Abstract
Purpose
We conducted a retrospective comparative-effectiveness study of best-corrected visual acuity (BCVA) and refractive error (RE) following immediate sequential (ISBCS) and delayed sequential (DSBCS) bilateral cataract surgery. We tested two hypotheses: (1) among DSBCS patients, 2nd eye outcomes were no different than 1st eye outcomes; (2) averaged between each patient’s two eyes, outcomes did not differ between ISBCS and DSBCS patients.
Design
Retrospective comparative-effectiveness study.
Subjects
Kaiser Permanente Northern California members who underwent non-complex bilateral cataract surgery during 2013 through June 30, 2015.
Methods
We performed an intention-to-treat analysis comparing ISBCS to DSBCS using conditional logistic regression analysis, accounting for surgeon and patient-level factors.
Main Outcome Measures
BCVA, RE.
Results
The analysis of visual outcomes included both eyes of 13,711 DSBCS and 3,561 ISBCS patients. Because of the large sample size, some statistical differences lacked clinical significance. Ocular comorbidities were slightly more prevalent in DSBCS patients. Postoperative BCVA was 20/20 or better in 48% of DSBCS 1st eyes, 49% of DSBCS 2nd eyes, 53% of ISBCS right eyes, and 51% of ISBCS left eyes. The within-person difference in postoperative BCVA averaged zero (0.00) between the 1st and 2nd DSBCS eyes, and between the ISBCS right and left eyes. After adjustment, average postoperative BCVA was better in ISBCS patients, although the difference was not statistically significant. (compared with 20/20 or better: odds ratio for worse than 20/20 was 0.91, 95% confidence interval 0.83–1.01). Emmetropia (spherical equivalent −0.5 to 0 D) was achieved in 61% of 1st DSBCS eyes, 61% of 2nd DSBCS eyes, 63% of ISBCS right eyes, and 62% of ISBCS left eyes. After adjustment, average postoperative RE was no different in ISBCS compared with DSBCS patients (compared with emmetropia: odds ratio for ametropia was 1.02, confidence interval 0.92–1.12). We confirmed one case of postoperative endophthalmitis in 10,494 ISBCS eyes (1.0 per 10,000 eyes), two cases in 38,736 DSBCS eyes (0.5 per 10,000 eyes) (p=0.6), and no patient had bilateral endophthalmitis.
Conclusion
Compared with DSBCS cataract surgery, we found no evidence that ISBCS surgery was associated with worse postoperative BCVA or RE, or with an increased complication risk.
Keywords: Cataract Surgery, Electronic Health Record, Immediate Sequential Bilateral Cataract Surgery, Delayed Sequential Bilateral Cataract Surgery, Surgical Complications, Visual Outcomes
INTRODUCTION
We conducted a retrospective comparative-effectiveness study to assess whether immediate sequential bilateral cataract surgery (ISBCS) cataract surgery offers similar visual outcomes to delayed sequential bilateral cataract surgery (DSBCS). We used the community-based population and electronic health record data of Kaiser Permanente Northern California to examine postoperative best-corrected visual acuity (BCVA), refractive error (RE), and surgical complications. We hypothesized that in DSBCS patients, the outcome of the 2nd eye was no different than in the 1st eye. This hypothesis was motivated by the assertion that DSBCS surgery offers the opportunity to improve refractive outcomes by allowing the surgeon to use the refractive outcome of the 1st eye to guide selection of the intraocular lens (IOL) for the 2nd eye.1, 2 In addition, we hypothesized that visual outcomes averaged between each patient’s two eyes did not differ between ISBCS and DSBCS patients. We also compared surgical complication rates between the two approaches.
METHODS
The study was approved by the Kaiser Foundation Research Institute’s institutional review board.
Setting
Kaiser Permanente Northern California is a community-based healthcare system that owns its hospitals and medical offices. For most patients, care is capitated (prepaid), and members receive comprehensive services. During 2013–15, clear cornea phacoemulsification using standardized phacoemulsification machines (Alcon, Irvine) and IOLs (Alcon, Irvine) was performed by 152 cataract surgeons at 22 surgical centers. In 2014, medical offices switched biometry equipment from IOLMaster to Lenstar, at which time biometry information became available for research. The only systemic practice modifications during the study period were the adoption of intracameral injection of antibiotic in 2013 and the increasing adoption of ISBCS. Otherwise, surgeons practiced according to the guidelines in their own department or according to their training and continuing education.
Study Population
The study included health plan members who underwent their first non-complex phacoemulsification for cataract (CPT codes 66984; ICD-9 codes 13.41, 13.71) during January 1, 2013 through June 30, 2015. As with past studies,3, 4 we excluded complex phacoemulsification cases and cases performed by glaucoma, oculoplastic or retinal specialists as well as procedures by any surgeon combined with corneal transplant (ICD-9 11.6, CPT4 codes 65710-65715) or glaucoma surgery (ICD-9 12.54, 12.64, 12.66, 12.69,-12.7, CPT4 codes 65850, 66170, 66172, 66180, 66185).
For the present analyses, we also excluded cases with previous endophthalmitis (ICD9 codes 360.00, 360.01, 360.03, 360.13, 360.19, 098.42) and we required information from manifest refractions for postoperative BCVA analysis.
The study focused on two cohorts of patients undergoing bilateral cataract surgery: ISBCS, with the right and left eyes performed back-to-back on the same day, and DSBCS, with the two eyes performed on separate days, the second eye within 1 year of the 1st. ISBCS surgeries were identified from a procedure code used by the health plan (bilateral surgery, code 1215493) and from a laterality variable recorded into structured operative data. Second surgeries that were performed >1 year after the 1st were not included because most did not represent planned bilateral surgeries. To characterize each patient’s history, we required at least 1 year of enrollment prior to cataract surgery on the 1st eye. We restricted the look-back to the 1 year before cataract surgery in the 1st eye to eliminate information bias that would have resulted had we given the DSBCS patients separate 1 year look-backs for each eye. A longer period of look-back in the DSBCS patients would have resulted in more diagnostic codes being written into the electronic medical record for mild visual complaints, including postoperative complaints recorded after the first surgery.
Data Collection
Visual Acuity and Refractive Error
Postoperative refractive error (RE) was calculated as the spherical equivalent (sphere + cylinder/2), measured in diopters (D), as recorded from manifest refractions performed by licensed optometrists. BCVA was obtained using Snellen charts projected by standardized equipment (Nikon, Tokyo) and was converted to logMAR equivalents. We did not include BCVA measurements from automated refractions, cycloplegic refractions, refractions obtained over contact lenses or glasses, retinoscopy, or “unaided acuity” because these represented <2% of the measurements and would have complicated the analysis.
