31.0) | 125.1 (29.7) | 114.9 (24.3) | |
| Pause length (s) | AM 6527†† | 1.7 (.6) | 2.3 (1.3) | 2.2 (.7) | 3.3 (1.4)** |
| Prefeeding†† | 1.6 (.5) | 2.1 (.8) | 2.4 (1.1) | 5.3 (3.7)** | |
| Quinine†† | 1.5 (.3) | 2.3 (1.1) | 2.4 (1.1)* | 4.7 (2.0)** | |
| Responses after pellet | AM 6527†† | 252.4 (182.1) | 169.2 (146.6) | 87.4 (66.8)** | 33.9 (34.2)** |
| Prefeeding†† | 212.2 (123.2) | 164.5 (153.6) | 106.3 (93.5)** | 46 (66.7)** | |
| Quinine†† | 256.3 (164.2) | 118.3 (78.3)** | 68.7 (49.1)** | 207.8 (184.8) | |
| Retrieval latency (ms) | AM 6527 | 632.3 (143.7) | 641.4 (133.1) | 630.3 (134.8) | 656.0 (147.5) |
| Prefeeding | 650.1 (146.2) | 654.7 (125.5) | 648.7 (147.6) | 646.3 (149.0) | |
| Quinine†† | 638.1 (143.8) | 640.3 (131.0) | 606.0 (147.7) | 482.8 (139.2)** |
Significant one-way repeated measures ANOVA of level (F(3,45) > 4.3; p < .01);
p < .05;
p < .01 post hoc test difference from control condition.
In contrast, number of ETEs differed by treatment (F(2,30) = 31.7, p < .001), level (F(3,45) = 7.7, p < .001), and produced a treatment × level interaction (F(6,90) = 3.8, p = .002). Simple main effects of level displayed in Table 1 suggest that ETEs decreased as a function of level for prefeeding and AM 6527, but were less affected by quinine, and appeared to increase (though not significantly over vehicle) in the 2.0 g/kg condition.
PRP length also produced a treatment × level interaction (F(6,90) = 4.5, p = .001), and a main effect of level (F(3,45) = 40.3, p < .001), but not of treatment (F(2,30) = 2.2, p = .131). Treatment and level also interacted for the length of both inter-response times (IRT; F(6,90) = 2.8, p = .016) and response pause length (PL; F(6,90) = 2.6, p = .022). Neither IRT (F(2,30) = 3.2, p = .054) nor PL (F(2,30) = 2.0, p = .151) produced a main effect of treatment. PL increased as a function of level (F(3,45) = 35.4, p < .001), but IRT did not (F(3,45) = 1.8, p = .154). Simple main effects of IRT indicate a significant increase only following AM 6527 treatment. These effects are shown in Table 1.
Lastly, the treatments were differentiated by two variables recorded after reinforcement had been delivered. Responses after pellet decreased by level (F(3,45) = 30.5, p < .001) but were not different by treatment (F(2,30) = 2.0, p = .149); however, a significant interaction (F(6,90) = 10.0, p < .001) indicates a linear effect for AM 6527 and prefeeding, and a biphasic effect for quinine, in which significant decreases were found at the low and medium but not high levels, relative to control. Main effects of treatment (F(2,30) = 13.1, p < .001) and level (F(3,45) = 3.0, p = .041) were found for RLat, and an interaction was also found (F(6,90) = 6.2, p < .001). As seen in Table 1, latencies to retrieve the pellet were little affected by prefeeding or AM 6527, but decreased as quinine concentrations increased.
Discriminant function analysis
In order to reduce the number of predictors, measures of TD and RD were not used because they failed to produce a significant treatment × level interaction. Box’s M was nonsignificant in all DFAs (p values > .19). Analysis was first conducted on all pre-feeding and quinine cases, collapsed across level, with fewer than 1142 responses, the lowest value in the control conditions, yielding 67 cases. Outliers were identified by finding Mahalanobis distances based on all predictors. Cases with distances greater than the critical chi-square at p = .001 were eliminated as outliers. This eliminated four cases (3 prefeeding and 1 quinine), resulting in a final data set of 63 cases, n = 27 for pre-feeding and n = 36 for quinine. Two outliers were eliminated from the AM 6527 treatment.
The stepwise procedure resulted in a function that highly significantly (Χ2(5) = 34.2, p < .001; Wilks’ λ = .557; canon. R2 = .442) discriminated prefeeding and quinine based on five predictors. Table 2 shows these predictors in order of absolute value of factor loadings. Observed power was computed as >.99 by entering parameters into the G*Power software package (Dusseldorf, Germany; Faul et al., 2007) as a MANOVA in which treatments and predictors were entered as independent and dependent variables, respectively.
