Abstract
Purpose
Intracameral injection is an effective method for preventing infection, but no controlled study has been published in the United States.
Design
We conducted an observational, longitudinal cohort study to examine the effect of topical and injected antibiotics on risk of endophthalmitis.
Participants
We identified 315 246 eligible cataract procedures in 204 515 members of Kaiser Permanente, California, 2005–2012.
Methods
The study used information from the membership, medical, pharmacy, and surgical records from the electronic health record.
Main Outcome Measures
The adjusted odds ratio (OR) and 95% confidence interval (CI) for the association of antibiotic prophylaxis (route and agent) with risk of endophthalmitis was estimated using logistic regression analysis.
Results
We confirmed 215 cases of endophthalmitis (0.07% or 0.7/1000). Posterior capsular rupture was associated with a 3.68-fold increased risk of endophthalmitis (CI, 1.89–7.20). Intracameral antibiotic was more effective than topical agent alone (OR, 0.58; CI, 0.38–0.91). Combining topical gatifloxacin or ofloxacin with intracameral agent was not more effective than using an intracameral agent alone (compared with intracameral only: intracameral plus topical, OR, 1.63; CI, 0.48–5.47). Compared with topical gatifloxacin, prophylaxis using topical aminoglycoside was ineffective (OR, 1.97; CI, 1.17–3.31).
Conclusions
Surgical complication remains a key risk factor for endophthalmitis. Intracameral antibiotic was more effective for preventing post-cataract extraction endophthalmitis than topical antibiotic alone. Topical antibiotic was not shown to add to the effectiveness of an intracameral regimen.
Endophthalmitis is a rare surgical site infection after cataract surgery with the potential for devastating loss of vision.1,2 There is no current single standard for prophylaxis in the United States or Canada.3,4 We conducted an observational comparative-effectiveness study, using the practice variation present in the Kaiser Permanente California program, to identify the most effective prophylaxis regimen from among those used in our system. The aims of the study were to assess the effectiveness of intracameral antibiotic injection with cefuroxime or moxifloxacin, as well as patient-instilled topical administration of gatifloxacin, ofloxacin, polymyxin-trimethoprim, moxifloxacin, or aminoglycoside (neomycin, gentamicin, tobramycin).
Methods
The study was approved by the local institutional review board of the Kaiser Foundation Research Institute.
Setting
Clear cornea phacoemulsification is performed in 21 surgical centers in Northern California and in 17 surgical centers in Southern California. Surgeons have autonomy in choosing their prophylactic route and agent, but a uniform practice included the topical application of povidone-iodine as a prep just before surgery. The health plan stores detailed information on office visits, pharmacy, surgery, and laboratory information in an electronic health record.
Study Population
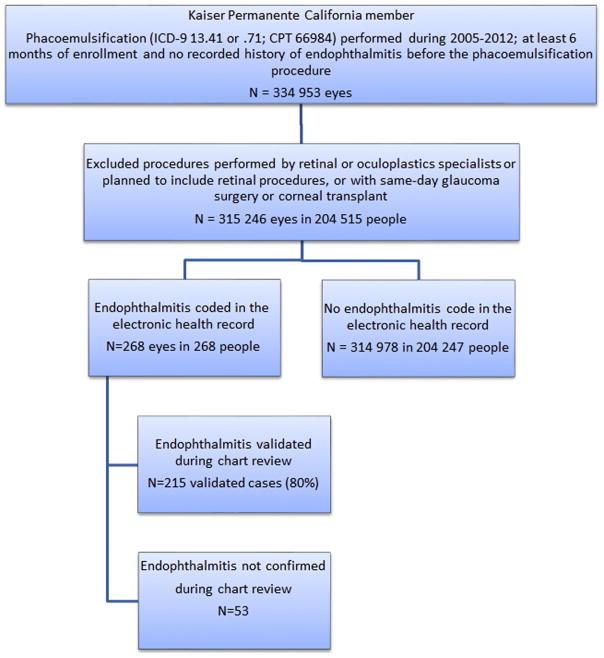
The present study included eyes from members with at least 6 months of health-plan enrollment before phacoemulsification between January 1, 2005, and December 31, 2012. We included procedures performed in a hospital, outpatient surgery center, or ophthalmology department procedure center. The study included phacoemulsifications assigned Current Procedural Terminology (CPT) code 66984 or International Classification of Diseases, 9th Edition (ICD-9) codes 13.41 or 13.71. These codes are assigned to procedures that were planned in advance to be simple, including procedures that ultimately were complicated by posterior capsular rupture (PCR). The study excluded phacoemulsification in <5% of procedures that were assigned CPT code 66982, defined in advance of surgery to be complex, because these procedures were too few in number to enable the more resource-intensive data validation that would have been required. We also excluded eyes with a diagnosis of endophthalmitis (ICD-9 codes: 360.00, 360.01, 360.03, 360.13, 360.19, 098.42) recorded before the first eligible procedure (Fig 1), procedures performed by retinal or oculoplastic specialists or involving planned retinal procedures, and procedures combined with corneal transplant (ICD-9 11.6) or glaucoma surgery (ICD-9 12.1–12.7).