We obtained preoperative BCVA from measurements recorded nearest the surgery date, up to 1 year before surgery. For postoperative BCVA and RE, we obtained the measurement recorded nearest the date of surgery during the interval 3 weeks to 1 year after surgery. The interval 3 weeks to 1 year was selected to optimize the completeness of postoperative data while providing time for vision to stabilize after surgery. We included refractions recorded as late as 1 year after surgery because patients with good postoperative visual acuity may not schedule an appointment for refraction for some time. We used the earliest measurement to minimize the late postoperative effects of ocular comorbidities and posterior capsular opacification.
Surgical Complications
We captured intraoperative posterior capsular rupture (PCR) and vitrectomy using natural language processing.3, 5 We identified cases with incident endophthalmitis recorded during the 120 days after the cataract surgery using ICD codes (Supplemental Material) that were then confirmed by a study ophthalmologist (NHS). We also captured postoperative macular edema cases during the 120 days after the first cataract surgery using ICD codes. In most patients, the second surgery was performed within 120 days of the first. We counted only one case of macular edema per patient because it was not possible based on coding alone to determine the laterality of the macular edema or whether the condition was unilateral or bilateral. To improve specificity,4 we required macular edema cases to have undergone ocular coherence tomography and to have filled a prescription for ophthalmic prednisolone during the 120 days after surgery.
Demographic Factors and Systemic Comorbidity
Patient age, sex, and race/ethnicity were obtained from self-reported membership information. Charlson comorbidities were calculated from diagnostic and procedure codes recorded during the year before surgery.
Ocular Comorbidity
Pre-existing ocular diseases were obtained from inpatient and outpatient data using the codes detailed in the Supplemental Material.
Medications
We obtained records for dispensed glaucoma medications including prostaglandin analogs, alpha agonists, and carbonic anhydrase inhibitors. Because exposure of oral alpha-1 agonists has been associated with floppy iris syndrome, we obtained medication records for up to 10 years before surgery.6
Biometry
Lenstar data were available for the final 12 months of the 30-month study period. For these patients, we obtained axial length (mm), anterior chamber depth (mm), and lens thickness (mm).
Data Analysis
We performed intention-to-treat analyses, in which patients scheduled for ISBCS who were converted to DSBCS were nonetheless retained in the ISBCS group.
Hypothesis (1) compared visual outcome between the 2nd and 1st eye of DSBCS patients to test whether the refractive error following implantation of the 2nd IOL was closer to emmetropia than the 1st. We examined the ISBCS cohort (left eye compared with right eye) as a negative control group. For this hypothesis, we tested whether there was a difference in the distributions of outcomes between DSBCS and ISBCS patients using a Chi-square test. For these analyses, it was not appropriate to adjust for surgeon- or patient-level factors because they did not vary within the patient.
Hypothesis (2) compared within-patient average visual outcome between the ISBCS and DSBCS cohorts. For this hypothesis, we dichotomized average postoperative BCVA as 20/20 or better versus worse than 20/20, because half of the patients achieved BCVA of 20/20 or better. Analysis of postoperative RE excluded patients with RE −2.1 or greater myopia because most were intended for near working distance. Emmetropia was defined as spherical error of −0.5 to 0 diopters (D), while eyes that were more myopic or hyperopic were defined as ametropic. We estimated the adjusted odds ratio (OR) and 95% confidence interval (CI) for the association of ISBCS versus DSBCS for each dichotomous outcome by fitting a conditional logistic regression model that stratified on surgeon. This approach assured adjustment for a variety of practice variations across surgeons, including choice of formula for IOL power calculations. To control for potential confounding by patient factors, the regression model also adjusted for patient-level variables including time to postoperative refraction.
In subgroup analyses, we examined biometric variables and patients without ocular comorbidities. We coded biometric variables into quartiles to obtain statistical efficiency. We also considered examining extreme values of axial length and anterior chamber depth, but the numbers of patients with extreme values were limited.
For surgical complications, we calculated 95% confidence intervals for the incidence rate using Fisher’s exact method. All analyses were performed in SAS 9.3.
RESULTS
Eligibility
Before exclusions, the number of ISBCS and DSBCS patients was 28,116. Among these, we excluded 2,521 (9%) who underwent one or both surgeries by a glaucoma, oculoplastics, or retinal specialist; 51 that had a concurrent cornea transplant or glaucoma surgery in one or both eyes, 26 with a prior diagnosis of endophthalmitis; and 903 (3%) that lacked ≥1 year enrollment preceding their surgery. After these exclusions, the study included 24,615 patients (5,247 ISBCS patients and 19,368 DSBCS patients). In this cohort, continuity of membership was 95% in the year following surgery. We estimated rates of postoperative endophthalmitis and macular edema in these 24,615 patients.
Among these 24,615 patients, 1,686 (32%) ISBCS patients and 5,657 (29%) DSBCS patients were missing information on postoperative BCVA for one or both eyes. Thus, the number available for the analysis of BCVA was 3,561 ISBCS patients (7,122 eyes) and 13,711 DSBCS patients (27,422 eyes). Among those with BCVA information, the number of ISBCS and DSBCS patients with bilateral information on RE was 3,396 (95%) and 13,423 (98%), respectively. Of these, 153 (5%) ISBCS and 706 (5%) DSBCS patients were RE −2.1 or more myopic and were excluded from the analysis of RE, although they were retained in the analysis of BCVA. Thus, the RE analysis included 3,243 ISBCS and 12,717 DSBCS patients. Among patients with information on postoperative BCVA, we obtained biometry data for subgroup analysis of 1,451 ISBCS (41%) and 2,596 DSBCS (19%) patients.
Characteristics of Patients
ISBCS and DSBCS patients with and without information on BCVA were nearly identical (Table 1), although ISBCS patients with short axial length were more likely to have BCVA recorded. ISBCS and DSBCS patients differed somewhat from each other. Compared with DSBCS patients, the ISBCS patients underwent their surgery during later years of the study, reflecting increasing adoption of ISBCS. They were less likely to receive a multifocal or toric lens. In addition, ISBCS patients had slightly lower prevalence of a pre-existing ocular comorbidity (Table 1). The groups were similar with respect to systemic comorbidities, use of alpha-1 agonists, ocular biometry, and prevalence of diabetic retinopathy.
Table 1.