Table 2.
Factor loadings of predictors that significantly differentiated treatments in discriminant function analyses.
| Discrimination of prefeeding and quinine
|
Discrimination of AM 6527, prefeeding, and quinine
|
||||||
|---|---|---|---|---|---|---|---|
| Predictors | Overall | Low | Medium | High | Predictors | Function 1 | Function 2 |
| Empty tray entries | .514 | .506 | .378 | .813 | Empty tray entries | .613 | −.172 |
| Retrieval latency | −.501 | −.129 | −.133 | −.511 | Retrieval latency | −.595 | −.154 |
| Time of first response | −.348 | −.646 | −.288 | −.260 | Inter-response Time | −.346 | .776 |
| Post-reinforcement Pause | .098 | .331 | .433 | −.080 | Post-reinforcement Pause | .050 | .624 |
| Pause length | .048 | .196 | .001 | −.091 | |||
Using these predictors, separate analyses were also performed at each level on all 16 animals. At the low level (i.e., 4.5 g pre-feeding and 0.4 g/kg quinine), a nonsignificant function (Χ2(5) = 5.0, p = .411; Wilks’ λ = .833; canon. R2 = .167) was constructed. However, at the medium level (9.0 g prefeeding and 1.0 g/kg quinine), the function was highly significant (Χ2(5) = 22.5, p < .001; Wilks’ λ = .441; canon. R2 = .560). The function at the high level (18.0 g prefeeding and 2.0 g/kg quinine) indicated a similar relationship between treatments and predictors (Χ2(5) = 23.1, p < .001; Wilks’ λ = .432; canon. R2 = .569). Factor loadings for these analyses are shown in Table 2.
Classification of AM 6527
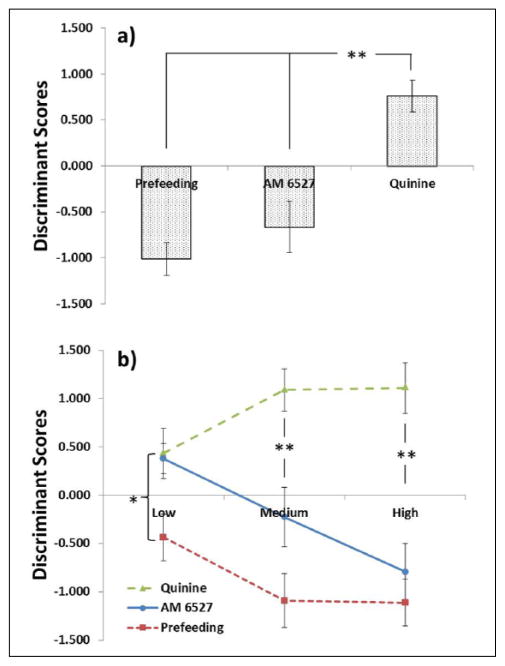
Discriminant scores were found by solving the discriminant function for each case based on linear combinations of the five predictors identified above. Average discriminant scores are shown for each treatment in Figure 3. The overall analysis produced significant differences (F(2,92) = 19.2, p < .001) between quinine, and both prefeeding and AM 6527. Prefeeding and AM 6527 were not different from one another, indicating similar effects (Figure 3(a)). The pattern was different when cases in the Low Levels were analyzed; a significant effect was found (Figure 3(b); F(2,45) = 4.3, p = .020), but post hoc testing revealed a difference only between prefeeding and quinine. Unlike the overall analysis, AM 6527 produced values closer to those of quinine, but no significant differences were found between AM 6527 and either of the non-drug treatments. However, both the medium (F(2,45) = 16.4, p < .001) and high (F(2,45) = 20.7, p < .001) levels matched the pattern found in the overall analysis, in that AM 6527 produced scores similar to those of prefeeding, and significantly different from quinine.
Figure 3.
Results of the discriminant function analysis are shown (a) for the overall analysis, collapsed across level, and (b) at each level. Y-axis represents scores resulting from a function composed of five predictors that significantly differentiated prefeeding and quinine conditions. *p < .05, **p < .01. Treatment differences via Tukey’s HSD post hoc analysis. X-axis categories: low = 0.5 mg/kg AM 6527, 4.5 g pellets prefeeding, 0.4 g/kg quinine; medium = 1.0 mg/kg AM 6527, 9 g pellets prefeeding, 1.0 g/kg quinine; high = 4.0 mg/kg AM 6527, 18 g pellets prefeeding, 2.0 g/kg quinine.
