Abstract
Purpose
To demonstrate the utility of a novel in vivo molecular imaging probe, HYPOX-4, to detect and image retinal hypoxia in real time, in a mouse model of retinal vein occlusion (RVO).
Methods
Retinal vein occlusion was achieved in adult mice by photodynamic retinal vein thrombosis (PRVT). One or two major retinal vein(s) was/were occluded in close proximity to the optic nerve head (ONH). In vivo imaging of retinal hypoxia was performed using, HYPOX-4, an imaging probe developed by our laboratory. Pimonidazole-adduct immunostaining was performed and used as a standard ex vivo method for the detection of retinal hypoxia in this mouse RVO model. The retinal vasculature was imaged using fluorescein angiography (FA) and isolectin B4 staining. Retinal thickness was assessed by spectral-domain optical coherence tomography (SD-OCT) analysis.
Results
By application of the standard ex vivo pimonidazole-adduct immunostaining technique, retinal hypoxia was observed within 2 hours post-PRVT. The observed hypoxic retinal areas depended on whether one or two retinal vein(s) was/were occluded. Similar areas of hypoxia were imaged in vivo using HYPOX-4. Using OCT, retinal edema was observed immediately post-PRVT induction, resolving 8 days later. Nominal preretinal neovascularization was observed at 10 to 14 days post-RVO.
Conclusions
HYPOX-4 is an efficient probe capable of imaging retinal hypoxia in vivo, in RVO mice. Future studies will focus on its use in correlating retinal hypoxia to the onset and progression of ischemic vasculopathies.
Keywords: retinal hypoxia, ischemia, retinal vein occlusion, in vivo imaging, HYPOX-4
Retinal vein occlusion (RVO) is the second most common condition affecting retinal blood vessels after diabetic retinopathy (DR).1 Retinal vein occlusion may produce ischemia, presumably leading to hypoxia, a known inducer of vascular endothelial cell growth factor (VEGF). VEGF regulates vascular permeability and angiogenesis associated with macular edema and pathologic ocular neovascularization (NV)2; previous studies indicate that it is elevated in mouse,3 rat,4 pig,5 and macaque models of RVO,6 and in human RVO patients.7–9 Neovascularization of the iris and retina, and macular edema, are vision-threatening consequences of RVO as well as other ischemia-induced retinopathies. Anti-VEGF therapies have proven efficacious against ocular NV in RVO.10 In recent years, the treatment approach for macular edema secondary to RVO, has shifted from “observation” to the administration of anti-VEGF therapies.11–13 However, it is uncertain whether RVO-related macular edema depends on hypoxia induction of VEGF to any extent.
Depending on the location of the occlusion, RVO can be broadly classified into two subgroups: occlusion of the central retinal vein at the level of the optic nerve, referred to as central retinal vein occlusion (CRVO), and occlusion at a more distal branch of the retinal vein, referred to as branch retinal vein occlusion (BRVO).14 According to Hayreh,15 RVO may be further classified as ischemic or nonischemic, each having distinct clinical features and prognosis. Nonischemic RVO is a comparatively benign disease that can become ischemic, translating to an increased morbidity.16 Some studies show that 20% of eyes with CRVO are ischemic and 45% of these develop neovascular glaucoma.16 In BRVO, NV commonly appears at the boundary between vascular and nonperfused (ischemic) retina. Because ischemia-induced hypoxia is considered to be a primary pathogenic component of RVO, its assessment is critical to the understanding of RVO pathogenesis. It is reasonable to hypothesize that the level of post-RVO hypoxia, is proportional to any developing VEGF-dependent neovascular response. Therefore, longitudinal monitoring of retinal hypoxia occurring in ischemic RVO could help guide the management of this disease during its early stages, pre-empting the onset and progression of a vision threatening neovascular response. Given these considerations, techniques capable of detecting and monitoring retinal hypoxia in the in vivo setting, to distinguish between ischemic and nonischemic RVO, are called for.