Characteristics of ISBCS and DSBCS patients with and without postoperative BCVA, Kaiser Permanente Northern California, 2013-June 2015, %
| ISBCS | DSBCS | DSBCS vs ISBCS (with BCVA data) | |||
|---|---|---|---|---|---|
| With BCVA data N=3,561 patients |
Missing BCVA data N=1,686 patients |
With BCVA data N=13,711 patients |
Missing BCVA data N=5,657 patients |
p-value | |
| Duration of enrollment, years | |||||
| <5 | 6 | 6 | 6 | 7 | Ref |
| 5–10 | 8 | 9 | 8 | 8 | 0.16 |
| >10 | 86 | 85 | 86 | 85 | 0.51 |
| Year of first surgery | |||||
| 2013 | 16 | 19 | 46 | 47 | Ref |
| 2014 | 48 | 48 | 40 | 40 | <0.0001 |
| 2015 | 36 | 33 | 13 | 13 | <0.0001 |
| Sex | |||||
| Female | 61 | 62 | 62 | 61 | Ref |
| Male | 39 | 38 | 38 | 39 | 0.48 |
| Age, years | |||||
| ≤74 | 54 | 55 | 51 | 53 | Ref |
| 75–79 | 22 | 21 | 23 | 22 | 0.76 |
| 80–84 | 15 | 15 | 17 | 16 | 0.09 |
| ≥85 | 9 | 8 | 9 | 9 | 0.99 |
| Race/ethnicity | |||||
| African-American | 5 | 5 | 5 | 5 | 0.43 |
| Asian-American | 13 | 12 | 15 | 15 | <0.01 |
| Latino/Hispanic | 12 | 12 | 10 | 10 | 0.02 |
| White | 64 | 65 | 63 | 63 | Ref |
| Multi-race | 5 | 5 | 6 | 5 | 0.24 |
| Other | 1 | 1 | 1 | 1 | 0.81 |
| Diabetes | 28 | 28 | 27 | 27 | 0.17 |
| Charlson-Deyo comorbidity index | |||||
| 0 | 41 | 41 | 41 | 41 | Ref |
| 1 | 18 | 19 | 19 | 19 | 0.31 |
| ≥2 | 41 | 40 | 40 | 40 | 0.26 |
| Preoperative medications | |||||
| Tamsulosin | 9 | 8 | 8 | 8 | 0.38 |
| Other alpha-1 agonist | 8 | 8 | 9 | 9 | 0.06 |
| Warfarin | 2 | 2 | 2 | 2 | 0.32 |
| Lens type | |||||
| Monofocal | 94.6 | 94.1 | 92.7 | 90.6 | Ref |
| Multifocal, one or both eyes | 0.7 | 0.8 | 1.5 | 2.5 | <0.001 |
| Toric, one or both eyes | 4.7 | 5.1 | 5.8 | 6.9 | 0.02 |
| Axial length* | |||||
| ≤22.9 | 25 | 18 | 25 | 23 | Ref |
| 23.0–23.9 | 37 | 40 | 37 | 37 | 0.74 |
| 24.0–24.9 | 21 | 28 | 21 | 21 | 0.90 |
| ≥25.0 | 18 | 14 | 18 | 19 | 0.86 |
| Cataract thickness* | |||||
| ≤4.3 | 33 | 30 | 31 | 35 | Ref |
| 4.4–4.6 | 27 | 28 | 26 | 23 | 0.21 |
| 4.7–4.8 | 16 | 15 | 18 | 17 | 0.20 |
| ≥4.9 | 23 | 27 | 26 | 25 | 0.18 |
| Anterior chamber depth* | |||||
| ≤2.8 | 26 | 29 | 28 | 25 | Ref |
| 2.9–3.1 | 29 | 26 | 29 | 32 | 0.72 |
| 3.2–3.4 | 26 | 30 | 25 | 24 | 0.75 |
| ≥3.5 | 19 | 15 | 18 | 19 | 0.29 |
| Preoperative ocular conditions | |||||
| Vitrectomy | 0.3 | 0.02 | 0.4 | 0 | 0.29 |
| Other retinovitreal procedures | 0.1 | 0.2 | 0.4 | 0.5 | 0.02 |
| Other ophthalmic procedures | 0.7 | 0.6 | 0.9 | 0.6 | 0.36 |
| Glaucoma w/o medication | 13 | 13 | 16 | 15 | <0.001 |
| Glaucoma w/medication | 3 | 3 | 4 | 3 | <0.01 |
| Pseudoexfoliation glaucoma | 0.1 | 0.1 | 0.3 | 0.3 | 0.06 |
| Diabetic retinopathy, NOS | 2 | 2 | 3 | 3 | 0.02 |
| Proliferative diabetic retinopathy | 1 | 1 | 1 | 1 | 0.28 |
| Nonproliferative diabetic retinopathy | 5 | 5 | 5 | 5 | 0.44 |
| Macular edema with diabetes | 0.7 | 0.7 | 1 | 1.3 | 0.03 |
| Macular edema without diabetes | 0.1 | 0.2 | 0.3 | 0.3 | 0.08 |
| Age-related macular degeneration | 9 | 9 | 11 | 11 | <0.001 |
| Posterior vitreous detachment | 4 | 4 | 5 | 5 | 0.002 |
| Epiretinal membrane | 3 | 3 | 5 | 5 | <0.001 |
| Retinal vein occlusion | 0.8 | 0.9 | 1.2 | 1.2 | 0.08 |
| Iritis or uveitis | 0.2 | 0.2 | 0.5 | 0.6 | 0.05 |
| Fuchs | 0.6 | 0.5 | 1.3 | 1.2 | 0.001 |
| Other corneal disorder | 2 | 2 | 3 | 3 | <0.01 |
| Blepharitis | 3 | 4 | 5 | 5 | <0.001 |
| Dry eye syndrome | 8 | 8 | 10 | 10 | 0.08 |
Biometry data was obtained from Lenstar and were available during the last 12 months of the study, which included 1,664 ISBCS patients and 2,347 DSBCS patients. BCVA was obtained from manifest refraction using Snellen charts projected by standardized equipment (Nikon, Tokyo). Postoperative BCVA was obtained from the earliest measurement recorded nearest the date of surgery during the interval 3 weeks to 1 year after surgery. Preoperative medications were obtained from automated pharmacy data. Preoperative medical conditions were obtained from diagnosis codes recorded into the electronic medical record.
Characteristics of ISBCS and DSBCS patients with and without postoperative BCVA, Kaiser Permanente Northern California, 2013-June 2015, %
Abbreviations: ISBCS immediate sequential bilateral cataract surgery; DSBCS delayed sequential bilateral cataract surgery; BCVA best-corrected visual acuity
Among DSBCS patients, the two surgeries were separated by a median of 37 days (quartiles: 23 and 66 days). The average time to refraction was 51 days for ISBCS, 65 days for the first DSBCS eye, and 48 days for the 2nd DSBCS eye. Preoperative BCVA averaged 20/53 (0.42 logMAR) among ISBCS patients and 20/55 (0.44 logMAR) among DSBCS patients. For DSBCS patients, and consistent with the practice of performing surgery on the worse-seeing eye first, preoperative BCVA averaged 20/63 (0.50 logMAR) in the 1st eye and 20/48 in the 2nd eye (0.38 logMAR).