Results of classifications are shown in Table 3. As expected, prefeeding (Χ2(1) = 17.3, p < .001) and quinine (Χ2(1) = 14.3, p < .001) cases had a strong tendency to be classified as such, rather than being misclassified as the other. AM 6527 exhibited a slight but significant tendency to be classified as prefeeding rather than quinine (Χ2(1) = 4.2, p = .040).
Table 3.
Classification of treatment cases as prefeeding-like or quinine-like.
| Overall | Predicted treatment
|
||
|---|---|---|---|
| Prefeeding | Quinine | ||
| Actual treatment | AM 6527* | 21 | 11 |
| Prefeeding* | 24 | 3 | |
| Quinine* | 8 | 28 | |
| Low | Prefeeding | Quinine | |
| AM 6527 | 5 | 11 | |
| Prefeeding | 10 | 6 | |
| Quinine | 5 | 11 | |
| Medium | Prefeeding | Quinine | |
| AM 6527 | 9 | 7 | |
| Prefeeding* | 14 | 2 | |
| Quinine* | 1 | 15 | |
| High | Prefeeding | Quinine | |
| AM 6527* | 13 | 3 | |
| Prefeeding* | 13 | 3 | |
| Quinine* | 2 | 14 | |
p < .05 significantly greater than chance classification of cases into either prefeeding or quinine.
Table 3 also shows results for all three treatments by level. For the low levels, more prefeeding cases were correctly classified than not, and both AM 6527 and quinine cases were more likely to be classified as quinine-like rather than prefeeding-like, although not significantly so. At the medium level, both prefeeding (Χ2(1) = 9.0, p = .003) and quinine (Χ2(1) = 12.3, p < .001) cases again tended to be correctly classified, and therefore easily discriminated, but AM 6527 cases were nearly evenly split in similarity to the two nondrug manipulations. On the other hand, a significant majority (Χ2(1) = 6.3, p = .012) of AM 6527 cases matched the pattern of the prefeeding group at the high level. Not surprisingly, both prefeeding (Χ2(1) = 6.3, p = .012) and quinine (Χ2(1) = 9.0, p = .003) cases were typically correctly classified.
Discriminant function analysis of AM 6527, prefeeding, and quinine
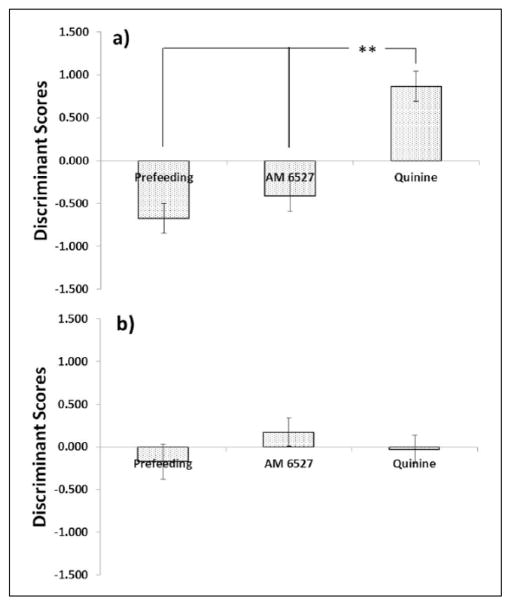
Excluding outliers and cases with over 1142 responses, there were 95 total cases from the AM 6527 (n = 32), prefeeding (n = 27), and quinine (n = 36) conditions. Stepwise DFA produced two functions significantly (Χ2(8) = 37.7, p < .001; Wilks’ λ = .660; canon. R2 = .327) relating the three treatments and four of the predictors; however, the second function was nonsignificant when the first was removed (Χ2(3) = 1.8, p = .627; Wilks’ λ = .981; canon. R2 = .019). Listed in order of factor loading onto Function 1 (Table 2), the included predictors were ETEs, RLat, IRT, and PRP. As can be seen in Figure 4, Function 1 of the analysis produced significant treatment differences (Figure 4(a); F(2,92) = 22.4, p < .001) in discriminant scores, but Function 2 did not (Figure 4(b); F(2,92) = 0.9, p = .412). Similar to the overall analysis, post hoc testing indicated that prefeeding and quinine were significantly different from each other, and AM 6527 was significantly different from quinine, but was not dissimilar from prefeeding.
Figure 4.