Figure 1.
Study population. CPT = Current Procedural Terminology; ICD-9 = International Classification of Diseases, 9th revision.
Data Collection
Acute, postoperative, infectious endophthalmitis was defined as occurring after the first postoperative day through the 90th day after phacoemulsification. Preliminary endophthalmitis was defined as having 1 or more ICD-9 diagnosis codes of 360.00, 360.01, 360.03, 360.13, 360.19, or 098.42, or having an aqueous or vitreous specimen submitted to microbiology within 90 days of phacoemulsification. Validated endophthalmitis was based on detailed medical record review by a trained medical record abstractor or ophthalmologist, including review of the operative report, first follow-up visit, visits to retinologists, microbiology results, and other pertinent information. We confirmed postoperative, infectious endophthalmitis when the diagnosis was recorded by a retina specialist within 90 days of surgery and treatment included injection of intravitreal antibiotics. We did not require microbiological confirmation, although positive cultures were noted and will be reported separately.
Topical antibiotic orders and dispensings were obtained from the computerized pharmacy information management system, from which we obtained details of the following ophthalmic antibiotic preparations: gatifloxacin, ofloxacin, polymyxin-trimethoprim, gentamicin, or tobramycin. Topical moxifloxacin was not on the formulary. We included medications dispensed up to 90 days before phacoemulsification. The intracameral agent was captured using natural language processing with validation by a manual review of the operative report; the positive predictive value was 99.9% (95% confidence intervals [CIs], 99.4–100); the negative predictive value was 99.9% (95% CI, 99.4–100).
Potential Confounding Factors
Patient age, sex, and race/ethnicity were obtained from membership data. Posterior capsular rupture was ascertained using natural language processing of the operative report; the positive predictive value of the algorithm was 94.4% (95% CI, 92.6–95.9), and the negative predictive value was 99.9% (95% CI, 99.3–100.0). Comorbidity was ascertained using data recorded into the electronic medical record during the 6 months preceding the surgery. Ocular comorbidity included diabetic retinopathy, glaucoma, and macular degeneration. Systemic comorbidity was coded using the Charlson Comorbidity Index, comprising cardiovascular and peripheral vascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, renal disease, cancer, and acquired immunodeficiency syndrome.5
Potential adverse events of antibiotic prophylaxis were determined from diagnostic codes recorded during the 120 days after the procedure and included retinal detachments and defects (ICD-9 361), cystoid macular edema (362.53), other retinal disorders (362 except 362.53), iritis (364), visual disturbances (368), keratitis and other corneal disorders (370–371), and disorders of the conjunctiva (372). Analyses of potential adverse events excluded patients with a history of the adverse event before their phacoemulsification.
Statistical Analysis
Multivariate logistic regression analysis was performed to examine the odds ratio (OR) and 95% CI for the association of antibiotic prophylaxis with the risk of endophthalmitis.6 All analyses were conducted using SAS version 9.3 (SAS Inc, Cary, NC). We performed subgroup analysis to assess topical agents, the most common topical and intracameral agents, the added effect of topical above intracameral, and effects in eyes with PCR.
Results
After excluding 5.9% of eyes with concurrent surgeries or complex procedures, the study included 315 246 eyes in 204 515 patients who underwent phacoemulsification and 215 validated cases of endophthalmitis. No patient developed endophthalmitis in both eyes. The crude incidence rate of endophthalmitis was 0.68 per 1000 phacoemulsification procedures. The frequency of PCR was 1.13%.
Compared with using topical agent alone, the adjusted OR for the association of endophthalmitis with intracameral injection (with or without topical agent) was 0.58 (95% CI, 0.38–0.91) (Table 1). We further observed that 4.5% of the cohort did not have a record of a dispensing of topical antibiotic or intracameral injection of antibiotic; the OR for this group was 1.95 (95% CI, 1.22–3.11) compared with using topical agent alone. These results were adjusted for year of surgery, patient age, Charlson comorbidity index, diabetic retinopathy, and PCR. Patient sex, race/ethnicity, history of glaucoma, and history of macular degeneration were not associated with the choice of antibiotic approach and did not confound the associations.