Hypothesis 1: Among DSBCS patients, outcomes in the 2nd eye were no different than in the 1st eye
Postoperative BCVA was 20/20 or better in 48% of DSBCS 1st eye, 49% of DSBCS 2nd eyes, 53% of ISBCS right eyes, and 51% of ISBCS left eyes (Figure 1a). Within-patient, the difference in postoperative BCVA (logMAR) between the 2nd and 1st eyes was zero (0.00) for 60% of DSBCS patients (Figure 1b). The difference between the left and right eyes was zero (0.00) for 68% of ISBCS patients. Among DSBCS patients, BCVA in the 2nd eye was better than the 1st for 20% and worse than the 1st for 19% of patients. Among ISBCS patients, the left eye had better acuity than the right for 15% and worse than the right for 17% of patients. These distributions differed between DSBCS and ISBCS patients in this large study (p<0.001). Because of the large sample size, some statistical differences lacked clinical significance.
Figure 1. Postoperative BCVA in 13,711 DSBCS patients and 3,561 ISBCS patients, Kaiser Permanente Northern California, 2013-June 2015.*.
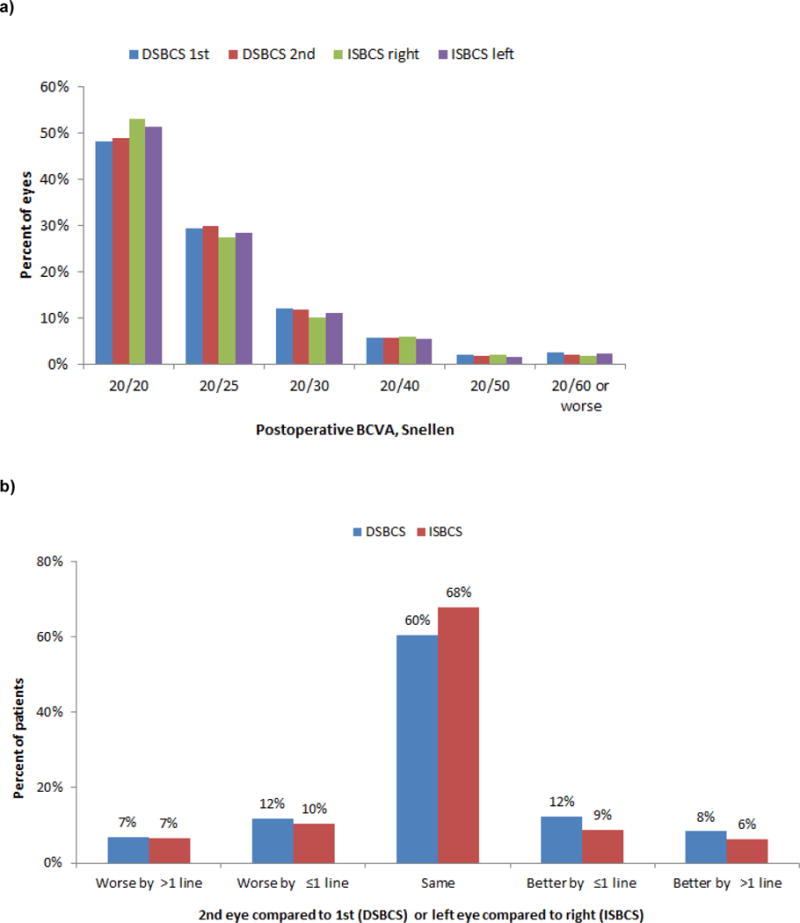
a) BCVA in each eye
b) Within-patient difference in BCVA (logMAR) between the two eyes**
*For DSBCS surgery, we report the first eye followed by the second eye. For ISBCS surgery, we report the right eye followed by the left eye, although the order of surgery was not obtained for the study.
**Chi-square, p<0.001.
Within-patient difference in BCVA (logMAR) between the two eyes
Abbreviations: ISBCS same-day bilateral cataract surgery; DSBCS different-day bilateral cataract surgery; BCVA best-corrected visual acuity
Refractive error averaged −0.38 D in the DSBCS 1st eye, −0.40 D in the DSBCS 2nd eye, −0.36 D in the ISBCS right eye, and −0.35 in the ISBCS left eye (Figure 2a). Emmetropia was achieved in 61% of DSBCS 1st and 61% of DSBCS 2nd eyes, and in 63% of ISBCS right and 63% of ISBCS left eyes. Within-person differences in emmetropia and ametropia between the 2nd and 1st eyes in DSBCS patients and between the left and right eyes in ISBCS patients are shown in Figure 2b. These distributions among DSBCS and ISBCS patients differed in this large study (p<0.001). Subgroup analysis focused on patients who received toric lenses in both eyes (ISBCS, N=170; DSBCS, N=415). Among these patients, the distributions of refractive results were similar in the two groups (p=0.31). In patients who received all types of lenses, postoperative anisometropia >2D occurred in 1.4% (N=44) of ISBCS and 1.7% (N=212) of DSBCS patients (p=0.21); in patients who received toric lenses in both eyes, anisometropia >2D occurred in 4.1% (N=7) of ISBCS and 2.4% (N=10) of DSBCS patients (N=0.26).
Figure 2. Postoperative refractive error in 12,669 DSBCS patients and 3,227 ISBCS patients, Kaiser Permanente Northern California, 2013-June 2015.*.
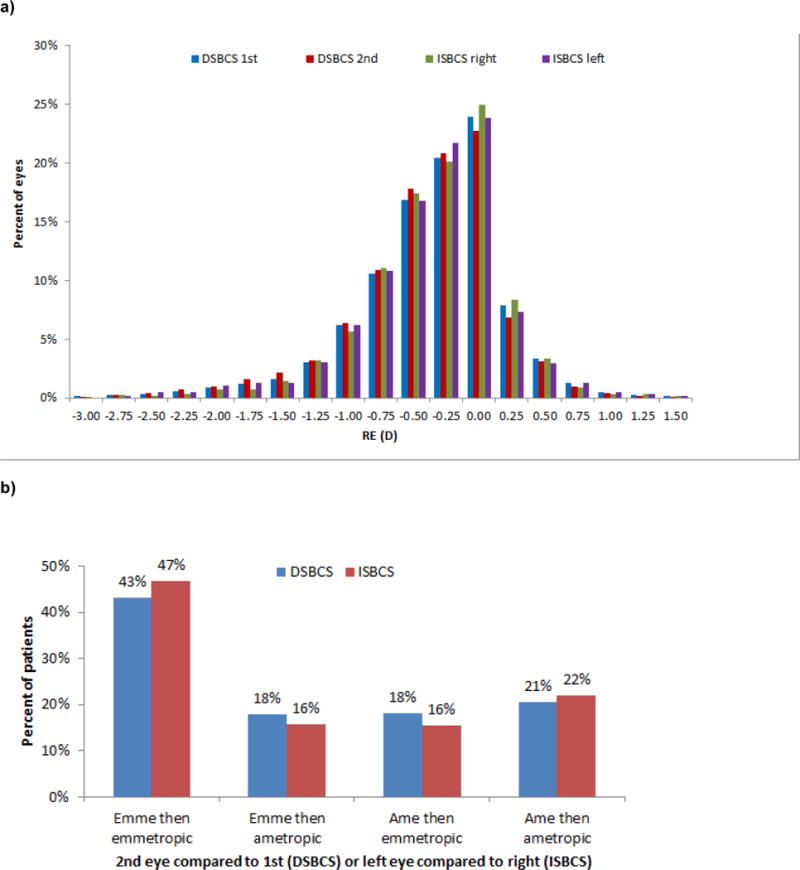
a) RE in each eye
b) Within-patient difference in RE (D) between the two eyes**
*For DSBCS surgery, we report the first eye then the second eye. For ISBCS surgery, we report the right eye then the left eye, although the actual order of surgery was not obtained for the study. Emmetropia was defined as −0.5 to 0 D.