Generally, it is assumed that retinal capillary nonperfusion provides some indirect measure of retinal hypoxia, and in the clinic, this is routinely assessed by fluorescein angiography (FA). The application of this technique provides conclusive findings of retinal capillary nonperfusion in approximately 50% to 60% of RVO cases. Therefore, the distinction between ischemic and nonischemic RVO is limited, if not totally unreliable.17 In many cases, the confirmation, the exact location, and the duration of the venous occlusion are uncertain making it difficult to assess any associated ischemia-induced hypoxia. Oxygen-dependent molecular phosphorescence quenching,18 oxygen sensitive electrodes,19 nuclear magnetic resonance (NMR),20 retinal oximetry,21 Doppler optical coherence tomography (D-OCT),22 visible-light OCT (vis-OCT),23 and immunohistochemical analysis,24 have been used to measure retinal oxygen levels, but each has its own drawbacks and none are suitable methods to assess retinal hypoxia secondary to ischemia in vivo and in real time.25–27
Molecular imaging techniques hold the potential, as powerful in vivo tools, to detect and image retinal hypoxia, perhaps allowing the use of hypoxia as a biomarker for the onset, progression, and resolution of ischemic retinopathies. Assessment of retinal hypoxia has been demonstrated by application of the ex vivo pimonidazole immunostaining technique. Pimonidazole contains the nitroimidazole moiety that mimics oxygen; nitroreductase enzymes in tissues with low-oxygen pressure reduce it, presumably causing its sequestration via pimonidazole-protein adduct formation. Detection and quantification of retinal hypoxia are performed by excising the tissue of interest, and staining it with antibodies that recognize these protein adducts.28 Recently, we have synthesized imaging probes incorporating hypoxia sensitive moieties and fluorescent dyes, and tested their capacities to detect and image hypoxia in vitro and in vivo.25,26 One of these, HYPOX-4, was tested in mouse oxygen-induced retinopathy (OIR), and we observed the induction of retinal hypoxia via HYPOX-4–dependent fluorescence emanating from the vasoattenuated zone (ischemia) in the central retina. HYPOX-4 demonstrated superior optical properties, optimal reactivity, and pharmacokinetic parameters for its application to the in vivo characterization of retinal hypoxia. To better understand the occurrence and the relationship of retinal hypoxia to the pathogenesis of RVO, we tested the ability of HYPOX-4 to image retinal hypoxia in vivo, in mouse RVO. We compared our results with those obtained by application of the standard ex vivo pimonidazole technique. Herein, we report the results.
Materials and Methods
Synthesis of HYPOX-4
The synthesis of HYPOX-4 was discussed in our previous report.25
Animal Procedures
C57BL/6J male mice 4 to 6 weeks of age were purchased from Charles River Laboratories (Chicago, IL, USA). All animal procedures used in this study were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Retinal Vein Occlusion in Mice
A detailed method for photodynamic retinal venous thrombosis (PRVT) in mice has been described in previous publications29,30 and was replicated in this study with slight modifications. Briefly, C57BL/6 male mice were anesthetized by intraperitoneal injection of a ketamine/xylazine (25/10 mg/kg) mixture. The eyes were dilated using 1% tropicamide; and Genteal lubricating eye gel (Alcon, Fort Worth, TX, USA) was used to keep the eyes moist. A PBS solution of 40 mM rose bengal (4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodo-fluorescein disodium salt, certified purity, 95%; Sigma-Aldrich Corp., St. Louis, MO, USA) was sterilized by filtration through a 0.22-μm filter. Mice received tail vein injections of rose bengal at a dose of 40 mg/kg. An argon laser photocoagulator (blue-green) mounted on a slit-lamp (Space Coast Laser, Inc., Palm Bay, FL, USA) was used to create RVO (50-μm spot size, 1-second duration, 50 mW). To completely stop the blood flow, two to three laser applications were required as determined by observing the fundus image with a slit-lamp microscope. In each fundus, one or two veins was/were photocoagulated to create retinal ischemia.
In Vivo and Ex Vivo Imaging of Retinal Hypoxia Using HYPOX-4 in RVO Mice
Two hours post-RVO, mice were injected intraperitoneally with HYPOX-4 (60 mg/kg body weight). Twenty-two hours post injection of HYPOX-4, in vivo HYPOX-4–dependent fluorescence imaging was performed. The mice were anesthetized with a ketamine/xylazine (25/10 mg/kg) mixture, dilated with 1% tropicamide, and placed on a warm platform, Genteal lubricating eye gel was used to keep the eyes moist. Fluorescent and bright field fundus images were acquired using the Micron IV retinal imaging system (Phoenix Research Laboratories, Pleasanton, CA, USA). Mice were killed, enucleated, and the globes were fixed in 10% neurtral buffered formalin (NBF). Retinas were dissected, stained with Alexa Fluor 647-conjugated isolectin B4 and flat-mounted on a microscope slide with Prolong Gold mounting medium (Life Technologies, Grand Island, NY, USA). Images were captured using an epifluorescence Nikon Eclipse Ti-E inverted microscope (Nikon, Melville, NY, USA).
Ex Vivo Detection of Retinal Hypoxia in RVO Mice Using Pimonidazole-Adduct Immunostaining
Two hours post-RVO, mice received intraperitoneal injections of pimonidazole hydrochloride (60-mg/kg body weight); 1 hour later they were killed and enucleated. The globes were fixed in 10% NBF for 2 hours; retinas were dissected and washed with tris-buffered saline (TBS); then they were blocked/permeabilized in 10% donkey serum with 1% Triton X-100/0.05% Tween 20 in TBS for 6 hours and stained with an antibody against pimonidazole-adducts (Hypoxyprobe, Burlington, MA, USA) followed by the secondary anti-rabbit IgG conjugated to Alexa Fluor 647- and Alexa Fluor 488-conjugated isolectin B4.The retinas were mounted on microscope slides with Prolong Gold mounting medium (Life Technologies). Ex vivo images were captured using an epifluorescence Nikon Eclipse Ti-E inverted microscope. Quantitative estimation of hypoxia in the RVO retinas were assessed using magic wand tool in Adobe image processing software (San Jose, CA, USA).