When discriminant function analysis was based on AM 6527 in addition to prefeeding and quinine, two functions were identified based on four predictors that contributed significant unique variability. (a) The first function was significant and indicated differences between quinine and both prefeeding and AM 6527, but not between AM 6527 and prefeeding. (b) The second function was not significant. **p < .01. Treatment differences via Tukey’s HSD post hoc analysis. X-axis categories: low = 0.5 mg/kg AM 6527, 4.5 g pellets prefeeding, 0.4 g/kg quinine; medium = 1.0 mg/kg AM 6527, 9 g pellets prefeeding, 1.0 g/kg quinine; high = 4.0 mg/kg AM 6527, 18 g pellets prefeeding, 2.0 g/kg quinine.
As AM 6527 cases were included in deriving the functions, cases could be classified into any of the three treatment groups in this analysis (Table 4). Both prefeeding (Χ2(2) = 9.6, p = .008) and quinine (Χ2(2) = 15.2, p < .001) cases were significantly likely to be correctly classified. When animals were treated with AM 6527, they were more likely to fit the pattern of prefeeding than either AM 6527 or quinine, although they were not significantly more frequently assigned to any one category.
Table 4.
Classification of treatment cases as AM 6527-like, prefeeding-like, or quinine-like.
| Predicted treatment
|
||||
|---|---|---|---|---|
| AM 6527 | Prefeeding | Quinine | ||
| Actual | AM 6527 | 11 | 14 | 7 |
| treatment | Prefeeding* | 10 | 15 | 2 |
| Quinine* | 7 | 6 | 23 | |
p < .05 significantly greater-than-chance classification of cases into one or more treatment groups.
Discussion
Effects of satiety and reduced reward value on food-maintained responding were significantly distinguished based on differential effects on ETEs, RLat, TFR, and to a lesser extent, PRP and PL. While characteristics of food-maintained lever pressing have been used to identify underlying similarities and differences between rate-altering manipulations (Hillhouse and Porter, 2014; Salamone et al., 1995), the present report may be the first multivariate analysis to identify the aspects of responding that are most relevant in differentiating satiety for the reinforcer from its orosensory qualities.
ETEs had the highest loading of any single predictor. Tray entries may therefore be of great importance in differentiating between these two processes: such appetitive behavior is inhibited when subjects are satiated, but when reward value of the reinforcer is lower, animals continue to nose poke for food even though lever pressing is diminished. Prefeeding also delayed TFR, similar to the manner in which it delays meal initiation (Ishii et al., 2003b). Where animals typically began responding within the first 10 s of the session under control conditions, even the lowest amount of the prefeeding condition delayed the average first response until 27 s into the session. A slight but significant increase in TFR in the quinine condition is difficult to explain, given that animals had no indication of reward value until after the first pellet of a session was delivered; however, animals were under standard food deprivation conditions and began responding much more quickly than in either prefeeding (Ishii et al., 2003a) or AM 6527 conditions. A similar phenomenon occurred at the end of the session: prefed animals ceased responding earlier, as evidenced by a treatment × level interaction in TLR.
On the other hand, RLat was reduced by the quinine manipulation, but was unaffected by prefeeding; animals took the same time to retrieve the food pellet after it was delivered regardless of satiety level, but surprisingly, responding for devalued reinforcement decreased the latency to retrieve it. Differences in PRP and PL also contributed sufficient unique variability to discriminate between prefeeding and quinine, but with much weaker loadings (<.1; Table 2). PRP produced stronger loadings in the low (.331) and medium (.433) level analyses and therefore may be more relevant when distinguishing treatments with more mild effects. While it has long been known that prefeeding increases PRP length (Sidman and Stebbins, 1954), reducing hedonic value in the low and medium levels increased PRP to a greater extent than prefeeding (Table 1).
An advantage of using DFA in evaluating the effects of AM 6527 is the creation of a single discriminant score based on linear combinations of predictors that significantly contributed unique variability, rather than interpreting individual measures in piecemeal fashion. As expected, prefeeding and quinine produced significantly different scores in all analyses (Figures 3 and 4). In the overall analysis (Figure 3(a)), AM 6527 produced scores similar to prefeeding and significantly different from quinine. Using the classification procedure, AM 6527 scores were also more likely to be classified as prefeeding-like, rather than quinine-like.
