Abstract
A series of novel, heteroaryl carboxylic acid conjugates of the sesquiterpene melampomagnolide-B (MMB, 3) has been evaluated as antitumor agents against an NCI panel of 64 human hematopoetic and solid tumor cell lines. The indole-3-acrylic acid conjugate 7j and the indole-3-carboxylic acid conjugate 7k were found to be the most potent analogs in the series. Compounds 7j and 7k exhibited remarkable growth inhibition, GI50 values in the range 0.03–0.30 μM and 0.04–0.28 μM, respectively, against the cell lines in the leukemia sub-panel, and exhibited GI50 values of 0.05–0.40 μM and 0.04–0.61 μM, respectively, against 90% of the solid tumor cell lines in the NCI panel. Compound 7a was particularly effective against the sub-panel of breast cancer cell lines with GI50 values in the range <0.01 to 0.30 μM. Compounds 7j, 7a and its water soluble analog 7p also exhibited potent anticancer activity against rat 9L-SF gliosarcoma cells in culture. Compound 7j was the most potent compound in the series in the M9-ENL1 AML cell assay with an lethal dose concentration, EC50 value of 720 nM, and exhibited the greatest cytotoxicity against a collection of primary AML stem cell specimens, which included a specimen that was unresponsive to PTL, affording EC50 values in the range 0.33 to 1.0 μM in three out of four specimens. The results from this study provide further evidence that analogs of the sesquiterpene MMB can be designed to afford molecules with significantly improved anticancer activity. Thus, both 7j and 7k are considered potential lead molecules in the search for new anticancer agents that can be used as treatments for both hematopoetic and solid tumors.
Keywords: Melampomagnolide B analogues, Indole carboxylic esters, Anti-cancer activity, Leukemia cell lines, 9L-SF gliosarcoma cells
Graphical abstract
1. Introduction
The sesquiterpene lactones are a noteworthy class of biologically active constituents of natural products that have been isolated from various medicinal plants. In recent years there has been an increasing interest in these 15-carbon terpenoids and studies on the synthesis of analogs of these natural products have been reported and their biological activities have been evaluated.[1–3] Parthenolide (PTL, 1, Figure 1), originally isolated from the medicinal herb Feverfew (Tanacetum parthenium), is one of the best-known members of the sesquiterpene lactones, having been identified as an anticancer agent that is effective against both hematological and solid tumors.[4–10]
Fig 1.
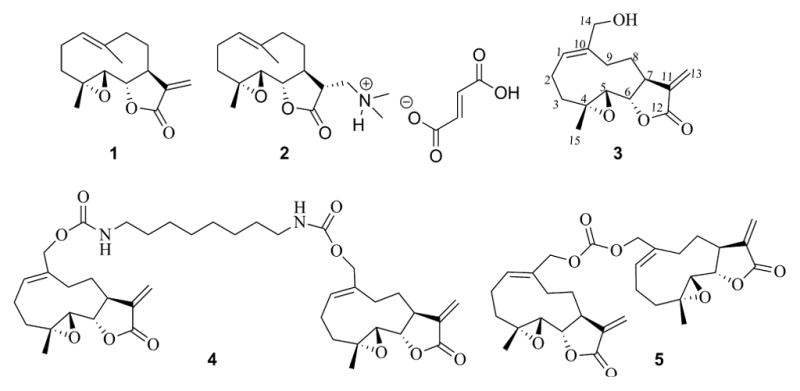
Anticancer sesquiterpene lactone PTL (1) and its derivatives (2–5).
PTL is a well-established antileukemic agent, but has poor drug-like properties and lacks clinical potential due to its poor oral bioavailability. Analogs have been recently designed in attempts to improve the drug-like properties of PTL while retaining its antitumor properties. One such analog, dimethlyamino parthenolide (DMAPT, 2, Figure 1), is currently in Phase I clinical studies for evaluation as a treatment for AML and other related leukemias.[11]
A structurally related sesquiterpene, melampomagnolide B (MMB, 3, Figure 1), a melampolide originally isolated from Magnolia grandiflora,[12] has similar antileukemic properties as PTL,[13] but is somewhat less potent. Both sesquiterpenes exhibit significantly reduced cytotoxicity against normal bone marrow stem cells when compared to their effect on AML stem cells.[13–15] The presence of the C-14 primary allylic hydroxyl group in the MMB molecule (3, Figure 1) allows the medicinal chemist the opportunity to examine the biological activity of a wide variety of new MMB derivatives via conjugation and functional group transformations. Utilizing this approach, C-14 carbamate and carbonate conjugates of MMB have recently been shown to possess improved cytotoxicity against hematological and solid tumor cell lines when compared to the parent compound,[16–18] and the dimeric MMB carbamate esters (4 and 5, Figure 1) have been shown to possess promising anticancer activities against a wide variety of both hematological and solid human tumor cell lines.[17] The symmetrical MMB carbonate dimer (5) also exhibits superior anticancer activity against 9LSF gliosarcoma and M9-EML1 AML cells when compared to PTL and DMAPT.[17]
In mechanistic studies, we have shown that MMB inhibits the phosphorylation of p65[13] in AML stem cells. In addition, a C-14 biotin-conjugated derivative of MMB has been prepared and used in elucidating the mechanism of action of MMB in AML stem cells[13, 14, 15]. Utilizing this probe, and carrying out subsequent streptavidine pull-down and LC/MS/MS peptide sequencing studies, the MMB-biotin probe pulls down IKKβ in both streptavidine/antibody and LC/MS/MS proteomic assays. Thus, the mechanism of action of MMB involves promotion of apoptosis by inhibition of the NFκB transcription factor complex, thereby down-regulating anti-apoptotic genes under NFκB control. MMB and PTL are believed to bind to both IKKβ and RelA/p65, leading to decreased phosphorylation of IκBα and reduced DNA binding of p65.[10, 14, 19–22]
Anticancer agents such as PTL and MMB also cause a reduction in cellular glutathione levels, and a selective increase in reactive oxygen species (ROS) in AML stem cells through inhibition of key enzymes involved in glutathione biosynthesis and catabolism.[14] In this respect, MMB-biotin pulls down important elements of the glutathione pathway, i.e. glutathione peroxidase (GPX1), glutathione ligase (both modulatory and catalytic units GCLC and GCLM, respectively), and thioredoxin.
Interestingly, the above pro-apoptotic actions of MMB are not observed in normal bone marrow stem cells.[13, 14] Thus, MMB exhibits two selective and synergistic anticancer activities, i.e. NF-κB inhibition and induction of oxidative stress through interaction with the glutathione pathway.
In the current study, we have prepared and evaluated several novel aryl carboxylic acid conjugates of MMB (7a–7o), and compared their anticancer properties to those of PTL. The results indicate that two indole carboxylic conjugates of MMB (7j and 7k) have been identified as strong leads in the development of potent sesquiterpene-derived anticancer agents. These compounds represent the most promising anticancer agents ever reported as structural analogs of MMB and PTL.
2. Results and discussion
2.1. Rational drug design and synthesis
Based on our previous work,[16–18] a series of novel heteroaromatic ester conjugates of MMB (7a–7o and 9) were prepared using standard EDC coupling conditions (Steglich type esterification) by reacting MMB (3) with an appropriate aryl carboxylic acid (6a–6o) in the presence of EDCI and DMAP in dichloromethane at room temperature for 8 h. The heteroaryl carboxylic acids 6a–6o were chosen from a variety of available indole (6a–6l), benzothiophene (6m–6n), and benzofuran carboxylic acids, (6o) to afford the corresponding MMB conjugates (7a–7o) in yields ranging from 54% to 74% (Scheme 1 and Table 1). Indole carboxylic acids 6e and 6f were prepared from the commercially available methyl esters of these compounds by alkaline hydrolysis to the corresponding acids.
Scheme 1.
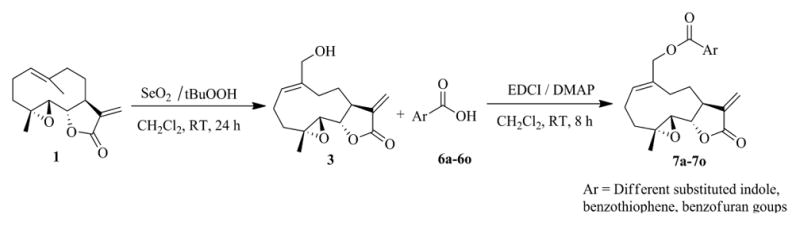
Synthesis of heteroaryl carboxylic acid esters of MMB (7a–7o)
Table 1.
Structures of carboxylic acid ester conjugates of melampomagnolide B (7a–7o), and product yields
| S. No | Heteroaromatic acid | Product | Isolated yield (%) |
|---|---|---|---|
| 1 |
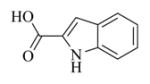 6a |
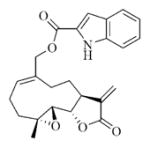 7a |
62 |
| 2 |
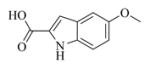 6b |
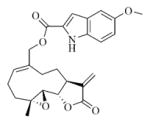 7b |
67 |
| 3 |
 6c |
 7c |
60 |
| 4 |
 6d |
 7d |
58 |
| 5 |
 6e |
 7e |
63 |
| 6 |
 6f |
 7f |
60 |
| 7 |
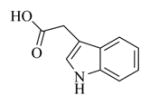 6g |
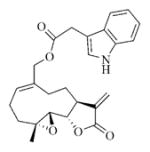 7g |
65 |
| 8 |
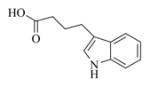 6h |
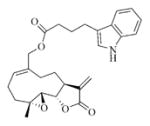 7h |
70 |
| 9 |
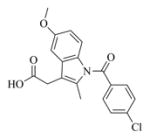 6i |
 7i |
62 |
| 10 |
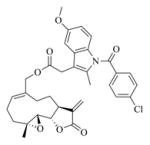
 6j |
 7j |
54 |
| 11 |
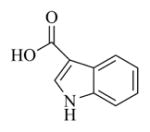 6k |
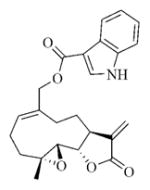 7k |
61 |
| 12 |
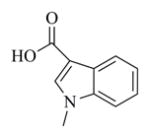 6l |
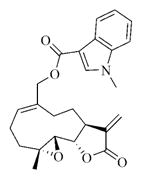 7l |
64 |
| 13 |
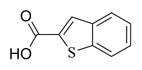 6m |
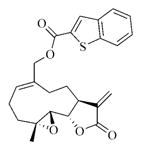 7m |
74 |
| 14 |
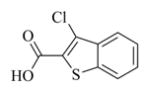 6n |
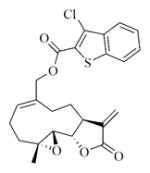 7n |
70 |
| 15 |
 6o |
 7o |
72 |
Conjugate 7p, a water-soluble analog of 7a, was synthesized by initial conversion of MMB (3) to the C-13 dimethylamino Michael adduct (8), followed by esterification of 8 with indole-2-carboxylic acid (5a) utilizing standard EDC coupling conditions to afford the MMB dimethylamino indole-2-carboxylate ester, 9, in 80% yield. Compound 9 was then treated with fumaric acid to afford compound 7p as the monofumarate salt (Scheme 2).
Scheme 2.