Table 1.
Associations with Endophthalmitis Risk, Kaiser Permanente California, 2005–2012
| Characteristic | Phacoemulsification Procedures (N=315 246), % | Endophthalmitis Cases (N=215), % | Adjusted* OR (95% CI) |
|---|---|---|---|
| Year of surgery | |||
| 2005–2007 | 22.3 | 31.6 | 1.0 (Ref) |
| 2008–2010 | 42.9 | 42.8 | 0.68 (0.49–0.94) |
| 2011–2012 | 34.8 | 25.6 | 0.57 (0.39–0.84) |
| Patient age, yrs | |||
| 18–69 | 35.3 | 33.5 | 1.0 (Ref) |
| 70–79 | 40.7 | 33.0 | 0.86 (0.62–1.20) |
| ≥80 | 24.0 | 33.5 | 1.46 (1.05–2.05) |
| Charlson Index | |||
| 0 | 40.4 | 37.2 | 1.0 (Ref) |
| ≥1 | 59.6 | 62.8 | 1.06 (1.00–1.14) |
| Diabetic retinopathy | |||
| No | 88.5 | 84.2 | 1.0 (Ref) |
| Yes | 11.5 | 15.8 | 1.31 (0.87–1.98) |
| PCR | |||
| No | 98.9 | 95.8 | 1.0 (Ref) |
| Yes | 1.1 | 4.2 | 3.68 (1.89–7.20) |
| Route of prophylaxis | |||
| Topical alone | 75.4 | 77.7 | 1.0 (Ref) |
| Intracameral with or without topical | 20.1 | 13.0 | 0.58 (0.38–0.91) |
| Neither intracameral nor topical | 4.5 | 9.3 | 1.95 (1.22–3.11) |
CI = confidence interval; OR = odds ratio; PCR = posterior capsular rupture.
Each variable was adjusted for every other variable shown in the table, with coding as shown.
In addition to findings related to the approach to prophylaxis, our study further observed an OR of 0.57 (95% CI, 0.39–0.84) for undergoing surgery in the latest years of the study (2011–2012) versus the earliest (2005–2007); an OR of 1.46 (95% CI, 1.05–2.05) for those aged ≥80 years versus <70 years; an OR of 1.31 (95% CI, 0.87–1.98) for patients with a history of diabetic retinopathy; and an OR of 3.68 (95% CI, 1.89–7.20) for surgeries complicated by PCR.
We further examined differences in the OR by antibiotic agent, with combinations of intracameral and topical agents, and in eyes with PCR (Table 2). Compared with using topical agent alone, the adjusted OR was 0.53 (95% CI, 0.30–0.95) for intracameral cefuroxime and 0.68 (95% CI, 0.36–1.33) for moxifloxacin (subgroup 2). In the subgroup of eyes that received topical but not intracameral agent, we observed no important or significant differences among gatifloxacin, ofloxacin, and polymyxin/trimethoprim; however, the OR with aminoglycoside (neomycin, gentamicin, tobramycin) was elevated (OR, 1.97; 95% CI, 1.17–3.31) and comparable to the level of risk without record of any use of antibiotic (subgroup 3). In the subgroup of eyes that received the most common topical antibiotic (i.e., topical fluoroquinolone), we observed no important differences between intracameral cefuroxime (OR, 0.58; 95% CI, 0.32–1.05) and moxifloxacin (OR, 0.74; 95% CI, 0.37–1.47) (subgroup 4). In the subgroup of eyes that received intracameral cefuroxime or moxifloxacin, the OR associated with adding concomitant topical gatifloxacin or ofloxacin was 1.63 (95% CI, 0.48–5.47), but could have resulted from chance (subgroup 5). In the relatively small group of eyes with PCR, the crude incidence of endophthalmitis was 2.40 per 1000 in those given topical antibiotic only and 2.48 in those given intracameral with or without topical; after adjustment, we could not draw a clear inference about the benefit of intracameral administration (intracameral ± topical compared with topical only: OR, 0.49; 95% CI, 0.08–3.09) (subgroup 6).
Table 2.