This effect was clarified when the analysis was repeated on each level. Figure 3(b) shows that AM 6527 scores were nearly identical to those of quinine at the low level (0.5 mg/kg), but moved closer to average prefeeding scores at the medium and high levels, which employed doses of 1.0 mg/kg and up. At these doses, post hoc tests indicated highly significant differences from quinine. Notably, AM 6527 moved toward prefeeding in a dose-dependent fashion, but did not perfectly overlap with the prefeeding condition at any dose, suggesting some greatly attenuated similarity with quinine.
As seen in Table 3 (low levels), a nonsignificant majority of AM 6527-treated cases better matched the pattern of performance under quinine. This may be largely due to the fact that TFR had the highest loading of the low level analysis and therefore best differentiated satiety from reduced hedonic value. When receiving 0.5 mg/kg AM 6527, animals began responding as early as they did for the lowest concentration of quinine pellets, and much earlier than when they were prefed 4.5 g (Table 1). As discussed, PRP differentiated prefeeding and quinine at the low and medium levels, but AM 6527 lengthened average PRP to a value between those of the nondrug manipulations. This may indicate either drug effect on both satiety and reduced hedonic value, or enough variability that this predictor may not best elucidate the mechanism of action of AM 6527. It may also explain why nearly as many (9 out of 16) 1.0 mg/kg AM 6527 cases were classified as matching the pattern of 9.0 g prefeeding, as 1.0 g/kg quinine (Table 3).
On the other hand, prefeeding- and quinine-induced effects on ETEs and RLat were better differentiated as level increased, and the effects of AM 6527 on these measures strongly implicate satiety as the main behavioral process affected, especially at higher doses. While TFR was less relevant based on a decreased predictor loading, perhaps due to increasing within-group variability, 4.0 mg/kg AM 6527 also delayed the first response to a similar extent as 18.0 g prefeeding. Strikingly, in terms of overall classification (Table 3), 4.0 mg/kg AM 6527 induced a behavioral profile as prefeeding-like as the 18 g prefeeding condition did. Taken together, as dose increased, performance under AM 6527 more clearly matched the pattern of satiated animals, and became more unlike responding for devalued reinforcers.
This conclusion was supported by the second DFA, in which discriminant functions were based on the AM 6527 condition, in addition to Prefeeding and Quinine. ETEs and RLat were once again the most relevant predictors in Function 1 (Table 2), which created the greatest separation between the prefeeding and quinine treatments (Figure 4(a)). Function 1 likely therefore represents the same pattern of behavior as the function derived from the previous analysis. Function 2 was based more upon IRT and PRP. Table 1 shows that AM 6527 dose-dependently lengthened IRT, a pattern found in neither of the nondrug treatments. Similarly, while all treatments lengthened PRP, AM 6527 did so to the greatest extent at its highest dose. Function 2 therefore most likely represents effects of AM 6527 that are unrelated to either satiety or reduced hedonic value, and may therefore be indicative of behavioral effects other than those on food motivation. In terms of classification (Table 4), quinine cases were typically assigned correctly, but strikingly, more AM 6527 cases were classified as prefeeding-like than AM 6527-like, and 10 of 27 prefeeding cases were classified as AM 6527-like.
Given that AM 6527 is a neutral CB1 antagonist, it is likely to have fewer non-motivational effects than CB1 inverse agonists such as rimonabant. For instance, AM 6527, unlike the CB1 inverse agonist AM 251, does not potentiate lithium-induced rejection reactions, a model of nausea (Limebeer et al., 2010). It should be noted, however, that if Function 2 represents effects of AM 6527 not accounted for by either satiety or changes in hedonic value of food, these effects are very mild. Function 2 was nonsignificant and accounted for less than 2% of the relationship between the predictors and Treatments (Figure 4(b)). The finding that AM 6527 reduced food-maintained responding via more than one mechanism is consistent with effects of rimonabant on a food-maintained, second order schedule; rimonabant decreased responding under both an “appetitive” initial FI-5 min phase and a “consummatory” FR5 phase, which was less affected by pre-feeding (Thornton-Jones et al., 2005). Similarly, the present results are in line with reports of numerous central and peripheral sites of CB1-mediated control of feeding and energy metabolism (Bellocchio et al., 2010; Cardinal et al., 2012; Cooper and Regnell, 2014; Cota et al., 2003; DiPatrizio and Simansky, 2008; DiPatrizio et al., 2011; Pagotto et al., 2006; Soria-Gómez et al., 2014; Tucci et al., 2004; Yoshida et al., 2010). This wide distribution may indicate redundancy (Bellocchio et al., 2013; Cota et al., 2003; Pagotto et al., 2006) among different sites in terms of the ability of CB1 antagonists to reduce feeding, and may partially explain contradictory findings. For instance, signaling from gut to brain is not necessary for the hypophagic effects of rimonabant (Madsen et al., 2009), but the novel, peripherally-restricted compound AM 6545 produces feeding effects similar to rimonabant (Cluny et al., 2010; Randall et al., 2010). Systemic administration of CB1 inverse agonists and antagonists reduces intake of several different kinds of food (McLaughlin et al., 2003, 2006; Verty et al., 2004), while administration of the endocannabinoid 2-arachidonoyl glycerol into the parabrachial nucleus in the pons increased consumption of palatable, but not unpalatable food (DiPatrizio and Simansky, 2008).