Ultra-High Resolution Optical Coherence Tomography Imaging
Optical coherence tomography of the retinas was performed using Bioptigen ultra-high resolution spectral-domain OCT (SD-OCT) imaging system with a mouse retinal bore (Bioptigen LLC, Morrisville, NC, USA). Before and at 2 hours after PRVT, retinas of RVO mice were visualized with the SD-OCT system. Mice were anesthetized by intraperitoneal injection of a ketamine/xylazine (25/10 mg/kg) mixture and a 1% tropicamide solution was used to dilate the eyes for the retinal imaging. Genteal lubricating eye gel was used to keep the eyes moist. The mice were wrapped in gauze, placed in a holding chamber, and the head position was stabilized with a bite bar. Images were acquired from the optic disc to approximately 1.4 mm of the superior retina including the occlusion. InVivoVue software (Bioptigen LLC) was used for morphometric analysis of the retina.
Fluorescein Angiography
Fluorescein angiography was performed according to the methods previously described.31 Briefly, mice were anesthetized with a ketamine/xylazine (25/10 mg/kg) mixture; dilated with 1% tropicamide, and placed on a warm platform; Genteal lubricating eye gel was used to keep the eyes moist. A 10% sodium fluorescein (AK-fluor; Akorn, Decatur, IL, USA) sterile solution was injected systemically at a dose of 60 mg/kg; fluorescent and bright-field fundus images and in some cases videos were acquired using the Micron IV retinal imaging system.
Statistics
Data are presented as mean ± SD. Student's t-tests were performed to compare two samples and, for comparison of more than two samples, 1-way ANOVA was performed using Prism 6 (Graph-Pad, San Diego, CA, USA). P less than or equal to 0.05 was considered as statistically significant.
Results
Retinal Vein Occlusion Causes Acute Retinal Hypoxia at an Early Stage
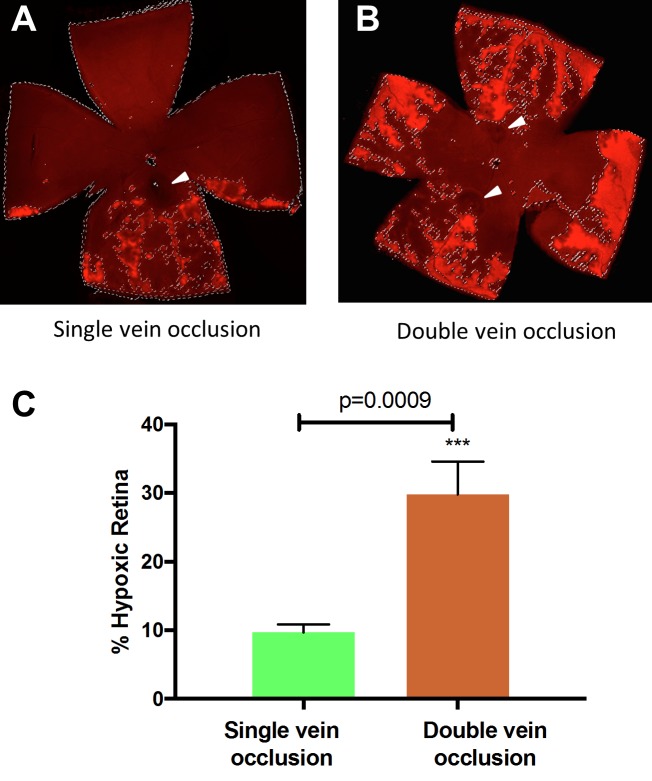
To evaluate retinal hypoxia in RVO mice, one or two of the major branch retinal vein(s) was/were occluded by PRVT.29 Retinal hypoxia was detected in mouse RVO using the standard ex vivo pimonidazole-adduct immunostaining technique.28 Total occlusion of the retinal vein was confirmed by FA at 2 hours after the laser treatment (Fig. 1). Retinal hypoxia was observed in all RVO mice within 2 hours post-PRVT. Photodynamic retinal venous thrombosis induced occlusion of one major retinal vein resulted in patchy areas of extravascular hypoxia adjacent to the occluded vein in the peripheral retina. Due to the dichotomous branching of the occluded retinal vein, regions of hypoxia were observed throughout one-half of the retina. Occlusion of temporal and nasal veins caused hypoxic regions to appear throughout the entire retina (Fig. 2). Based on pimonidazole-adduct immunostaining, we estimate that approximately 12% and 30% of the mouse retina becomes hypoxic following one or two vaso-occlusive events (nasal and temporal), respectively (Fig. 3). There were no significant changes in the retinal hypoxia of the nasal vessels after temporal vein occlusion. In transverse retinal sections from these RVO mice, hypoxia was localized to the cells in the inner nuclear layer with processes extending to the inner plexiform layer and retinal ganglion cell layer (Supplementary Fig. S4). After PRVT, the venous diameter downstream to the occlusion site is threadlike and is barely appreciable by FA (Fig. 1). Also, upstream from the occlusion site, the vein becomes dilated as seen in the fluorescein angiography. Supplementary Movie S1 illustrates an example of the venous blood circulation after RVO induction, with blood flow redirected toward the central retina presumably through collateral circulation,32 as evidenced by increased fluorescence observed in FA.