Subgroup Analysis: Associations of Antibiotic Prophylaxis with Endophthalmitis Risk
| Subgroup* | Contrast | No. of Endophthalmitis Cases/No. of Phacoemulsification Procedures | Crude Incidence Rate, per 1000 | Adjusted* OR (95% CI) |
|---|---|---|---|---|
| 1. All eyes | Topical alone | 167/237 709 | 0.70 | 1.0 (Ref) |
| Intracameral ± topical | 28/63 241 | 0.44 | 0.58 (0.38–0.91) | |
| No antibiotic | 20/14 296 | 1.40 | 1.95 (1.22–3.11) | |
| 2. Eyes receiving antibiotic | Topical alone | 167/237 709 | 0.70 | 1.0 (Ref) |
| Intracameral cefuroxime ± topical | 14/35 781 | 0.39 | 0.53 (0.30–0.95) | |
| Intracameral moxifloxacin ± topical | 10/21 150 | 0.47 | 0.68 (0.36–1.33) | |
| Intracameral other† ± topical | 4/6310 | 0.63 | 0.93 (0.34–2.55) | |
| 3. Eyes receiving topical but not intracameral agent | Gatifloxacin | 68/114 247 | 0.60 | 1.00 (Ref) |
| Ofloxacin | 68/95 021 | 0.72 | 1.19 (0.85–1.67) | |
| Polymyxin/trimethoprim | 10/13 768 | 0.73 | 1.12 (0.58–2.19) | |
| Aminoglycoside‡ | 20/13 086 | 1.53 | 1.97 (1.17–3.31) | |
| 4. Eyes receiving topical fluoroquinolone ± intracameral | Topical gatifloxacin or ofloxacin | 139/213 993 | 0.65 | 1.0 (Ref) |
| Intracameral cefuroxime ± topical | 14/35 781 | 0.39 | 0.58 (0.32–1.05) | |
| Intracameral moxifloxacin ± topical | 10/21 150 | 0.47 | 0.74 (0.37–1.47) | |
| 5. Eyes receiving intracameral cefuroxime or moxifloxacin | Without topical gatifloxacin or ofloxacin | 3/11 001 | 0.27 | 1.0 (Ref) |
| With topical gatifloxacin or ofloxacin | 21/45 930 | 0.46 | 1.63 (0.48–5.47) | |
| 6. Eyes with PCR | Topical only | 6/2496 | 2.40 | 1.0 (Ref) |
| Intracameral ± topical | 2/805 | 2.48 | 0.49 (0.08–3.09) |
CI = confidence interval; OR = odds ratio; PCR = posterior capsular rupture.
Six separate models were run, 1 for each subgroup. Every model was adjusted using the variables and coding provided in Table 1.
Vancomycin or unspecified.
Aminoglycosides included neomycin, gentamicin, and tobramycin.
The incidence rates of potential adverse events occurring through postoperative day 120 are shown in Table 3 together with the ORs and CIs for the association with intracameral compared with topical antibiotic. Intracameral injection was associated with a lower risk of retinal detachment (OR, 0.80; 95% CI, 0.67–0.96) and conjunctival disorders (OR, 0.84; 95% CI, 0.78–0.91). Intracameral injection was associated with slightly higher risks of “other retinal disorders” (OR, 1.51; 95% CI, 1.31–1.74), iritis (OR, 1.14; 95% CI, 1.08–1.21), and keratitis and other cornea disorders (OR, 1.10; 95% CI, 1.03–1.19).
Table 3.
Associations of Prophylaxis (Intracameral Compared with Topical) with Potential Adverse Events
| Diagnosis (ICD-9 code) | Topical (N=237 709), % | Intracameral (N=63 241), % | Adjusted OR* | 95% CI | |
|---|---|---|---|---|---|
| Retinal detachment | 361 | 0.37 | 0.31 | 0.80† | 0.67–0.96 |
| Diabetic retinopathy | 362.0 | 0.31 | 0.21 | 0.74‡ | 0.60–0.91 |
| Cystoid macular edema | 362.53 | 1.94 | 1.93 | 0.97 | 0.90–1.05 |
| Macular degeneration | Other 362.5 | 0.83 | 0.79 | 0.98 | 0.87–1.10 |
| Other retinal disorders | Other 362 | 0.33 | 0.69 | 1.51† | 1.31–1.74 |
| Iritis | 364 | 3.19 | 3.91 | 1.14† | 1.08–1.21 |
| Keratitis and other cornea disorders | 370–371 | 1.74 | 2.30 | 1.10† | 1.03–1.19 |
| Conjunctival disorders | 372 | 1.81 | 1.74 | 0.84† | 0.78–0.91 |
CI = confidence interval; ICD-9 = International Classification of Diseases, 9th revision; OR = odds ratio.
Adjusted for patient age, year of surgery, Charlson comorbidity index, history of diabetic retinopathy, and PCR.
P ≤ 0.05.
P ≤ 0.005.