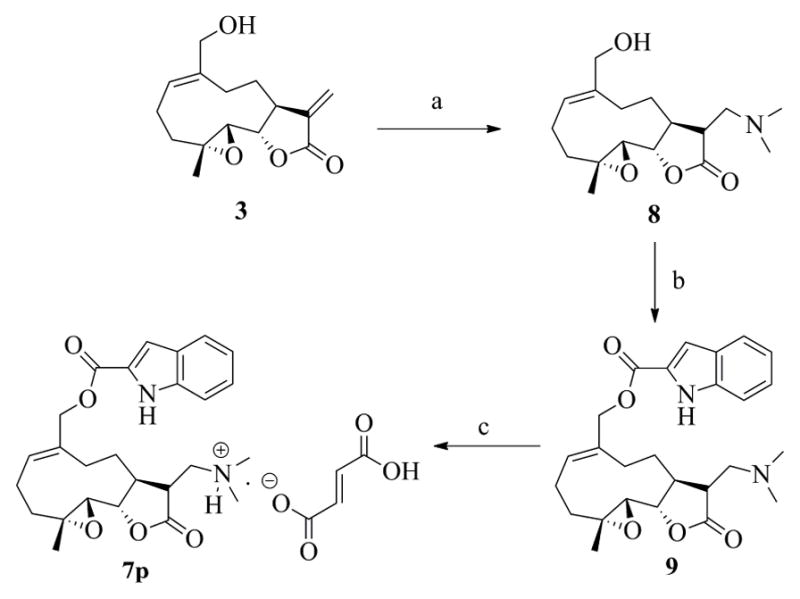
Synthesis of compound 7p, a water-soluble analog of the indole-2-carboxylic acid ester of MMB (7a). Reagents: a) dimethylamine, methanol, RT, 6 h; b) 6a, EDCI, DMAP, DCM, RT, 8 h; c) Fumaric acid, ethanol.
2.2. Biological Evaluation
2.2.1. In vitro growth inhibition exhibited by heteroaryl carboxylic acid esters of MMB against a panel of human cancer cell lines
MMB ester conjugates 7a–7o and dimethylamine MMB ester conjugate (7p) were submitted to the National Cancer Institute (NCI) for anticancer screening. The compounds were first screened at a single concentration of 10−5 M against a panel of 60 human cancer cell lines derived from nine human cancer cell types grouped into sub-panels that contain leukemia, lung, colon, central nervous system (CNS), melanoma, renal, ovary, breast and prostate cancer cell lines. Compounds that showed more than 60% growth inhibition in at least eight of the 60 cell lines in the panel were selected for a complete dose response study utilizing the procedure described by Rubinstein et al.,[23] at five different concentrations over the range 10−4 to 10−8 M. From the initial single dose screening data, ten analogs, 7a–7d, 7h, 7j–7m, 7o and 7p, were selected for five dose testing for evaluation of cancer cell growth inhibitory properties (GI50). Dose-response curves were generated by plotting cytotoxic effect against the log10 of the drug concentration for each cell line. Cytotoxic effects of each compound were determined as GI50 values, which represent the molar drug concentration required to cause 50% growth inhibition. The results are given in Table 2.
Table 2.
Growth inhibition (GI50) dataa for MMB heteroaryl ester conjugates 7a–7d, 7h, 7j–7m and 7o, and dimethylamine MMB ester conjugate 7p against a panel of 60 human cancer cell lines
| Panel/cell line | 1 | 7a | 7b | 7c | 7d | 7h | 7j | 7k | 7l | 7m | 7o | 7p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| (μM)b | (μM)b | (μM)b | (μM)b | (μM)b | (μM)b | (μM)b | (μM)b | (μM)b | (μM)b | (μM)b | (μM)b | |
| Leukemia | ||||||||||||
| CCRF-CEM | 9.16 | 0.28 | 0.34 | 0.91 | 0.39 | 0.75 | 0.07 | 0.05 | 0.21 | 0.40 | 0.33 | 0.32 |
| HL-60(TB) | 5.71 | 0.54 | 0.32 | 2.07 | 0.44 | 1.63 | 0.25 | 0.27 | 1.54 | 1.89 | 1.22 | 0.24 |
| K-562 | 22.6 | 0.32 | 0.56 | 2.24 | 1.14 | 2.60 | 0.27 | 0.27 | 0.40 | 0.81 | 1.15 | 0.63 |
| MOLT-4 | 14.3 | 1.51 | 1.32 | 2.26 | 0.55 | 1.53 | 0.30 | 0.28 | 0.57 | 1.88 | 1.77 | 1.51 |
| RPMI-8226 | 8.20 | 0.26 | 0.50 | 2.00 | 0.81 | 2.31 | 0.03 | 0.04 | 0.21 | 0.56 | 0.71 | 0.28 |
| SR | NA | 1.08 | NA | 2.03 | 0.77 | 1.73 | 0.27 | 0.28 | 0.34 | 0.52 | 1.15 | 1.59 |
| Lung Cancer | ||||||||||||
| A549/ATCC | 91.8 | 2.06 | 1.66 | 3.08 | 1.69 | 5.47 | 1.27 | 1.50 | 1.82 | 2.04 | 2.91 | 2.44 |
| EKVX | 17.3 | NA | 1.50 | 1.58 | 1.40 | 2.02 | 0.23 | 0.31 | 0.96 | 1.69 | 2.02 | 3.36 |
| HOP-62 | 49.0 | NA | 1.64 | 2.92 | 2.22 | 6.54 | 0.82 | 0.82 | 1.81 | 1.81 | 2.48 | 2.20 |
| HOP-92 | 13.5 | 0.48 | 0.44 | 1.38 | 1.25 | 1.68 | 0.15 | 0.16 | 0.36 | 1.68 | 1.44 | 1.40 |
| NCI-H226 | 20.5 | 1.47 | 1.57 | 1.76 | 1.80 | 2.04 | 0.23 | 0.44 | 1.48 | 2.00 | 1.74 | 1.98 |
| NCI-H23 | 20.2 | 1.69 | 1.49 | 1.90 | 1.63 | 1.72 | 1.25 | 1.63 | 1.93 | 1.74 | 1.98 | 1.96 |
| NCI-H322M | 21.7 | 1.68 | 1.88 | 2.69 | 4.00 | 13.8 | 1.73 | 1.85 | 3.01 | 1.98 | 5.52 | 10.8 |
| NCI-H460 | 18.8 | 1.73 | 1.83 | 3.04 | 1.72 | 4.54 | 1.01 | 1.10 | 1.73 | 2.21 | 2.00 | 5.85 |
| NCI-H522 | 5.47 | 0.75 | 0.70 | 1.65 | 1.59 | 1.57 | 0.18 | 0.19 | 0.39 | 0.98 | 0.68 | 1.22 |
| Colon Cancer | ||||||||||||
| COLO 205 | 14.5 | NA | 1.18 | 1.64 | 1.72 | 1.86 | 0.22 | 0.24 | 1.24 | 1.71 | 1.55 | 1.98 |
| HCC-2998 | 24.1 | 2.14 | 1.54 | 2.02 | 1.40 | 1.79 | 0.27 | 0.32 | 1.81 | 1.75 | 2.47 | 1.72 |
| HCT-116 | 10.8 | 0.19 | 0.42 | 1.50 | 0.70 | 0.96 | 0.18 | 0.19 | 1.02 | 0.59 | 0.32 | 0.29 |
| HCT-15 | 10.2 | 0.21 | 0.32 | 1.15 | 1.00 | 1.61 | 0.18 | 0.25 | 0.53 | 0.40 | 1.07 | 0.29 |
| HT29 | 23.5 | 0.27 | 0.35 | 1.13 | 1.33 | 1.71 | 0.19 | 0.24 | 0.52 | 0.49 | 0.82 | 0.43 |
| KM12 | 16.2 | 2.05 | 1.54 | 1.98 | 2.15 | 1.89 | 0.40 | 0.47 | 1.83 | 1.78 | 2.02 | 2.24 |
| SW-620 | 14.5 | 0.25 | 0.64 | 1.43 | 1.40 | 1.84 | 0.18 | 0.23 | 0.30 | 0.51 | 0.66 | 1.70 |
| CNS Cancer | ||||||||||||
| SF-268 | 25.1 | 1.59 | 1.66 | 1.62 | 1.44 | 1.69 | 0.22 | 0.29 | 1.30 | 1.54 | 1.56 | 1.48 |
| SF-295 | 28.9 | 1.71 | 2.42 | 7.10 | 3.18 | 5.69 | 1.62 | 1.94 | 2.41 | 3.33 | 5.90 | 3.95 |
| SF-539 | 18.9 | 1.32 | 1.02 | 1.55 | 1.44 | 1.63 | 0.26 | 0.26 | 0.74 | 1.60 | 1.45 | 2.51 |
| SNB-19 | 70.9 | 1.60 | 1.57 | 5.04 | 2.91 | 2.59 | 0.64 | 1.30 | 1.59 | 2.09 | 3.16 | 11.9 |
| SNB-75 | 48.4 | 1.01 | NA | 11.1 | 0.57 | 12.6 | 0.17 | 0.20 | 1.09 | 3.52 | 1.34 | 3.21 |
| U251 | 41.5 | 1.89 | 1.60 | 1.82 | 1.75 | 1.95 | 0.21 | 0.61 | 1.42 | 1.52 | 1.63 | 2.61 |
| Melanoma | ||||||||||||
| LOX IMVI | 8.24 | 0.18 | 0.30 | 1.05 | 0.81 | 1.55 | 0.05 | 0.04 | 0.22 | 0.20 | 1.42 | 0.17 |
| MALME-3M | 13.8 | 1.56 | 1.61 | 1.67 | 1.63 | 1.79 | 0.28 | 0.32 | 1.62 | 1.82 | 1.66 | 2.01 |
| M14 | NA | 0.81 | 1.27 | 1.89 | 1.47 | 1.66 | 0.22 | 0.22 | 1.41 | 1.75 | 1.43 | 1.57 |
| MDA-MB-435 | NA | 0.76 | 0.62 | 1.50 | 1.24 | 1.73 | 0.22 | 0.24 | 1.35 | 1.33 | 1.43 | 1.28 |
| SK-MEL-2 | 16.2 | 1.92 | 1.60 | 1.95 | 1.73 | 1.96 | 0.20 | 0.23 | 1.38 | 1.75 | 1.77 | 2.10 |
| SK-MEL-28 | 21.0 | 1.29 | 1.23 | 1.79 | 1.58 | 1.70 | 0.33 | 0.36 | 1.55 | 1.76 | 1.75 | 2.21 |
| SK-MEL-5 | 17.4 | 1.26 | 1.74 | 1.85 | 1.91 | 1.74 | 0.20 | 0.20 | 1.42 | 1.75 | 1.52 | 1.85 |
| UACC-257 | 14.4 | 1.61 | 1.61 | 2.28 | 1.70 | 2.03 | 0.35 | 0.36 | 1.32 | 1.77 | 1.52 | 1.47 |
| UACC-62 | 16.8 | 1.47 | 1.38 | 1.89 | 1.67 | 1.84 | 0.17 | 0.18 | 1.26 | 1.85 | 1.68 | 1.65 |
| Ovarian Cancer | ||||||||||||
| IGROV1 | 21.1 | NA | 1.48 | 1.44 | 1.38 | 1.63 | 0.22 | 0.25 | 0.69 | 1.76 | 1.39 | 2.07 |
| OVCAR-3 | 17.4 | NA | 0.93 | 1.20 | 1.25 | 1.53 | 0.17 | 0.20 | 0.38 | 0.44 | 0.48 | 0.35 |
| OVCAR-4 | 21.5 | NA | 1.63 | 1.67 | 1.33 | 1.66 | 0.20 | 0.25 | 1.14 | 2.08 | 1.56 | 1.36 |
| OVCAR-5 | 34.1 | 1.14 | 1.77 | 1.77 | 1.53 | 1.89 | 0.31 | 0.40 | 1.56 | 2.02 | 1.88 | 2.38 |
| OVCAR-8 | 17.1 | 1.99 | 1.58 | 2.17 | 1.34 | 3.38 | 0.19 | 0.28 | 0.80 | 1.80 | 2.43 | 1.68 |
| NCI/ADR-RES | 15.0 | 2.20 | 1.68 | 1.98 | 1.45 | 2.90 | 0.35 | 0.39 | 2.15 | 2.14 | 2.59 | 1.42 |
| SK-OV-3 | 54.7 | 1.65 | 4.90 | 11.6 | 10.2 | 17.3 | 1.88 | 2.24 | 4.33 | 13.9 | 16.7 | 2.19 |
| Renal Cancer | ||||||||||||
| 786-0 | NA | 0.37 | 1.27 | 1.78 | 1.51 | 1.60 | 0.22 | 0.32 | 1.46 | 1.52 | 1.81 | 1.71 |