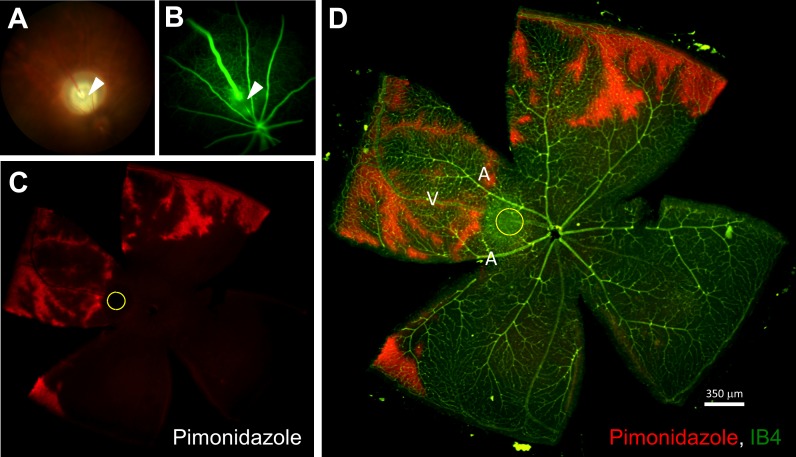
Figure 1.
Detection of retinal hypoxia in mouse RVO retina 2 hours post-PRVT (1 occlusion). (A) Fundus photograph of a mouse eye after PRVT-induced occlusion of a single vein (arrowhead) near the optic disk. (B) Fluorescein angiogram indicating complete nonperfusion of the fluorescent dye downstream from the occlusion. (C) Hypoxia was confirmed in RVO mouse retinas by pimonidazole-adduct immunostaining (red). (D) IB4 staining (green) merged with pimonidazole-adduct immunostaining (red); arrowhead and the yellow circle indicating the site of PRVT. A and V correspond to arteries and veins, respectively.
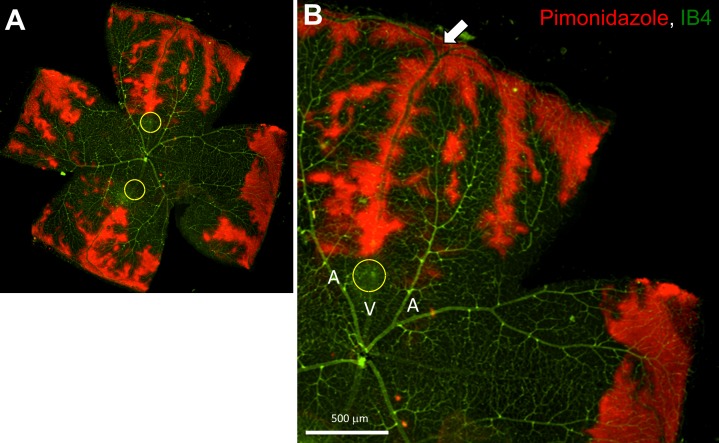
Figure 2.
Occurrence of retinal hypoxia in a mouse retina after occlusion of the temporal and nasal veins, 2 hours post-PRVT (2 occlusions). (A) Hypoxia was confirmed in RVO mice retina by pimonidazole-adduct immunostaining (red); IB4 staining (green) merged with pimonidazole-adduct immunostaining (red); Yellow circles indicate the PRVT-induced photocoagulation site. (B) Higher magnification of A. White arrow shows the dichotomous branching points.
Figure 3.
Quantitative analysis of hypoxia in RVO retinas by pimonidazole-adduct immunostaining. (A) After the occlusion of one major retinal vein (arrowhead), approximately 12 ± 3% of the retina becomes hypoxic. (B) Occlusion of two major retinal veins (arrowheads) causes approximately 30 ± 7% of the total retina becomes hypoxic. Double occlusion resulted in focal regions of hypoxia in the periphery, throughout the entire retinal circumference. Student's t-test was performed and P < 0.001 is considered as highly significant.
Retinal Vein Occlusion Leads to Retinal Edema, Increased Vascular Tortuosity and Neovascularization
Retinal vein occlusion causes focal retinal tissue damage that was clearly visible in the b-scan and en face images obtained from an SD-OCT imaging system (Supplementary Fig. S1). We observed changes in retinal thickness shortly after PRVT-induced occlusion that most likely result from increased permeability of the occluded vein. This proposition is supported because we applied the same amount of energy via PRVT to retinal areas located between two major vessels without significantly affecting retinal thickness. We also examined the retinal thickness at later times post-PRVT revealing that the edema observed earlier, had resolved to normal levels by day 8 (Supplementary Fig. S2). Retinal hemorrhages and increased vascular tortuosity were also commonly observed shortly after the vaso-occlusive event. Similar to other studies,3 in these RVO mice, the occluded vein spontaneously recannulated approximately 1 week post-PRVT treatment, as demonstrated by FA. Interestingly, nominal NV was commonly observed in these mice approximately 10 to 14 days post-PRVT (Fig. 4; Supplementary Fig. S3).