Discussion
We conducted a controlled, observational cohort study of 315 246 phacoemulsification procedures to assess the effectiveness of intracameral antibiotic administration, compared with topical administration, for preventing acute postoperative endophthalmitis. We also compared various antibiotic agents with one another after accounting for route of administration. Important findings include a 42% reduction in risk associated with intracameral administration, an approximate doubling of risk in the 4.5% of eyes that had no evidence of antibiotic prophylaxis, and lack of effectiveness of topical aminoglycosides. The study did not detect differences in risk between intracameral cefuroxime and intracameral moxifloxacin or between intracameral agent alone and intracameral plus topical agent. Furthermore, among those not exposed to intracameral antibiotic, the study did not detect differences among topical gatifloxacin, ofloxacin, and polymyxin/trimethoprim. Posterior capsular rupture was associated with a 3.7-fold increased risk of endophthalmitis.
Strengths of the study included the large, well-defined, community-based cohort; multiple treatment arms; outstanding follow-up; detailed information on potential confounding factors; and generalizability to other community-based settings.7,8 A key concern with observational comparative effectiveness studies is the potential for confounding by indication, in which the surgeon’s decision to use one or another practice is based on the risk of endophthalmitis. In this study, the use of intracameral antibiotic required compounding, and the surgeon prepared for the day using routine workflows that are unrelated to the risk of each case, so that confounding by indication is unlikely. However, if it were to occur, and surgeons chose intracameral injection only for high-risk eyes, this would result in underestimation of the true benefit of intracameral injection. To minimize confounding by indication, we sought to exclude eyes planned in advance for complex surgery.
Another potential limitation was the absence of information on factors such as patient adherence to their drops once the prescriptions were dispensed, although this was not likely to differ systematically among eyes receiving various prophylactic regimens. Exclusion of patients who were assigned CPT code 66982, whose surgery was performed by a retinologist or oculoplastic surgeon, or who underwent multiple, concurrent surgeries may have reduced the generalizability of the study somewhat. However, validation of diagnostic coding and surgical histories, planned procedures, and surgical complications proved to be highly resource-intensive in these patients and was not feasible for the current study.
Finally, we did not obtain information about environmental cleaning, equipment sterilization, or wound construction and management; over time, quality control in these areas has improved, and the result was largely captured by adjustment for year of surgery. We also examined the surgeon’s experience as a covariate in the analysis, but it did not confound the association with antibiotic approach, although it should be noted that the number of endophthalmitis cases was nearly the same as the number of surgeons, so analysis of surgeon’s experience lacked statistical power. We plan a separate report of surgeon experience and other factors in relation to the risk of PCR, which is more common than endophthalmitis and therefore provides a feasible end point for examining surgeon factors.
Because the majority of patients filled their prescription for topical antibiotic in advance of their surgery, we could not ascertain, at the patient level, preoperative and postoperative administrations. However, we conducted a survey in 2011 asking each surgeon about his/her topical antibiotic orders, and 70% routinely prescribed preoperative use and 97% routinely prescribed postoperative use. Only 28% reported administering antibiotic drops in the preoperative holding area.
The risk of endophthalmitis in patients without intra-cameral agent was lower in this study than in past reports (risk in intracameral group, 0.044%; risk in topical group, 0.070%). In other ways, our study results are generally consistent with the European Society of Cataract & Refractive Surgeons randomized controlled trial (risk in intracameral group, 0.05%; risk in control group, 0.35%),9 with the population-based cohort study of Lundstrom et al10 (0.048%, 0.35%), and with several studies that used historical controls,11 including our own (0.14%, 0.31%).12 To our knowledge, our study is the first to report associations of intracameral injection with risk of potential adverse events, although this was not a primary aim of the study, the analysis was exploratory, the events were not validated using chart review, and the study was not designed specifically to optimize the validity of these comparisons. The absolute risks of adverse events generally were very low, and because of the size of the study, even small differences were statistically significant. We recommend confirmation of these findings.