| A498 | 7.04 | 1.74 | 1.63 | 3.35 | 2.65 | 11.9 | 0.27 | 0.26 | 1.59 | 10.1 | 12.9 | 13.1 |
| ACHN | NA | 0.34 | 0.81 | 1.46 | 1.35 | NA | 0.20 | 0.21 | 0.82 | 1.50 | 1.44 | 0.67 |
| CAKI-1 | 9.35 | 1.08 | 1.71 | 1.20 | 1.19 | NA | NA | NA | NA | 1.46 | NA | 1.11 |
| RXF 393 | 12.2 | 1.07 | 1.14 | 1.47 | 1.38 | 1.46 | 0.17 | 0.19 | 0.58 | 1.23 | 1.43 | 1.14 |
| SN12C | 22.8 | 1.74 | 1.77 | 1.85 | 1.70 | 1.90 | 0.19 | 0.23 | 0.71 | 2.06 | 1.78 | 2.21 |
| TK-10 | NA | 1.39 | 1.28 | 1.83 | 1.75 | 1.64 | 0.27 | 0.28 | 1.10 | 1.55 | 3.01 | 2.12 |
| UO-31 | 41.9 | 0.88 | 1.12 | 1.20 | 1.21 | 1.41 | 0.15 | 0.16 | 0.77 | 1.40 | 1.46 | 0.52 |
| Prostate Cancer | ||||||||||||
| PC-3 | NA | 1.42 | 1.83 | 1.70 | 2.42 | 1.92 | 0.32 | 0.38 | 1.47 | 1.83 | 1.76 | 2.37 |
| DU-145 | NA | 1.21 | 1.03 | 1.67 | NA | 1.67 | 0.39 | 0.38 | 1.01 | 1.05 | 1.18 | 1.49 |
| Breast Cancer | ||||||||||||
| MCF7 | 16.4 | 0.30 | 0.34 | 1.02 | 0.86 | 1.42 | 0.22 | 0.26 | 0.40 | 0.38 | 0.39 | 0.61 |
| MDA-MB-231/ATCC | NA | 1.14 | 1.58 | 1.59 | 1.32 | 0.17 | 0.19 | 1.05 | 1.97 | 1.64 | 1.74 | |
| HS 578T | NA | NA | 3.09 | 2.95 | 2.25 | 3.19 | 0.35 | 0.61 | 2.39 | 2.89 | 2.91 | 3.03 |
| BT-549 | NA | <0.01 | 1.06 | NA | 1.31 | 1.37 | 0.17 | 0.17 | 0.51 | NA | 1.48 | 1.57 |
| T-47D | NA | <0.01 | 1.30 | 1.56 | 1.34 | 2.17 | 0.20 | 0.40 | 1.19 | 1.47 | 1.35 | 2.11 |
| MDA-MB-468 | NA | 0.30 | 1.13 | 1.86 | 1.89 | 1.76 | 0.19 | 0.19 | 0.44 | 1.63 | 1.50 | 1.12 |
NA: Not analyzed;
GI50 values <1 μM are bolded;
GI50: 50% growth inhibition defined as concentration of drug resulting in a 50% reduction in net protein increase compared with control cells.
All ten conjugates tested in the NCI 60 human cancer cell screen 5-dose assays showed significantly improved anticancer activity when compared to PTL (Table 2). Compounds 7j and 7k, exhibited the most potent growth inhibition in the series against both hematological and solid tumor cell lines in the human tumor cell panel, affording GI50 values in the range 0.03–0.30 μM and 0.04–0.28 μM, respectively, against the cell lines in the leukemia sub-panel, and exhibiting GI50 values of 0.05–0.40 μM and 0.04–0.61 μM, respectively, against 90% of the solid tumor cell lines in the panel. Remarkable GI50 potency ranges were exhibited by 7j in the renal cancer (0.15–0.27 μM), breast cancer (0.17–0.35 μM), prostate cancer (0.32–0.39 μM), ovarian cancer (0.17–0.35 μM), melanoma (0.05–0.35 μM), and colon cancer (0.18–0.40 μM) sub-panels. Similarly, compound 7k afforded GI50 potency ranges of 0.16–0.32 μM, 0.17–0.61 μM, 0.20–2.24 μM, 0.04–0.36 μM, and 0.19–0.47 μM in the renal cancer, breast cancer, ovarian cancer, melanoma, and colon cancer sub-panels, and exhibited a GI50 value of 0.38 μM against PC-3 and DU-145 cell lines in the prostate cancer sub-panel. Of note, compounds 7j and 7k both exhibited low nanomolar growth inhibition against CCRF-CFM and RPMI-8226 leukemia cell lines with GI50 values of 70 nM and 30 nM, and 50 nM and 40 nM, respectively. Compounds 7j and 7k also potently inhibited the growth of the melanoma cell line, LOX IMVI, with GI50 values of 50 nm and 40 nM, respectively, and were particularly effective against the drug resistant breast cancer cell lines MDA-MB-231/ATCC (GI50 = 170 nM and 190 nM, respectively) and MDA-MB-468 (190 nM and 190 nM, respectively). Although compound 7a was generally less potent than either 7j or 7k in the five-dose study, it exhibited remarkable potency against two of the cell lines in the breast cancer cell sub-panel, affording GI50 values of <10 nM for the BT-549 and T-47D cell lines. Compound 7a was also an effective anticancer agent against the drug resistant breast cancer cell line MDA-MB-468 (GI50 = 300 nM). However, the water soluble dimethylamino analog of 7a, compound 7p, which was evaluated as its fumarate salt, was somewhat less potent than 7a in the human cancer cell panel assays (Table 2), and did not exhibit the low nanomolar potency of 7a against breast cancer cell lines BT-549, T-47D and MDA-MB-468. Nevertheless, compound 7p did show good potency against the cell lines in the leukemia sub-panel (EC50 range 0.24–1.59 μM) and in the colon cancer sub-panel (EC50 range 0.29–2.24 μM).
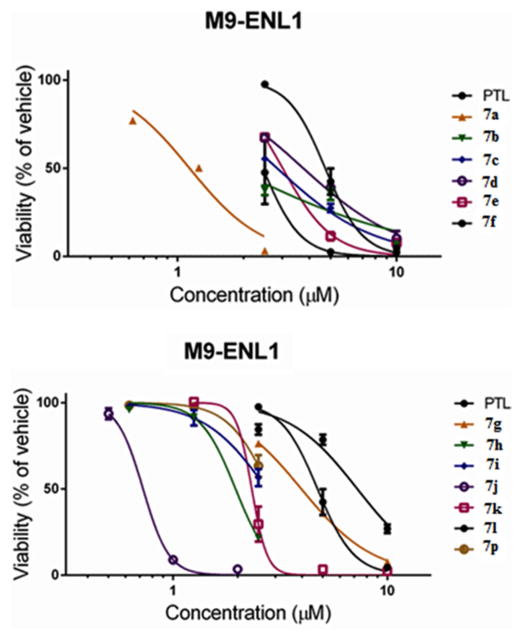
2.3. Antileukemic activity of heteroaryl carboxylic acid esters of MMB (7a–7o) and dimethylamine MMB ester conjugate 7p against M9-ENL1 AML and primary AML stem cell specimens
To investigate further the antileukemic activity of the different heteroaryl MMB ester conjugates (7a–7p), these compounds were tested for antileukemic activity against M9-ENL1 acute myelogenous leukemia cells (Table 3, Figure 2). This cell line was obtained from transduction of human blood cells with the leukemia-derived MLL-ENL translocation gene. Selected MMB conjugates 7a, 7b, 7j were also screened against primary AML stem cell specimens, which were obtained with informed consent from human patients (Table 4; Figure 3). Dose-response curves were generated over the concentration range 0.5–10 μM to determine the concentration resulting in 50% efficacy (EC50). Evaluations were performed after 24 h of drug exposure using flow cytometric analysis by labeling with 240 Annexin V and 7-aminoactinomycin D (7-AAD) to identify apoptotic cell populations. Evaluations were performed using viability labeling with trypan blue dye, as well as flow cytometric labeling with Annexin V and propidium iodide to determine dead cell populations. For all studies, PTL and/or MMB were included as a reference control.
Table 3.
EC50 (μM)a values for PTL (1), MMB (3), MMB heteroaryl ester conjugates 7a–7o, and dimethylamine MMB ester conjugate 7p against M9-ENL1 AML cells
| Entry | MMB Conjugate | M9-ENL1 AML Viability
|
|
|---|---|---|---|
| EC50 (μM) | 95% Cl | ||
| 1 | 1 | 5.5 | [5.1–5.9] |
| 2 | 7a | 1.1 | [0.90–1.4] |
| 3 | 7b | 1.8 | [0.90–3.3] |
| 4 | 7c | 2.8 | [2.5–3.2] |
| 5 | 7d | 3.8 | [3.5–4.0] |
| 6 | 7e | 3.0 | [2.8–3.2] |
| 7 | 7f | 2.5 | [2.2–2.7] |
| 8 | 7g | 4.0 | [3.9–4.1] |
| 9 | 7h | 2.0 | [1.8–2.1] |
| 10 | 7i | 2.7 | [2.5–3.2] |
| 11 | 7j | 0.7 | [0.68–0.77] |
| 12 | 7k | 1.9 | [1.9–2.8] |
| 13 | 7l | 7.2 | [6.3–8.3] |
| 14 | 7m | 2.7 | [2.3–3.1] |
| 15 | 7n | 2.1 | [2.0–2.3] |
| 16 | 7o | 2.9 | [2.7–3.2] |
| 17 | 7p | 2.8 | [2.6–3.1] |
EC50 values below 2μM are bolded.
Fig 2.
EC50 (95% CI) values for PTL (1), MMB (3), and MMB heteroaryl ester conjugates 7a–7o and dimethylamine MMB ester conjugate (7p) against M9-ENL1 AML cells
Table 4.