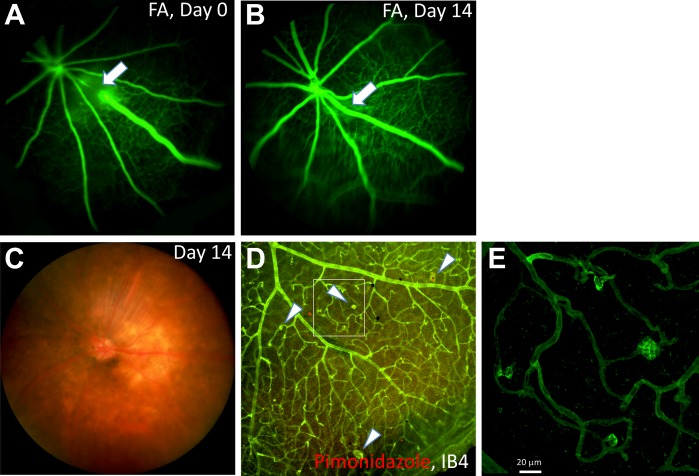
Figure 4.
Ex vivo analysis of a RVO mouse retina at 14 days post-PRVT showing retinal NV. (A) Fluorescein angiogram taken at an early stage post-PRVT, indicating complete occlusion of the vein (white arrow). (B) At day 14, the occluded vein had spontaneously recannulated as indicated by the FA (white arrow). (C) Fundus photograph of the mouse eye at day 14 post-PRVT of a major vein near the optic disk. (D) IB4 immunostaining of the same retina showed the occurrence of neovascular tufts in the peripheral retina adjacent to the occluded vein (arrowheads). The level of hypoxia was minimal at this time (red). (E) Higher magnification of the NV shown in (D).
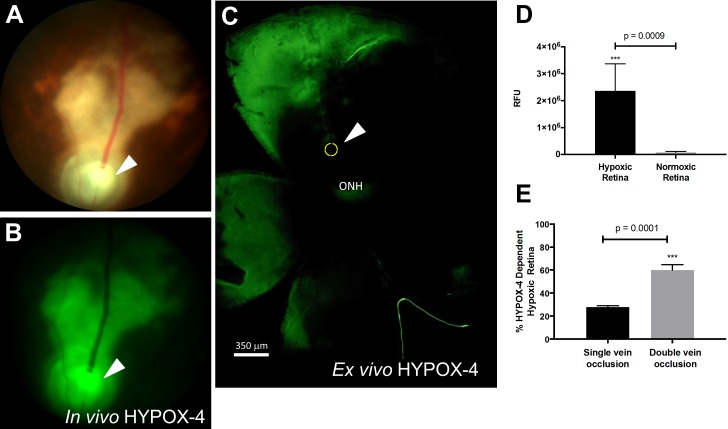
In Vivo and Ex Vivo Detection of Retinal Hypoxia in RVO Using HYPOX-4
In vivo imaging of retinal hypoxia was performed in C57BL/6 RVO mice using HYPOX-4. HYPOX-4 was systemically administered 2 hours after the PRVT; 22 hours later, HYPOX-4–dependent fluorescence was detected, indicating hypoxia upstream from the photocoagulation site (Fig. 5). Ex vivo analysis of HYPOX-4 fluorescence from the same retinal tissues, as measured by computer aided analysis (ImageJ software, http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) agreed with the in vivo findings. The patterns of retinal hypoxia observed by HYPOX-4–dependent imaging were generally the same as those obtained by pimonidazole-adduct immunostaining. HYPOX-4 produced more confluent images of retinal hypoxia, whereas the pimonidazole-adduct staining patterns appeared patchy. We did not observe any change in autofluorescence in mouse retinal tissues due to PRVT-induced RVO (Supplementary Fig. S5). Also, HYPOX-4 was cleared from ocular tissues within 24 hours after injection (Supplementary Fig. S6). These results clearly demonstrate that vascular occlusion can induce retinal hypoxia, in addition to demonstrating the utility of HYPOX-4 to detect it in the in vivo setting. The results suggest that in vivo HYPOX-4–dependent retinal imaging could provide useful information about the role of hypoxia in RVO.
Figure 5.
HYPOX-4–dependent in vivo and ex vivo imaging of retinal hypoxia in a RVO mouse. HYPOX-4 was administered by intraperitoneal injection 2 hours post-PRVT. In vivo imaging was performed 22 hours post injection. (A) Bright-field fundus photograph of a RVO mouse. The arrowhead and yellow circle show the position of the vein occlusion. (B) In vivo HYPOX-4–dependent fluorescence activity was detected upstream from the occlusion, indicating retinal hypoxia (green). (C) Ex vivo HYPOX-4–dependent fluorescence activity detected in the same retina (green). (D) Relative fluorescence intensity was measured using ImageJ software from hypoxic and normoxic retina in (B) and represented as RFU. (E) Quantitative analysis of HYPOX-4–dependent fluorescence in RVO retinas receiving single or double vein occlusion. Student's t-test was performed and P < 0.001 is considered as highly significant. ONH, optic nerve head.