We previously reported a preliminary study, using historical controls, that was based in only 1 of our medical centers, the Diablo Service Area, and included 16 264 phacoemulsification procedures compared with the 315 246 procedures included in this study.12 The prior study observed a decline in the rate (per 1000) of endophthalmitis from 3.13 (95% CI, 1.43–5.93) in 2007 to 0.14 (95% CI, 0–0.78) in 2010 to 2011 after full implementation of intracameral injection. In the present analysis, the average rate of endophthalmitis in eyes given intracameral injection was 0.44 per 1000 (N = 28 cases). To enable direct comparison of the present study with the earlier report, we conducted a subgroup analysis restricted to the Diablo Service Area. In this 1 facility, the adjusted OR for the benefit of intracameral injection, compared with topical alone, was 0.42 (95% CI, 0.11–1.62), which is somewhat lower than the OR of 0.58 (95% CI, 0.38–0.91) estimated for all of the service areas combined. After accounting for intracameral injection, our study observed a substantial 43% reduced risk of endophthalmitis over the 8-year study period, suggesting broad improvements in surgical safety separate from intracameral use. This declining rate may be due to improvements in wound construction, stromal hydration, environmental cleaning, and surgeon experience, among others.13,14
We observed a greater than 350% increased risk associated with PCR, a 46% increased risk for those 80 years of age and older, and a 31% increased risk for those with diabetic retinopathy. These results are consistent with past studies.10,15–18
One advantage of an observational study over a randomized controlled trial is the ability to evaluate a multiplicity of treatment arms, including treatments that were not envisioned to be important at the time of study design. This observational study identified 2 important opportunities for quality improvement within our setting that likely translate to other community-based settings. We observed that 4.2% of the eyes that underwent phacoemulsification received topical aminoglycoside. Aminoglycosides compared poorly with other topical drug classes in preventing endophthalmitis. Although aminoglycosides are effective against Staphylococci, the most common infective organism in post-cataract surgery endophthalmitis, evidence shows that they do not penetrate well into the anterior chamber.19–21
We further observed prescribing errors and patient non-adherence to orders for topical prophylaxis, resulting in 4.5% of the cohort not receiving any antibiotic prophylaxis. In 2013, we implemented intracameral injection as a standard practice in our Northern California program, and our rate of endophthalmitis is now on the order of 1 per 10 00022; other Kaiser Permanente regions are actively implementing intracameral injection as well. At present, 97% of our surgeons use postoperative topical antibiotic, although elimination of topical drops is under discussion.
During phacoemulsification, infective agents might enter the eye through surgical instruments handled by operating room staff or by influx from the patient’s own eyelid or conjunctiva.2 Povidone-iodine has been shown to be effective in killing organisms on the patient’s eyelid and conjunctiva.23 Subconjunctival and topical antibiotics are intended to kill organisms on the ocular surface; however, aqueous concentrations might not be adequate to kill the most common causative organism, coagulase-negative Staphylococcus aureus.11,24 In contrast, intracameral antibiotics achieve concentrations that are several times greater than the concentration needed to kill 90% of most bacterial isolates.24–27 An incompetent, leaky wound may be more susceptible to infection by organisms present on the eyelid or from patients’ hands or eye dropper tips after the effective concentration of antibiotic from intracameral injection has dropped.28,29 Our study was not large enough to detect a difference between intracameral antibiotic alone with intra-cameral antibiotic combined with a topical agent. However, it has been argued that elderly patients have trouble instilling their eye drops30 and may “fish mouth” the wound by direct contact of an eye dropper tip to the eye near the wound, thereby increasing the risk of infection. A benefit of intracameral antibiotic is lack of dependence on the patient. Our study did not measure whether the patients correctly used their topical agent; thus, we could not determine whether incorrect administration of the topical agent was a factor in our results, although studies have shown a wide variability in tear film and anterior chamber antibiotic concentrations due to anatomic and instillation differences.11
Intracameral Cefuroxime versus Moxifloxacin
We did not find convincing evidence for a difference in effectiveness between intracameral cefuroxime and moxifloxacin. We previously described the 2-step process we use to compound cefuroxime,31 whereas moxifloxacin can be used straight out of the bottle or diluted with equal parts balanced salt solution to achieve a concentration of 250 micrograms per 0.1 ml. In addition, we have reported an intracameral agent compounding error, in which 9 mg of cefuroxime was administered to 13 eyes of 11 patients, resulting in acute macular edema that resolved within 1 week without further adverse consequences.32 As a consequence of the compounding error, we now contract with a single US Food and Drug Administration–registered outsourcing facility. However, accidental substitution of a moxifloxacin product (Moxeza, Alcon Laboratories Inc., Fort Worth, TX) that contains inactive ingredients has been associated with toxic anterior segment syndrome.33 Increasing resistance to fluoroquinolones might argue in favor of cefuroxime, particularly in patients who have been exposed to topical fluoroquinolones in the past. However, intracameral injection of any antibiotic drug may be less subject to emerging resistance because it is a single, highly concentrated dose in a relatively confined space, in contrast to repeated application of lower, less-effective doses. Van-comycin also has been injected for infection prophylaxis and is effective against gram-positive bacteria, but because of concerns about resistance, it is currently reserved in our setting for the approximately 1% of patients who are allergic to both penicillin/cephalosporin and a fluoroquinolone.34
In conclusion, we recommend intracameral injection of cefuroxime or moxifloxacin in all phacoemulsification procedures. Use of topical agent alone is less effective and is subject to prescribing errors and nonadherence. Topical aminoglycosides seem to be ineffective as antibiotic prophylaxis for endophthalmitis.