EC50 (95% CI, μM) valuesa for PTL (1), and MMB heteroaryl ester conjugates 7a, 7b, and 7j against primary AML cell specimens
| Com. | AML01 | AML02 | AML03 | AML04 |
|---|---|---|---|---|
|
| ||||
| EC50 | EC50 | EC50 | EC50 | |
| 1 | 5.2 [4.6–6.0] | 6.3 [5.6–7.0] | 1.9 [1.6–2.2] | 13 [11–15] |
| 7a | 3.5 [3.1–4.0] | 2.7 [2.6–2.8] | 2.9 [2.1–3.8] | 2.2 [2.0–2.5] |
| 7b | 3.0 [2.7–3.2] | 3.3 [3.0–3.6] | 2.2 [1.7–3.0] | 3.3 [3.0–3.8] |
| 7j | 1.3 [1.2–1.5] | 0.91 [0.82–1.1] | 0.32[0.24–0.53] | 1.0[0.83–1.4] |
EC50 values ≤1μM are bolded.
Fig 3.
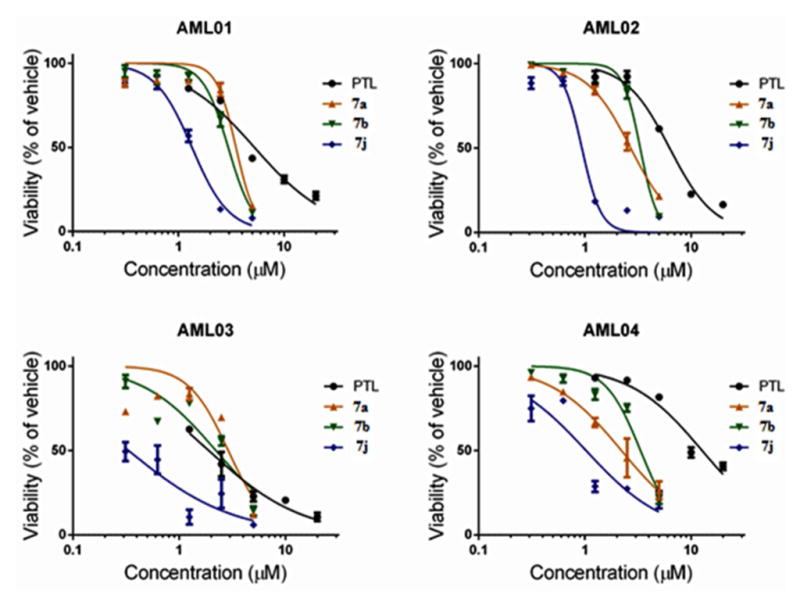
Dose-response curves for PTL (1) and MMB heteroaryl ester conjugates (7a, 7b and 7j) with primary AML cell specimens. MMB esters 7a, 7b, and 7j exhibited potent antileukemic activity with EC50 values in the range 0.3–3.5 μM against four specimens of primary AML cells.
The data from the M9-ENL1 AML cell assay indicated that the heteroaryl carboxylic acid esters of MMB (7a–7o) were 2- to 20-fold more cytotoxic than MMB, and were generally more cytotoxic than PTL in this assay. The most potent compound in the series was the indole-3-acrylic acid ester conjugate of MMB, 7j (EC50 = 0.72 μM), which was approximately 9-fold more potent compared to PTL (EC50 = 5.5μM) and greater than 20-fold more potent when compared to the parent sesquiterpene, MMB (EC50 = 15 μM), in this assay. Other compounds of interest were 7a, 7b, and 7k, which exhibited EC50 values of 1.1 μM, 1.8 μM and 1.9 μM, respectively. It is noteworthy to mention that compound 7p, the water soluble analog of 7a, afforded an EC50 value of 2.8 μM against the M9-ENL1 AML cell line.
Based on the above antileukemic properties of conjugates 7a–7o in the M9-ENL1 AML assay, compounds 7a, 7b, and 7j were selected for evaluation of their cytotoxicity against four primary AML cell specimens (Figure 3). Evaluations were performed after 24 h of drug exposure using flow cytometric analysis by labeling with 240 Annexin V and 7-aminoactinomycin D (7-AAD) to identify apoptotic cell populations. PTL was included as a reference control in these assays. Dose-response curves were generated over the concentration range 0.5–10 μM to determine the concentration resulting in 50% efficacy (EC50). In three of these AML specimens all four MMB conjugates exhibited improved cytotoxicity when compared to PTL. The most potent compound in the group was 7j, which was more effective than PTL in all four primary AML specimens, affording EC50 values over the range 0.30–1.3 μM. Compound 7j was particularly effective (EC50 = 1.0 μM) against primary specimen AML04, which was obtained from an AML patient after disease relapse, and was more resistant to the action of PTL (EC50 = 13 μM).
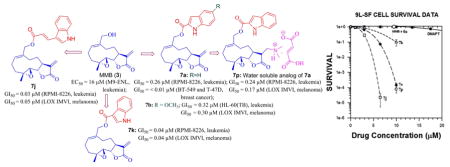
2.4. Anticancer activity of heteroaryl carboxylate esters of MMB (7a–7o) and dimethylamine MMB ester conjugate 7p against rat 9L-SF gliosarcoma cells
The intriguing results with the MMB conjugates 7a–7o and dimethylamine MMB ester conjugate 7p against hematological and solid tumor cell lines directed our attention to their potential cytotoxicity against rat 9L-SF gliosarcoma cells in culture. For this purpose we initially examined the activity of conjugates 7a–7d, 7j, 7m and 7p against 9L-SF cells. Initial screening results demonstrated that conjugates 7a, 7b, 7j and 7p were effective cytotoxic agents against this solid tumor cell type. Prior experience with the natural sesquiterpene PTL and its synthetic analog DMAPT demonstrated that these compounds had potent toxicity against cell lines derived from solid tumors when such cells were treated at low cell densities (less than 2.7 × 104 cells/cm2)[17] but were far less toxic when these drugs were used to treat confluent cell monolayers.[17] Consequently, the above MMB conjugates were screened visually (microscopic observation) for their ability to inhibit proliferation or cause cell death in 50% confluent monolayers of rat 9L-SF gliosarcoma cells. The MMB-Indole composite drugs that were most effective at inducing visually detectable cell detachment and necrosis at concentrations less than 20 μM were 7a and 7j. The MMB conjugates 7a, 7b, and 7j were tested for their ability to render 9L-SF cells clonogenically nonviable using the colony formation assay (Figure 4). In addition, the clonogenic toxicity of the two components of 7a, i.e. MMB (3) and indole-2-carboxylic acid (6a) were also tested, along with the sesquiterpene analog, DMAPT (2); the combination of MMB plus indole-2-carboxylic acid were tested to determine if the toxicity of 7a could be replicated by treating the cells with MMB (3) or indole-2-carboxylic acid (6a) alone, or in combination.
Fig 4.
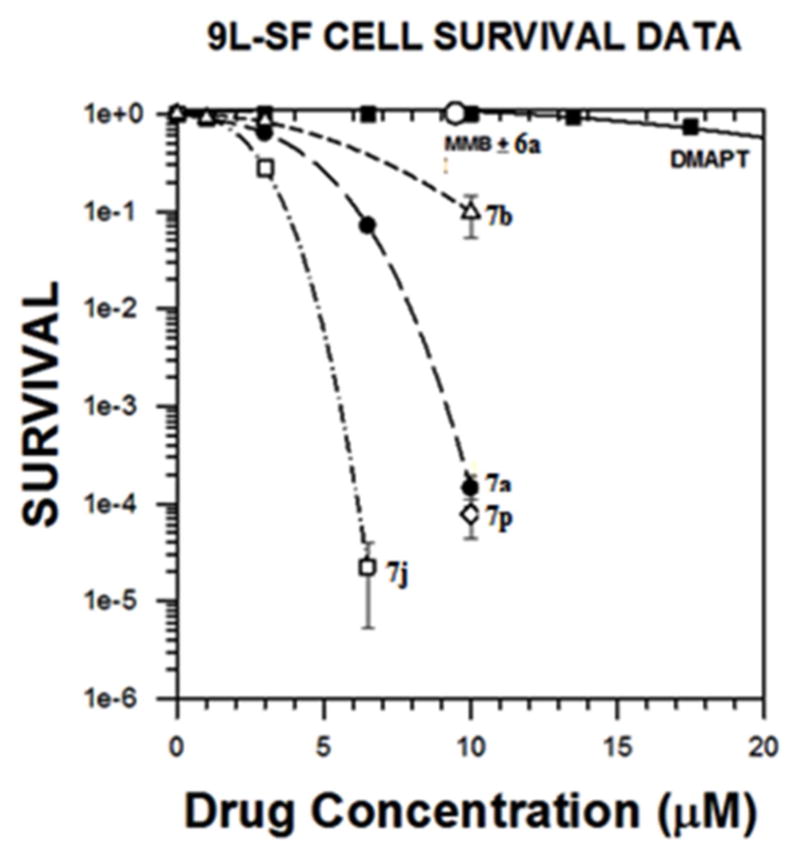
Dose-response Curves for Compounds 7a, 7b, 3+6a, 7p, 7j and DMAPT against rat gliosarcoma cells (9L-SF): cell survival as a function of drug concentration for cells treated for 48 h with the indicated drug. Each datum point represents the mean plus standard error of the mean for three experiments.
3.0. SAR and mechanism of action studies
Useful data to base structure-activity information on can be gleaned from the NCI 60 human cancer cell panel results. From these data, it can clearly be seen that of the bicyclic aromatic carboxylic acid conjugates of MMB (7a–7p) tested, the indole-2-carboxylic acid (7a), indole-3-carboxylic acid (7k) and indole-3-acrylic acid (7j) esters were potent anticancer agents, with 7j being the most active compound in the series. Conjugation of MMB (3) with indole-5- or indole-6-carboxylic acids (6e and 6f, respectively) led to failure of the resulting esters 7e and 7f to qualify for 5-dose testing. Incorporating methoxy, fluoro or chloro substituents at the 5-position of the indole-2-carboxylate moiety (i.e. 7b, 7c, and 7d, respectively) led to MMB conjugates with similar properties to the unsubstituted indole-2-carboxylic acid conjugate 7a. N-Methylation of the indole-3-carboxylic ester of MMB afforded 7l, which had lower overall cytotoxicity than 7k against the human cancer cells in the NCI screening panel. The MMB conjugate of indole-3-acetic acid (7g) and the structurally related indomethacin-MMB conjugate 7i both failed to qualify for 5-dose testing, whereas the indole-3-butyric acid conjugate 7h was considerably less potent than 7k, indicating that insertion of methylene moieties between the carboxylate moiety and the indole 3-position is detrimental to anticancer potency. The use of benzothiophene-2-carboxylic acid instead of indole-2-carboxyic acid afforded MMB conjugate 7m, which qualified for 5-dose testing but exhibited quite low potency against most of the cells in the NCI panel. The related 3-chlorobenzothiophene-2-carboxylic acid ester of MMB (7n) did not qualify for 5-dose testing. Interestingly, the benzofuran-2-carboxylic acid ester of MMB (7o) was only slightly less cytotoxic than the indole-2-carboxylic acid ester 7a in the NCI cancer screen. The most potent MMB conjugate in the series was 7j, in which the indole-3-carboxyl moiety in 7k has been replaced with an indole-3-acrylic acid moiety. This structural change introduces a second Michael acceptor moiety into the structure of 7j. MMB conjugates 7j and 7k were the most active compounds in the NCI screen. The design of the dimethylamino Michael adduct 7p as a water-soluble analog of 7a is based on a similar approach that was utilized previously[24] to afford DMAPT, a water soluble analog of PTL. DMAPT is currently in Phase 1 clinical trials for treatment of AML.[11] As is the case with DMAPT, 7p retains the cytotoxic properties of the parent compound 7a, but has somewhat less potency.