**Chi-square, p<0.001.
Postoperative refractive error in 12,717 DSBCS patients and 3,243 ISBCS patients
Abbreviations: ISBCS, immediate sequential bilateral cataract surgery; DSBCS, delayed sequential bilateral cataract surgery; emme, emmetropia; ame ametropia.
Hypothesis 2: Averaged between the two eyes, outcomes do not differ between DSBCS and ISBCS patients
Average postoperative BCVA (average of within-patient averages) was 20/20 or better in 49% of DSBCS patients and 52% of ISBCS. Average postoperative RE was −0.39 D in DSBCS and −0.36 D in ISBCS patients. Emmetropia was achieved in 61% of DSBCS eyes and 62% of ISBCS eyes. Logistic regression analyses for Hypothesis 2 are shown in Table 2. Model 1 examines BCVA and is shown in the center of the table. Model 2 examines RE and is shown on the right side of the table. The odds of having average postoperative BCVA worse than 20/20 (compared with 20/20 or better) was higher in older patients, women, African-Americans, and in patients with specific systemic and ocular comorbidities. Comparing outcomes from the ISBCS to the DSBCS group, we found no evidence for differences in postoperative BCVA worse than 20/20 (odds ratio [OR], 0.91; 95% confidence interval [CI] 0.83–1.01) or with ametropia (OR, 1.02; CI 0.92–1.12).
Table 2.
Hypothesis 2: Adjusted* odds ratio (95% confidence interval) for the association of ISBCS vs DSBCS surgery with postoperative visual outcomes (within-patient average between the two eyes), Kaiser Permanente Northern California, 2013-June 2015.
| MODEL 1 BCVA worse than 20/20 vs 20/20 or better (Includes 3,561 ISBCS and 13,711 DSBCS patients) |
MODEL 2 RE ametropia vs emmetropia (Includes 3,243 ISBCS and 12,717 DSBCS patients) |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Surgery | ||||||
| ISBCS | 0.91 | 0.83–1.01 | 0.07 | 1.02 | 0.92–1.12 | 0.75 |
| DSBCS | 1.00 | Ref. | 1.00 | Ref. | ||
| Year of surgery | ||||||
| 2013 | 1.00 | Ref. | 1.00 | Ref. | ||
| 2014 | 1.03 | 0.96–1.11 | 0.77 | 1.02 | 0.95–1.10 | 0.90 |
| 2015 | 1.08 | 0.98–1.20 | 0.16 | 1.05 | 0.96–1.16 | 0.40 |
| Patient age, years | ||||||
| ≤74 | 1.00 | Ref. | 1.00 | Ref. | ||
| 75–79 | 1.91 | 1.76–2.07 | <0.0001 | 0.93 | 0.86–1.01 | <0.001 |
| 80–84 | 2.80 | 2.54–3.09 | <0.0001 | 1.04 | 0.94–1.14 | 0.83 |
| 85+ | 5.18 | 4.46–6.00 | <0.0001 | 1.22 | 1.09–1.38 | <0.001 |
| Patient sex | ||||||
| M | 1.00 | Ref. | 1.00 | Ref. | ||
| F | 1.12 | 1.04–1.20 | 0.003 | 1.07 | 1.00–1.15 | 0.05 |
| Patient race/ethnicity | ||||||
| White | 1.00 | Ref. | 1.00 | Ref. | ||
| African-American | 1.30 | 1.11–1.53 | 0.91 | 1.20 | 1.02–1.40 | 0.02 |
| Asian-American | 1.82 | 1.64–2.02 | <0.0001 | 1.06 | 0.96–1.17 | 0.38 |
| Hispanic | 1.23 | 1.10–1.38 | 0.21 | 0.98 | 0.88–1.09 | 0.34 |
| Other | 1.32 | 1.15–1.51 | 0.92 | 0.90 | 0.79–1.02 | 0.01 |
| Charlson comorbidity | ||||||
| 0 | 1.00 | Ref. | 1.00 | Ref. | ||
| 1 | 1.08 | 0.98–1.18 | 0.24 | 0.93 | 0.85–1.02 | 0.09 |
| ≥2 | 1.28 | 1.17–1.40 | <0.0001 | 1.00 | 0.92–1.09 | 0.34 |
| Clinical history (yes/no) | ||||||
| Diabetes | 1.08 | 0.98–1.18 | 0.12 | 0.92 | 0.84–1.00 | 0.05 |
| Flomax | 1.03 | 0.91–1.17 | 0.65 | 0.93 | 0.83–1.06 | 0.27 |
| Warfarin | 1.13 | 0.88–1.44 | 0.34 | 0.93 | 0.74–1.16 | 0.53 |
| AMD | 2.15 | 1.90–2.43 | <0.0001 | 1.01 | 0.91–1.13 | 0.80 |
| Corneal disease | 2.71 | 2.16–3.40 | <0.0001 | 1.37 | 1.13–1.66 | 0.001 |
| DMR | 1.80 | 1.55–2.01 | <0.0001 | 1.11 | 1.00–1.28 | 0.13 |
| ERM | 1.95 | 1.64–2.32 | <0.0001 | 1.01 | 0.87–1.18 | 0.88 |
| Glaucoma | 1.22 | 1.12–1.33 | <0.0001 | 1.03 | 0.96–1.12 | 0.45 |
| Retinovitreal procedure | 3.88 | 1.78–8.46 | 0.0007 | 1.04 | 0.60–1.81 | 0.90 |
| PCR | 1.60 | 1.20–2.13 | 0.001 | 1.26 | 1.00–1.63 | 0.09 |
| Average time to refraction | ||||||
| 3–4 weeks | 1.00 | Ref. | 1.00 | Ref. | ||
| 5–7 weeks | 0.91 | 0.83–1.01 | 0.16 | 1.03 | 0.93–1.14 | 0.80 |
| 8+ weeks | 0.92 | 0.82–1.03 | 0.40 | 1.08 | 0.96–1.21 | 0.18 |
The logistic regression analyses were stratified on the identity of the surgeon and included every variable shown in the table, as coded in the table. RE and BCVA were obtained from manifest refraction using Snellen charts projected by standardized equipment (Nikon, Tokyo). Analysis of postoperative RE excluded patients with RE −2.1 or greater myopia. Emmetropia was defined as spherical error of −0.5 to 0 diopters (D), while eyes that were more myopic or hyperopic were defined as ametropic. Postoperative BCVA was obtained from the earliest measurement recorded nearest the date of surgery during the interval 3 weeks to 1 year after surgery. Tamsulosin dispensings were obtained from automated pharmacy data recorded up to 10 years before the first cataract surgery date. Preoperative medical conditions were obtained from diagnosis codes recorded into the electronic medical record.