Acknowledgments
The authors thank Marilyn Foley, Medical Record Abstractor.
Abbreviations and Acronyms
- CI
confidence interval
- CPT
Current Procedural Terminology
- ICD-9
International Classification of Diseases, 9th revision
- OR
odds ratio
- PCR
posterior capsular rupture
Footnotes
Financial Disclosure(s):
The author(s) have made the following disclosure(s): L.J.H.: Funded by research contracts – MedImmune unrelated to the present project.
K.L.W.: Research grant – Pfizer and provided consulting to Pfizer, UCB, AbbVie, Genentech, Janssen, and Merck Serono; none of his activities were related to the present project.
D.S.F.: Grant support – Allergan and served as a consultant for ThromboGenics and NightstaRx; Allergan manufactures Zymaxid (gatifloxacin).
Funded by a grant from the National Eye Institute (R21EY022989). L.J.H. and N.H.S. were also funded by a Kaiser Permanente Community Benefit grant.
Author Contributions:
Conception and design: Herrinton, Shorstein, Liu, Chang
Data collection: Herrinton, Shorstein, Paschal, Liu, Contreras
Analysis and interpretation: Herrinton, Shorstein, Paschal, Liu, Contreras, Winthrop, Chang, Melles, Fong
Obtained funding: Herrinton, Shorstein
Overall responsibility: Herrinton, Shorstein, Paschal
References
- 1.Javitt JC, Tielsch JM, Wang Q, et al. National outcomes of cataract extraction. Retinal detachment and endophthalmitis after outpatient cataract surgery. Cataract Patient Outcomes Research Team. Ophthalmology. 1994;101:100–5. doi: 10.1016/s0161-6420(13)31251-2. [DOI] [PubMed] [Google Scholar]
- 2.Friling E, Lundstrom M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39:15–21. doi: 10.1016/j.jcrs.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Ophthalmology. Cataract in the Adult Eye; Preferred Practice Patterns. San Francisco, CA: American Academy of Ophthalmology; 2011. [Accessed August 25, 2015]. Available at: http://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-october-2011. [Google Scholar]
- 4.Ndegwa S, Cimon K, Severn M. Intracameral antibiotics for the prevention of endophthalmitis post-cataract surgery: review of clinical and cost-effectiveness and guideline. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health; Oct, 2010. [Accessed May 4, 2011]. Rapid Response Report: Peer-Reviewed Summary with Critical Appraisal. Available at: External link, https://www.cadth.ca/media/pdf/M0019_Intracameral_Antiobiotics_L3_e.pdf. [Google Scholar]
- 5.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 6.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 7.Gordon NP. [Accessed February 20, 2014];Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2007 California Health Interview Survey Report. 2012 Jan 4; Available at: http://www.dor.kaiser.org/external/DORExternal/mhs/special_reports.aspx?ekmensel=f5948e6e_166_172_btnlink.