4. Conclusions
Evaluation of a novel series of heteroaryl esters of the melampolide sesquiterpene MMB (7a–7p) has identified two remarkably potent anticancer agents with activities against a wide variety of hematological and solid human tumor cell lines. These two compounds, the indole-3-acrylic acid conjugate 7j and the indole-3-carboxylic acid conjugate 7k were evaluated in studies utilizing an NCI panel of 64 human cancer cell lines, M9-ENL1 AML cells, primary AML stem cell specimens obtained with informed consent from AML patients, and also in studies with San Francisco variant 9L rat gliosarcoma cells (9L-SF) cells. Compounds 7j and 7k exhibited remarkable GI50 values in the range 0.03–0.27 μM and 0.04–0.28 μM, respectively, against the cell lines in the NCI leukemia sub-panel, and exhibited GI50 values of 0.05–0.40 μM and 0.04–0.61 μM, respectively, against 90% of the solid tumor cell lines in the NCI panel. 7j was the most potent compound in the M9-ENL1 AML cell assay with an EC50 value of 720 nM, and exhibited the greatest cytotoxicity against a small collection of primary AML stem cell specimens, which included a specimen from a patient that was that was unresponsive to PTL. Compounds 7j and 7a were found to be 60,000-fold and 10,000-fold, respectively, more toxic than DMAPT (2) against rat 9L-SF gliosarcoma cells in culture, and 7a exhibited remarkable potency against breast cancer cell lines BT-549 and T-47D with GI50 values of <10 nM for each cell line. It is also important to note that 7p, the water-soluble analog of 7a, was shown to be equipotent with 7a in the 9L-SF colony formation assay, indicating that a water- soluble analog of 7a can be designed without loss of anticancer activity in this solid tumor cell line.
The results from this study provide further evidence that analogs of the melampolide MMB can be designed to afford molecules with significantly improved anticancer activity. Thus, both 7j and 7k are considered potential lead molecules in the search for new anticancer agents that can be used as treatments for both hematopoetic and solid tumors.
5. Experimental Section
5.1. Chemistry
Melting points were recorded on a Kofler hot-stage apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Varian 400 MHz spectrometer equipped with a Linux workstation operating on vNMRj software. All the spectra were phased, baseline was corrected where necessary, and solvent signals (CDCl3) were used as reference for both 1H and 13C spectra. The chemical shifts given as δ values in parts per million (ppm) and coupling constants (J) are given in hertz (Hz). HRMS data were obtained on an Agilent 6210 LCTOF instrument operated in multimode. Thin-layer chromatographic (TLC) separations were carried out on pre-coated silica gel plates (F 254 Merck). The purities of all the final compounds were determined to be ≥95% as determined by high pressure liquid chromatography (HPLC) on a Model 1290 Agilent HPLC-Diode Array unit utilizing an Altima-C18 column (4.6 × 250 349mm, 5 μm), a mobile phase of acetonitrile containing 30% Milli Q water at a flow rate of 0.8 mL/min. Detection was at 210 nm. If required, synthetic products were purified by flash column chromatography (silica gel; methanol/dichloromethane).
5.2. Methodology for the in vitro 60 human cancer cell screen
The methodology for the anticancer activity screening assay was carried out at the National Cancer Institute utilizing a reported literature procedure.[23] Briefly, RPMI 1 medium with 5% fetal bovine serum and 2 mM L-glutamine was used for growing the NCI-60 human tumor cells. Initially, the tumor cells were inoculated into 96-well microtiter plates in 100 μL of medium at plating densities starting from 5,000 to 40,000 cells per well. The range of cell numbers is dependent on the doubling time of the individual tumor cell lines. The plates were then incubated at 37 °C for 24 h prior to the addition of the submitted compounds. After 24 h, two microtiter plates of each tumor cell line were fixed in situ with TCA. The optical density reading at this point represents the cell population for each tumor cell line at the time of compound addition (ODtzero). The MMB conjugates were dissolved in DMSO at 400-fold concentration to the desired final maximum test concentration and stored at −80 °C. Then, an aliquot of the frozen concentrate was thawed and diluted to 10−4 M with medium containing 50 μg/mL of gentamicin. A control sample with just DMSO was also prepared. Aliquots of 100 μL of the different heteroaryl MMB ester conjugate dilution controls were added to the appropriate microtiter wells containing 100 μL of medium, resulting in the required final drug concentrations of 10−5 M and 0 M (control). Once the MMB conjugates were added, the microtiter plates were incubated for 48 h at 37 °C and 100% relative humidity. Cold TCA was used to terminate the assay for adherent cells. Cells were fixed by the addition of 50 μL of cold 50% (w/v) TCA and further incubated for 1 h at 4 °C. The supernatant was discarded, and the microtiter plates were splashed five times with water and air-dried. Sulforhodamine B (SRB) solution (100 μL) at 0.4% (w/v) in 1% CH3COOH was added to each well, and the plates were incubated for another 10 min at room temperature. After SRB staining, free SRB was removed by washing five times with 1% acetic acid. Bound SRB stain was successively dissolved with 10 mM trizma base, and the optical density was measured at a wavelength of 515 nm. The growth inhibitory or cytotoxicity effects of the MMB conjugates in the above cellular assay was measured by determining the percentage of cell growth (PG) inhibition. Optical density (OD) measurements of SRB-derived color just before exposing the cells to the test compound (ODtzero) and after 48 h exposure to the test compound (ODtest) or the control vehicle (ODctrl) were recorded.
5.3. Methodology for the colony formation assay with 9L-SF gliosarcoma Cells
San Francisco variant 9L, rat gliosarcoma cells (9L-SF)[25] were cultured in DMEM/F12 medium containing 10% iron-supplemented calf serum and grown in a humidified 37.0 °C incubator with atmospheric air supplemented to 5% CO2. The 9L-SF cells were seeded into 60 mm culture dishes such that they were 50% confluent 24 h later (i.e. cell density of 6.65 × 104 cells/cm2). The medium content in each dish was 0.313 mL/cm2 of growth surface area. Compounds 7a, 7b and 7j were dissolved in DMSO at a concentration of 12 mM and then diluted serially into the culture medium. The maximum DMSO concentration to which the drug- treated cells were exposed to was 0.05% (for the 10 μM concentration), and some cells were also treated with 0.05% DMSO only. The fumarate salt of DMAPT, which is a water-soluble analog of PTL, was used in these experiments. A 2.93 mM stock solution of DMAPT was made in normal saline, and this was diluted serially to produce the different concentrations of DMAPT used for the clonogenic assay to test for DMAPT toxicity. MMB (3) and indole-2-carboxylic acid (6a) were each dissolved in DMSO to afford a concentration of 5.0 mM and serially diluted to 10 μM in cell culture medium for a single concentration testing for clonogenic toxicity. Compound 7p, a water-soluble analog of 7a, was also included in the assay and was utilized as the monofumarate salt. This compound was dissolved in normal saline at a concentration of 5 mM and diluted serially to 10 μM for comparative single concentration testing of its clonogenic toxicity. All cultures were treated with their respective drugs for 48 h. Cells were then trypsinized from the dishes, diluted appropriately and seeded into 25 cm2 flasks for the colony formation. Cell density in the assay flasks was maintained at 2,000 cells/cm2 by adding lethally irradiated (25 Gy), nonclonogenic 9L-SF feeder cells. All experiments were repeated three times using three replicate flasks to determine the toxicity of each tested drug concentration.
Following treatment, drug-containing medium was removed from the culture dishes, and cells were rinsed three times with fresh medium before the cells were trypsinized from the culture dish surface, diluted appropriately, and their concentration determined using an electronic cell counter. A previously determined number of cells were seeded into 25 cm2 or 75 cm2 culture flasks to determine cell survival by means of colony formation (flasks seeded in triplicate). When the seeded cell number would result in a cell density of less than 2,000 cells/cm2, the cells were placed into a flask that was seeded 24 h earlier with lethally irradiated feeder cells at a density of 2,000 feeders/cm2.[26] No feeders were used when the number of seeded experimental cells yielded a cell density in the flask greater than 2,000 cells/cm2. The seeded flasks were incubated long enough to permit formation of distinct colonies with a longer incubation time required following drug treatments that resulted in lower survival levels. Cell survival was normalized to the plating efficiency (number of colonies divided by the number of seeded cells) exhibited by control, non-treated 9L-SF cells.
5.4. Methodology for M9-ENL1 AML cells and primary AML cell Assays with MMB conjugates 7a–7o and the fumarate salt of the indole-2-carboxylic acid ester of dimethylamino MMB, 7p
Compounds 7a–7p were screened for cytotoxicity against the M9-ENL1 AML cell line, and the most active compounds then screened against primary AML specimens as follows: M9-ENL1 AML cells were plated at a density of 106 cells/mL in alpha-MEM culture media (Invitrogen) supplemented with 5% of human plasma, 20% of FBS, and the cytokines SCF, IL-3, IL-7, and FLT3 (Peprotech). For primary AML specimens, cells were obtained from volunteer donors. Informed consent was obtained in accordance with the Declaration of Helsinki. Cells were isolated from the samples using Ficoll-Paque (GE Healthcare Bio-Sciences, Pittsburgh, PA) density gradient separation. In some cases, cells were cryopreserved in freezing medium of Iscove modified Dulbecco medium (IMDM), 40% of fetal bovine serum (FBS), and 10% of dimethyl sulfoxide (DMSO) or in CryoStor CS-10 (VWR,West Chester, PA). Cells were cultured in serum-free medium (SFM), prepared with Iscove’s MDM supplemented with 20% of BIT 9500 serum substitute (StemCell Technologies), LDL, and beta mercaptoethanol for 1 h before the addition of PTL or the MMB conjugates. Drugs were diluted from a DMSO stock into PBS such that the final concentration of DMSO did not exceed 0.5%. Flow cytometric analysis was performed by co-staining with Annexin V and 7-AAD, according to manufacturer specifications, to identify the percentage of non-apoptotic cells, which was defined as the population with negative staining for both labels. The percentage of non-apoptotic cells observed was normalized to that of the vehicle control, and dose-response curves were analyzed using GraphPad Prism software to determine EC50 values. All analyses were conducted in triplicate.
5.5. Comparison of NCI Screening and the Colony Formation Assay
It should be noted that the NCI drug screening methodology and the colony formation assay measure very different cancer-relevant end points of metabolic and growth inhibition and clonogenic death, respectively. Because of this, both assays can facilitate the selection of compounds that should be translated forward into testing in animal models for specific cancer types, e.g., glioblastoma, hepatocellular carcinoma, and so forth. The NCI cancer screening program encompasses a larger number of compounds and identifies which molecules have the greatest toxicity for each cancer cell type. Once investigators decide which cancer type to study in animal models, the smaller number of compounds that are highly toxic to this cancer type, can be tested in vitro using the colony formation assay. Arguably, the compounds that are most effective in rendering the cells clonogenically dead will have the greatest likelihood of treating ectopic or orthotopic tumors successfully, and these molecules are likely the ones that should progress to animal studies.