Discussion
Increased ocular VEGF has been reported in the mouse and macaque in response to PRVT-induced RVO.3,6 Because VEGF is hypoxia-dependent, and ischemia is present in these models, it is reasonable to assume that it is an indicator of retinal hypoxia. Oxygen-electrode measurements in miniature pigs with PRVT-induced RVO, revealed preretinal hypoxia over the occluded vein, thus providing a direct measure of retinal hypoxia due to RVO.33
Similar to pimonidazole, the structure of HYPOX-4 incorporates the nitroimidazole moiety, serving as a biomarker of tissue hypoxia. In tissues with low oxygen pressure, azo- and nitroreductases are hypothesized to reduce the nitroimidazole causing it to become protein bound and sequestered. Using standard optical imaging equipment, Oregon Green dye also incorporated into the HYPOX-4 structure, enables the detection of zones of retinal hypoxia by its fluorescence activity. We have demonstrated here, through the application of HYPOX-4, that retinal hypoxia develops after the onset of RVO-induced retinal ischemia in living mice and in real time. Heretofore, these types of measurements have not been reported in animal models of RVO; which may be largely due to the limitations of existing technologies. The observed patterns of retinal hypoxia determined by HYPOX-4–dependent fluorescence, are consistent with those that would be expected, overlapping significantly with the retinal areas that are drained by the vein prior to the occlusive event (Fig. 5). Our data show that retinal hypoxia increases with the number of PRVT-induced occlusions. Patterns of hypoxia determined by HYPOX-4 fluorescence activity were generally similar to those obtained by the ex vivo pimonidazole-adduct immunofluorescence technique. Although, they tended to be nominally more diffuse, probably owing to the greater bioavailability of HYPOX-4. The pimonidazole immunoreactivity provided an alternative hypoxia assessment and confirmed HYPOX-4 as a potential in vivo hypoxia-imaging agent.
We observed retinal edema and NV in our RVO mice; both are vision-threatening sequelae to RVO. In patients, retinal edema may produce a serious distortion of vision if the macula is involved. It has been presumed that venous occlusion results in increased fluid pressure, causing the affected vein to swell, compromising its structural integrity, allowing fluid to leak into the retinal parenchyma, and finally resulting in macular edema. Notably, clinical and experimental data demonstrate the efficacy of anti-VEGF therapies against macular edema secondary to RVO.13 This immediately raises the suspicion of hypoxia as a pathogenic component, although other molecular signaling mechanisms that raise tissue levels of VEGF are known. Interestingly, if hypoxia does indeed factor into the pathogenesis of RVO-associated macular edema, then its in vivo imaging would offer distinct advantages. In this study, we observed retinal edema 2 hours post-PRVT that resolved by day 8 (Supplementary Fig. S2). And this finding is consistent with the results of other studies in the mouse, rat, and pig (Supplementary Fig. S1).3,34,35 At this early time-point, the observed retinal edema is most likely due to increased permeability of the distended and structurally compromised occluded retinal vein, rather than hypoxia. This is reasonable because this time of assessment is too short for de novo protein synthesis to occur (e.g., hypoxia-induced VEGF or other vasoactive factors). We observed retinal NV in all of the eyes of these mice 10 to 14 days post-PRVT (Fig. 4; Supplementary Fig. S3). These results are generally consistent with those of previous studies; retinal NV, secondary to RVO, was observed at 2 weeks in the mouse,29 3 weeks in miniature pigs,35 2 weeks in albino rats,36 and 10 to 12 weeks in pigmented rats.34 In each of these studies, recannulization of occluded retinal veins in experimental subjects was reported. We observed recannulization of the affected retinal vein by post-PRVT day 8, in almost all of our RVO mice. Recannulization clearly increases blood flow, oxygenating the retina, and conceivably reducing any hypoxia-dependent VEGF. Therefore, it is reasonable to consider whether the retinal NV that others and we have observed is indeed hypoxia-dependent. After laser-induced venous occlusion, blood flow and oxygen diffusion to the perivascular tissue ceases, and by our HYPOX-4 assessments, triggering the onset of hypoxia. Additionally, destructive processes occurring secondary to venous occlusion may ensue, creating focal regions of retinal hypoxia due to vasoregression.3,29 This may offset the oxygenation effects of recannulization, resulting in a hypoxia-induced vasoproliferative response. In the current study, we assessed retinal hypoxia at 9 (HYPOX-4 in vivo) to 10 days (Pimonidazole ex vivo) prior to the development of retinal NV. Our data do not support or refute a link between hypoxia and retinal NV in this mouse model of RVO. Furthermore, we do not discount the possibility that ischemia-reperfusion injury or inflammation may raise VEGF levels contributing to the observed retinal NV. Future studies are aimed at better defining the hypoxia/NV relationship in experimental RVO using HYPOX-4 as a molecular probe to detect and quantify retinal hypoxia.29,32 The development of a durable RVO model in rodents will be critical to these studies and we are currently pursuing this goal.