Hypothesis 2: Adjusted* odds ratio (95% confidence interval) for the association of ISBCS vs DSBCS surgery with postoperative visual outcomes (within-patient average between the two eyes), Kaiser Permanente Northern California, 2013-June 2015.
Abbreviations: ISBCS Same-day bilateral cataract surgery; DSBCS different-day bilateral cataract surgery; BCVA best-corrected visual acuity; RE refractive error; IOL intraocular lens; D diopters; PCR post capsular rupture; AMD age-related macular degeneration; DMR diabetic macular retinopathy; ERM epiretinal membrane
Lenstar data were available for 1,451 ISBCS patients and 2,596 DSBCS patients. In this subgroup of patients, before adjustment for biometry, the OR for the association of ISBCS versus DSBCS for postoperative BCVA worse than 20/20 was 0.99 (CI 0.82–1.18) and for ametropia was 1.14 (CI 0.95–1.37). After adjustment for biometry (axial length, cataract thickness, anterior chamber depth) the ORs changed only negligibly for both BCVA worse than 20/20 (OR, 1.00; CI 0.84–1.21) and for ametropia (OR, 1.15; CI 0.96–1.38) (see Supplemental Material).
Subgroup Analysis Restricted to Patients Without an Ocular Comorbidity
In subgroup analysis restricted to patients without an ocular comorbidity (2,412 ISBCS and 8,343 DSBCS patients with postoperative BCVA), the OR for the association of ISBCS with BCVA worse than 20/20 was 0.95 (CI: 0.84–1.07). The OR for the association of ISBCS with ametropia was 0.98 (CI: 0.86–1.10) (see Supplemental Material).
Surgical Complications
Twenty-five cases (0.7%) were converted from ISBCS to DSBCS because surgery in the first eye did not proceed as planned and the second eye surgery was aborted that day. Review of the operative report showed that 6 were cases with PCR/vitrectomy; 4 with patient agitation or blood pressure elevation; 3 with a delay in surgery, e.g., because of an equipment problem; 3 iris-related problems; 2 in which the IOL had to be removed; 2 concerns about intraoperative corneal edema; 2 zonular dehiscence; 1 “floppy capsular bag”; 1 anterior capsular tear; and 1 case with intraoperative bleeding. We found 6 other cases (0.2%) of ISBCS in which the first eye had a small posterior capsular rent or underwent a planned vitrectomy, and the surgeon nonetheless completed the second eye as a ISBCS.
The incidence rates of PCR and vitrectomy did not differ between ISBCS and DSBCS, or between the DSBCS 1st and 2nd eyes (Table 3). In 10,494 ISBCS eyes (5,247 patients), we confirmed 1 postoperative endophthalmitis case (rate, 1 per 10,000 eyes), while in 38,736 DSBCS eyes (19,368 patients) we confirmed 2 endophthalmitis cases (rate, 0.5 per 10,000 eyes; p=0.32). No patient had bilateral endophthalmitis (upper 95% confidence interval, 17 bilateral cases per 10,000 patients). In the ISBCS cohort, we observed 29 patients with macular edema (rate, 0.55%), while in the DSBCS cohort, we observed 165 macular edema patients (rate, 0.85%, p=0.03).
Table 3.
Incidence of intraoperative complications, Kaiser Permanente Northern California, 2013–June 2015, %
| Complication | ISBCS and DSBCS: average between the two eyes
|
DSBCS: 1st eye vs 2nd eye
|
||||
|---|---|---|---|---|---|---|
| ISBCS N=5,247 |
DSBCS N=19,368 |
P value | 1st eye N=19,368 | 2nd eye N=19,368 | P value | |
| Posterior capsular rupture | 0.84 | 0.67 | 0.23 | 0.66 | 0.67 | 0.94 |
| Vitrectomy | 0.42 | 0.45 | 0.82 | 0.48 | 0.42 | 0.42 |
|
| ||||||
| Either of the above | 0.93 | 0.88 | 0.79 | 0.91 | 0.85 | 0.61 |
Posterior capsular rupture and vitrectomy were obtained from natural language processing of operative reports.
Incidence of intraoperative complications, %
Abbreviations: ISBCS same-day bilateral cataract surgery; DSBCS different-day bilateral cataract surgery
Unintended IOL implantation occurred in 1 ISBCS eye and in 3 DSBCS eyes. In the ISBCS patient, a 22.0D IOL intended for the 2nd eye was implanted into the first eye in which a 22.5D (same model) lens was intended.
DISCUSSION
Kaiser Permanente surgeons began offering ISBCS surgery in 2010. To assess whether outcomes obtained from ISBCS surgery were similar to those from DSBCS surgery, we compared postoperative visual outcomes and the risk of complications between the two procedures.
Effectiveness of ISBCS Surgery
Averaging between the two eyes, we found no evidence for an important or convincing difference in BCVA or RE between ISBCS and DSBCS patients after accounting for surgeon differences and patient baseline characteristics, most importantly, ocular comorbidities. Some have argued that one potential disadvantage of ISBCS surgery is the loss of the opportunity to adjust the IOL power of the 2nd eye when the postoperative refraction from the 1st eye differs from the intended target.7 This is based on studies showing improved target accuracy when adjusting the 2nd IOL power by half of the difference between the expected and actual refraction of the 1st eye.2, 8 Recent formulae improve estimates of effective lens position by incorporating parameters such as anterior chamber depth and lens thickness that increase accuracy and reduce the need for adjustments to the target for the 2nd eye.1 The clinically relevant benefit of adjustments are felt to be less significant with the advent of newer generation, more accurate formulae. Furthermore, contemporary IOLs are available in minimum 0.5D increments, making smaller adjustments in power infeasible.9 We compared RE between the 1st and 2nd DSBCS eyes, and found no evidence for a difference.