5.6. General synthetic procedure for the synthesis of MMB ester conjugates 7a–7o
To the stirred solution of an appropriate heteroaryl carboxylic acid (0.15 mmol) in dry dichloromethane (5 mL) under an argon atmosphere at 0 °C was added EDCI (0.181 mmol), and the mixture allowed to stir for 15 min. Then a catalytic amount of DMAP (0.045 mmol) was added, followed by addition of MMB (0.151 mmol) in dry dichloromethane (3 mL) at 0 °C and the resulting mixture stirred for 8 h at room temperature. After completion of the reaction (monitoring by TLC), the reaction mixture was washed with water (10 ml) and the dichloromethane layer separated, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by silica gel column chromatography using 2 to 5% methanolic dichloromethane as eluent to afford the corresponding heteroaryl esters of MMB (7a–7o) as solids in 56 to 74%. Analytical data for the above MMB esters are provided below.
5.6.1 Indole-2-carboxylic acid ester of MMB (7a)
Pale yellow solid, mp:141–143 °C; 1H NMR (CDCl3, 400 MHz): δ 8.85 (s, 1H), 7.70 (d, J = 8.4 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.34 (t, J = 7.2 Hz, 1H), 7.22 (s, 1H), 7.18 (t, J = 7.6 Hz, 1H), 6.25 (s, 1H), 5.82 (t, J = 8.8 Hz, 1H), 5.55 (s, 1H), 4.96 (d, J = 12.8 Hz, 1H), 4.75 (d, J = 12.8 Hz, 1H), 3.90 (t, J = 9.2 Hz, 1H), 2.98 (brs, 1H), 2.92 (d, J = 9.2 Hz, 1H), 2.53–2.17 (m, 6H), 1.74 (t, J = 8.0 Hz, 1H), 1.56 (s, 3H), 1.14 (t, J = 13.2 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.4, 161.7, 138.7, 137.1, 134.9, 130.9, 127.5, 126.7, 125.9, 122.8, 121.2, 120.6, 112.0, 109.2, 81.1, 67.0, 63.4, 60.1, 42.8, 36.7, 25.9, 24.5, 24.0, 18.1 ppm; HRMS m/z calcd. for C24H26NO5, (M+H)+: 408.1806, found: 408.1809.
5.6.2. 5-Methoxyindole-2-carboxylic acid ester of MMB (7b)
White solid, mp:130–132 °C; 1H NMR (CDCl3, 400 MHz): δ 8.85 (s, 1H), 7.33 (d, J = 8.8 Hz, 1H), 7.13 (s, 1H), 7.06 (s, 1H), 7.00 (d, J = 8.8 Hz, 1H), 6.25 (s, 1H), 5.81 (t, J = 8.4 Hz, 1H), 5.55 (s, 1H), 4.94 (d, J = 12.4 Hz, 1H), 4.74 (d, J = 12.4 Hz, 1H), 3.90 (t, J = 9.6 Hz, 1H), 3.85 (s, 3H), 2.99 (brs, 1H), 2.89 (d, J = 9.2 Hz, 1H), 2.52–2.15 (m, 6H), 1.73 (t, J = 8.8 Hz, 1H), 1.62 (s, 3H), 1.16 (t, J = 12.4 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.5, 161.7, 154.9, 138.7, 134.9, 132.5, 130.8, 127.8, 127.1, 120.6, 117.6, 112.9, 108.8, 102.6, 81.1, 66.9, 63.4, 60.1, 55.8, 42.8, 36.7, 25.8, 24.5, 23.9, 18.1 ppm; HRMS (ESI) m/z calcd. for C25H28NO6, (M + H)+: 438.1911, found: 438.1920.
5.6.3. 5-Fluroindole-2-carboxylic acid ester of MMB (7c)
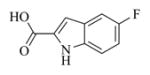
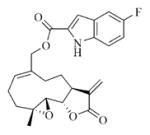
White solid, mp: 127–129 °C; 1H NMR (CDCl3, 400 MHz): δ 8.89 (brs, 1H), 7.37–7.30 (m, 2H), 7.17 (s, 1H), 7.13 (t, J = 9.6 Hz, 1H), 5.81 (s, 1H), 5.79 (t, J = 8.0 Hz, 1H), 5.55 (s, 1H), 4.95 (d, J = 12.8 Hz, 1H), 4.75 (d, J = 12.4 Hz, 1H), 3.90 (t, J = 9.6 Hz,1H), 2.97 (brs, 1H), 2.91 (d, J = 9.2 Hz, 1H), 2.53–2.16 (m, 6H), 1.76 (t, J = 4.4 Hz, 1H), 1.57 (s, 3H), 1.17 (t, J = 12.8 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.4, 161.4, 159.5, 157.2, 138.7, 134.7, 133.6, 131.0, 128.3, 127.7, 127.6, 120.6, 115.2, 114.9, 113.0, 112.9, 109.0, 109.0, 107.1, 106.8, 81.1, 67.1, 63.4, 60.1, 42.8, 36.7, 25.8, 24.5, 24.1, 18.1 ppm; HRMS calcd VWR,West Chester, PA VWR,West Chester, PA for C24H24NO5FNa, (M+Na)+: 448.1531, found: 448.1546.
5.6.4. 5-Chloroindole-2-carboxylic acid ester of MMB (7d)
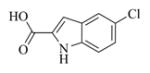
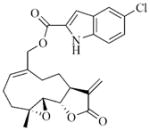
White solid, mp: 145–147 °C; 1H NMR (CDCl3, 400 MHz): δ 9.13 (s, 1H), 7.65 (s, 1H), 7.38 (d, J = 8.8 Hz, 1H), 7.29 (d, J = 8.8 Hz,1H), 7.14 (s, 1H), 6.25 (s, 1H), 5.78 (t, J = 8.8 Hz,1H), 5.55 (s, 1H), 4.95 (d, J = 12.8 Hz,1H), 4.76 (d, J = 12.4 Hz,1H), 3.90 (t, J = 8.8 Hz, 1H), 2.95 (brs, 1H), 2.90 (d, J = 8.8 Hz, 1H), 2.53–2.19 (m, 6H), 1.76–1.61 (m, 1H), 1.55 (s, 3H), 1.26–1.15 (m, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.5, 161.5, 138.7, 135.4, 134.7, 130.9, 128.3, 128.1, 126.7, 126.3, 121.8, 120.6, 113.3, 108.4, 81.1, 67.2, 63.4, 60.1, 42.8, 36.7, 25.8, 24.4, 23.9, 18.1 ppm; HRMS calcd. for C24H24NO5NaCl, (M+Na)+: 464.1235, found: 464.1233.
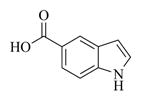
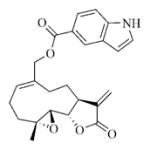
5.6.5. Indole-5-carboxylic acid ester of MMB (7e)
White solid, mp: 134–136 °C; 1H NMR (CDCl3, 400 MHz): δ 8.54 (s, 1H), 8.38 (s,1H), 7.89 (d, J = 8.8 Hz, 1H), 7.42 (d, J = 8.8 Hz, 1H), 7.30 (t, J = 2.8 Hz, 1H), 6.65 (s, 1H), 6.21 (s, 1H), 5.80 (t, J = 8.8 Hz, 1H), 5.52 (s, 1H), 4.93 (d, J = 12.4 Hz, 1H), 4.75 (d, J = 12.4 Hz, 1H), 3.88 (t, J = 9.6 Hz, 1H), 3.04 (brs, 1H), 2.95 (d, J = 9.6 Hz, 1H), 2.49–2.16 (m, 6H), 1.65–1.64 (m, 1H), 1.56 (s, 3H), 1.17 (t, J = 12.8 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.6, 167.4, 138.7, 138.6, 135.4, 130.4, 127.6, 125.9, 123.9, 123.4, 121.5, 120.7, 111.0, 104.1, 81.2, 66.9, 63.4, 60.2, 42.9, 36.8, 26.0, 24.8, 24.0, 18.1 ppm; HRMS calcd. for C24H26NO5, (M+H)+: 408.1806, found: 408.1805.
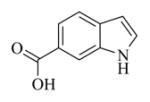
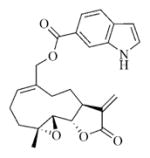
5.6.6. Indole-6-carboxylic acid ester of MMB (7f)
White solid, mp 129–131 °C; 1H NMR (CDCl3, 400 MHz): δ 8.62 (brs, 1H), 8.09 (s,1H), 7.92 (d, J = 8.8 Hz, 1H), 7.66 (d, J = 8.4 Hz, 1H), 7.39 (s, 1H), 6.19 (s, 1H), 5.82 (t, J = 8.4 Hz, 1H), 5.49 (s, 1H), 4.93 (d, J = 12.4 Hz, 1H), 4.75 (d, J = 12.4 Hz, 1H), 3.92 (t, J = 9.2 Hz, 1H), 3.14 (t, J = 8.4 Hz, 1H), 2.96 (d, J = 12.0 Hz, 1H), 2.54-2.16 (m, 6H), 1.73 (t, J = 7.2 Hz, 1H), 1.56 (s, 3H), 1.16 (t, J = 12.0 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.8, 167.4, 138.8, 135.4, 135.2, 131.9, 130.8, 128.0, 120.9, 120.8, 120.6, 120.5, 113.6, 103.1, 81.3, 67.3, 63.4, 60.1, 43.0, 36.8, 25.9, 25.0, 24.0, 18.1 ppm; HRMS calcd. for C24H26NO5, (M+H)+: 408.1806, found: 408.1810.
5.6.7. Indole-3-acetic acid ester of MMB (7g)
White solid; mp: 67–69 °C; 1H NMR (CDCl3, 400 MHz): δ 8.16 (s, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.39 (d, J = 8.4 Hz, 1H), 7.21 (t, J = 7.2 Hz, 1H), 7.11 (d, J = 6.8 Hz, 1H), 7.07 (s, 1H), 6.13 (s, 1H), 5.61 (t, J = 7.6 Hz, 1H), 5.32 (s, 1H), 4.70 (d, J = 12.0 Hz,1H), 4.41 (d, J = 12.4 Hz,1H), 3.83 (q, J = 16.0 Hz, J = 3.6 Hz, 2H), 3.70 (d, J = 9.6 Hz,1H), 2.59–2.55 (t, J = 12.0 Hz, 1H), 2.29–2.01 (m, 7H), 1.48–1.44 (m, 4H), 0.91 (t, J = 12.4 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 171.8, 169.9, 139.2, 136.4, 134.9, 131.8, 127.1, 123.2, 122.6, 120.0, 119.8, 118.9, 111.8, 108.4, 81.0, 67.1, 63.0, 59.9, 42.6, 36.5, 32.0, 25.8, 25.3, 23.9, 18.1 ppm; HRMS calcd. for C25H27NO5Na, (M+Na)+: 444.1781, found: 444.1788.
5.6.8. Indole-3-butyric acid ester of MMB (7h)
White solid, mp: 74–76 °C; 1H NMR (CDCl3, 400 MHz): δ 8.20 (s, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.17 (t, J = 6.8 Hz, 1H), 7.09 (t, J = 7.2 Hz, 1H), 6.95 (s, 1H), 6.17 (s, 1H), 5.64 (t, J = 8.4 Hz,1H), 5.44 (s, 1H), 4.61 (d, J = 12.0 Hz,1H), 4.42 (d, J = 12.4 Hz,1H), 3.83 (d, J = 9.2 Hz, 1H), 2.83–2.77 (m, 4H), 2.38–2.14 (m, 8H), 2.09 (p, J = 7.2 Hz, J = 14.4 Hz, 2H), 1.60–1.59 (m, 1H), 1.56 (s, 3H), 1.09 (t, J = 11.6 Hz, 1H) ppm; 13C NMR (CDCl3, 200 MHz): δ 173.4, 169.6, 138.7, 136.4, 134.9, 130.8, 127.4, 121.9, 121.7, 120.4, 119.1, 118.7, 115.1, 111.3, 81.1, 66.8, 63.2, 60.1, 42.7, 36.6, 33.8, 25.7, 25.3, 24.6, 24.5, 23.8, 18.0 ppm; HRMS calcd. For C27H32NO5, (M+H)+: 450.2346, found: 450.2344.