In conclusion, we have demonstrated that retinal hypoxia and edema appear within 2 hours, and retinal NV at 10 to 14 days post-PRVT in RVO mice. To our knowledge, this is the first report describing the occurrence of retinal hypoxia and edema at this early time-point.37 This study also demonstrates that, HYPOX-4, a new optical imaging probe, has high potential for the detection of retinal hypoxia in vivo in experimental RVO, before the onset of physiological changes leading to retinal cell damage and NV. We propose that the level of retinal hypoxia produced by a PRVT-induced occlusive event in the retina is proportional to the intensity of a developing vasoproliferative response. In future studies, we will test the ability of HYPOX-4–dependent imaging of hypoxia to determine this relationship and hopefully use it to predict the potential severity of an incipient vasculopathy.
Supplementary Material
Acknowledgments
Supported in part by research Grants from National Institutes of Health (Bethesda, MD, USA) R01EY23397, R01EY07533, and R01EY23639 (JSP), a grant from the Knights Templar Eye Foundation, Inc., Flower Mound, TX, USA (MIU), MMPC MICROMouse U24DK076169 (to AJ), a grant from the Carl Marshall Reeves & Mildred Almen Reeves Foundation, Inc., Columbus, IN, USA (JSP), Vanderbilt Diabetes Research and Training Center Core grant (P30 DK020593-34-39; Bethesda, MD, USA), Vanderbilt Vision Research Center National Eye Institute Core Grant (P30 EY008126; Bethesda, MD, USA), and an Unrestricted Grant from Research to Prevent Blindness, Inc. (New York, NY, USA).
Disclosure: M.I. Uddin, P; A. Jayagopal, P; G.W. McCollum, None; R. Yang, None; J.S. Penn, P
References
- 1. Petrella RJ,, Blouin J,, Davies B,, Barbeau M. Incidence and characteristics of patients with visual impairment due to macular edema secondary to retinal vein occlusion in a representative Canadian cohort. J Ophthalmol. 2012; 2012: 723169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng EW,, Shima DT,, Calias P,, Cunningham ET, Jr,, Guyer DR,, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006; 5: 123–132. [DOI] [PubMed] [Google Scholar]
- 3. Dominguez E,, Raoul W,, Calippe B,, Sahel JA,, Guillonneau X,, Paques M,, Sennlaub F. Experimental branch retinal vein occlusion induces upstream pericyte loss and vascular destabilization. PLoS One. 2015; 10: e0132644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rehak M,, Hollborn M,, Iandiev I,, et al. Retinal gene expression and Müller cell responses after branch retinal vein occlusion in the rat. Invest Ophthalmol Vis Sci. 2009; 50: 2359–2367. [DOI] [PubMed] [Google Scholar]
- 5. McAllister IL,, Vijayasekaran S,, Chen SD,, Yu DY. Effect of triamcinolone acetonide on vascular endothelial growth factor and occludin levels in branch retinal vein occlusion. Am J Ophthalmol. 2009; 147: 838–846. e2. [DOI] [PubMed] [Google Scholar]
- 6. Zhao T,, Lu Q,, Tao Y,, Liang XY,, Wang K,, Jiang YR. Effects of apelin and vascular endothelial growth factor on central retinal vein occlusion in monkey eyes intravitreally injected with bevacizumab: a preliminary study. Mol Vis. 2011; 17: 1044–1055. [PMC free article] [PubMed] [Google Scholar]
- 7. Noma H,, Funatsu H,, Mimura T,, Harino S,, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009; 116: 87–93. [DOI] [PubMed] [Google Scholar]
- 8. Pe'er J,, Folberg R,, Itin A,, Gnessin H,, Hemo I,, Keshet E. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998; 105: 412–416. [DOI] [PubMed] [Google Scholar]
- 9. Aiello LP,, Avery RL,, Arrigg PG,, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 10. Adamis AP,, Shima DT,, Tolentino MJ,, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a non-human primate. Arch Ophthalmol. 1996; 114: 66–71. [DOI] [PubMed] [Google Scholar]
- 11. Brown DM, Campochiaro PA, Singh RP, et al.; for the CRUISE Investigators. . Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117: 1124–1133. e1. [DOI] [PubMed] [Google Scholar]
- 12. Campochiaro PA,, Brown DM,, Awh CC,, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011; 118: 2041–2049. [DOI] [PubMed] [Google Scholar]
- 13. Campa C,, Alivernini G,, Bolletta E,, Parodi MB,, Perri P. Anti-VEGF therapy for retinal vein occlusions. Curr Drug Targets. 2016; 17: 328–336. [DOI] [PubMed] [Google Scholar]
- 14. Ehlers JP,, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011; 56: 281–299. [DOI] [PubMed] [Google Scholar]