Because of this, it is not surprising that AM 6527 in the current study may have both reduced the hedonic value of the palatable operant pellets at low doses, and also induced satiety in a dose-related fashion. This may explain reports of both an increase in satiety (Ameloot et al., 2010; McLaughlin et al., 2003, 2006; Thornton-Jones et al., 2007; Verty et al., 2004) as well as reduced hedonic value of food (De Vry et al., 2004; Higgs et al., 2003; Jarrett et al., 2005, 2007; Rasmussen et al., 2012; Sanchis-Segura et al., 2004; Simiand et al., 1998). Recent evidence indicates that food deprivation enhances odor perception that is mediated by olfactory bulb CB1 receptors (Soria-Gómez et al., 2014). Our findings with AM 6527 may also explain why 1.0 mg/kg AM 251 reduced hedonic reactions to sucrose (Jarrett et al., 2007), but 2.5 mg/kg rimonabant, a more potent drug, did not alter reactions to sucrose per se (Jarrett et al., 2005).
It should be noted that there are also likely within-session changes in food-motivated behavior, as have been demonstrated (Davis and Levine, 1977; Higgs et al., 2003), although the predictors in the present analysis were session-wide averages of behavior. This approach was undertaken to maximize the number of predictors that could discriminate the manipulations in an interpretable manner. As combinations of averages were sufficient to significantly discriminate behavioral patterns, as has been shown previously (McLaughlin et al., 2010), future studies can explore changes in predictors over time.
The results presented here can be used to clarify the behavioral processes of other drug and nondrug manipulations that affect operant responding in rats, a widely-used and sensitive assay. The CB1 neutral antagonist AM 6527 not only reduced food-maintained responding, but did so by both reducing reward value and producing satiety at 0.5 mg/kg, and at higher doses, better matched a satiety-like profile of behavior, suggesting that CB1 blockade may work at moderate to high doses by inducing the perception of fullness. Future research may utilize these results in evaluation of peripherally-restricted or locally-applied CB1 antagonists to better understand how the distribution of this receptor contributes to its effects on energy intake, as well as the mechanism by which these processes inhibit food-maintained responding. These techniques may also be relevant to the discovery of novel treatments for obesity and metabolic disorders.
Acknowledgments
The first two authors contributed equally to this work. The authors thank Dr. Jaime Lecker (Bio-Serv) for designing the quinine operant pellets, and Dr. Ronald A. Craig for statistical consultation.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Ameloot K, Janssen P, Scarpellini E, et al. Endocannabinoid control of gastric sensorimotor function in man. Aliment Pharmacol Ther. 2010;31:1123–1131. doi: 10.1111/j.1365-2036.2010.04259.x. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Lafenetre P, Cannich A, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13:281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Soria-Gómez E, Quarta C, et al. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. Proc Natl Acad Sci U S A. 2013;110:4786–4791. doi: 10.1073/pnas.1218573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Seiden LS. Inter-response time changes as a function of water deprivation and amphetamine. J Pharmacol Exp Ther. 1975;193:701–712. [PubMed] [Google Scholar]
- Cardinal P, Bellocchio L, Clark S, et al. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology. 2012;153:4136–4143. doi: 10.1210/en.2012-1405. [DOI] [PubMed] [Google Scholar]
- Carriero D, Aberman J, Lin SY, et al. A detailed characterization of the effects of four cannabinoid agonists on operant lever pressing. Psychopharmacology. 1998;137:147–156. doi: 10.1007/s002130050604. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, et al. A novel peripherally restricted cannabinoid antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacology. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ME, Regnell SE. The hepatic cannabinoid 1 receptor as a modulator of hepatic energy state and food intake. Br J Clin Pharmacol. 2014;77:21–30. doi: 10.1111/bcp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza MC, Afifi AA. Comparison of stopping rules in forward stepwise discriminant analysis. J Am Statist Assoc. 1979;74:777–785. [Google Scholar]