5.6.9. Indomethacin ester of MMB (7i)
Pale yellow solid, mp: 95–97 °C; 1H NMR (CDCl3, 400 MHz): δ 7.67 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 8.4 Hz, 2H), 6.95 (s, 1H), 6.84 (d, J = 8.8 Hz, 1H), 6.68 (d, J = 9.2 Hz,1H), 6.19 (s, 1H), 5.62 (t, J = 8.4 Hz,1H), 5.39 (s, 1H), 4.66 (d, J = 12.4 Hz,1H), 4.51 (d, J = 12.4 Hz,1H), 3.83 (s, 3H), 3.79 (t, J = 9.2 Hz, 1H), 3.69 (s, 2H), 2.73–2.71 (m, 1H-), 2.69 (d, J = 9.2 Hz, 1H), 2.40 (s, 3H), 2.36–2.10 (m, 6H), 1.54–1.53 (m, 1H), 1.50 (s, 3H), 1.30–1.28 (m, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 170.6, 169.4, 168.4, 156.2, 139.5, 138.7, 136.2, 134.7, 133.8, 131.4, 130.9, 130.7, 130.6, 129.4, 120.4, 115.3, 112.4, 111.4, 101.9, 81.0, 67.1, 63.3, 60.0, 55.9, 53.6, 42.7, 36.7, 30.7, 25.7, 24.6, 23.9, 18.2 ppm; HRMS calcd. for C34H34NO7NaCl, (M+H)+: 626.1916, found: 626.1908.
5.6.10. Indole-3-acrylicacid ester of MMB (7j)
White solid, mp: 137–139 °C; 1H NMR (CDCl3, 400 MHz): δ 8.61 (brs, 1H), 7.93 (d, J = 15.6 Hz, 1H), 7.88 (brs, 1H),7.50 (d, J = 2.8 Hz, 1H), 7.42 (brs, 1H), 7.29–7.27 (m, 2H), 6.45 (d, J = 16.0 Hz,1H), 6.26 (d, J = 3.6 Hz, 1H), 5.79 (t, J = 8.4 Hz,1H), 5.58 (d, J = 3.2 Hz,1H), 4.81 (d, J = 12.4 Hz,1H), 4.62 (d, J = 12.8 Hz,1H), 3.88 (t, J = 6.04 Hz,1H), 3.09–3.07 (m, 1H), 2.94 (d, J = 9.2 Hz, 1H), 2.48–2.20 (m, 6H), 1.72–1.69 (m, 1H), 1.58 (s, 3H), 1.42–1.33 (m, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.8, 168.1, 139.5, 138.7, 137.3, 135.4, 130.5, 129.9, 125.2, 123.4, 121.5, 120.7, 120.4, 113.3, 112.2, 112.1, 81.4, 66.8, 63.4, 60.3, 42.8, 36.7, 26.0, 24.8, 23.9, 18.1 ppm; HRMS calcd. for C26H27NO5Na, (M+Na)+: 456.1781, found: 456.1790.
5.6.11. Indole-3-carboxylic acid ester of MMB (7k)
White solid, mp:140–142 °C; 1H NMR (CDCl3, 400 MHz): δ 8.82 (s, 1H) 8.16–8.14 (m, 1H), 7.91 (s,1H), 7.45–7.43 (m, 1H), 7.29–7.27 (m, 2H), 6.18 (s, 1H), 5.80 (t, J = 8.4 Hz, 1H), 5.49 (s, 1H), 4.92 (d, J = 12.4 Hz, 1H), 4.80 (d, J = 12.8 Hz, 1H), 3.89 (t, J = 9.2 Hz, 1H), 3.03 (t, J = 9.2 Hz, 1H), 2.95 (d, J = 9.2 Hz, 1H), 2.51–2.14 (m, 6H), 1.70–1.65 (m, 1H), 1.56 (s, 3H), 1.14 (t, J = 13.2 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.5, 164.6, 138.8, 137.3, 135.7, 135.4, 130.1, 126.6, 123.1, 122.2, 121.5, 120.4, 110.1, 106.5, 81.2, 65.8, 63.4, 60.1, 42.8, 36.8, 25.9, 24.8, 23.9, 18.1 ppm; HRMS (ESI) m/z calcd. for C24H25NO5Na (M + Na)+: 430.1625, found: 430.1620.
5.6.12. N-Methylindole-3-carboxylic acid ester of MMB (7l)
White solid, mp: 109–111 °C; 1H NMR (CDCl3, 400 MHz): δ 8.14 (d, J = 7.2 Hz, 1H), 7.75 (s, 1H), 7.37–7.30 (m, 3H), 6.18 (s, 1H), 5.80 (t, J = 8.4 Hz, 1H), 5.50 (s, 1H), 4.92 (d, J = 12.4 Hz, 1H), 4.75 (d, J = 12.4 Hz, 1H), 3.88 (m, 4H), 3.05 (t, J = 9.2 Hz, 1H), 2.92 (d, J = 9.2 Hz, 1H), 2.51–2.15 (m, 6H), 1.70–1.68 (m, 1H), 1.55 (s, 3H), 1.15 (t, J = 13.2 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.5, 164.6, 138.8, 137.3, 135.6, 135.4, 130.0, 126.6, 123.1, 122.2, 121.5, 120.4, 110.0, 106.5, 81.2, 65.8, 63.4, 60.1, 42.8, 36.8, 33.6, 25.9, 24.8, 23.9, 18.1 ppm; HRMS (ESI) m/z calcd. for C25H27NO5Na, (M + Na)+: 444.1781, found: 444.1777.
5.6.13. Benzothiophene-2-carboxylic acid ester of MMB (7m)
White solid, mp: 71–73 °C; 1H NMR (CDCl3, 400 MHz): δ 8.06 (s, 1H), 7.89–7.85 (m, 2H), 7.49–7.40 (m, 2H), 6.24 (d, J = 2.8 Hz, 1H), 5.83 (t, J = 8.0 Hz,1H), 5.55 (d, J = 2.0 Hz, 1H), 4.93 (d, J = 12.0 Hz,1H), 4.77 (d, J = 12.8 Hz,1H), 3.90 (t, J = 9.6 Hz,1H), 2.99 (brs, 1H), 2.92 (d, J = 9.2 Hz, 1H), 2.53–2.17 (m, 6H), 1.73–1.69 (m, 1H), 1.57 (s, 3H), 1.17 (t, J = 13.2 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.53, 162.7, 142.4, 138.8, 138.8, 134.8, 131.4, 131.1, 127.4, 125.8, 125.3, 122.9, 120.7, 81.2, 67.7, 63.5, 60.2, 43.0, 36.8, 25.9, 24.77, 24.10, 18.2 ppm; HRMS calcd. for C24H25O5S, (M+H)+: 403.0909, found: 403.0917.
5.6.14. 3-Chlorobenzothiophene-2-carboxylic acid ester of MMB (7n)
White solid, mp: 69–71 °C; 1H NMR (CDCl3, 400 MHz): δ 7.99 (d, J = 6.8 Hz, 1H), 7.83 (d, J = 7.6 Hz, 1H), 7.54–7.50 (m, 2H), 6.23 (d, J = 3.6 Hz, 1H), 5.86 (t, J = 8.4 Hz, 1H), 5.55 (d, J = 2.8 Hz, 1H), 4.91 (dd, J = 12.4 Hz, J = 27.2 Hz, 2H), 3.90 (d, J = 9.6 Hz, 2H), 2.99 (t, J = 3.2 Hz, 1H), 2.92 (d, J = 9.2 Hz,1H), 2.53–2.17 (m, 6H), 1.76 (m, 1H), 1.57 (s, 3H), 1.18 (t, J = 12.8 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.5, 161.2, 138.8, 138.8, 137.2, 134.6, 131.7, 128.7, 128.0, 125.9, 125.5, 124.2, 123.0, 120.7, 81.2, 67.9, 63.5, 60.2, 43.0, 36.8, 25.9, 24.7, 24.1, 18.2 ppm; HRMS calcd. for C24H24O5SCl, (M+H)+: 459.1028, found: 459.1035.
5.6.15. Benzofuran-2-carboxylic acid ester of MMB (7o)
White solid, mp: 59–61 °C; 1H NMR (CDCl3, 400 MHz): δ 7.69 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.55 (s, 1H), 7.49 (t, J = 6.8 Hz, 1H), 7.33 (t, J = 7.6 Hz,1H), 6.22 (d, J = 3.6 Hz,1H), 5.85 (t, J = 8.4 Hz, 1H), 5.54 (d, J = 3.2 Hz,1H), 5.0 (d, J = 12.4 Hz, 1H), 4.75 (d, J = 12.0 Hz, 1H), 3.91 (t, J = 9.2 Hz, 1H), 3.09 (brs, 1H), 2.95 (d, J = 6.8 Hz, 1H), 2.54–2.25 (m, 6H), 1.75 (t, J = 9.6 Hz, 1H), 1.57 (s, 3H), 1.18 (t, J = 12.4 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 169.6, 159.5, 156.0, 145.2, 138.9, 134.7, 132.2, 128.2, 127.0, 124.2, 123.1, 120.5, 114.7, 112.6, 81.2, 68.0, 63.5, 60.2, 43.0, 36.8, 26.1, 25.0, 24.1, 18.2 ppm; HRMS calcd. for C24H25O6, (M+H)+: 409.1646, found: 409.1648.
5.7. Synthesis of dimethylaminomelampomagnolide B (8)
Melampomagnolide B (1 mmol), was dissolved in methanol (5 ml) at room temperature. Added dimethylamine solution in methanol (0.18 ml, 2.5 M in methanol) and stirred the reaction mass at room temperature for 6 hrs, until the completion of reaction by TLC (TLC mobile phase, dichloromethane: methanol= 98:2). Reaction mass was concentrated under reduced pressure to remove methanol. Obtained crude product was purified by silica column chromatography using 2% methanolic dichloromethane. Gummy mass; 1H NMR (CDCl3, 400 MHz): δ 5.60 (t, J = 7.6 Hz, 1H), 4.16 (dd, J = 12.8 Hz, J = 32.0 Hz, 2H), 3.88 (t, J = 9.2 Hz, 1H), 2.82 (d, J = 9.2 Hz, 1H), 2.76–2.70 (m, 2H), 2.51–2.42 (m, 6H), 2.30–2.15 (m, 7H), 2.15–2.08 (m, 2H), 1.64–1.61 (m, 1H), 1.52 (s, 3H), 1.11 (t, J = 11.2 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 176.7, 140.9, 127.4, 81.6, 66.2, 63.9, 59.9, 57.3, 45.5, 44.1, 42.1, 36.9, 27.5, 25.8, 23.7, 17.9 ppm. HRMS calcd. for C17H28NO4, (M+H)+: 310.2047, found: 310.2037.