- 15. Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005; 24: 493–519. [DOI] [PubMed] [Google Scholar]
- 16. Hayreh SS. Classification of central retinal vein occlusion. Ophthalmology. 1983; 90: 458–474. [DOI] [PubMed] [Google Scholar]
- 17. Hayreh SS,, Klugman MR,, Beri M,, Kimura AE,, Podhajsky P. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990; 228: 201–217. [DOI] [PubMed] [Google Scholar]
- 18. Shahidi M,, Shakoor A,, Blair NP,, Mori M,, Shonat RD. A method for chorioretinal oxygen tension measurement. Curr Eye Res. 2006; 31: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linsenmeier RA,, Braun RD,, McRipley MA,, et al. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci. 1998; 39: 1647–1657. [PubMed] [Google Scholar]
- 20. Berkowitz BA,, Penn JS. Abnormal panretinal response pattern to carbogen inhalation in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1998; 39: 840–845. [PubMed] [Google Scholar]
- 21. Hardarson SH,, Harris A,, Karlsson RA,, et al. Automatic retinal oximetry. Invest Ophthalmol Vis Sci. 2006; 47: 5011–5016. [DOI] [PubMed] [Google Scholar]
- 22. Dai C,, Liu X,, Zhang HF,, Puliafito CA,, Jiao S. Absolute retinal blood flow measurement with a dual-beam Doppler optical coherence tomography. Invest Ophthalmol Vis Sci. 2013; 54: 7998–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soetikno BT,, Yi J,, Shah R,, et al. Inner retinal oxygen metabolism in the 50/10 oxygen-induced retinopathy model. Sci Reports. 2015; 5: 16752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scott A,, Fruttiger M. Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye. 2010; 24: 416–421. [DOI] [PubMed] [Google Scholar]
- 25. Uddin MI,, Evans SM,, Craft JR,, et al. In vivo imaging of retinal hypoxia in a model of oxygen-induced retinopathy. Sci Reports. 2016; 6: 31011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uddin MI,, Evans SM,, Craft JR,, Marnet LJ,, Uddin MJ,, Jayagopal A. Applications of azo-based probes for imaging retinal hypoxia. ACS Med Chem Lett. 2015; 6: 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans SM,, Kim K,, Moore CE,, et al. Molecular probes for imaging of hypoxia in the retina. Bioconjug Chem. 2014; 25: 2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varia MA,, Calkins-Adams DP,, Rinker LH,, et al. Pimonidazole: a novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecol Oncol. 1998; 71: 270–207. [DOI] [PubMed] [Google Scholar]
- 29. Zhang H,, Sonoda KH,, Qiao H,, Oshima T,, Hisatomi T,, Ishibashi T. Development of a new mouse model of branch retinal vein occlusion and retinal neovascularization. Jpn J Ophthalmol. 2007; 51: 251–257. [DOI] [PubMed] [Google Scholar]
- 30. Ebneter A,, Agca C,, Dysli C,, Zinkernagel MS. Investigation of retinal morphology alterations using spectral domain optical coherence tomography in a mouse model of retinal branch and central retinal vein occlusion. PLoS One. 2015; 10: e0119046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hui F,, Nguyen CT,, Bedggood PA,, et al. Quantitative spatial and temporal analysis of fluorescein angiography dynamics in the eye. PLoS One. 2014; 9: e111330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henkind P,, Wise GN. Retinal Neovascularization, collaterals, and vascular shunts. Br J Ophthalmol. 1974; 58: 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pournaras CJ,, Tsacopoulos M,, Strommer K,, Gilodi N,, Leuenberger PM. Experimental retinal branch vein occlusion in miniature pigs induces local tissue hypoxia and vasoproliferative microangiopathy. Ophthalmology. 1990; 97: 1321–1328. [DOI] [PubMed] [Google Scholar]
- 34. Saito Y,, Park L,, Skolik SA,, et al. Experimental preretinal neovascularization by laser-induced venous thrombosis in rats. Curr Eye Res. 1997; 16: 26–33. [DOI] [PubMed] [Google Scholar]
- 35. Danis RP,, Yang Y,, Massicotte SJ,, Boldt HC. Preretinal and optic nerve head neovascularization induced by photodynamic venous thrombosis in domestic pigs. Arch Ophthalmol. 1993; 111: 539–543. [DOI] [PubMed] [Google Scholar]
- 36. Ham DI,, Chang K,, Chung H. Preretinal neovascularization induced by experimental retinal vein occlusion in albino rats. Korean J Ophthalmol. 1997; 11: 60–64. [DOI] [PubMed] [Google Scholar]
- 37. Fukuda S,, Okuda K,, Kishino G,, et al. In vivo retinal and choroidal hypoxia imaging using a novel activatable hypoxia-selective near-infrared fluorescent probe. Graefes Arch Clin Exp Ophthalmol. 2016; 254: 2373–2385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.