- 8.Koebnick C, Langer-Gould AM, Gould MK, et al. Socio-demographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–88. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Lundstrom M, Wejde G, Stenevi U, et al. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology. 2007;114:866–70. doi: 10.1016/j.ophtha.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Barry P, Cordovés L, Gardner S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract Surgery: Data, Dilemmas and Conclusions. European Society of Cataract and Refractive Surgeons; Dublin, Ireland: 2013. [Accessed April 21, 2015]. Available at: http://www.sbop.com.br/conteudo/OK%202013%20ESCRS%20Endophthalmitis-Guidelines.pdf. [Google Scholar]
- 12.Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39:8–14. doi: 10.1016/j.jcrs.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda S, Kawana K, Yasuno Y, Oshika T. Wound architecture of clear corneal incision with or without stromal hydration observed with 3-dimensional optical coherence tomography. Am J Ophthalmol. 2011;151:413–9. doi: 10.1016/j.ajo.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Masket S, Belani S. Proper wound construction to prevent short-term ocular hypotony after clear corneal incision cataract surgery. J Cataract Refract Surg. 2007;33:383–6. doi: 10.1016/j.jcrs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Hatch WV, Cernat G, Wong D, et al. Risk factors for acute endophthalmitis after cataract surgery: a population based study. Ophthalmology. 2009;116:425–30. doi: 10.1016/j.ophtha.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Kamalarajah S, Ling R, Silvestri G, et al. Presumed infectious endophthalmitis following cataract surgery in the UK: a case-control study of risk factors. Eye (Lond) 2007;21:580–6. doi: 10.1038/sj.eye.6702368. [DOI] [PubMed] [Google Scholar]
- 17.Menikoff JA, Speaker MG, Marmor M, Raskin EM. A case-control study of risk factors for postoperative endophthalmitis. Ophthalmology. 1991;98:1761–8. doi: 10.1016/s0161-6420(91)32053-0. [DOI] [PubMed] [Google Scholar]
- 18.Javitt JC, Vitale S, Canner JK, et al. National outcomes of cataract extraction: endophthalmitis following inpatient surgery. Arch Ophthalmol. 1991;109:1085–9. doi: 10.1001/archopht.1991.01080080045025. [DOI] [PubMed] [Google Scholar]
- 19.Cagini C, Piccinelli F, Lupidi M, et al. Ocular penetration of topical antibiotics: study on the penetration of chloramphenicol, tobramycin and netilmicin into the anterior chamber after topical administration. Clin Experiment Ophthalmol. 2013;41:644–7. doi: 10.1111/ceo.12087. [DOI] [PubMed] [Google Scholar]
- 20.Yao K, Zhang Z, Yang YH, Wu XD. Aqueous humor penetration of topically applied ofloxacin, ciprofloxacin and tobramycin. Zhonghua Yan Ke Za Zhi. 2003;39:736–9. [PubMed] [Google Scholar]
- 21.Gonzalez LS, 3rd, Spencer JP. Aminoglycosides: a practical review. Am Fam Physician. 1998;58:1811–20. [PubMed] [Google Scholar]
- 22.Carnahan MC, Chang WJ, Shorstein NH, Herrinton LJ. New benchmark in preventing phacoemulsification-related endophthalmitis. J Cataract Refract Surg. 2014;40:1568. doi: 10.1016/j.jcrs.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769–75. doi: 10.1016/s0161-6420(91)32052-9. [DOI] [PubMed] [Google Scholar]
- 24.Matsuura K, Suto C, Akura J, Inoue Y. Comparison between intracameral moxifloxacin administration methods by assessing intraocular concentrations and drug kinetics. Graefes Arch Clin Exp Ophthalmol. 2013;251:1955–9. doi: 10.1007/s00417-013-2294-7. [DOI] [PubMed] [Google Scholar]
- 25.Montan PG, Wejde G, Setterquist H, et al. Prophylactic intracameral cefuroxime. Evaluation of safety and kinetics in cataract surgery. J Cataract Refract Surg. 2002;28:982–7. doi: 10.1016/s0886-3350(01)01270-6. [DOI] [PubMed] [Google Scholar]
- 26.Wejde G, Samolov B, Seregard S, et al. Risk factors for endophthalmitis following cataract surgery: a retrospective case-control study. J Hosp Infect. 2005;61:251–6. doi: 10.1016/j.jhin.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Suto C, Morinaga M, Yagi T, et al. Conjunctival sac bacterial flora isolated prior to cataract surgery. Infect Drug Resist. 2012;5:37–41. doi: 10.2147/IDR.S27937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taban M, Sarayba MA, Ignacio TS, et al. Ingress of India ink into the anterior chamber through sutureless clear corneal cataract wounds. Arch Ophthalmol. 2005;123:643–8. doi: 10.1001/archopht.123.5.643. [DOI] [PubMed] [Google Scholar]
- 29.Sarayba MA, Taban M, Ignacio TS, et al. Inflow of ocular surface fluid through clear corneal cataract incisions: a laboratory model. Am J Ophthalmol. 2004;138:206–10. doi: 10.1016/j.ajo.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 30.An JA, Kasner O, Samek DA, Lévesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014;40:1857–61. doi: 10.1016/j.jcrs.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen ET, Shorstein NH. Preparation of intracameral antibiotics for injection. J Cataract Refract Surg. 2013;39:1778–9. doi: 10.1016/j.jcrs.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong DC, Waxman MD, Herrinton LJ, Shorstein NH. Transient macular edema after intracameral injection of moderately elevated dose of cefuroxime during phacoemulsification surgery. JAMA Ophthalmol. 2015 Jul 30; doi: 10.1001/jamaophthalmol.2015.2421. http://dx.doi.org/10.1001/jamaophthalmol.2015.2421 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 33.Braga-Mele R, Chang DF, Henderson BA, et al. Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40:2134–42. doi: 10.1016/j.jcrs.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14:73–156. doi: 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]