Nearly one-third of patients in our study did not have a BCVA or RE measurement during the period 21 days to one year after their surgery, and this is a potential study limitation. To assess the potential magnitude of this limitation, we compared patients with and without these data, finding their baseline characteristics to be nearly identical (Table 1). Based on this comparison of patients with complete and missing data, we believe the missing data were missing at random and did not bias the study.
The surgeon’s intended refractive targets is recorded onto the consent form, which is accessible at the clinician-facing front-end of the electronic medical record, but not the back-end where the research team extracts data using batch processing. For analysis of postoperative refractive error, we were unable to efficiently and reliably ascertain the surgeons’ intended refractive targets due to this limitation. We therefore uniformly assigned target refraction was between 0 to −0.5D and we excluded patients with a postoperative RE < −2.1D, which are more likely to have been targeted for near or arms-length working distances. Nor could we exclude intentional mini-monovision in which the second eye was targeted from −0.75 to −1.50. While systematic differences between the ISBCS and DSBCS groups in the proportion of eyes targeted for working distances other than emmetropia may exist, Figure 2a gives no evidence for this. Moreover, we could not reliably ascertain whether eyes had received a corneal refractive procedure before cataract surgery.
Comparative effectiveness studies are subject to confounding by indication. This would occur, for example, if patients selected for ISBCS vs DSBCS had fewer ocular comorbidities and we could not account for this difference using statistical adjustment, either because we did not have the information, or because we lacked adequate sample size to perform the adjustment. The results in Table 1 indicate that, indeed, patients selected for ISBCS were slightly less likely to have an ocular comorbidity recorded in the year before their surgery. This is consistent with some surgeon practices to exclude from ISBCS surgery those patients with ocular comorbidities potentially predisposing to complications or adverse events. Fortunately, because the sample size was large and detailed information was available from the electronic medical record, we could carefully adjust for the occurrence of these diagnoses. We then extended the data analysis to assess whether differences in the severity of ocular comorbidities were important. In this subgroup analysis, we restricted the cohort to patients without any baseline ocular comorbidity. The results of this subgroup analysis were very similar to the main results (BCVA: entire population, OR 0.91; subgroup without ocular comorbidity, OR 0.95) and argue in favor of study validity.
In 2015, Malvankar-Mehta and colleagues10 published a systematic review contrasting postoperative BCVA following ISBCS and DSBCS surgery. The review, which included 10 randomized and observational studies of 3,657 subjects, reported that improvement in BCVA after ISBCS and DSBCS surgeries was similar.10 The results of our study are consistent with this conclusion. The populations studied in the reports reviewed by Malvankar-Mehta and colleagues differed somewhat from one another and from our own cohort. However, two of the reviewed studies appeared to be based in populations that were similar to ours with respect to the severity of cataract, as indicated by preoperative BCVA. Average preoperative BCVA in our study population (mean, 0.42 logMAR) was similar to a 2011 Finnish study population (mean, 0.42 logMAR)11 and a 2009 multicenter study population located in New Zealand, Australia, and Japan (mean, 0.48 logMAR).12 We note that average postoperative BCVA was somewhat better in our cohort than these two past studies (0.08 logMAR compared with 0.13 and 0.20 logMAR, respectively), most likely because of recent advances in surgical techniques.
Our study is also consistent with others1, 2 in refuting the assertion that DSBCS surgery leads to improved outcomes in the 2nd eye. If surgeons did indeed make adjustments to the refractive target for the 2nd eye based on the results from the 1st eye, these adjustments did not translate into improved outcomes for the 2nd eye. One reason for this may be the excellent outcomes that are achieved with contemporary biometry equipment and later-generation formulae for the IOL power calculation.
Key strengths of our findings for the effectiveness of ISBCS surgery include the diversity of the population; the size of population, which enabled detailed adjustment and subgroup analysis; and the ability to account for differences in outcomes across surgeons. Our population was community-based and generalizable. The study was innovative in reporting not only postoperative BCVA but also RE, including anisometropia.
Safety of ISBCS Surgery
We found no evidence for differences in the rates of PCR or vitrectomy between ISBCS and DSBCS surgeries. This is consistent with past reports.11–13 Many surgeons who perform ISBCS abort surgery for the 2nd eye when PCR occurs in the 1st to give the eye and the patient time to heal. For this reason, we recommend obtaining the patient’s agreement on which eye is to be operated on first (generally the worse seeing eye) and recording the agreement onto the consent form that is signed by the patient. In addition, to prevent unintended IOL errors, each eye’s IOL type (multifocal, toric, monofocal) and working distance should be clearly designated on the consent form. We also recommend that the consent form, indicating IOL type and working distance, and biometry printout be present in the operating room for only one eye at a time.
The rate of endophthalmitis in our study was very low (1 in 10,000 patients) and did not differ between ISBCS and DSBCS cohorts. We observed no case of bilateral endophthalmitis following ISBCS surgery among 5,247 patients (upper 95% confidence interval, 17 per 10,000 patients). An editorial by Li and colleagues14 describes four cases of bilateral endophthalmitis following ISBCS surgery, all stemming from inadequate sterilization and lack of separating instrument trays between the two eyes. The use of intracameral antibiotic together with clinical workflow changes to reduce infection and toxic anterior segment syndrome, such as performing inspections of the surgical venue and cleaning and sterilization processes, are important aspects of risk reduction.15 Practice recommendations for ISBCS16 merit further consideration given the publication of new studies and evidence.15
DSBCS patients had about twice the rate of ophthalmology and optometry utilization in the weeks following their surgery compared to ISBCS patients. The higher rate of macular edema in DSBCS versus ISBCS patients (0.85% vs 0.55%, p=0.03) may have resulted from the DSBCS patients’ greater opportunity, consequent to their higher utilization, to receive a diagnosis in response to reporting a minor postoperative visual complaint. Because they are rare, serious complications are difficult to study. Differences in complication rates will remain a salient research topic until larger sample sizes can be obtained in settings such as ours and in meta-analyses.17
Implications
ISBCS surgery has been shown to save health care resources in other parts of the world18, 19 and to halve the cost of bilateral cataract surgery.20 Lower costs could increase access to care. Despite the potential economic benefit to payers, patients, and society,21, 22 the current Medicare reimbursement model is a barrier to widespread adoption of ISBCS surgery in the U.S.23 due to a reduction in total reimbursement compared to DSBCS.24 Future research to investigate the impact on the patient’s experience and access to cataract surgery may demonstrate that, combined with high-quality clinical processes, ISBCS surgery is a valuable modality to improving the effectiveness, safety, and experience of care.
Supplementary Material
Acknowledgments
This research was supported by a grant from the Kaiser Permanente Garfield Fund. Drs. Herrinton, Liu, and Shorstein were also funded by grants from the Kaiser Permanente Community Benefit program and from the National Eye Institute (R21EY022989).