- Cota D, Marsicano G, Tschöp M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Levine MW. A model for the control of ingestion. Psychol Rev. 1977;84:379–412. [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Eckel G, et al. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol. 2004;483:55–63. doi: 10.1016/j.ejphar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulate feeding of palatable foods in rats. J Neurosci. 2008;28:9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Astarita G, Schwartz G, et al. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci U S A. 2011;108:12904–12908. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ. Cholecystokinin and gut-brain signaling. Regul Pept. 2009;155:6–10. doi: 10.1016/j.regpep.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Ettinger RH, Staddon JE. Operant regulation of feeding: a static analysis. Behav Neurosci. 1983;97:639–653. doi: 10.1037//0735-7044.97.4.639. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Skjoldager PD, Liao R, et al. Distinguishing between haloperidol’s and decamethonium’s disruptive effects on operant behavior in rats: use of measurements that complement response rate. J Exp Anal Behav. 1991;56:239–260. doi: 10.1901/jeab.1991.56-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MJ, Cain ME. Effects of satiety on operant responding in rats raised in enrichment. Behav Pharmacol. 2011;22:40–48. doi: 10.1097/FBP.0b013e3283425a86. [DOI] [PubMed] [Google Scholar]
- Higgs S, Williams CM, Kirkham TC. Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after delta(9)-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology. 2003;165:370–377. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH. Ketamine, but not MK-801, produces antidepressant-like effects in rats responding on a differential-reinforcement-of-low-rate operant schedule. Behav Pharmacol. 2014;25:80–91. doi: 10.1097/FBP.0000000000000014. [DOI] [PubMed] [Google Scholar]
- Hilltop Lab Animals, Inc. [accessed 24 September 2015];SD rats growth chart. n.d Available at: http://hilltoplabs.com/public/sdgrowth.html.
- Hodge J, Bow JP, Plyler KS, et al. The cannabinoid CB1 receptor inverse agonist AM 251 and antagonist AM 4113 produce similar effects on the behavioral satiety sequence in rats. Behav Brain Res. 2008;193:298–305. doi: 10.1016/j.bbr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann A, Dietrich A, Mathar D, et al. Slave to habit? Obesity is associated with decreased behavioural sensitivity to reward devaluation. Appetite. 2015;87:175–183. doi: 10.1016/j.appet.2014.12.212. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. 8. Washington, DC: National Academy Press; 2011. [Google Scholar]
- Ishii Y, Blundell JE, Halford JCG, et al. Palatability, food intake and the behavioural satiety sequence in male rats. Physiol Behav. 2003a;80:37–47. doi: 10.1016/s0031-9384(03)00207-5. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JCG, et al. Effects of systematic variation in presatiation and fasting on the behavioural satiety sequence in male rats. Physiol Behav. 2003b;79:227–238. doi: 10.1016/s0031-9384(03)00066-0. [DOI] [PubMed] [Google Scholar]
- Jarrett MM, Limebeer CL, Parker LA. Effect of Δ9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol Behav. 2005;86:475–479. doi: 10.1016/j.physbeh.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Jarrett MM, Scantlebury J, Parker LA. Effect of Δ9-tetrahydrocannabinol on quinine palatability and AM251 on sucrose and quinine palatability using the taste reactivity test. Physiol Behav. 2007;90:425–430. doi: 10.1016/j.physbeh.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and Analysis: A Researcher’s Handbook. 4. Englewood Cliffs, NJ: Prentice-Hall; 2004. [Google Scholar]
- Limebeer CL, Vemuri VK, Bedard H, et al. Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol. 2010;161:336–349. doi: 10.1111/j.1476-5381.2010.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ. Reports of the death of CB1 antagonists have been greatly exaggerated: Recent preclinical findings predict improved safety in the treatment of obesity. Behav Pharmacol. 2012;23:537–550. doi: 10.1097/FBP.0b013e3283566a8c. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Lu D, Winston KM, et al. Behavioral effects of the novel cannabinoid full agonist AM 411. Pharmacol Biochem Behav. 2005a;81:78–88. doi: 10.1016/j.pbb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Qian L, Wood JT, et al. Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM 1387. Pharmacol Biochem Behav. 2006;83:396–402. doi: 10.1016/j.pbb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, et al. The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology. 2005b;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Swezey LA, et al. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and the food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Swezey LA, et al. Detailed analysis of food-reinforced operant lever pressing distinguishes effects of a cannabinoid CB1 inverse agonist and dopamine D1 and D2 antagonists. Pharmacol Biochem Behav. 2010;96:75–81. doi: 10.1016/j.pbb.