5.8. Synthesis of the indole-2-carboxylic acid ester of dimethylamino MMB (9)
To a stirred solution of indole-2-carboxylic acid (6a, 0.15 mmol) in dry dichloromethane (5 mL) under an argon atmosphere at 0 °C was added EDCI (0.181 mmol), and the mixture allowed to stir for 15 min. Then a catalytic amount of DMAP (0.045 mmol) was added, followed by addition of dimethylaminomelampomagnolide B (8, 0.15 mmol) in dry dichloromethane (3 mL) at 0 °C and the resulting mixture stirred for 8 h at room temperature. After completion of reaction (monitoring by TLC), the reaction mixture was washed with water (10 ml) and the dichloromethane layer separated, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by silica gel column chromatography using 2 to 5% methanolic dichloromethane as eluent to afford the corresponding indole-2-carboxylic acid ester of dimethylamino MMB (9) as white solid, mp: 114–116 °C; 1H NMR (CDCl3, 400 MHz): δ 8.92 (s, 1H), 7.70 (d, J = 8.4 Hz, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.35 (t, J = 7.2 Hz, 1H), 7.23 (s, 1H), 7.18 (t, J = 7.6 Hz, 1H), 5.72 (t, J = 8.8 Hz, 1H), 5.03 (d, J = 12.8 Hz, 1H), 4.85 (d, J = 12.8 Hz, 1H), 3.90 (t, J = 9.6 Hz, 1H), 2.83 (d, J = 9.2 Hz,1H), 2.79 (dd, J = 4.8 Hz, J = 12.8 Hz, 1H), 2.69 (dd, J = 5.2 Hz, J = 12.8 Hz, 1H), 2.54–2.44 (m, 4H), 2.38–2.28 (m, 2H), 2.24 (s, 6H) 2.23–2.13 (m, 2H), 1.65–1.61(m, 1H), 1.56 (s, 3H), 1.13 (t, J = 12.4 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 177.0, 161.6, 137.0, 135.7, 128.8, 127.5, 127.1, 125.7, 122.7, 121.1, 112.0, 109.0, 81.3, 66.8, 64.0, 60.0, 58.3, 45.8, 44.5, 43.0, 37.0, 31.7, 27.0, 24.7, 23.9, 18.1 ppm; HRMS calcd. for C26H33N2O5, (M+H)+: 453.2384, found: 453.2336.
5.9. The fumarate salt of the indole-2-carboxylic acid ester of dimethylamino MMB (7p)
Compound 9 (50 mg, 0.11 mmol) was dissolved in ethanol under an argon atmosphere, and fumaric acid (0.11 mmol in 0.5 ml ethanol) in ethanol solution was added drop- wise while stirring the reaction mixture for 30 minutes at room temperature. The resulting white precipitate was filtered and dried under vacuum to afford 7p (37 mg) in 60% yield. White solid, mp: 184–186 °C; 1H NMR (CD3OD, 400 MHz): δ 7.65 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.20 (s, 1H), 7.10 (t, J = 7.6 Hz, 1H), 6.70 (s, 2H), 5.79 (t, J = 8.0 Hz, 1H), 5.02 (d, J = 12.4 Hz, 1H), 4.76 (d, J = 12.8 Hz, 1H), 4.18 (t, J = 9.2 Hz, 1H), 3.30–3.23 (m, 2H), 3.01–3.00 (m, 1H), 2.91 (d, J = 9.2 Hz, 1H), 2.78 (s, 6H), 2.64–2.39 (m, 4H), 2.25–2.14 (m, 3H), 1.80–1.75 (m, 1H) 1.58 (s, 3H), 1.10 (t, J = 13.2 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ 178.0, 170.4, 163.3, 139.2, 136.6, 135.9, 131.0, 128.6, 128.3, 126.2, 123.2,, 121.5, 113.3, 109.7, 83.4, 67.8, 64.5, 61.5, 57.4, 44.6, 44.5, 44.0, 43.1, 37.8, 26.8, 25.3, 24.5, 17.9 ppm; CHN analysis calcd. for C30H38N2O10.H2O; C, 61.42%, H, 6.53%, N, 4.78%, O, 27.27%; Found: C, 61.25%, H, 6.56%, N, 4.43%, O, 27.27%.
Supplementary Material
Design and synthesis of novel MMB-indole carboxylic acid ester analogs
Screening of analogs for anticancer activity against NCI-60 cancer cell panel
Potent growth inhibitory effects of analogs on human cancer cell lines
Potent anti-leukemic activity of analogs against cell specimens from AML patients
Potent antiproliferative effects against rat 9L-SF gliosarcoma cells
Acknowledgments
We are grateful to the NIH/National Cancer Institute (grant # R01 CA158275), the UAMS Translational Research Institute (TRI), grant UL1TR000039 through the NIH National Center for Research Resources, the National Center for Advancing Translational Sciences, the UAMS Department of Radiology for partial support of this research, to the Arkansas Research Alliance for an Arkansas Scholar award, and to the NCI drug-screening program. The authors also thank Laura J. Bernock, M.S. for her technical assistance.
Appendix A. Supplementary data
1H-NMR, 13C NMR, and HRMS spectra for synthetic products are provided in the Supplementary Information. Supplementary data related to this article can be found at http://....
Footnotes
Conflict of Interest Statement
The authors declare the following competing financial interest(s): The University of Arkansas for Medical Sciences (UAMS) holds patents on the molecules described in this study. A potential royalty stream to SB, JP, NRP, VJ, CTJ, and PAC may occur consistent with UAMS policy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woods JR, Mo H, Bieberich AA, Alavanja T, Colby DA. Fluorinated amino-derivatives of the sesquiterpene lactone, parthenolide, as (19)f NMR probes in deuterium-free environments. J Med Chem. 2011;54:7934–7941. doi: 10.1021/jm201114t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai JD, Li D, Long J, Zhang HL, Lin JP, Qiu CJ, Zhang Q, Chen Y. Biomimetic semisynthesis of arglabin from parthenolide. J Org Chem. 2012;77:7103–7107. doi: 10.1021/jo300888s. [DOI] [PubMed] [Google Scholar]
- 3.Ma WW, Shi QQ, Ding YH, Long J, Zhang Q, Chen Y. Synthesis of micheliolide derivatives and their activities against AML progenitor cells. Molecules. 2013;18:5980–5992. doi: 10.3390/molecules18055980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight DW. Feverfew: chemistry and biological activity. Nat Prod Rep. 1995;12:271–276. doi: 10.1039/np9951200271. [DOI] [PubMed] [Google Scholar]
- 5.Skalska J, Brookes PS, Nadtochiy SM, Hilchey SP, Jordan CT, Guzman ML, Maggirwar SB, Briehl MM, Bernstein SH. Modulation of cell surface protein free thiols: a potential novel mechanism of action of the sesquiterpene lactone parthenolide. PLoS One. 2009;4:e8115. doi: 10.1371/journal.pone.0008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Becker MW, Bennett JM, Sullivan E, Lachowicz JL, Vaughan A, Sweeney CJ, Matthews W, Carroll M, Liesveld JL, Crooks PA, Jordan CT. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasim S, Crooks PA. Antileukemic activity of aminoparthenolide analogs. Bioorg Med Chem Lett. 2008;18:3870–3873. doi: 10.1016/j.bmcl.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 9.Oka D, Nishimura K, Shiba M, Nakai Y, Arai Y, Nakayama M, Takayama H, Inoue H, Okuyama A, Nonomura N. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-kappaB. Int J Cancer. 2007;120:2576–2581. doi: 10.1002/ijc.22570. [DOI] [PubMed] [Google Scholar]
- 10.Hewamana S, Alghazal S, Lin TT, Clement M, Jenkins C, Guzman ML, Jordan CT, Neelakantan S, Crooks PA, Burnett AK, Pratt G, Fegan C, Rowntree C, Brennan P, Pepper C. The NF-kappaB subunit Rel A is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukemia and represents a promising therapeutic target. Blood. 2008;111:4681–4689. doi: 10.1182/blood-2007-11-125278. [DOI] [PubMed] [Google Scholar]
- 11.https://globenewswire.com/news-release/2009/01/28/391631/158480/en/Leuchemix-Provides-Update-On-Cancer-Trial.html
- 12.El-Feraly FS. Melampolides from Magnolia grandiflora. Phytochemistry. 1984;23:2372–2374. [Google Scholar]
- 13.Nasim S, Pei S, Hagen FK, Jordan CT, Crooks PA. Melampomagnolide B: a new antileukemic sesquiterpene. Bioorg Med Chem. 2011;19:1515–1519. doi: 10.1016/j.bmc.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 14.Pei S, Minhajuddin M, Callahan KP, Balys M, Ashton JM, Neering SJ, Lagadinou ED, Corbett C, Ye H, Liesveld JL, O’Dwyer KM, Li Z, Shi L, Greninger P, Settleman J, Benes C, Hagen FK, Munger J, Crooks PA, Becker MW, Jordan CT. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J Biol Chem. 2013;288:33542–33558. doi: 10.1074/jbc.M113.511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei S, Minhajuddin M, D’Alessandro A, Nemkof T, Stevens BM, Adane B, Khan N, Hagan FK, Yadav VK, De S, Ashton JM, Hanson KC, Gutman JA, Pollyea DA, Crooks PA, Smith C, Jordan CT. Rational design of a parthenolide-based drug regimen that selectively eradicates acute myelogenous leukemia stem cells. J Biol Chem. 2016;291:21984–22000. doi: 10.1074/jbc.M116.750653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janganati V, Penthala NR, Madadi NR, Chen Z, Crooks PA. Anti-cancer activity of carbamate derivatives of melampomagnolide B. Bioorg Med Chem Lett. 2014;24:3499–3502. doi: 10.1016/j.bmcl.2014.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janganati V, Ponder J, Jordan CT, Borrelli MJ, Penthala NR, Crooks PA. Dimers of Melampomagnolide B Exhibit Potent Anticancer Activity against Hematological and Solid Tumor Cells. J Med Chem. 2015;58:8896–8906. doi: 10.1021/acs.jmedchem.5b01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albayati ZA, Janganati V, Chen Z, Ponder J, Breen PJ, Jordan CT, Crooks PA. Bioorg Med Chem. 2016;25:1235–1241. doi: 10.1016/j.bmc.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 20.Dai Y, Guzman ML, Chen S, Wang L, Yeung SK, Pei XY, Dent P, Jordan CT, Grant S. The NF (Nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol. 2010;151:70–83. doi: 10.1111/j.1365-2141.2010.08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 22.Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett. 1997;402:85–90. doi: 10.1016/s0014-5793(96)01502-5. [DOI] [PubMed] [Google Scholar]
- 23.Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82:1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 24.Neelakantan S, Nasim S, Guzman ML, Jordan CT, Crooks PA. Aminoparthenolides as novel anti-leukemic agents: Discovery of the NF-kappaB inhibitor, DMAPT (LC-1) Bioorg Med Chem Lett. 2009;19:4346–4349. doi: 10.1016/j.bmcl.2009.05.092. [DOI] [PubMed] [Google Scholar]
- 25.Henderson SD, Kimler BF, Morantz RA. Radiation therapy of 9L rat brain tumors. Int J Radiat Oncol Biol Phys. 1981;7:497–502. doi: 10.1016/0360-3016(81)90136-x. [DOI] [PubMed] [Google Scholar]
- 26.Borrelli MJ, Thompson LL, Dewey WC. Evidence that the feeder effect in mammalian cells is mediated by a diffusible substance. Int J Hyperthermia. 1989;5:99–103. doi: 10.3109/02656738909140436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.