Financial Support: This project was funded by the Garfield Memorial Fund, Kaiser Permanente and the National Eye Institute R01 EY027329. The project also used products developed under earlier research grants provided by NEI R21 EY022989 and Kaiser Permanente’s Community Benefit program. These sponsors had no role in the design or conduct of this research.
Abbreviations and acronyms
- ISBCS
Immediate sequential bilateral cataract surgery
- DSBCS
delayed sequential bilateral cataract surgery
- BCVA
best-corrected visual acuity
- RE
refractive error
- IOL
intraocular lens
- D
diopters
- PCR
post capsular rupture
- AMD
age-related macular degeneration
- DMR
diabetic macular retinopathy
- ERM
epiretinal membrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Kaiser Permanente potentially derives a benefit from improvements in operating room and clinic efficiency and implementation of immediate sequential bilateral cataract surgery, and this represents a potential conflict of interest.
This article contains additional online–only material. The following should appear online-only: Material labeled “Supplemental Material.”
LITERATURE CITED
- 1.Olsen T. Use of fellow eye data in the calculation of intraocular lens power for the second eye. Ophthalmol. 2011;118:1710–15. doi: 10.1016/j.ophtha.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 2.Jivrajka RV, Shammas MC, Shammas HJ. Improving the second-eye refractive error in patients undergoing bilateral sequential cataract surgery. Ophthalmol. 2012;119:1097–1101. doi: 10.1016/j.ophtha.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmol. 2016;123:287–94. doi: 10.1016/j.ophtha.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shorstein NH, Liu L, Waxman MD, Herrinton LJ. Comparative effectiveness of three prophylactic strategies to prevent clinical macular edema after phacoemulsification surgery. Ophthalmol. 2015;122:2450–6. doi: 10.1016/j.ophtha.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Shorstein NH, Amsden LB, Herrinton LJ. Natural language processing to ascertain two key variables from operative reports in ophthalmology. Pharmacoepidemiol Drug Saf. 2017 doi: 10.1002/pds.4149. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang DF, Braga-Mele R, Mamalis N, et al. ASCRS Cataract Clinical Committee ASCRS White Paper: clinical review of intraoperative floppy-iris syndrome. J Cataract Refractive Surg. 2008;34:2153–62. doi: 10.1016/j.jcrs.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Henderson BA, Schneider J. Same-day cataract surgery should not be the standard of care for patients with bilateral visually significant cataract. Surv Ophthalmol. 2012;57:580–3. doi: 10.1016/j.survophthal.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Covert DJ, Henry CR, Koenig SB. Intraocular lens power selection in the second eye of patients undergoing bilateral, sequential cataract extraction. Ophthalmol. 2010;117:49–54. doi: 10.1016/j.ophtha.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Arshinoff SA. IOL power prediction. Ophthalmol. 2012;119:2646. doi: 10.1016/j.ophtha.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Malvankar-Mehta MS, Chen YN, Patel S, et al. Immediate versus delayed sequential bilateral cataract surgery: A systematic review and meta-analysis. PLoS One. 2015;29(10):e0131857. doi: 10.1371/journal.pone.0131857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarikkola AU, Uusitalo RJ, Hellstedt T, et al. Simultaneous bilateral versus sequential bilateral cataract surgery: Helsinki Simultaneous Bilateral Cataract Surgery Study Report 1. J Cataract Refract Surg. 2011;37:992–1002. doi: 10.1016/j.jcrs.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Chung J, Park SH, Lee WJ, Lee SJ. Bilateral cataract surgery: a controlled clinical trial. Japanese J Ophthalmol. 2009;53:107–113. doi: 10.1007/s10384-008-0627-6. [DOI] [PubMed] [Google Scholar]
- 13.Serrano-Aguilar P, Ramallo-Farina Y, Cabrera-Hernandez JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refractive Surg. 2012;38:1734–1742. doi: 10.1016/j.jcrs.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Li O, Kapetanakis V, Claoue C. Simultaneous bilateral endophthalmitis after immediate sequential bilateral cataract surgery: what’s the risk of functional blindness? Am J Ophthalmol. 2014;157:749–751. doi: 10.1016/j.ajo.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Shorstein NH, Lucido C, Carolan J, Liu L, Slean G, Herrinton LJ. Failure modes and effects analysis for bilateral same-day cataract surgery. J Cataract Refractive Surg. doi: 10.1016/j.jcrs.2016.12.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arshinoff S, Claoue C, Johansson B. iSBCS General Principles for Excellence in ISBCS 2009. 2009 http://www.isbcs.org/wp-content/uploads/2011/03/2010-07-20-FINAL-ISBCS-SBCS-suggestions-from-ESCRS-Barcelona.pdf. Accessed Dec 6, 2016.
- 17.Kessel L, Andresen J, Erngaard D, et al. Immediate sequential bilateral cataract surgery: A systematic review and meta-analysis. J Ophthalmol. 2015;2015:912481. doi: 10.1155/2015/912481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lansingh VC, Eckert KA, Strauss G. Benefits and risks of immediately sequential bilateral cataract surgery: a literature review. Clin Exp Ophthalmol. 2015;43:666–72. doi: 10.1111/ceo.12527. [DOI] [PubMed] [Google Scholar]
- 19.Arshinoff SA, Chen SH. Simultaneous bilateral cataract surgery: Financial differences among nations and jurisdictions. J Cataract Refract Surg. 2006;32:1355–1360. doi: 10.1016/j.jcrs.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 20.Malvankar-Mehta MS, Filek R, Iqbal M, et al. Immediately sequential bilateral cataract surgery: a cost-effective procedure. Can J Ophthalmol. 2013;48:482–8. doi: 10.1016/j.jcjo.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Rush SW, Gerald AE, Smith JC, et al. Prospective analysis of outcomes and economic factors of same-day bilateral cataract surgery in the United States. J Cataract Refract Surg. 2015;41:732–739. doi: 10.1016/j.jcrs.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 22.Neel ST. A cost-minimization analysis comparing immediate sequential cataract surgery and delayed sequential cataract surgery from the payer, patient, and societal perspectives in the United States. JAMA Ophthalmol. 2014;132:1282–1288. doi: 10.1001/jamaophthalmol.2014.2074. [DOI] [PubMed] [Google Scholar]
- 23.Singh R, Dohlman TH, Sun G. Immediately sequential bilateral cataract surgery: advantages and disadvantages. Curr Opin Ophthalmol. 2017;28:81–86. doi: 10.1097/ICU.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 24.Neel ST. A cost and policy analysis comparing immediate sequential cataract surgery and delayed sequential cataract surgery from the physician perspective in the United States. JAMA Ophthalmol. 2014;132:1359–1362. doi: 10.1001/jamaophthalmol.2014.3335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