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen AN, Jelsing J, van der Wall EHEM, et al. Rimonabant induced anorexia in rodents is not meditated by vagal or sympathetic gut afferents. Neurosci Lett. 2009;449:20–23. doi: 10.1016/j.neulet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Mathes CM, Gregson JR, Spector AC. The selective serotonin reuptake inhibitor paroxetine decreases breakpoint of rats engaging in a progressive ratio licking task for sucrose and quinine solutions. Chem Senses. 2013;38:211–220. doi: 10.1093/chemse/bjs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagotto U, Cervino C, Vicennati V, et al. How many sites of action for endocannabinoids to control energy metabolism? Int J Obes. 2006;30:S39–S43. doi: 10.1038/sj.ijo.0803277. [DOI] [PubMed] [Google Scholar]
- Randall PA, Vemuri VK, Segovia KN, et al. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacol Biochem Behav. 2010;97:179–184. doi: 10.1016/j.pbb.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen EB, Reilly W, Buckley J, et al. Rimonabant reduces the essential value of the food in the genetically obese Zucker rat: an exponential demand analysis. Physiol Behav. 2012;105:734–741. doi: 10.1016/j.physbeh.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Kurth P, McCullough LD, et al. The effects of nucleus accumbens dopamine depletions on continuously reinforced operant responding: contrasts with the effects of extinction. Pharmacol Biochem Behav. 1995;50:437–443. doi: 10.1016/0091-3057(94)00294-s. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Cline BH, Marsicano G, et al. Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology. 2004;176:223–232. doi: 10.1007/s00213-004-1877-8. [DOI] [PubMed] [Google Scholar]
- Scheen AJ, Finer N, Hollander P, et al. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomized controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- Sharma MK, Murumkar PR, Barmade MA, et al. A comprehensive patents review on cannabinoid 1 receptor antagonists as antiobesity agents. Expert Opin Ther Pat. 2015;25:1093–1116. doi: 10.1517/13543776.2015.1064898. [DOI] [PubMed] [Google Scholar]
- Sidman M, Stebbins WC. Satiation effects under fixed-ratio schedules of reinforcement. J Comp Physiol Psychol. 1954;47:114–116. doi: 10.1037/h0054127. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, et al. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Sink K, McLaughlin PJ, Wood JT, et al. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Wood J, et al. Oral bioavailability of the novel cannabinoid CB1 antagonist AM6527: effects on food-reinforced behavior and comparisons with AM4113. Pharmacol Biochem Behav. 2009;91:303–313. doi: 10.1016/j.pbb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Gómez E, Bellocchio L, Reguero L, et al. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014;17:407–415. doi: 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]
- Tam J, Cinar R, Liu J, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16:167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Kennett GA, Vickers SP, et al. A comparison of the effects of the CB1 receptor antagonist SR141716A, pre-feeding and changed palatability on the microstructure of ingestive behaviour. Psychopharmacology. 2007;193:1–9. doi: 10.1007/s00213-007-0745-8. [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology. 2005;179:452–460. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- Tucci SA, Rogers EK, Korbonits M, et al. The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intra-hypothalamic ghrelin. Br J Pharmacol. 2004;143:520–523. doi: 10.1038/sj.bjp.0705968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Vann RE, Wise LE, et al. Effects of antipsychotic drugs on operant responding after acute and repeated administration. Psychopharmacology. 2002;160:182–191. doi: 10.1007/s00213-001-0969-y. [DOI] [PubMed] [Google Scholar]
- Verty ANA, McGregor IS, Mallet PE. Consumption of high carbohydrate, high fat, and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci Lett. 2004;354:217–220. doi: 10.1016/j.neulet.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Wills KL, Vemuri K, Kalmar A, et al. CB1 antagonism: interference with affective properties of acute naloxone-precipitated morphine withdrawal in rats. Psychopharmacology. 2014;231:4291–4300. doi: 10.1007/s00213-014-3575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F, Rodgers R. Low dose naloxone attenuates the pruritic but not the anorectic response to rimonabant in male rats. Psychopharmacology. 2013;226:415–431. doi: 10.1007/s00213-012-2916-5. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Ohkuri T, Jyotaki M, et al. Endocannabinoids selectively enhance sweet taste. Proc Natl Acad Sci U S A. 2010;107:935–939. doi: 10.1073/pnas.0912048107. [DOI] [PMC free article] [PubMed] [Google Scholar]